Abstract
The amygdala is vulnerable to stress-dependent disruptions in neural development. Animal models have shown that stress increases dendritic arborization leading to larger amygdala volumes. Human studies of early stress and amygdala volume, however, remain inconclusive. This study compared amygdala volume in adults with childhood maltreatment to healthy controls. Eighteen participants from a longitudinal cohort and 33 cross-sectional controls (17M/35F, 25.4 ±3.1 years) completed a structural magnetic resonance imagining scan and the Maltreatment and Abuse Chronology of Exposure scale. Random forest regression with conditional trees was used to assess relative importance of exposure to adversity at each age on amygdala, thalamic or caudate volume. Severity of exposure to adversity across age accounted for 27% of the variance in right amygdala volume. Peak sensitivity occurred at 10–11 years of age, and importance of exposure at this time was highly significant based on permutation tests (p=0.003). The regression model showed that exposure during this sensitive period resulted in steep dose-response function with maximal response to even modest levels of exposure. Subjects in the highest exposure quartile (MACE-11, range 11 – 54) had a 9.1% greater right amygdala volume than subjects in the lowest exposure quartile (MACE-11, < 3.5). No associations emerged between age of exposure and volume of left amygdala or bilateral caudate or thalamus. Severity of adversity experienced at age 10–11 contributed to larger right but not left amygdala volume in adulthood. Results provide preliminary evidence that the amygdala may have a developmental sensitive period in preadolescence.
Keywords: Amygdala, Adversity, Maltreatment, Abuse and Neglect, Sensitive Periods, Early Life Stress
1. Introduction
Childhood adversity is a major risk factor for psychopathology associated with 30–70% of the population attributable risk fraction for depression, suicide attempts, anxiety disorders and substance abuse (see (1) for review). Early adversity may increase risk through excessive release of glucocorticoids and epigenetic modifications that alter critical developmental processes such as neurogenesis, synaptogenesis and myelination (2). The amygdala may be particularly vulnerable due to high glucocorticoid receptor density (3) and postnatal developmental trajectory characterized by rapid initial growth followed by more sustained growth to peak volumes between 9–11 years and gradual pruning (4, 5). Aberrant amygdala volume and function have been reported in psychiatric disorders marked by affective dysregulation (6–9).
Translational studies show that psychological stressors (i.e., immobilization) and administration of stress hormones stimulates dendritic arborization and formation of new spines in the amygdala and increases volume (10, 11). Using a parallel human-mice model, Cohen and colleagues (12) showed that manipulating type and timing of stressor to parallel early orphanage experiences in humans led to early and persistent alterations in amygdala development and function. Interestingly, this pattern is opposite to stress-induced hippocampal atrophy and is less reversible even when the stressor is removed (12, 13). The consequences of early life stress on the human amygdala and the underlying causes (e.g., dendritic arborization), however, remain inconclusive. In fact, many studies have reported no differences in amygdala volume following adversity (14–24). Yet, increased amygdala volume was found in children who had experienced prolonged institutional deprivation (25, 26) or rearing by chronically depressed mothers (27). In contrast, smaller amygdala volumes were reported among adults with childhood trauma and diagnoses of Borderline Personality (28, 29) or Dissociative Identity Disorders (30, 31).
Several factors may contribute to these inconsistencies. First, stress-related effects on the amygdala may change over development, leading to hypertrophy in childhood followed by shrinkage in adolescence or adulthood. Second, the amygdala may show a differential response to stress, enlarging in the face of neglect or insufficient human interaction, as with prolonged institutional deprivation, or shrinking with exposure to the types of intense abuse often reported by individuals with borderline personality or dissociative identity disorders. Third, the timing of adversity may be critical (17, 32). Given the amygdala’s developmental trajectory, it may be particularly sensitive to structural changes during early childhood when it is growing at a rapid rate, and again during preadolescence when growth peaks and pruning takes over, as observed in the hippocampus (33).
To explore these factors, we recruited adults from a 30-year longitudinal sample who were followed since infancy and had experienced significant levels of childhood adversity during different developmental stages to take part in a magnetic resonance imaging (MRI) study. Control subjects were healthy adults with no or very low levels of childhood adversity. It was hypothesized that adults who experienced childhood adversity would show: (1) increased stress-related symptoms, and (2) increased amygdala volume. We also explored whether exposure to adversity during particular developmental windows was associated with larger effects on amygdala volume.
Consistent with previous research (e.g., (26)), the caudate and thalamus were selected a priori as control structures that should be less susceptible to periadolescent stress due to their developmental trajectory and lower glucocorticoid receptor density (34–36).
2. Materials and Methods
2. 1 Participants
The study was approved by the Harvard Medical School, Cambridge Hospital, and McLean Hospital IRBs. Subjects provided informed written consent and were reimbursed $100 for their time. Two groups were enrolled: 18 longitudinal participants with early and continued life stress (ELS: 8M/10F, 29.33±0.49 years) and 33 cross-sectional healthy controls (HC: 9M/24F, 23.43±1.45 years) with no or very low exposure to childhood maltreatment and no history of psychopathology.
HC subjects were participants in a larger study of maltreatment-related effects on psychopathology and neurobiology. We were aware when designing this study that HC subjects would be about 6 years younger than ELS subjects, as HC subjects needed to be between 20–25 years of age at the time of recruitment into the original study. We concluded that this difference would be acceptable as amygdala volume should be stable within the age range of the sample (5). Moreover, if volume did decline slightly with age, then this would be tolerable, as it would bias results in the opposite direction given our prediction of greater amygdala volume in the older ELS group.
ELS participants were first recruited as infants (8.5±5.6 months) for a study on social risk factors and child development (37). The initial cohort consisted of 76 families who were at or below 200% of federal poverty line. Extensive home and lab-based observations of mother-infant dyads were conducted to characterize the quality of maternal caregiving and infant attachment. Cognitive, affective and physical development was assessed during multiple waves in infancy, childhood, and late adolescence (age 20 years) (37–39), with a 74% follow-up rate. At age 29, 33 participants were relocated and screened for inclusion in the study. Eighteen ELS adults met inclusion criteria and participated in the MRI study.
Participants in the ELS group were quite representative of the larger longitudinal cohort from which they were recruited and whose developmental outcomes have been well characterized from infancy to adulthood (e.g. 37–39). Specifically, ELS participants did not differ from the larger cohort in family demographic characteristics (effect sizes: family income µ = .04, ns; gender … = .14, ns; mother single parent … = .02, ns; mother high school only … = .03, ns; ethnic minority status … = .05, ns); or in quality of parent-child interaction in infancy, childhood, or adolescence (η = .01 – .05, all ns); or in severity of childhood maltreatment (η = .01, ns); or in extent of Axis I or Axis II psychopathology on the SCID in adulthood (.00 – .00, all ns).
