Abstract
Malignant melanoma is associated with poor clinical prognosis; however, novel molecular and immune therapies are now improving patient outcomes. Almost 50% of melanomas harbor targetable activating mutations of BRAF which promote RAS-RAF-MEK-ERK pathway activation and melanoma proliferation. Recent evidence also indicates that melanomas bearing mutant BRAF may also have altered immune responses, suggesting additional avenues for treatment of this patient group. The small molecule inhibitors selective for mutant BRAF induce significant but short-lived clinical responses in a proportion of patients, but also lead to immune stimulatory bystander events, which then subside with the emergence of resistance to inhibition. Simultaneous BRAF and MEK inhibition, and especially combination of BRAF inhibitors with new immunotherapies such as checkpoint blockade antibodies, may further enhance immune activation, or counteract immunosuppressive signals. Pre-clinical evaluation and ongoing clinical trials should provide novel insights into the role of immunity in the therapy of BRAF-mutant melanoma.
Keywords: BRAF mutations, immune suppression, BRAF inhibitors, Melanoma/skin cancers, Protein tyrosine kinases, Phase I-III Trials, Antibody immunotherapy, Cellular responses to anticancer drugs, Kinase and phosphatase inhibitors, Tumor Immunobiology, Immune responses to cancer, Immunomodulation, Tumor resistance to immune response
Therapeutic Approaches for Melanoma
Cutaneous melanoma is an aggressive and potentially lethal form of skin cancer originating from the malignant transformation of melanocytes in the basal layer of the epidermis of the skin. The incidence of malignant melanoma has trebled worldwide since the 1970s (1). In 20% of cases, patients develop locoregional or distant metastases and historic median survival for those diagnosed with distant metastases is only 6-9 months (2). Treatment options for malignant melanoma have been limited until recently. For metastatic disease, chemotherapeutic agents such as Dacarbazine (DTIC) were the standard of care for over 30 years, but do not significantly improve median overall survival (OS). Immunotherapy with high dose Interferon (IFNα2b) is approved in the adjuvant setting and for resected advanced disease, but suffers from significant associated toxicities and only a small portion of patients derive clinical benefits. Due to associated severe toxicities, high dose IL-2 is only administered for stage IV disease in limited numbers of specialized centers worldwide, with around 5% of the patients achieving long-term clinical responses (3).
The clinical landscape has been transformed with the approval of new therapies in the last two years, which include pathway inhibitor drugs and a monoclonal antibody. Vemurafenib, approved by the Food and Drug Administration (FDA) in 2011, is a kinase inhibitor selective for the commonest mutant form of the BRAF kinase (BRAFV600E) designed to target transformation and proliferation of melanoma cells. Two new pathway inhibitor drugs Dabrafenib (also an inhibitor of mutant BRAF) and Trametinib (a MEK inhibitor) were approved in 2013. Vemurafenib, Dabrafenib and Trametinib are all associated with potent, but often short-term responses (4-6). Ipilimumab, an anti-CTLA-4 (cytotoxic T-lymphocyte antigen 4) monoclonal antibody blocking a T cell regulatory pathway designed to promote activated immunity was approved in 2011 (7) as a second line treatment for advanced melanoma. More recently it has been approved for first-line therapy. Ipilimumab treatment is characterized by durable responses but only in a minority of patients.
The success of Ipilimumab has enhanced appreciation of the potential of immune responses to influence patient outcomes. Importantly, emerging studies suggest that BRAF mutant melanomas and BRAF inhibition can also alter immune inflammatory mechanisms associated with tumors. Here we review evidence of associations between BRAF mutant melanoma and BRAF pathway inhibition with immunity and discuss their potential translational implications, including exploring the merits of combination strategies to strengthen immune responses or to counteract tumor-associated immune escape mechanisms.
Activating Immune Responses
Melanoma elicits immune responses, a notion supported by clinical and experimental evidence such as partial regressions in some melanoma lesions, T cell infiltration in tumors correlating with better clinical outcomes, higher incidence of melanoma in immunosuppressed individuals, and the discovery of melanoma-specific antigens and spontaneous T cell and antibody responses against melanoma-associated antigens in patients (8). However, immune activation is counteracted by immune evasion mechanisms orchestrated by tumors on multiple levels. These may include recruitment of regulatory T cells (Treg), secretion of immunosuppressive mediators such as IL-10, Vascular Endothelial Growth Factor (VEGF) and Transforming Growth Factor (TGFβ) and redirecting T and B cell responses in lesions and the circulation (9-13). Through re-educating their environment, tumors may recruit immune suppressive cells such as regulatory T cells (Treg), alternatively activated (M2d) macrophages and myeloid-derived suppressor cells (MDSC) but also promote exhaustion, reduce anti-tumoral functions and suppress maturation of important immune sentinels such as dendritic cells (DC), cytotoxic T cells (CTL) and macrophages (14-16).
Various therapeutic strategies have been based on the premise that immune responses could be directed against melanoma to restrict tumor growth, if immune escape mechanisms can be counteracted or neutralized. Immunotherapy has made considerable advances in the past years with a diverse range of “immune potentiators” developed for therapy. The cytotoxic T-lymphocyte antigen 4 (CTLA-4) and the programmed cell death 1 (PD-1) are transmembrane proteins on T cells that transduce inhibitory signals and reduce antigen-specific T cell responses. The monoclonal antibodies Ipilimumab and Nivolumab bind to CTLA-4 and PD-1, respectively, designed to reverse these checkpoint mechanisms in T cells (17). In a Phase III trial, Ipilimumab treatment at 3 mg/kg doses resulted in a median overall survival of 10 months, and of 10.1 months when given in combination with a gp100 peptide, while the median overall survival for patients given gp100 treatment alone was 6.4 months (18). In a subsequent Phase III trial, overall survival with high-dose Ipilimumab (10 mg/kg) plus Dacarbazine (11.2 months) was higher than Dacarbazine treatment alone (9.1 months). High dose (10 mg/kg) treatments are reported to result in four-year survival rates of 19.7% - 28.4% in previously-treated patients, and 37.7% - 49.5% in treatment-naive patients (19). Ipilimumab treatment is thus characterized by slow onset but durable response rates in a proportion of patients. Treatment is also associated with immune-related toxic side-effects arising from the universal activation of CTLA-expressing T cells irrespective of antigen specificity. These toxicities are observed in approximately 50-60% of patients and include mainly inflammatory skin and gastrointestinal colitis symptoms which can be managed with corticosteroid treatment. Despite associated toxicities and long-term survival benefits in only subsets of patients, antibodies blocking negative immune signals via CTLA-4, PD-1 and other molecules (e.g. CD40 and CD137) have demonstrated that it is possible that clinical benefits could be harnessed with activation of immunity in the context of cancer. The emergence of such antibodies has reinvigorated interest in the translation of cancer immunotherapies to the clinic.
Constitutively-activated BRAF
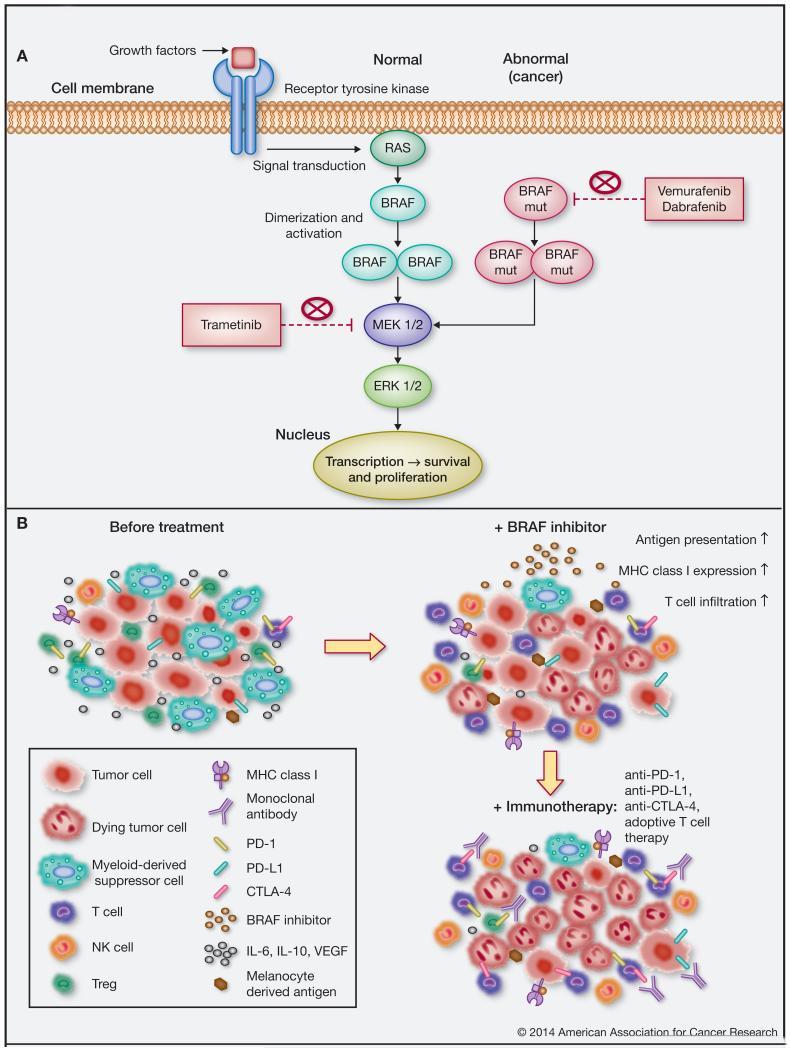
Melanoma is one of the richest cancers with respect to mutations per mb of DNA and exhibits a versatile genetic profile across patients and anatomic locations of tumors (20). The B-raf gene is mutated in up to 66% of human malignant melanomas. Its protein product, the BRAF kinase, is a key player in the RAS-RAF-MEK-ERK proliferative pathway (Fig. 1A) which is widely dysregulated in various cancers, including melanoma (21, 22). B-raf activating mutations are located in the kinase domain; this is also the case for the common amino acid substitution at position V600E, a valine (V) to a glutamic acid (E), the mutant form targeted by Verumafenib therapy (23, 24). B-raf activating mutations may lead to a disrupted conformation of the kinase domain, which dramatically enhances BRAF activity and leads to constitutive ERK activation (25). This mechanism was proposed based on X-ray crystal structure data of the wild type and mutant (BRAFV600E) forms of BRAF in their inactive conformations as part of the complex with a non-specific BRAF inhibitor (Sorafenib). A later study revealed the crystal structure of BRAFV600E in a complex with a selective inhibitor in active conformation and suggested another model for constitutively activated BRAFV600E. This model was based on a negatively charged glutamate at position 600, mimicking the conformation of the phosphorylated wild type protein, which is necessary for kinase activation (26). This was thought to result in constitutively activated BRAF kinase, which is likely to promote RAS-RAF-MEK-ERK network-supported proliferation and tumor growth. This led to the concept that oncogenic mutations in the RAS-RAF-MEK-ERK pathway may provide therapeutic opportunities to target the mutant forms of molecules like BRAFV600E in melanoma (22).
Figure 1.
A, The RAS-RAF-MEK-ERK cellular signaling cascade couples extracellular signals to transcription factors, regulating gene expression. Extracellular signal molecules (i.e. growth factors) bind to their respective receptor tyrosine kinases which in turn recruit and activate the GTPase RAS. RAS phosphorylates and promotes the dimerization and activation of the RAF family (ARAF, BRAF and CRAF) of protein kinases. Activated RAF is responsible for the subsequent signal transmission through MEK1/2 and ERK1/2 and the transcription of genes involved in cell cycle regulation. Mutations in the B-raf gene cause disruptions in the kinase domain and constitutive activation of the BRAF kinase, promoting cell cycle dysregulation, cell survival and proliferation. Kinase inhibitors (Vemurafenib, Dabrafenib) specific for mutant BRAF result in high response rates but short overall median survival of patients with melanoma due to the emergence of resistance. MEK inhibitors (e.g. Trametinib) constitute a strategy in battling BRAF inhibitor resistance. B, BRAF inhibitor treatment may condition tumor microenvironments in favor of immune activation. Top left: Melanoma tumors may promote conditions contributing to ineffective anti-tumoral immunity, e.g.: infiltration of immunosuppressive cells (e.g. Treg, MDSC); immunosuppressive cytokines (IL-6, IL-10, VEGF); reduced MHC Class I and tumor-specific antigen expression; effector cell exhaustion (e.g. detected by expression of PD-1, CTLA-4 on immune cells and PD-L1 on tumor cells). Top right: BRAF inhibitors cause tumor cell death, reducing the associated immunosuppressive signals: favoring infiltration of T- and NK-cells, decreasing immunosuppressive cells and restoring tumor antigen expression and presentation via MHC class I. Bottom right: BRAF inhibitor treatment may condition tumor microenvironments in support of immunotherapy: maintain enhanced immune cell activation in adoptive T cell therapy; counteract T cell exhaustion signals with anti-PD-1, anti-PD-L1, anti-CTLA-4 blockers; maintain loss of immunosuppressive elements with anti-CTLA-4 blockade of Treg infiltrates.
Treatments with Small Molecule Inhibitors to BRAF Mutant Forms
One of the first BRAF inhibitors tested in clinical trials was Sorafenib, a multi-kinase inhibitor, which does not distinguish between mutant and wild type BRAF. Although combined Sorafenib and DTIC treatment resulted in improved response rates and progression-free survival in early trials, it failed to meet expectations in a Phase III clinical trial when compared to standard chemotherapy (27, 28). After Sorafenib, a new generation of BRAF inhibitors selective for mutant BRAF was designed. Vemurafenib, a V600E/K mutation-selective BRAF inhibitor (Fig. 1A) was approved by the FDA in 2011 and by the European Medicines Agency (EMEA) in 2012. In the pivotal Phase III trial of Vemurafenib compared to DTIC, Vemurafenib increased the median overall survival (OS) from 9.6 months for DTIC-treated patients to 13.2 months, and was associated with a response rate of 48%, compared to 5% with standard chemotherapy (DTIC). However, progression-free survival (PFS) was only 5.3 months due to the appearance of drug resistance (29, 30). In an extended follow up study, the agent was shown to improve survival for patients with the most common V600E as well as those with the less common V600K BRAF mutant forms (31).
Resistance is thought to be attributed to a number of factors including induction of alternative splice variants of BRAF or de novo mutations in NRAS or MEK. Up-regulation of signaling through receptor tyrosine kinases in alternative proliferative pathways such as the PI3K/AKT pathway are also thought to be associated with both innate as well as acquired resistance (32). Additionally, even following therapy, constitutively-active BRAF has been reported to still activate the MAPK pathway through dimerizing with CRAF (33-36). BRAF inhibition is also associated with dermatological side effects such as skin photosensitivity, rashes, squamoproliferative lesions including keratoacanthomas and squamous-cell carcinomas or, more rarely, de novo primary melanomas and secondary melanomas. Cases of NRAS-leukaemia and KRAS-mutant colorectal cancer have also been reported (35). These paradoxical oncogenic effects of BRAF inhibitor treatment, often manageable by careful monitoring, are thought to arise from inhibitor recognition of wild type BRAF. This may lead to BRAF-CRAF dimerization along with enhanced RAS, resulting in MAPK pathway activation. In cells with either mutant RAS acquired from external stimuli such as UV exposure or when wild type RAS is activated through external growth factor signals, BRAF inhibition may also support proliferation and migratory properties through focal adhesion kinase (FAK/ERK) signaling (37).
Dabrafenib, a small molecule BRAFV600E kinase inhibitor approved by the FDA in 2013, also acts similarly to Vemurafenib (4), but has a different side-effect profile, in particular reduced phototoxicity (38). As is the case with Vemurafenib however, most patients go on to develop resistance to Dabrafenib (median PFS – 5.1 months). Trametinib is a MEK1/2 kinase inhibitor which functions downstream of BRAF in the same pathway, triggering G1 cell cycle arrest, apoptosis and reduced cell proliferation (39). In a Phase III clinical trial, the agent demonstrated favorable progression-free survival (4.8 months) compared to Dacarbazine (1.5 months) and overall survival rates of 81% compared to 67% with Dacarbazine (40).