In the present study, subjects were excluded if they reported a significant medical or neurological condition, substance abuse in the past six months, or did not meet MRI safety criteria. With the exception of one ELS participant, all individuals were right-handed. Compared to healthy controls, ELS participants were older, less likely to be single, and less likely to have a college degree (Table 1). Age and gender served as covariates in initial models. In the ELS group, three participants were diagnosed with current anxiety disorder (Generalized Anxiety Disorder = 1; Social Phobia = 1; Panic Disorder = 1) and three subjects met criteria for mood disorder (Major Depression = 2; Dysthymia/MDD = 1) at the time of the study. Two subjects were previously diagnosed with Attention-deficit-hyperactivity disorder (ADHD) but were not in treatment or taking medication at the time of the scan. Eight participants reported a history of substance abuse or dependence. At the time of the scan, four subjects were taking medication for past opioid dependence (subutex = 1; suboxone = 2, methadone = 1). Current use of cannabis was endorsed by two subjects.
Table 1.
Demographics for participants in the healthy control (HC) and adversity (ELS) groups.
| Characteristics | ELS (n=18) |
HC (n=33) |
Statistical Value | p-levels |
|---|---|---|---|---|
| Age, mean±SD | 29.3±0.49 | 23.42±1.45 | t =16.71 | .001 |
| Females, N (%) | 10 (55.60) | 24 (72.70) | x2=1.55 | .21 |
| White, N (%) | 14 (77.80) | 23 (62.20) | x2=2.82 | .42 |
| Single, N (%) | 11 (61.10) | 31 (93.90) | x2=8.64 | .003 |
| College degree, N (%) | 2 (11.10) | 28 (84.80) | x2=26.15 | .001 |
2.2 Measures
2.2.1 Maltreatment Exposure and Current Symptomatology
Timing and severity of exposure to childhood maltreatment was assessed using the Maltreatment and Abuse Chronology of Exposure scale (MACE; Teicher and Parigger, unpublished). This new instrument assesses exposure to ten types of maltreatment during each year of childhood from ages 6 to 18, including childhood sexual abuse, parental verbal abuse, non-verbal emotional abuse and physical abuse, witnessing of intra-parental physical violence and violence toward siblings, plus peer verbal abuse, peer physical abuse, emotional neglect, and physical neglect. Items within each category were selected using item response theory, and category scores were summed to provide a total score. Excellent test-retest reliability across all ages (r=0.894, n = 60) was demonstrated by comparing severity scores for each subject on test versus retest. Test-retest reliability at specific ages ranged from r = 0.808 at age 12 to 0.901 at age 16. Reliability at age 6 (r = 0.857) was not significantly different than reliability at older ages. The MACE showed good convergent validity as the instrument correlated 0.741 (95% CI 0.697–0.780, t=23.74, df = 462, p < 10–16) with Childhood Trauma Questionnaire (CTQ) scores and 0.705 (95% CI 0.677–0.731, t=26.21, df=1323, p < 10–16) with Adverse Childhood Experiences (ACE) scores. However, MACE scores, on average, accounted for 2.28-fold and 2.04-fold more of the variance in symptom ratings (depression, anxiety, somatization, anger-hostility, dissociation, ‘limbic irritability’ and suicidal ideation) than CTQ or ACE scores, respectively. The Parental Bonding Inventory (PBI) (40) was used to assess Maternal Care (warmth/affection vs. coldness/rejection) and Maternal Overprotection (intrusion/control vs. encouragement/autonomy) until age 16.
Lifetime histories of psychopathology were assessed using the Structured Clinical Interview for DSM disorders (SCID; (41)). The Perceived Stress Scale (PSS) was used as a global measure of subjective stress experienced during the past month (42), the Symptom Questionnaire (SQ) (43) assessed symptoms of depression, anxiety, anger-hostility and somatic complaints during the last week, and the State version of the State-Trait Anxiety Inventory (44) assessed existing anxiety levels immediately before and after MRI.
2.2.2 Magnetic Resonance Imaging
Data was collected using a 3T TIM Trio scanner (Siemens AG, Erlangen, Germany) with a 32-channel head coil using a T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) pulse sequence (TE: 2.25ms; TR: 2100ms; FA = 12; FOV: 256mm; slice number: 128; voxel size: 1.0×1.0×1.3mm; slice thickness: 1.33mm) in the sagittal plane (scan duration: 6 min).
2.2.3 Image Processing
Gray matter volume (GMV) in amygdala, caudate and thalamus were semi-automatically assessed using FreeSurfer 5.1 (http://surfer.nmr.mgh.harvard.edu). Although this program originated as a method for assessing cortical structure (45–48), it has evolved to include robust tools for subcortical volume analyses (49, 50). Voxels within subcortical regions are labeled using an elaborate process based on both a subject-independent probabilistic atlas derived from a hand-labeled training set and on subject-specific measures (50). This procedure has been found to label the brain in a manner that is statistically indistinguishable from those provided by experienced manual raters (49). Overall, FreeSurfer provides one of the most reliable automated brain segmentation methods for assessing the amygdala and these measures correlate highly with expert hand tracings (51). Automated segmentation eliminates differences between studies and facilitates replication by other investigators (52, 53). The authors (CMA, PP) visually inspected all T1-weighted and automated images. Manual adjustments were not required. Regional volumes and total GMV were extracted and exported into SPSS 19.0 and R (54) for statistical analysis.
2.3 Statistical Analysis
2.3.1 Analysis of Group Differences
Between group differences in exposure to maltreatment between ages 6–18 years and the influence of gender on exposure were assessed using linear mixed effect models with group and gender as fixed effects (R packages lme4, and LMERConvenienceFunctions). Between group differences in symptom ratings were assessed using independent t-tests with Welch’s correction for unequal variance. Hypothesis testing of overall group differences in neuroimaging measures were assessed using linear mixed effect models with group and hemispheres as fixed effects and age, gender and total GMV as covariates.
As we had hypothesized a priori that childhood maltreatment would be associated with increased GMV in the amygdala but would not be associated with alteration in GMV in caudate or thalamus, we did not adjust the significance of amygdala measures for these planned negative control comparisons.