BRAF inhibitors thus seem to induce significant but short-term clinical responses. Clinical trials testing alternative BRAF inhibitors are also underway (Table 1), and further in-depth analyses of resistance mechanism pathways and strategies to counteract these are needed.
Table 1.
Registered clinical trials in the United States and Europe for BRAF inhibitors alone or in combination with alternative kinase inhibitors (selected from the following sources: http://www.clinicaltrials.gov; http://public.ukcrn.org.uk; https://www.clinicaltrialsregister.eu)
| Category | Drug/ Intervention | Drug type | Sequence of drug administration | Stage/Cancer type | Identifier | Phase |
|---|---|---|---|---|---|---|
| BRAF inhibitor only | RO5212054 (PLX3603) | BRAFV600 kinase inhibitor | N/Aa | Advanced Solid Tumors | NCT01143753 (US) | I |
| LGX818 | BRAFV600 kinase inhibitor | N/Aa | Locally Advanced or Metastatic Melanoma | NCT01436656 (US) | I | |
| CEP-32496 | BRAFV600E kinase inhibitor | N/Aa | Advanced Solid Tumors (Phase I) Advanced Melanoma and Metastatic Colorectal Cancer (Phase II) | NCT01877811 (US) | I/II | |
| RAF265 (CHIR-265) | BRAF and VEGFR-2 inhibitor | N/Aa | Locally Advanced or Metastatic Melanoma | NCT00304525 (US) | I/II | |
| LGX818 | BRAFV600 kinase inhibitor | N/Aa | Stage IV or Unresectable Stage III Melanoma | NCT01894672 (US) | II | |
| GSK2118436 (Dabrafenib) | BRAFV600E/K kinase inhibitor | N/Aa | Metastatic Melanoma to the Brain | NCT01266967 (US) | II | |
| Vemurafenib | BRAFV600 kinase inhibitor | N/Aa | Metastatic Melanoma to the Brain | NCT01378975 (US) | II | |
| Vemurafenib | BRAFV600 kinase inhibitor | N/Aa | Surgically incurable and unresectable Stage IIIC or Stage BRAF V600 mutation-positive melanoma | NCRN324 BRIM-P (UK) (Paediatric patients) (US) | I | |
| GSK2118436 (Dabrafenib) | BRAFV600E/K kinase inhibitor | N/Aa | Previously treated metastatic (Stage IV) BRAF V600E/K mutation-positive cutaneous melanoma | 2009-015297-36 (EU) | II | |
| Vemurafenib | BRAFV600 kinase inhibitor | N/Aa | High-risk BRAF V600 mutation-positive cutaneous melanoma (Stage IIC or III) after surgical resection | NCRN442 BRIM 8 (UK) | III | |
| Vemurafenib | BRAFV600 kinase inhibitor | N/Aa | BRAFV600 mutation-positive unresectable or metastatic melanoma | NCRN530 ZeSS (UK) | IV | |
| BRAF inhibitor + alternative kinase inhibitor | XL281 (1) +/− Famotidine (2) | Multiple RAF kinase inhibitor (1) H2 receptor antagonist (2) |
XL281 administered once daily, Famotidine administered concomitantly in a single dose during weeks 2, 3 and 4 of first cycle of trial | Non-small-cell Lung Cancer, Colorectal Cancer, Papillary Thyroid Cancer, Melanoma | NCT00451880 (US) | I |
| Vemurafenib (1) + GDC-0973 (Cobimetinib) (2) |
BRAFV600 kinase inhibitor (1) MEK1 inhibitor (2) |
Vemurafenib – oral repeated dose; GDC-0973 – oral repeated dose | Locally-Advanced/Unresectable or Metastatic Melanoma | NCT01271803 (US) | I | |
| PLX3397 (1) + Vemurafenib (2) | RTK inhibitor of KIT, CSF1R and FLT3 (1) BRAFV600 inhibitor (2) |
PLX3397 administered once daily; Vemurafenib administered twice daily | Unresectable or Metastatic Melanoma | NCT01826448 (US) | I | |
| PX-866 (1) + Vemurafenib (2) | PI-3K inhibitor (1) BRAFV600 kinase inhibitor (2) |
PX-866 and Vemurafenib co-administered daily in 28-day cycles | BRAF-mutant Cancers Including Advanced Melanoma | NCT01616199 (US) | I/II | |
| Vemurafenib (1) + P1446A-05 (2) |
BRAFV600 kinase inhibitor (1) Cyclin-dependent kinase 4 inhibitor (2) |
Vemurafenib twice daily; co-administered with P1446A-05 once daily | Advanced or Inoperable Malignant Melanoma | NCT01841463 (US) | I/II | |
| GSK2141795 (1) + Dabrafenib (2) | Akt inhibitor (1) BRAFV600E/K kinase inhibitor (2) |
GSK2141795 once daily co-administered with Dabrafenib twice daily on days 1-28 | BRAF-mutant Cancer Including Recurrent, Stage IIIC and Stage IV Melanoma | NCT01902173 (US) | I/II | |
| LEE011 (1) + LGX818 (2) | Cyclin-dependent kinase 4/6 inhibitor (1) BRAFV600 kinase inhibitor (2) |
LEE011 administered once daily for 21 consecutive days followed by a 7-day break (28 day-cycle); LGX818 administered once daily on a continuous dosing schedule (28-day cycle) | Locally Advanced or Metastatic Melanoma | NCT01777776 (US) | I/II | |
| Dabrafenib (1) + Trametinib (2) |
BRAFV600E/K kinase inhibitor (1) MEK I/II inhibitor (2) |
Dabrafenib administered twice daily on days 1-28; Trametinib added on days 15-28, followed by surgery on days 28-30 | Pre-Surgical Model of Advanced, Operable Melanoma | NCT01701037 (US) | II | |
| Dabrafenib (1) + Trametinib (2) |
BRAFV600E/K kinase inhibitor (1) MEK I/II inhibitor (2) |
Dabrafenib administered twice daily; Trametinib administered once daily | Metastatic Melanoma Which is Refractory or Resistant to BRAF inhibitor | NCT01619774 (US) | II | |
| Dabrafenib (1) + Trametinib (2) |
BRAFV600E/K kinase inhibitor (1) MEK I/II inhibitor (2) |
Dabrafenib administered twice daily; Trametinib administered once daily | Metastatic Melanoma Which is Refractory or Resistant to BRAF inhibitor | NCT01619774 | II | |
| Dabrafenib (1) + Trametinib (2) |
BRAFV600E/K kinase inhibitor (1) MEK I/II inhibitor (2) |
Dabrafenib administered twice daily; Trametinib administered once daily; repeated in 3-week cycles | Unresectable Stage III and Stage IV Melanoma | NCT01726738 (US) | II | |
| Dabrafenib (1) + Trametinib (2) |
BRAFV600E/K kinase inhibitor (1) MEK I/II inhibitor (2) |
Single dose of Dabrafenib alone on Day 1; Continuous repeat doses of Trametinib on Days 2-15; Second single dose of Dabrafenib administered concomitantly with Trametinib on Day 15; No medication administered on Days 16-28 (washout period) | Metastatic Melanoma | NCT01072175 (US) | II | |
| LGX818 (1) + MEK162 (2) vs LGX818 (1) + LEE011 (3) vs LGX818 (1) + BGJ398 (4) vs LGX818 (1) + BKM120 (5) vs LGX818 (1) + INC280 (6) |
BRAFV600 kinase inhibitor (1) MEK1/2 inhibitor (2) Cyclin-dependent kinase 4/6 inhibitor (3) FGFR inhibitor (4) PI-3K inhibitor (5) c-Met inhibitor (6) |
Single agent treatment with LGX818 followed by ‘rational combination’ with other agents following disease progression on LGX818 alone | Locally Advanced or Metastatic Melanoma | NCT01820364 (US) | II | |
| Dabrafenib (1) + Trametinib (2) |
BRAFV600E/K kinase inhibitor (1) MEK I/II inhibitor (2) |
Dabrafenib administered twice daily; Trametinib administered once daily for 12 months | High-risk Melanoma After Surgical Resection | NCT01682083 (US) | III | |
| Dabrafenib (1) + Trametinib (2) vs Dabrafenib (3) monotherapy |
BRAFV600E/K kinase inhibitor (1) MEK I/II inhibitor (2) BRAFV600E/K kinase inhibitor (3) |
Dabrafenib administered twice daily; Trametinib administered once daily | Unresectable (Stage IIIC) or Metastatic (Stage IV) Melanoma | NCT01584648 (US) | III | |
| LGX818 (1) + MEK162 (2) vs LGX818 monotherapy vs Vemurafenib (3) monotherapy |
BRAFV600 kinase inhibitor (1) MEK1/2 inhibitor BRAFV600 kinase inhibitor (3) |
LGX818 administered once daily; MEK162 administered twice daily | Unresectable or Metastatic Melanoma | NCT01909453 (US) | III | |
| Dabrafenib (1) + Trametinib (2) vs Vemurafenib (3) monotherapy |
BRAFV600E/K kinase inhibitor (1) MEK I/II inhibitor (2) BRAFV600 kinase inhibitor (3) |
Dabrafenib administered twice daily; Trametinib administered once daily | Unresectable (Stage IIIc) or Metastatic (Stage IV) Melanoma | NCT01597908 (US) | III | |
| Vemurafenib (1) + GDC-0973 (Cobimetinib) (2) vs Vemurafenib monotherapy |
BRAFV600 kinase inhibitor (1) MEK1 inhibitor (2) |
Vemurafenib administered twice daily on days 1-28 of each 28-day cycle; GDC-0973 administered once daily on days 1-21 of each 28-day cycle | Unresectable Locally Advanced or Metastatic Melanoma | NCT01689519 (US) | III | |
| GSK2118436 (Dabrafenib) (1) + GSK1120212 (Trametinib) (2) vs GSK2118436 (Dabrafenib) + Placebo |
BRAFV600E/K kinase inhibitor (1) MEK I/II inhibitor (2) |
N/Sb | Previously treated advanced, unresectable (Stage IIIC) or metastatic (Stage IV) BRAF V600E/K mutation-positive cutaneous melanoma | 2012-005569-10 (EU) | II | |
| LGX818 (1) + MEK162 (2) vs LGX818 (1) + LEE011 (3) vs LGX818 (1) + BGJ398 (4) vs LGX818 (1) + BKM120 (5) vs LGX818 (1) + INC280 (6) |
BRAFV600 kinase inhibitor (1) MEK1/2 inhibitor (2) Cyclin-dependent kinase 4/6 inhibitor (3) FGFR inhibitor (4) PI-3K inhibitor (5) c-Met inhibitor (6) |
N/Sb | Locally Advanced or Metastatic Melanoma | 2012-004798-17 (EU); NCT01820364 (USA) | II | |
| Dabrafenib (1) + Trametinib (2) vs Vemurafenib (3) monotherapy |
BRAFV600E/K kinase inhibitor (1) MEK I/II inhibitor (2) BRAFV600 kinase inhibitor (3) |
N/Sb | Unresectable (Stage IIIC) or metastatic (Stage IV) BRAF V600E/K mutation-positive cutaneous melanoma | NCRN423 COMBI-V (UK - Closed – in follow-up); 2011-006088-23 (EU - Ongoing) | III | |
| Dabrafenib (1) + Trametinib (2) vs Placebo |
BRAFV600E/K kinase inhibitor (1) MEK I/II inhibitor (2) |
N/Sb | High-risk BRAF V600E/K mutation-positive cutaneous melanoma after surgical resection | NCRN427 COMBI-AD (UK - Ongoing); 2012-001266-15 (EU – Ongoing) | III | |
| Vemurafenib (1) + GDC-0973 (Cobimetinib) (2) vs Vemurafenib monotherapy |
BRAFV600 kinase inhibitor (1) MEK1 inhibitor (2) |
N/Sb | Previously untreated BRAFV600 mutation-positive, unresectable locally advanced (Stage IIIC) or metastatic (Stage IV) melanoma | NCRN510 CO-BRIM (UK) | III | |
| GSK2118436 (Dabrafenib) (1) + GSK1120212 (Trametinib) (2) vs GSK2118436 (Dabrafenib) monotherapy |
BRAFV600E/K kinase inhibitor (1) MEK I/II inhibitor (2) |
N/Sb | Unresectable (Stage IIIC) or metastatic (Stage IV) BRAF V600E/K mutation-positive cutaneous melanoma | NCRN286 COMBI-d (UK-Closed – in follow-up); 2011-006087-49 (EU -Ongoing) | III | |
| LGX818 (1) + MEK162 (2) vs LGX818 monotherapy vs Vemurafenib (3) monotherapy |
BRAFV600 kinase inhibitor (1) MEK1/2 inhibitor BRAFV600 kinase inhibitor (3) |
N/Sb | Locally advanced, unresectable or metastatic BRAF V600 mutant cutaneous melanoma | 2013-001176-38 (EU) | III |
N/A: Not applicable;
N/S: Not stated;
Note: Trials are on-going unless where otherwise stated.
Mutant BRAF and Immune Responses
Evidence for immunogenicity of the mutant BRAF protein in melanoma
A number of studies suggest that mutant forms of BRAF may be recognized by host immunity and could be involved in anti-tumor immune responses. Ex vivo stimulation of lymphocytes derived from patients with melanoma with a synthetic BRAF peptide carrying the V600E mutation led to the generation of MHC class II-restricted CD4+ T cells specific for this peptide; these cells recognized tumor cells expressing mutant BRAF (41). Andersen and colleagues also reported the presence of HLA-B*2705-restricted CTL (Cytotoxic T Lymphocyte) responses in the blood of patients with melanoma against a synthetic mutant but not against the wild-type BRAF (42). Another study reported that stimulation with BRAF peptides carrying the V600E mutation in vitro induced HLA-A*0201-restricted proliferation of T cells derived from patients with BRAFV600E-positive melanomas, these sensitized T cells were cytotoxic against BRAFV600E/HLA-A*0201 positive melanoma cells. Furthermore, HLA-A*0201-restricted BRAFV600E peptides stimulated proliferation of T cells from HLA-A2-positive patients with BRAFV600E-positive melanoma and cytotoxicity against BRAFV600E-positive melanoma cells. T cells from healthy controls and patients with BRAFV600E-negative lesions did not respond to mutated epitope challenge (43). As the HLA-A*0201 haplotype is present in 50% of patients with melanoma, vaccination studies aimed at activating immunity against the mutant BRAFV600E form in this patient group were suggested. In concordance with these findings, blocking of the BRAF-MAPK pathway in BRAF signaling-addicted melanoma cells in vitro triggered enhanced recognition of tumor cell antigens by tumor-infiltrating T lymphocytes and the authors suggested that BRAF blockade and adoptive T cell therapies may confer synergistic effects (44).
Taken together these studies suggest that immune responses against the mutant form of BRAF may be present or harnessed in melanoma and that the selective immunogenicity of the mutant forms may provide the basis for the development of new strategies to overcome immunological tolerance.