2.3.2 Sensitive Period Analysis
The presence of a potential ‘sensitive period’, during which exposure to childhood maltreatment might be more strongly associated with alterations in amygdala volume, was assessed using random forest regression with conditional inference trees (‘cforest‘ in R package party (55)). This is a form of machine learning in which a large number of unpruned decision trees are generated and their results aggregated. Random forest regression has the advantage of high accuracy, no restrictions regarding the distribution and scaling properties of the data, high tolerance for multicolinearity and a novel means of determining variable importance (56, 57). We used a variant of Breiman’s approach with conditional trees as the base learners to avoid a potential problem with biased estimates that can emerge when variables differ in range or number of categories (55). Conditional forest regression indicates importance by assessing the decrease in accuracy, as noted by the increase in mean square error (MSE), of the forest’s fit following permutation of a given predictor variable. Permutation of important predictor variables produces a large increase in MSE, whereas permutation of unimportant predictors produces little or no increase in MSE.
While random forest regression is well-suited to identify ages when severity of exposure has the most important predictive effect on morphology (58, 59), we also sought to determine whether the magnitude of importance at peak periods could have occurred by chance. Hence, we used a re-randomization test in which we calculated the maximal increase in MSE with severity of exposure at any age in the original data set, and then tested for this degree of increase in MSE in 10,000 alternative random forests regressions in which the association between regional volume and exposure histories was randomly reshuffled.
Finally, we evaluated whether the importance of exposure during the sensitive period exceeded the importance of overall exposure across childhood (i.e., composite score based on whether or not specific events were experienced at any time during the subject’s first 18 years). This was accomplished by computing conditional forest regression including overall severity as an additional predictor. Significance of differences in importance of exposure during a specific age versus childhood in general was assessed using bootstrap resampling (60).
For these analyses we used regional measures of GMV in amygdala, thalamus and caudate nuclei as dependent variables and adjusted these measures for the subject’s total GMV. Age and gender were not included as additional covariates as the mixed effect models showed that their influence was well accounted for by total GMV (resulting in their non-significant contributions) and more parsimonious models were produced by their exclusion. MACE severity scores at each age (between 6 – 18 years) were used as the predictor variables. Regional GMV was centered and scaled for each region to provide an arbitrary mean of 100 and SD of 10, to facilitate comparisons between regions in importance of exposure at each age using the increase in MSE criteria. Each forest consisted of 500 trees with four variables randomly selected for evaluation at each node.
3. Results
3.1 Maltreatment Exposure and Current Symptomatology
As expected, ELS subjects reported more severe exposure to childhood maltreatment across development than HC (F(1,611) = 48.89, p < 10−11) (Figure 1). Exposure was slightly higher in females than males (F(1,611) = 4.46, p < 0.04). ELS subjects reported at least a moderate degree of exposure to 2.8 ± 2.1 different types of maltreatment (range 0 – 8) versus 0.4 ± 0.5 types in HC (range 0 – 1) (t(18.078) = −4.93, p = 0.0001). Compared to HC, ELS subjects experienced greater severity of exposure to emotional neglect (t(20.36) = 4.26, p < 0.0004), parental verbal abuse (t(20.76) = 4.06, p < 0.0006) and physical neglect (t(22.86) = 3.31, p < 0.004). Both groups reported low exposure to sexual abuse, non-verbal emotional abuse, witnessing interparental violence and peer physical abuse yielding no group differences. The ELS group also reported lower levels of maternal care (p = .002) but not maternal overprotection (p = .31) (Table 2).
Figure 1.
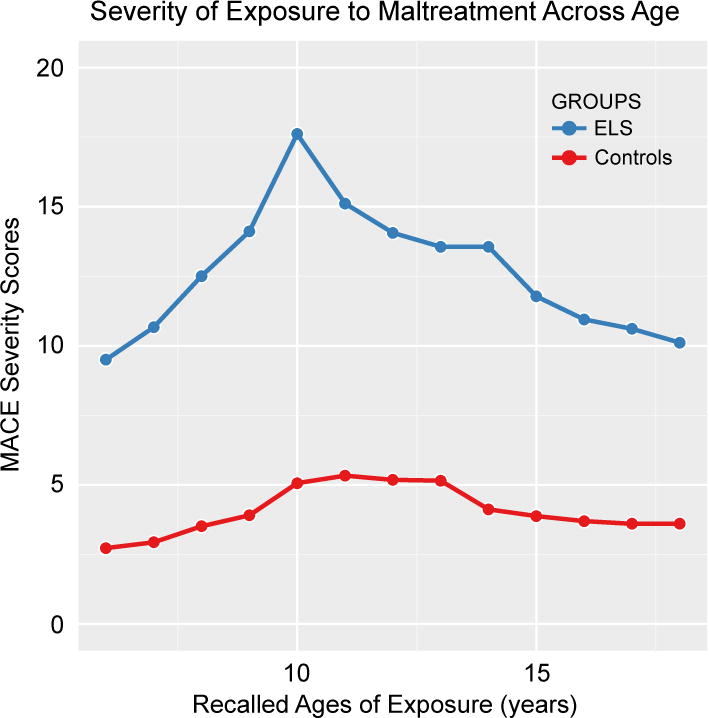
Retrospectively reported severity of exposure to childhood maltreatment during different childhood ages in longitudinally-followed participants with early life stress versus healthy controls.
Table 2.
Self-report measures of parental care and current symptomatology (mean±standard deviation) for participants in the healthy control (HC) and adversity (ELS) groupsa.
| Measures | ELS (n=18) | HC (n=33) | Statistic (t-test) | p-levels |
|---|---|---|---|---|
| PBI: Care | 24.00±9.02 | 31.92±4.42 | −4.23 | .002 |
| PBI: Overprotection | 12.28±7.90 | 10.24±6.07 | 1.03 | .31 |
| PSS | 21.72±7.74 | 15.69±6.08 | 3.05 | .004 |
| STAI: Pre-Scan | 31.78±7.43 | 29.41±5.06 | 1.34 | .24 |
| STAI: Post-Scan | 28.39±7.72 | 30.50±7.42 | −.95 | .35 |
| SQ: Anxiety | 10.44±3.31 | 7.24±4.28 | 2.75 | .008 |
| SQ: Depression | 8.89±3.53 | 7.00±4.13 | 1.64 | .11 |
| SQ: Somatic | 6.89±3.31 | 6.67±3.86 | .21 | .84 |
| SQ: Anger-Hostility | 10.94±3.47 | 6.15±4.43 | 4.0 | .001 |
PBI=Parental Bonding Instrument, PSS=Perceived Stress Scale; STAI=Spielberger State-Trait Anxiety Inventory (State version), SQ=Symptom Questionnaire.
In support of our first hypothesis, the ELS group reported higher levels of perceived stress in the past month than HC (p = .004) and more current symptoms of anxiety (p = .008) and anger-hostility (p < .001) (Table 2).