BRAF mutant forms and immune escape mechanisms
In addition to immunogenic responses against mutated BRAF, a number of studies suggest that the mutant BRAF protein kinase may conversely be associated with tumor-induced immune escape mechanisms. Increased expression of immunosuppressive mediators (IL-6, IL-10, VEGF) by BRAFV600E melanoma cells have been reported. These cytokines can promote recruitment of inflammatory cell subsets such as Myeloid Derived Suppressor Cells (MDSC) and regulatory T cells (Treg) in tumor microenvironments. These effects were reduced through targeted mutant BRAF protein inhibition or through RNA interference strategies, implying that eliminating mutant BRAF-expressing tumor cells could result in some control of tumor-associated immunosuppressive signals (45). Additionally, it was demonstrated that under these conditions, BRAFV600E melanoma cells could impair maturation of DC, and suppress their capacity to produce IL-12 and TNFα (45). Another study reported increased transcription of IL-1 (IL-1α and IL-1β) by melanoma cell lines transduced to express BRAFV600E. IL-1α/β-stimulated tumor-associated fibroblasts could suppress melanoma-specific cytotoxic T cell functions by upregulation of the checkpoint ligand molecules COX-2, PD-L1 and PD-L2. IL-1 protein overexpression was reversed by targeted BRAFV600E inhibition both in cell lines and in tumors from patients treated with the BRAFV600E inhibitor Vemurafenib (46). Although IL-1 cytokines play immunostimulatory as well as immunosuppressive functions in different contexts, these findings suggest that BRAF inhibition could influence these immune signals in tumors. Another immunosuppressive effect of mutant BRAF may relate to the downregulation of MHC class I molecules by melanoma cells. MHC class I expression is reduced in A375 melanoma cells over-expressing mutant BRAFV600E, and MHC class I and II expression triggered by IFNγ and IFNα2b can be enhanced after BRAF inhibition with Vemurafenib only in homozygous, but not in heterozygous, BRAFV600E mutant cells (47).
In spite of evidence to support that immune responses against mutant BRAF could be triggered under certain contexts, associations of BRAFV600E-expressing melanoma cells with immunosuppressive signals indicate that melanomas may employ adaptive mechanisms to avoid immune clearance (Fig. 1B).
Could BRAF inhibitors be toxic to host immunity?
An important question relates to the potential of BRAF inhibitors to exert toxic effects on immune cells. Several studies have indicated that BRAF inhibitors do not appear to have direct adverse effects on lymphocytes. Comin-Anduix et al. reported that clinical concentrations of the BRAFV600E inhibitor Vemurafenib which are cytotoxic to melanoma cells do not affect the viability or function of lymphocytes from healthy donors or from patients with melanoma. Importantly, there was a significant gap between the therapeutic concentrations of the inhibitor and the concentrations needed to observe any toxic effects of the agent on lymphocytes (48). Additionally, treatment of different melanoma cells lines and primary melanoma tumor digests with a selective BRAFV600E inhibitor resulted in enhanced expression of melanocyte differentiation antigens, important for immune recognition (e.g. gp100, MART-1), while not adversely affecting melanoma patient T cell function (48-50). Another study confirmed that a BRAF inhibitor selective for BRAFV600E, BRAFV600K and BRAFV600G did not affect cancer patients’ immunity in relation to a number of clinical parameters, such as serum cytokine levels, PBMC counts and frequencies of different leukocyte subsets (B cells, T cells, NK cells, monocytes, DC, Treg) (51). A subsequent study reported no adverse effects of Vemurafenib on cytokine production by CD4+ and CD8+ T cells in a BRAF wild type (BRAFWT) mouse model, and no deleterious effects on T cell-mediated anti-tumoral functions.
However, in spite of significant evidence to suggest that BRAF inhibitors do not have a deleterious effect on host immune cells, Hooijkaas et al. reported some adverse effects of Vemurafenib on the immune response in BRAFV600E-positive melanoma mouse models. Treatment was associated with significant decreases in tumor infiltrating T cells, NK cells, monocytes and myeloid derived suppressor cells (MDSC), and infiltration of these cells could not be rescued by addition of an anti-CTLA-4 antibody. The same study reported that combination treatments with anti-CTLA-4 antibodies did not confer additional tumor restriction above those observed with Vemurafenib alone. The findings may be interpreted as a negative impact of Vemurafenib on immune responses in the local tumor microenvironment (52). An alternative explanation may be that Vamurafenib-induced tumor cell death leads to reduced immunosuppressive cytokine production and reduced numbers of immunoregulatory cell infiltrates such as Treg or MDSCs. The latter may explain the absence of additive tumor restrictive effects with anti-CTLA-4 antibody treatment, which is thought to also function by targeting immune effector cell responses against CTLA-4-expressing tumor-associated Treg (53, 54).
BRAF inhibitor influence on host immune responses
Although BRAF inhibitor drugs are not designed to directly activate anti-tumor immune responses, there is increasing evidence to indicate enhanced anti-tumor immunity with use of these agents and correlation with clinical responses. Wilmott et al. demonstrate increased CD4+ and CD8+ T cell infiltration in melanoma patient biopsies from patients in the early stages of treatment with Vemurafenib and Dabrafenib (55). Immune cell infiltrate rates in biopsies from patients who relapsed following treatment resembled those observed in pre-treatment samples, and correlated with the appearance of resistance against BRAF inhibitor treatment. These observations imply BRAF inhibitors appear to reverse some tumor-associated immunosuppressive signals and that the immunostimulatory effects observed with response to treatments subside with disease progression. Another study also revealed increases in the frequency of tumor antigen-specific CD8+ T cells and modest increases in circulating levels of tumor necrosis factor α (TNFα) associated with BRAFV600 inhibitors (51). Furthermore, BRAFV600E inhibition has been reported to restore maturation of DC and production of TNFα and IL-12 without any adverse effects on DC viability or capacity to prime T cells (45, 56). BRAF inhibitor treatment of patients with metastatic melanoma resulted in reduced generation and differentiation of MDSC, known to be promoted by tumor-induced expression of immunosuppressive cytokines such as IL-6 (57).
In a BRAFV600E/Pten inducible mouse model of melanoma, the immunosuppressive effects of BRAFV600E melanoma tumors were manifested through accumulation of immunoregulatory cell subsets such as FoxP3+ Tregs and CD11b+/Gr-1+ MDSCs, reduced frequencies of CD4 T cells producing IFNγ, TNFα and IL-2 and lower expression of the co-stimulatory ligand CD40L (58). Compromized CD40L-CD40 signaling was linked to loss of maturation signals necessary for antigen presentation, resulting in concentration of immature DCs, and of macrophages featuring alternatively activated M2 phenotypes in tumors. BRAFV600E inhibitor treatment reduced tumor growth and increased CD8 and CD4 T cell infiltration. Importantly, enhanced expression of CD40L and production of IFNγ by CD4 T cells along with reduced frequencies of FoxP3+ Tregs were observed and specific blocking of IFNγ and CD40L signaling were individually shown to impede BRAFV600E inhibitor effects. These findings support key contributions of host immunity to BRAF inhibitor functions. In a mouse model of BRAFV600E melanoma, Vemurafenib treatment decreased expression of the regulatory Chemokine (C-C motif) Ligand 2 (CCL2) by melanoma cells and this was associated with tumor growth reduction. Reduced tumor growth was associated with higher NK cell infiltrates and increases in the CD8+ T cell to Treg ratios in tumors (59). In the same study it was shown that combining BRAFV600E-targeted therapy with antibodies to immune modulatory molecules such as CD137 and CCL2 with could confer additional benefits in restricting tumor growth and in suppressing de novo tumorigenesis. These findings further support the notion that activating immunity alongside pathway inhibition could be beneficial (Fig. 1B).
In summary, some studies suggest that BRAF-MAPK pathway inhibition may not have a negative impact on the immune system of cancer patients, but may influence host immune responses systemically and in tumor microenvironments in multiple ways, counteracting immunosuppressive pathways and often favoring immune activation (Fig. 1B). These effects may be directly attributed to reduced tumor cell viability and consequently to reduced tumor-induced immunomodulation. The subsequent appearance of resistance to BRAF inhibitors and restoration of tumor growth and the tumor-induced immunosuppressive equilibrium at the time of disease progression support a link between mutant BRAF dysregulation and alterations in immune signals in cancer (46, 47).
Combination Treatments: Possible Synergistic Benefits with Enhancing Immunity?
Pathway inhibitor combinatory approaches
It has been suggested that combination therapies with BRAF and alternative inhibitors of the MAPK pathway might be a strategy to overcome resistance and to prolong patient progression-free survival, with the aim of improving the short-lived clinical benefits of BRAF-targeted monotherapies (35). BRAF inhibitor-resistant melanomas have been reported to feature elevated expression of the checkpoint molecule ligand PD-L1, which could be reduced with subsequent treatment with MEK and PI3K inhibitors (60). These findings may indicate additive effects of combined pathway inhibitors on known checkpoint immune modulatory mechanisms. Results of an open-label combination phase I/II clinical trial between the newly FDA-approved BRAFV600E/K inhibitor Dabrafenib and the MEK1/2 inhibitor Trametinib provided some optimism with a non-significantly reduced frequency of cutaneous squamous-cell carcinomas detected, possibly due to reduction of MAPK signaling which may counteract the effects of BRAF inhibitors on wild type cells. The study showed significantly-increased median PFS and response rates compared to those with single agents (61); however, clinical efficacy for combination treatments remains to be determined. A potential future direction may entail the use of inhibitors to RAF and ERK alongside BRAF blockade, which may also prevent the paradoxical activation of the MAPK pathway in wild type cells. Immune response activation may also be enhanced in these combinatory strategies, as hallmarks of immunosuppression associated with resistance to BRAF-targeted therapy characterized by lower CD8+ T cell infiltration and reduced tumor antigen expression at the time of progression were reversed with BRAF and MEK combination therapies (62). Tumor biopsies from patients with metastatic melanoma treated with either a BRAF inhibitor (Vemurafenib) alone or BRAF plus MEK inhibitor combinations (Dabrafenib + Trametinib) were taken before and during therapy and at the point of relapse. BRAF alone and BRAF plus MEK combination treatments were associated with increased tumor antigen expression, CD8+ T cell infiltrates and reduced inflammatory cytokine levels (IL-6, IL-8). In biopsies from patients treated with BRAF inhibitor treatment alone, upregulation of the exhaustion markers PD-1 and TIM-3 in T cells and of PD-L1 (PD-1 ligand) on melanoma cells (62) might help explain subsequent loss of immune activatory signals and reversal towards immune suppression in BRAF inhibitor-resistant disease. Clinical trials of combination therapies with a BRAF inhibitors and different MEK, Cdk, PI3K and Akt inhibitors may provide novel clinical and mechanistic insights in future (Table 1).
Pathway inhibitors and immunotherapy: aiming to overcome immune suppression
BRAF inhibitors may initially condition the tumor microenvironment to favor immune activation, perhaps rendering these agents synergistic partners to strategies specifically targeting the immune response. The proposition that the clinical efficacy observed with small molecule inhibitors might be enhanced if combined with immunotherapies is currently under investigation in a number of pre-clinical and clinical settings (Fig. 1B; Table 2).
Table 2.
Registered clinical trials in the United States and Europe for BRAF inhibitors in combination with immunotherapies with or without different kinase inhibitors and/or chemotherapeutic agents (selected from the following sources: http://www.clinicaltrials.gov; http://public.ukcrn.org.uk; https://www.clinicaltrialsregister.eu)
| Drug/Intervention | Drug type | Sequence of drug administration | Stage/Cancer type | Identifier | Phase |
|---|---|---|---|---|---|
| Ipilimumab (1) +/− Dabrafenib (2) +/− Trametinib (3) | anti-CTLA-4 monoclonal antibody (1) BRAFV600E/K kinase inhibitor (2) MEK I/II kinase inhibitor (2) |
Oral Dabrafenib twice daily for 25 days; +/− Oral Trametinib once daily for 25 days; +/− IV Ipilimumab repeated every 3 weeks for 4 courses | Unresectable or Metastatic Melanoma | NCT01940809 (US) | I |
| Vemurafenib (1) + Young TILsa (2) + Cyclophosphamide (3) + Fludarabine (4) + Aldesleukin (5) |
BRAFV600 kinase inhibitor (1) Tumor infiltrating lymphocytes (2) Chemotherapeutic agent (3) Chemotherapeutic agent (4) IL-2 (5) |
Once cryopreserved, autologous TILa available, patients commence oral Vemurafenib administered twice daily; IV Cyclophosphamide on Days -7 and -6; IV Fludarabine on Days -5 through -1; Infusion of 1 × 109- 2 × 1011 young TILa on Day 0; followed by IV infusion of high dose Aldeseukin | Metastatic Melanoma | NCT01585415 (US) | I |
| Dabrafenib (1) +/− Trametinib (2) + Ipilimumab (3) |
BRAFV600E/K kinase inhibitor (1) MEK I/II kinase inhibitor (2) anti-CTLA-4 monoclonal antibody (3) |
Oral Dabrafenib administered twice daily; +/− Oral Trametinib administered once daily; IV Ipilimumab repeated every 3 weeks for 4 courses | Unresectable or Metastatic Melanoma | NCT01767454 (US) | I |
| Ipilimumab (1) + Dabrafenib (2) + Trametinib (3) vs Ipilimumab (1) + Trametinib (3) vs Ipilimumab (1) + Dabrafenib (2) vs Ipilimumab (1) monotherapy | anti-CTLA-4 monoclonal antibody (1) BRAFV600E/K kinase inhibitor (2) MEK I/II inhibitor (3) |
Oral Dabrafenib twice daily for 25 days; Oral Trametinib once daily for 25 days; followed by IV Ipilimumab repeated every 3 weeks for 4 courses | Unresectable or Metastatic Melanoma | NCT01938703 (US) | I |
| Vemurafenib (1) + Interleukin-2 + Interferon Alpha-2b | BRAFV600 kinase inhibitor (1) | Oral Vemurafenib administered twice daily for a 21 day cycle; IV Interleukin-2 administered on Days 2-5 of a 21 day cycle; Subcutaneous Interferon Alpha-2b administered on Days 1-5 of a 21 day cycle | Metastatic Melanoma | NCT01603212 (US) | I/II |
| Ipilimumab (1) + Vemurafenib (2) | anti-CTLA-4 monoclonal antibody (1) BRAFV600 kinase inhibitor (2) |
Oral Vemurafenib administered twice daily; IV Ipilimumab repeated every 3 weeks | Metastatic Melanoma | NCT01400451 (US) | I/II |
| Lymphodepletion using Fludarabine (1) and Cyclophosphamide (2) ACTb with TILa Infusion + Vemurafenib (3) HDc Interleukin-2 (Aldesleukin) | Chemotherapeutic agent (1) Chemotherapeutic agent (2) BRAFV600 kinase inhibitor (3) |
Combination of Vemurafenib followed by lymphodepletion with Fludarabine and Cyclophosphamide; ACTb with TILa infusion followed by high dose IL-2 | Metastatic Melanoma | NCT01659151 (US) | II |
| Vemurafenib (1) + Aldesleukin (2) |
BRAFV600 kinase inhibitor (1) IL-2 (2) |
Oral Vemurafenib administered twice daily; IV infusion of Aldesleukin administered as per study protocol | Metastatic or Unresectable Melanoma | NCT01754376 (US) | II |
| HDc Interleukin-2 (IL-2) + Vemurafenib (1) | BRAFV600 kinase inhibitor (1) | Initial course of Vemurafenib followed by high dose IL-2 (patients discontinue Vemurafenib prior to treatment with IL-2 and resume dosing afterward) | Metastatic Melanoma | NCT01683188 (US) | IV |
| Vemurafenib (1) + Pegylated Interferon Alpha-2b + Interleukin-2 | BRAFV600 kinase inhibitor (1) | N/Se | Unresectable Stage III or Stage IV BRAF mutation-positive cutaneous melanoma | 2013-000773-71 (EU) | II |
| Chemotherapyd + Interferon Alpha-2b + Vemurafenib (1) vs Chemotherapy + Interferon Alpha-2b | BRAFV600 kinase inhibitor (1) | N/Se | Unresectable (Stage III) or metastatic (Stage IV) BRAF mutation-positive melanoma vs Unresectable (Stage III) or metastatic (Stage IV) BRAF mutation-negative melanoma | 2013-000280-84 (EU) | II |
TIL: Tumor infiltrating lymphocytes;
ACT: Adoptive Cell Therapy;
HD: High dose;
Unspecified chemotherapy;
N/S: Not stated;
Note: Trials are on-going unless where otherwise stated
Preclinical studies showed that BRAF inhibitors may enhance the potency of adoptive immune cells by promoting tumor antigen expression, antigen recognition and T cell infiltration in tumors (44). In a mouse model of melanoma, Vemurafenib in combination with adoptive lymphocyte transfer therapy resulted in enhanced tumor cytotoxicity and cytokine secretion by tumor-infiltrating adoptively-transferred T cells (63). In another in vivo model, Vemurafenib treatment resulted in increased T cell infiltration into tumors and was attributed to loss of VEGF expression by destruction of melanoma cells. In this study, the findings were consistent with reduced VEGF expression in biopsy samples of patients treated with BRAFV600E inhibitor therapy (64). These findings provide rationale for synergistic effects when combining BRAF inhibition and T cell immune activatory therapy strategies and inspired early clinical trials for this concept.