3. 2 Neuroimaging
3.2.1 Childhood Adversity and Amygdala Volume
Consistent with the second hypothesis, mixed effect models showed that there was a significant effect of group (F(1,46) = 4.48, p < 0.04) on amygdala volume. There was also an effect of hemisphere (F(1,46) = 21.48, p < 0.0001) but no significant group × hemisphere (G×H) interaction (F(1,46) = 0.004, p > 0.9) (Figure 2A). On average, least square mean adjusted amygdala volume was 3.8% greater in ELS versus HC subjects. In contrast, there were no significant effects of group, or G×H interactions on caudate (Group: F(1,46) = 0.34, p > 0.5; G×H: F(1,46) = 0.55, p > 0.4) or thalamus (Group: F(1,46) = 2.36, p = 0.14; G×H: F(1,46) = 0.09, p > 0.7).
Figure 2.
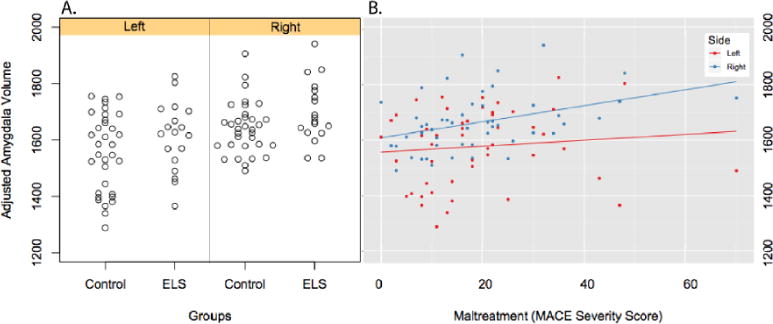
A. Scatter plot showing differences in amygdala volume adjusted for age, gender and total gray matter volume in ELS and HC subjects. B. Linear regression graphs illustrating relationship between severity of exposure to adversity during childhood and adjusted amygdala volume.
There was also a significant linear association between severity of exposure to adversity across childhood (MACE sum score) and adjusted volume of the right (r = 0.38, p < 0.006), but not left (r = 0.10, p > 0.4), amygdala (Figure 2B). A multiple regression analysis confirmed that the significant regressive relationship between severity of exposure to adversity and right amygdala was not confounded by differences in education, martial status, medication use, clinical diagnosis, current symptoms of depression and anxiety or recent stress (Table 3). There were no significant linear associations between MACE sum score and adjusted right and left caudate or thalamic volumes. Increased right but not left amygdala volume was also marginally associated with increased anxiety symptoms (SQ: r=.27, p=.07).
Table 3.
Multiple linear regression analysis showing effects of maltreatment severity, total gray matter volume and potential confounding factors on right amygdala volume.
| Coefficients | Estimate | Standard Error | t value | Probability |
|---|---|---|---|---|
| (Intercept) | 299.50 | 365.30 | 0.82 | 0.42 |
| MACE Severity | 4.55 | 1.74 | 2.61 | 0.013 |
| SCID Anxiety | −111.1 | 108.70 | −1.02 | 0.31 |
| Current Anxiety | 5.94 | 5.20 | 1.14 | 0.26 |
| SCID Major Depression | −93.62 | 100.00 | −0.94 | 0.36 |
| Current Depression | −2.90 | 5.14 | −0.56 | 0.58 |
| SCID Substance Abuse | 63.21 | 87.80 | 0.72 | 0.48 |
| Perceived Stress Scale | −0.53 | 2.97 | −0.18 | 0.86 |
| Married (yes/no) | 0.43 | 49.32 | 0.01 | 0.99 |
| Education (Years) | −4.00 | 10.43 | −0.38 | 0.70 |
| Current Medication Use | −11.08 | 95.82 | −0.12 | 0.91 |
| Sex | 19.17 | 50.79 | 0.38 | 0.71 |
| Age | −3.99 | 7.90 | −0.51 | 0.62 |
| Total Gray Matter Volume | 0.002 | 0.0004 | 5.61 | 0.0000003 |
3.2.2 Sensitive Periods for Maltreatment Exposure
Finally, exploratory analyses to assess potential sensitive periods indicated that severity of exposure at 10–11 years of age was the most important predictor of right amygdala volume (Figure 3). On balance, severity of exposure at 10–11 years of age accounted for 27% of adjusted volume (Table 4). Eliminating age 11 from the analysis (by permutation) produced a 12.7 (e.g., 1.27 SD) mean decrease in accuracy (i.e., increase in MSE) of the regression. The probability of obtaining a peak of at least this height was quite low (p = 0.0033), as was the probability of obtaining an overall fit with as low a MSE (p = 0.023) (Table 4).
Figure 3.
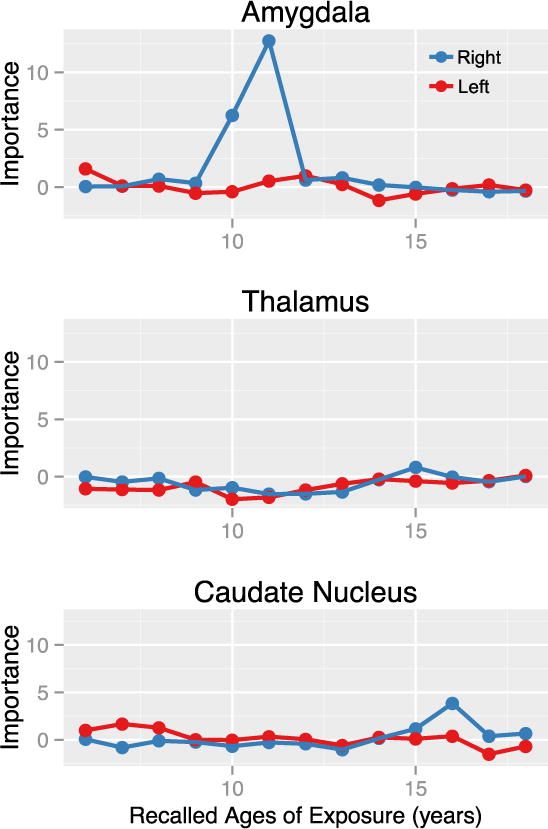
Results of random forest regression with conditional trees indicating importance of exposure to early life stress from 6 – 18 years of age on amygdala, thalamic, and caudate volumes. Importance is indicated by degradation in fit, as indicated by increase in mean square error, following effective elimination of each age from the model by permutation.
Table 4.