Another approach would be to combine the increased intra-tumoral immune activity following BRAF inhibitor administration with the functions of checkpoint blockade inhibitor antibodies to overcome T cell exhaustion (65). However, a Phase I clinical trial featuring concurrent treatment with Ipilimumab (anti-CTLA-4 antibody) and Vemurafenib (BRAFV600E/K inhibitor) reported asymptomatic hepatotoxicity effects reversible upon discontinuation of the drug combinations or with glucocorticoid treatment, resulting in termination of the study (66). Phase I clinical trials with the BRAFV600E/K inhibitor Dabrafenib with or without Trametinib (MEK1/2 inhibitor) in combination with Ipilimumab are on-going (Table 2). Other suggested treatment partners of BRAF inhibitors include IFNα2b, IL-2, antibodies to PD-L1, CD137 and IL-1 blockers which may act as adjuvants (47, 59). Clinical trials to test different combinations of Vemurafenib with IL-2 or IFNα2b are on-going and are expected to shed light on the merits of future combination therapies (Table 2). However, since IFNα2b or IL-2 monotherapies are associated with high reported toxicities, a cautious approach to such combinatory strategies would be paramount.
Translational Considerations and the Future of Combination Treatments
A number of factors, relating to efficacy and safety, may be taken into account when considering implementation of combination strategies; these may include dose, timing and sequence of administration. For these there is currently little precedence, but perhaps understanding and taking into account host immune responses may be important in this context.
It has been proposed that patients with mutant BRAF-expressing tumors with highly symptomatic disease, in particular those with acutely life threatening metastases such as brain metastases, should be prescribed a BRAF inhibitor treatment based on capacity to trigger quick clinical responses. Considering the quick impact on tumor growth restriction and the positive overall effect of BRAF inhibitors on immunity, treatment with a BRAF inhibitor might perhaps be followed by immunotherapy such as a checkpoint blocker to neutralize T cell inhibitory signals (67). On the other hand, a case for immunotherapy preceding BRAF inhibitor therapy may be supported by early reports that significant proportions (around half) of patients who do not respond to BRAF inhibitor treatment generally have a worse clinical outlook compared to untreated patients. It is therefore worth exploring whether identifying and treating these patients with immunotherapy such as with Ipilimumab as early as possible may be beneficial (68, 69). It may also be reasonable for asymptomatic patients to be treated with immunotherapy, because of the potential to confer long-term, durable responses. The same patients may then receive BRAF inhibitor treatment as a salvage therapy on disease progression (70). This however may not be widely practical, since in some countries, including the United Kingdom, Ipilimumab is not yet routinely funded for first line therapy for metastatic melanoma, therefore patients with early disease may not be able to access treatment.
In future, the clinical landscape may feature pathway inhibitor simultaneous combinations with immunotherapies, including checkpoint blockade agents. Treatment combinations may be informed by elucidating tumor escape mechanisms associated with pathway inhibitors and by designing complementary immune intervention strategies to overcome these. With increasing numbers of patients now treated with pathway inhibitor drugs, the critical mechanisms of immune response cross-talk with BRAF mutant melanomas and BRAF inhibitors and how these are linked to the almost inevitable rise of resistant disease require further in-depth investigation. Under the selective pressure of human immune responses and pathway blockade interventions, tumors may evolve to activate alternative downstream interacting signaling pathways and can escape destruction through clonal selection. This may give rise to BRAF-resistant melanomas with enhanced capacity to manipulate immunity.
Patients with mutant BRAF or NRAS melanomas at later disease stages (III and IV) -the cohort most likely to be offered pathway inhibitor drugs- have a worse prognosis when compared to other patient groups (71, 72). BRAF-resistant melanomas are able to maintain or reactivate important signaling pathways MAPK and PI3K. Indeed, genomic analyses identified mutant BRAF amplification, alternative splice variants and de novo RAS gene alterations as well as mutations in the PI3K/Pten/Akt pathway associated with BRAF-resistant tumors (73, 74). Under the selective pressure of pathway inhibitor drugs, MAPK pathway-promoted immune suppressive mediators VEGF, IL-6 and IL-10 could constitute particularly important tools for emerging resistant tumors to maintain or re-establish command of their microenvironments and to re-educate host immunity (45). Enhanced expression of PD-L1 by reactivated MAPK pathway in BRAF inhibitor-resistant melanomas would also support capability to reclaim suppression of host immune sentinels like T cells (60). It is therefore possible that BRAF mutant melanomas constitute more aggressive tumors better able to establish effective suppression of host immunity along with BRAF inhibitor-resistance.
Exploring resistance as a function of pathway network dysregulation rather than in relation to particular mutations on individual molecules, together with monitoring immune suppressive or activatory signals, may help elucidate specific signatures associated with disease progression and lead to the identification of targets for immunotherapy. For instance, if tumor escape is associated with enhanced VEGF production and PD-L1 expression by T cells, possible combinations of VEGF and/or PD-L1 blockade strategies with pathway inhibitors may prevent or restrict melanoma progression. Furthermore, therapeutics that could support or complement pathway inhibitor functions by “waking up” dormant immune activating signals such as enhancing CD40-CD40L interactions to promote antigen-presenting cell activation may help target “Achilles heel” elements of melanomas and augment pathway inhibitor drug effects. Toxicities observed in the Ipilimumab plus Vemurafenib combinatory trial, perhaps due to paradoxical MAPK pathway activation of wild-type cells alongside autoimmune effects of Ipilimumab in the presence of enhanced BRAF inhibitor-induced antigen presentation, mandate careful design of therapeutic strategies less likely to attack healthy tissues. Combinations with tumor antigen-specific antibodies or T cells that selectively target tumor cells, or attacking modulatory elements in tumor microenvironments associated with tumor resistance to BRAF inhibitors such as the PD-1/PD-L1 axis, may merit consideration. The complexities of the interactions between resistance to pathway inhibition and immunomodulation may be addressed with emerging and novel bioinformatics tools. Activatory immunological signatures associated with specific melanoma subtypes have already been shown to predict more favorable prognosis. Furthermore, immune suppressive molecular signatures predict worse clinical outcomes (71, 75). These indicate double-edged sword roles for immunity in melanoma disease progression, but equally support the rationale for monitoring immunity alongside clinical course and clinical responses to treatments.
Concluding Thoughts
Novel insights from the laboratory and the clinic support links between pathway dysregulation with different components of immune responses. BRAF inhibitors exhibit immune activating functions, which alone, may not be sufficient to counteract tumor-associated escape mechanisms. Thus combination treatments with different inhibitors of the RAS-RAF-MEK-ERK proliferative pathway, and also with immunotherapies may help enhance these circumscribed immunological and clinical responses. Future translational directions may take advantage of dysregulated pathway molecules such as BRAF being recognized by host immunity and of molecular pathway cross-talk with specific molecules associated with immune suppression to develop rational targeted immunotherapies such as vaccines and therapeutic antibodies. Bioinformatics tools such as gene clustering and pathway analyses in large patient datasets are revealing differential classification of melanomas, including specific immunological signatures associated with good or bad prognoses. As both checkpoint blockade antibodies and small molecule inhibitors are in clinical use, it is important now to elucidate whether we can link clinical responses with immune activation or with counteracting immune suppressive signals in order to improve treatment. New approaches may consider molecular heterogeneity and pathway dysregulation together with monitoring immunological parameters such as antigen presentation, effector cell activation or immunosuppressive elements in tumors before and during therapy and while in remission. These could in future provide additional criteria with which to predict clinical benefits, facilitate stratification and guide optimal monotherapy or combinatory approaches for different subsets of patients.