Sensitive period analysis of volumes of key regions using random forest regression with conditional trees indicating significance of peak height and fit based on randomized resampling.
| Region | Coefficient of Determination | Mean Square Error (MSE) | Significance MSE | Peak Age | Peak Height Importance* | Significance Peak Height |
|---|---|---|---|---|---|---|
| Right Amygdala | 0.27 | 74.0 | P = 0.023 | 11 | 12.7 | P = 0.0033 |
| Left Amygdala | 0.13 | 87.7 | P > 0.8 | 6 | 1.59 | P > 0.5 |
| Right Thalamus | 0.19 | 88.1 | P > 0.8 | 16 | 1.22 | P > 0.6 |
| Left Thalamus | 0.15 | 91.0 | P > 0.9 | 14 | 0.82 | P > 0.7 |
| Right Caudate | 0.16 | 84.8 | P > 0.5 | 16 | 4.08 | P > 0.2 |
| Left Caudate | 0.14 | 85.7 | P > 0.5 | 8 | 2.05 | P > 0.5 |
Decrease in mean accuracy of the fit, as indexed by the increase in mean square error, following effective elimination by permutation of that age from the regression analysis.
In contrast, the same analysis applied to the left amygdala showed that severity of exposure across this age range accounted for only 13% of adjusted volume, and the most prominent peak had low importance. The likelihood of obtaining a peak with this importance by chance was high (p = 0.59), as was the probability of obtaining this degree of fit (p > 0.8) (Table 4). Similarly, no evidence emerged for significant sensitive periods for exposure to ELS on caudate or thalamic volume in this sample.
Comparing the importance of exposure at specific ages versus all of childhood indicated that exposure at age 10 (t(71.98) = 4.46, p < .0001) and 11 (t(62.45) = 9.13, p < 10−12) were substantially more important than overall childhood exposure in this sample. Adjusted right amygdala volume was 9.12% [95% CI 3.9% – 14.3%] greater in the highest versus lowest exposure quartiles at age 11 (1757.6±105.8 vs.1610. 7±72.7; t(17.29) = −3.89, p = 0.001).
Random forest regression does not assume a linear relationship between predictor variables and outcomes. Figure 4 shows the nature of the modeled relationship between degree of exposure at ages 10 and 11 and scaled right amygdala volume. Interestingly, the model delineated a sharp, nearly stair-step, dose-response relationship between exposure and right amygdala volume. Right amygdala volume remained low up to MACE scores of 5, then rose rapidly to a new plateau level 0.23 SD larger at MACE score = 10 for exposure at age 10 (holding exposure at age 11 constant and below threshold), and 0.35 SD larger at MACE score = 12 for exposure at age 11 (holding exposure at age 10 constant and below threshold).
Figure 4.
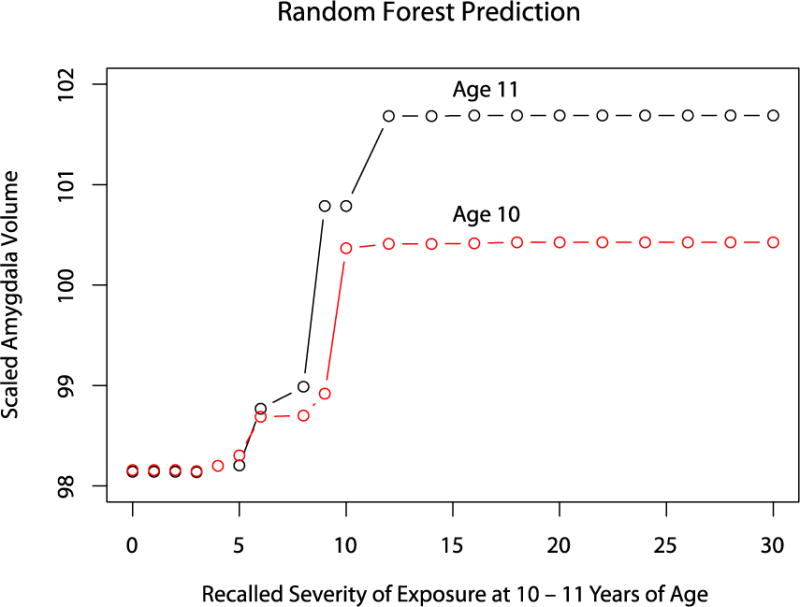
Dose-response relation between recalled severity of exposure at 10–11 years of age and scaled right amygdala volume as predicted by the random forest regression model. Exposure at ages 6–9 and 12–18 held constant at mean value for all subjects. Exposure at age 10 (or 11) held constant at subthreshold MACE score of 4 while exposure at age 11 (or 10) varied across × – axis.
The types of maltreatment that were positively and significantly associated with right amygdala volume included parental verbal abuse, physical maltreatment, non-verbal emotional abuse, witnessing sibling assault, and peer verbal abuse at 10–11 years (Table 5). No associations between abuse type and left amygdala volume were found. With the exception of a modest positive correlation between thalamic volume and parental verbal abuse, no other correlations were found between type of abuse at age 10 or 11 and regional volumes.
Table 5.
Partial correlations between types of maltreatment experienced at age 10 and 11 and regional volumes adjusted for age, gender, and total gray matter volumea.
| PVA | NVEA | PhysM | IPV | WSibA | PeerVA | PeerPhys | EN | PN | |
|---|---|---|---|---|---|---|---|---|---|
| Right | |||||||||
| Amygdala | .26* | .36** | .32** | .23 | .44*** | .28** | .22 | .16 | −.15 |
| Thalamus | .29* | .19 | −.002 | .11 | −.26 | −.05 | .15 | −.06 | −.007 |
| Caudate | .13 | .03 | −.002 | .02 | −.04 | .05 | .12 | .05 | −.10 |
| Left | |||||||||
| Amygdala | −.03 | .06 | −.05 | .02 | .07 | −.07 | .17 | −.09 | −.19 |
| Thalamus | .24 | .12 | −.07 | .07 | −.21 | −.19 | .16 | −.04 | −.02 |
| Caudate | .07 | −.03 | .05 | −.07 | −.14 | −.08 | .08 | −.02 | −.07 |
Note. N=51.
PVA= parental verbal abuse, NVEA = non-verbal emotional abuse, PhysM = physical maltreatment, IPV = witnessed intra-parental violence, WsibA = witnessed sibling assault, PeerVA = peer verbal abuse, PeerPhys = peer physical abuse, EN = emotional neglect, PN = physical neglect. No participant reported sexual abuse at 10–11 years of age.
p < .10,
p ≤ .05,
p ≤ .01
3.2.3 Hippocampal Sensitive Period Comparison
As the sensitive period for the right amygdala at 11 years (Figure 3) roughly corresponded to the time of peak exposure (10 years, Figure 1) the question arouse as to whether this ‘sensitive period’ was an artifact of more severe exposure or greater variability in exposure occurring during this time window. To help rule out this possibility we assessed whether the hippocampus showed the same sensitive period in this population. Prior work strongly indicated that the hippocampus should have an earlier and possibly later sensitive period (17).
Right but not left hippocampal volume correlated with MACE severity (F1,34 = 6.54, p = 0.015) after controlling for total gray matter volume, age, gender, education, martial status, medication use, clinical diagnosis, current symptoms of depression and anxiety and recent stress. Exploratory analyses with random forest regression indicated that severity of exposure at 7 and 14 years of age were the most important predictor of right hippocampal volume (Figure 5). Severity of exposure across age accounted for 27% of adjusted volume. Eliminating age 7 from the analysis (by permutation) produced an 8.2 point decrease in accuracy (i.e., increase in MSE) of the regression. The probability of obtaining a peak of at least this height was low (p = 0.035), as was the probability of obtaining an overall fit with as low a MSE (p = 0.034)
Figure 5.
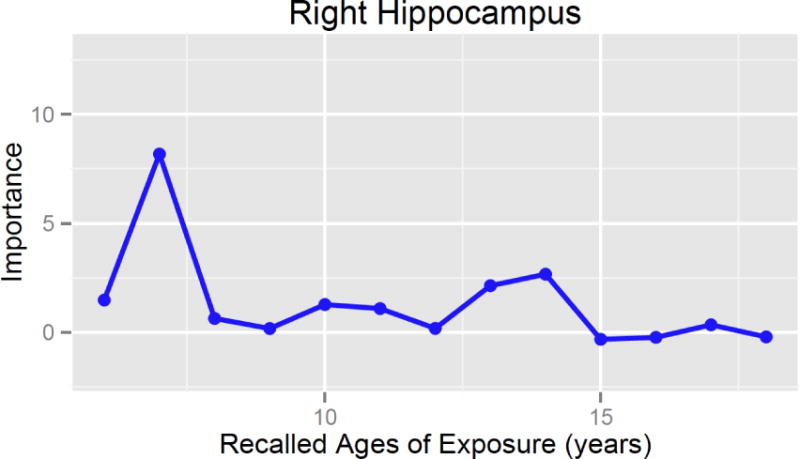
Results of random forest regression with conditional trees indicating importance of exposure to early life stress from 6 – 18 years of age on right hippocampal volume.
4. Discussion
This study provides initial evidence for enlarged amygdala volume associated with ELS in an adult sample. In particular, in relation to the right amygdala, we found a dose-response relation between severity of exposure and volume. The results further add to our understanding by identifying important differences in right versus left amygdala sensitivity to the type and timing of ELS.
Previous studies have also observed a specific association between ELS and anatomical changes in the right amygdala (25, 61). For example, Metha et al. (25) found increased right but not left amygdala volume in adolescents who experienced early institutionalization. Buss and colleagues (61) showed that higher maternal cortisol in early gestation was associated with increased right amygdala volume in girls at age seven which mediated the relationship between maternal cortisol and affective problems. Similarly, right-lateralized amygdala hyper-reactivity to negative stimuli was found in adults with maltreatment histories (20). Interestingly, Dannlowski and colleagues (62) showed that even at a level of automatic emotion processing, childhood maltreatment was associated with hyper-reactivity of the right but not left amygdala in response to subliminally presented negative faces. The right amygdala increases in volume over a longer time period than the left amygdala, potentially leaving it more vulnerable to stress-related effects during later periods of development (5). In contrast, the left amygdala appears to develop more rapidly in the first few years of life, suggesting a more prominent role in early development. Furthermore, a recent longitudinal study showed that only left amygdala growth but not right amygdala growth following childhood maltreatment is mediated by adolescent psychopathology (63). Functionally, the amygdala has been proposed to be associated with self-referential processing of negative emotional stimuli on the right and positive emotional stimuli on the left (64). Stimulation of right amygdala in patients with temporal lobe epilepsy has been shown to produce greater intensity of emotions such as fear, compared to left-sided stimulation (65).
In addition, evidence for a developmental sensitive period between 10–11 years of age was strong for the right amygdala. Random forest regression indicated that the importance of exposure at 11 years of age exceeded that for all other ages (except 10 years) by at least 5.7-fold. Further, the importance of exposure at 11 years was 3.5-fold greater than for overall exposure during the first 18 years. Increased amygdala GMV was associated with exposure to several active forms of abuse at age 10–11, but was not associated with degree of exposure to physical or emotional neglect at those ages. Analysis of the random forest model further revealed a steep, near stair-step, dose-response function during the sensitive period, suggesting a threshold effect at a relatively modest level of exposure.
Given the similar peak age of amygdala sensitivity and the age of most severe maltreatment exposure, it could be argued that amygdala development is sensitive to severity. However, the highest mean exposure level was at age 10 while maximal importance was at age 11. Further, severity of exposure was very similar at 8, 11 and 14 years (i.e., 14.1, 15.1, 14.1, respectively), but importance attributable to these ages was markedly different (0.34, 12.74 and 0.63, respectively). In addition, the conditional random forest technique used is not biased by independent variable parameters, such as variability, range or mean (55). Perhaps, most convincingly, applying the same technique to the hippocampus delineated a completely different sensitive period. This provides strong evidence that sensitive periods detected are unrelated to developmental differences in overall exposure level. This principle was further confirmed using simulated data (not shown).
These results provide several possible explanations for some of the discrepant findings in previous studies. For example, previous studies of children exposed to adversity have reported increased amygdala volume in children with institutional deprivation or rearing by chronically depressed mothers (25–27). However, studies of childhood adversity focusing on adults have either found no significant differences (14–20) or smaller amygdala volumes in borderline personality or dissociative identity disorder subjects (28–31). Results of the present study suggest, first, that timing of exposure may be extremely important, and overall exposure levels across childhood may be misleading if the maltreatment occurred before or after the sensitive period. Second, the association between maltreatment and volume appears to be lateralized, and may be missed by studies looking for bilateral differences (e.g., (17)). Perhaps most importantly, the random forest regression model suggests that the right amygdala appears to be vulnerable to even modest levels of adversity during the sensitive period, and appears to be affected by types of maltreatment (e.g., peer verbal abuse, witnessing violence to siblings) not detected by other assessments. Hence, detecting an ELS effect on the right amygdala may require comparison with a control group with extremely low levels of exposure.
Further research is needed to determine what factors may lead to increased or decreased amygdala volume (e.g., 63), keeping in mind that this may not be a cause and effect relationship with ELS. For example, it is possible that reduced amygdala volume may be a preexisting risk factor for certain forms of psychopathology (53), and may appear as a maltreatment-related consequence in these samples. Based on randomized animal models, possible neurobiological mechanisms relating childhood stress to increased amygdala volume include inadequate pruning or stress-related overproduction of new spines in the amygdala. Several cortical and subcortical regions overproduce axons, dendrites, synapses, and receptors (66). Typically, these overproduced elements are extensively pruned in the period between onset of puberty and emergence of adulthood (67, 68). Increased amygdala volume may therefore be a result of stress secondary to adversity inhibiting the pruning process. Alternatively, direct administration of stress hormones in animals has shown to increase dendritic arborization and formation of new spines in the amygdala (10, 11). Thus, volumetric increases in the amygdala may be a direct consequence of stress-related arborization during preadolescence. Future research will need to deconstruct these potential pathways.
Despite these intriguing findings, the interpretation of morphometric change remains challenging as multiple factors contribute to structural volume (e.g., size of neurons and glial cells, density, vascularity) (69). Although previous research has found higher levels of anxiety associated with increased right amygdala volume (70), the present study only showed a trend-level association. Future studies will need to assess whether functional response shows a similar sensitive period given that amygdala hyper-reactivity to emotional stimuli has been frequently reported in individuals with ELS (20, 71–73).
4.1 Implications
Identifying sensitive periods when adversity will have a particularly harmful impact on amygdala development is pivotal to linking childhood experiences to later psychopathology. Unlike reversible hippocampal atrophy, the effects of adversity on the amygdala persist even after the termination of adversity, making this subcortical region more resistant to recovery (13). In addition, changes in amygdala volume have been proposed as a vulnerability factor for psychiatric illness (53). Sensitive periods are also likely windows of opportunity during which clinical interventions may provide maximal benefits (33) to minimize or preempt long-term consequences of abuse.
4.2 Limitations
Several limitations should be acknowledged. First, timing and severity of maltreatment were reported retrospectively. Although prospective longitudinal data confirmed the presence of adversity in the ELS group (e.g., 37, 38, 39), the degree and timing of specific types of maltreatment across both ELS and HC groups was reported at age 29. Self-reports have been shown to be reliable and sensitive to levels of maltreatment unlikely to come to clinical attention. Subjective evaluations of the severity of the abuse are especially relevant for an individual’s level of stress response and consequent impact on neural development (74). It could be argued that retrieving information on the exact timing of abuse in childhood may be challenging for the adult. However, maltreatment experiences are salient events in an individual’s life that are often a vital part of an individual personal narrative. The excellent test-retest reliability of the MACE also provides support that adults are very consistent in their recall of the timing of maltreatment experiences. The MACE is a new instrument specifically designed for studying sensitive period effects. In addition to the assessment of exposure at each age, it has the potential advantages, including the assessment of more types of early adversity, the selection of items based on item-response theory, and very high test-retest reliability, as noted. Indeed, overall exposure and exposure at specific ages meet Bland and Altman’s (75) stringent criteria for reliability/reproducibility. However, there are limits to recollection even of traumatic events, which are almost certainly modified during the passage of time (e.g., (76)).
Second, conclusions about the shape of the sensitive exposure period and the impact of different types of abuse on amygdala development are preliminary given that the ELS sample, on average, experienced relatively moderate levels of exposure throughout development. While exposure at 10–11 years was most predictive in this sample, it is likely that severe exposure outside the sensitive period would also have impact.
Third, six individuals with ELS met current diagnostic criteria for a mood or anxiety disorder, which is typical for subjects with moderate levels of exposure to early adversity (1). Selecting samples in which all exposed subjects meet criteria for a specific disorder, such as PTSD, or who are all free of psychopathology might have produced a somewhat different outcome. It is our perspective that selecting subjects based on experience regardless of outcome provides the most generalizable information on the consequences of exposure. Selecting subject who all meet criteria for a specific disorder may bias results by recruiting subjects who are susceptible in the same way, and provides results that are most applicable to that subgroup. Similarly, selecting maltreated subjects with no history of psychopathology may provide results that are most applicable to relatively resilient individuals.
Finally, we cannot infer causality from this study because the data were correlational and crossectional. Future work should include prospective longitudinal designs and early MRI scanning of infants or young children, a relatively novel procedure that was not available when the current participants were infants. Despite support for changes in amygdala development, analytic methods in humans are limited in identifying the underlying mechanism causing atrophies or hypertrophies following early life stress. Understanding ‘normative’ brain development, and divergences thereof, continue to be a challenging task for future research.
5. Conclusion
To explore sensitive periods during which the amygdala is susceptible to early and continued life stress, we recruited adults from a 30-year longitudinal sample who were followed from infancy and who experienced adversity during different developmental stages. Random forest regression revealed that severity of adversity experienced at age 10–11 contributed to a larger right but not left amygdala volume in adulthood. Results provide preliminary evidence that the amygdala may have a developmental sensitive period extending into preadolescence. Most critically, sensitive periods are likely windows of opportunity during which clinical interventions may provide maximal benefits to minimize or preempt long-term consequences of childhood adversity.
Highlights.
Adults with childhood maltreatment show increased amygdala volume.
Severity of adversity accounts for 27% of variance in right amygdala volume.
Adversity at age 10–11 contributes to larger right but not left amygdala volume.
Results suggest a potential sensitive period of the amygdala in preadolescence.
Clinical interventions during sensitive periods may help limit sequelae of abuse.
Acknowledgments
This research was supported by funding received from the Harvard Catalyst/Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758) and the Frederick Leonhardt Foundation awarded to KLR, MHT, and PP and R01 MH091391 awarded to MHT. The authors would like to sincerely thank Sarah Richardt and Cynthia McGreenery for their important contributions to participant recruitment and data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary data from this research was presented as a poster at the 68th annual scientific meeting of the Society of Biological Psychiatry in San Francisco.
Financial Disclosures:
None of the authors report any biomedical financial interests or potential conflicts of interest relevant to the subject matter of the manuscript.
References
- 1.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2013.12070957. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 3.Peiffer A, Barden N, Meaney MJ. Age-related changes in glucocorticoid receptor binding and mRNA levels in the rat brain and pituitary. Neurobiol Aging. 1991;12:475–479. doi: 10.1016/0197-4580(91)90076-v. [DOI] [PubMed] [Google Scholar]
- 4.Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus. 2010;20:922–935. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One. 2012;7:e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Saleh K, Carballedo A, Lisiecka D, Fagan AJ, Connolly G, Boyle G, et al. Impact of family history and depression on amygdala volume. Psychiatry Res. 2012;203:24–30. doi: 10.1016/j.pscychresns.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med. 2004;34:1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- 9.Schmahl CG, Vermetten E, Elzinga BM, Douglas Bremner J. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 10.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Cohen MM, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci U S A. 2013;110:18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse–a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brambilla P, Soloff PH, Sala M, Nicoletti MA, Keshavan MS, Soares JC. Anatomical MRI study of borderline personality disorder patients. Psychiatry Res. 2004;131:125–133. doi: 10.1016/j.pscychresns.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 16.van Harmelen AL, van Tol MJ, van der Wee NJ, Veltman DJ, Aleman A, Spinhoven P, et al. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol Psychiatry. 2010;68:832–838. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Andersen SL, Tomoda A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012;72:57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59:975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 21.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 22.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 23.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 24.Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 25.Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 26.Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci U S A. 2011;108:14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 29.Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Research-Neuroimaging. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 30.Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD. Hippocampal and amygdalar volumes in dissociative identity disorder. Am J Psychiatry. 2006;163:630–636. doi: 10.1176/appi.ajp.163.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weniger G, Lange C, Sachsse U, Irle E. Reduced amygdala and hippocampus size in trauma-exposed women with borderline personality disorder and without posttraumatic stress disorder. J Psychiatry Neurosci. 2009;34:383–388. [PMC free article] [PubMed] [Google Scholar]
- 32.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J Psychiatr Res. 2000;34:383–392. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 35.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 36.Hasan KM, Walimuni IS, Abid H, Frye RE, Ewing-Cobbs L, Wolinsky JS, et al. Multimodal quantitative magnetic resonance imaging of thalamic development and aging across the human lifespan: implications to neurodegeneration in multiple sclerosis. J Neurosci. 2011;31:16826–16832. doi: 10.1523/JNEUROSCI.4184-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyons-Ruth K, Connell DB, Grunebaum HU, Botein S. Infants at social risk: maternal depression and family support services as mediators of infant development and security of attachment. Child Dev. 1990;61:85–98. doi: 10.1111/j.1467-8624.1990.tb02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyons-Ruth K, Easterbrooks MA, Cibelli CD. Infant attachment strategies, infant mental lag, and maternal depressive symptoms: predictors of internalizing and externalizing problems at age 7. Dev Psychol. 1997;33:681–692. doi: 10.1037//0012-1649.33.4.681. [DOI] [PubMed] [Google Scholar]
- 39.Obsuth I, Brumariu L, Lyons-Ruth K. Disorganized behavior in adolescent-parent interactions: Relations to attachment state of mind, partner abuse, and psychopathology. Child Dev. doi: 10.1111/cdev.12113. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker G. The Parental Bonding Instrument: Psychometric properties reviewed. Psychiatric Developments. 1989;4:317–335. [PubMed] [Google Scholar]
- 41.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 42.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 43.Kellner R. A symptom questionnaire. Journal of Clinical Psychiatry. 1987;48:268–273. [PubMed] [Google Scholar]
- 44.Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 45.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 46.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 47.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 48.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 50.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 51.Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bergouignan L, Chupin M, Czechowska Y, Kinkingnehun S, Lemogne C, Le Bastard G, et al. Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? Neuroimage. 2009;45:29–37. doi: 10.1016/j.neuroimage.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 53.Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatry. 2012;69:1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 55.Strobl C, Boulesteix AL, Zeileis A, Hothorn T. Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinformatics. 2007;8:25. doi: 10.1186/1471-2105-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Breiman L. Random Forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- 57.Cutler DR, Edwards TC, Jr, Beard KH, Cutler A, Hess KT, Gibson J, et al. Random forests for classification in ecology. Ecology. 2007;88:2783–2792. doi: 10.1890/07-0539.1. [DOI] [PubMed] [Google Scholar]
- 58.Tomoda A, Polcari A, Anderson CM, Teicher MH. Reduced Visual Cortex Gray Matter Volume and Thickness in Young Adults Who Witnessed Domestic Violence during Childhood. PLoS One. 2012;7:e52528. doi: 10.1371/journal.pone.0052528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi J, Jeong B, Polcari A, Rohan ML, Teicher MH. Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage. 2012;59:1071–1079. doi: 10.1016/j.neuroimage.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Efron B, Tibshirani R. An Introduction to the Bootstrap. Boca Raton: Chapman and Hall//CRC; 1993. [Google Scholar]
- 61.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A. 2012;109:E1312–1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Human Brain Mapping. 2013;34:2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whittle S, Dennison M, Vijayakumar N, Simmons JG, Yücel M, Lubman DI, et al. Childhood maltreatment and psychopathology affect brain development during adolescence. J Am Acad Child Adolesc Psychiatry. 2013;52:940–952. doi: 10.1016/j.jaac.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Yoshimura S, Ueda K, Suzuki S, Onoda K, Okamoto Y, Yamawaki S. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain Cogn. 2009;69:218–225. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 65.Gloor P, Olivier A, Quesney LF, Andermann F, Horowitz S. The role of the limbic system in experiential phenomena of temporal lobe epilepsy. Ann Neurol. 1982;12:129–144. doi: 10.1002/ana.410120203. [DOI] [PubMed] [Google Scholar]
- 66.Rakic P. Development of the primate cerebral cortex. In: Lewis M, editor. Child and Adolescent Psychiatry. Baltimore: Williams and Wilkins; 1991. [Google Scholar]
- 67.Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- 68.Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- 69.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 70.Juranek J, Filipek PA, Berenji GR, Modahl C, Osann K, Spence MA. Association between amygdala volume and anxiety level: magnetic resonance imaging (MRI) study in autistic children. J Child Neurol. 2006;21:1051–1058. doi: 10.1177/7010.2006.00237. [DOI] [PubMed] [Google Scholar]
- 71.Grant MM, Cannistraci C, Hollon SD, Gore J, Shelton R. Childhood trauma history differentiates amygdala response to sad faces within MDD. J Psychiatr Res. 2011;45:886–895. doi: 10.1016/j.jpsychires.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, et al. Heightened neural reactivity to threat in child victims of family violence. Current Biology. 2011;21:R947–R948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 73.van Harmelen AL, van Tol MJ, Demenescu LR, van der Wee NJ, Veltman DJ, Aleman A, et al. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Browne C, Winkelman C. The effect of childhood trauma on later psychological adjustment. J Interpers Violence. 2007;22:684–697. doi: 10.1177/0886260507300207. [DOI] [PubMed] [Google Scholar]
- 75.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 76.Wessely S, Unwin C, Hotopf M, Hull L, Ismail K, Nicolaou V, et al. Stability of recall of military hazards over time. Evidence from the Persian Gulf War of 1991. Br J Psychiatry. 2003;183:314–322. doi: 10.1192/bjp.183.4.314. [DOI] [PubMed] [Google Scholar]