Acknowledgments
Grant Support
The authors acknowledge support from Cancer Research UK (C30122/A11527; C30122/A15774) (S.N. Karagiannis; D.H Josephs; J.F. Spicer); KCL Experimental Cancer Medicine Centre, jointly funded by Cancer Research UK, the National Institute for Health Research, Welsh Assembly Government, HSC R&D Office for Northern Ireland and Chief Scientist Office, Scotland (J.F. Spicer; S.N. Karagiannis; F.O. Nestle); CR UK/EPSRC/MRC/NIHR KCL/UCL Comprehensive Cancer Imaging Centre (C1519/A10331) (J.F. Spicer; D.H. Josephs; S.N. Karagiannis); the Medical Research Council (MR/L023091/1) (S.N. Karagiannis; F.O. Nestle); and the Dermatrust (K.E. Lacy; I.U. Egbuniwe; F.O. Nestle). The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London (S.N. Karagiannis; P. Karagiannis; F.O. Nestle; K.E. Lacy; K.M. Ilieva, I. Correa). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The authors are solely responsible for study design, data collection, analysis, decision to publish, and preparation of the manuscript.
Abbreviations
- CTLA-4
Cytotoxic T-lymphocyte antigen 4
- PD-1
Programmed cell death 1
- PD-L
Programmed cell death Ligand
- DTIC
Dacarbazine
- IFN
Interferon
- EMEA
European Medicines Agency
- FDA
Food and Drug Administration
- Treg
regulatory T cells
- VEGF
Vascular Endothelial Growth Factor
- TGFβ
Transforming Growth Factor
- MDSC
myeloid-derived suppressor cells
- DC
dendritic cells
- CTL
cytotoxic T cells
- TNFα
Tumor Necrosis Factor α
- OS
Overall survival
- PFS
progression-free survival
- C-C motif
Chemokine
- CCL2
Ligand 2
- TIL
Tumor infiltrating lymphocytes
- ACT
Adoptive Cell Therapy
- HD
High dose
Footnotes
Disclosures of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27:3–9. doi: 10.1016/j.clindermatol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauschild A. Adjuvant interferon alfa for melanoma: new evidence-based treatment recommendations? Curr Oncol. 2009;16:3–6. doi: 10.3747/co.v16i3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballantyne AD, Garnock-Jones KP. Dabrafenib: first global approval. Drugs. 2013;73:1367–76. doi: 10.1007/s40265-013-0095-2. [DOI] [PubMed] [Google Scholar]
- 5.Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K, et al. Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov. 2012;11:873–86. doi: 10.1038/nrd3847. [DOI] [PubMed] [Google Scholar]
- 6.Wright CJ, McCormack PL. Trametinib: first global approval. Drugs. 2013;73:1245–54. doi: 10.1007/s40265-013-0096-1. [DOI] [PubMed] [Google Scholar]
- 7.Sznol M. Advances in the treatment of metastatic melanoma: new immunomodulatory agents. Semin Oncol. 2012;39:192–203. doi: 10.1053/j.seminoncol.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Shimanovsky A, Jethava A, Dasanu CA. Immune alterations in malignant melanoma and current immunotherapy concepts. Expert Opin Biol Ther. 2013;13:1413–27. doi: 10.1517/14712598.2013.827658. [DOI] [PubMed] [Google Scholar]
- 9.Fujimura T, Ring S, Umansky V, Mahnke K, Enk AH. Regulatory T cells stimulate B7-H1 expression in myeloid-derived suppressor cells in ret melanomas. J Invest Dermatol. 2012;132:1239–46. doi: 10.1038/jid.2011.416. [DOI] [PubMed] [Google Scholar]
- 10.Song S, Wang Y, Wang J, Lian W, Liu S, Zhang Z, et al. Tumour-derived IL-10 within tumour microenvironment represses the antitumour immunity of Socs1-silenced and sustained antigen expressing DCs. Eur J Cancer. 2012;48:2252–9. doi: 10.1016/j.ejca.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert AE, Karagiannis P, Dodev T, Koers A, Lacy K, Josephs DH, et al. Monitoring the systemic human memory B cell compartment of melanoma patients for anti-tumor IgG antibodies. PLoS One. 2011;6:e19330. doi: 10.1371/journal.pone.0019330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karagiannis P, Gilbert AE, Josephs DH, Ali N, Dodev T, Saul L, et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. The Journal of clinical investigation. 2013;123:1457–74. doi: 10.1172/JCI65579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayaraman P, Parikh F, Lopez-Rivera E, Hailemichael Y, Clark A, Ma G, et al. Tumor-expressed inducible nitric oxide synthase controls induction of functional myeloid-derived suppressor cells through modulation of vascular endothelial growth factor release. Journal of immunology (Baltimore, Md: 1950) 2012;188:5365–76. doi: 10.4049/jimmunol.1103553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, Ge Y, Xiao M, Lopez-Coral A, Azuma R, Somasundaram R, et al. Melanoma-derived conditioned media efficiently induce the differentiation of monocytes to macrophages that display a highly invasive gene signature. Pigment Cell Melanoma Res. 2012;25:493–505. doi: 10.1111/j.1755-148X.2012.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs JF, Nierkens S, Figdor CG, de Vries IJ, Adema GJ. Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy? Lancet Oncol. 2012;13:e32–42. doi: 10.1016/S1470-2045(11)70155-3. [DOI] [PubMed] [Google Scholar]
- 16.Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. Journal of immunology (Baltimore, Md: 1950) 2012;189:5602–11. doi: 10.4049/jimmunol.1201018. [DOI] [PubMed] [Google Scholar]
- 17.Simeone E, Ascierto PA. Immunomodulating antibodies in the treatment of metastatic melanoma: the experience with anti-CTLA-4, anti-CD137, and anti-PD1. J Immunotoxicol. 2012;9:241–7. doi: 10.3109/1547691X.2012.678021. [DOI] [PubMed] [Google Scholar]
- 18.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, et al. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol. 2013;24:2174–80. doi: 10.1093/annonc/mdt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. The New England journal of medicine. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 21.Keshet Y, Seger R. The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol. 2010;661:3–38. doi: 10.1007/978-1-60761-795-2_1. [DOI] [PubMed] [Google Scholar]
- 22.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 23.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–8. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6:313–9. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 25.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 26.Xie P, Streu C, Qin J, Bregman H, Pagano N, Meggers E, et al. The crystal structure of BRAF in complex with an organoruthenium inhibitor reveals a mechanism for inhibition of an active form of BRAF kinase. Biochemistry. 2009;48:5187–98. doi: 10.1021/bi802067u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisen T, Marais R, Affolter A, Lorigan P, Robert C, Corrie P, et al. Sorafenib and dacarbazine as first-line therapy for advanced melanoma: phase I and open-label phase II studies. British journal of cancer. 2011;105:353–9. doi: 10.1038/bjc.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:2823–30. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 29.Chapman PB, Hauschild A, Robert C, Larkin JMG, Haanen JBAG, Ribas A, et al. Updated overall survival (OS) results for BRIM-3, a phase III randomized, open-label, multicenter trial comparing BRAF inhibitor vemurafenib (vem) with dacarbazine (DTIC) in previously untreated patients with BRAFV600E-mutated melanoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012:8502. ASCO Annual Meeting2012. [Google Scholar]
- 30.da Rocha Dias S, Salmonson T, van Zwieten-Boot B, Jonsson B, Marchetti S, Schellens JH, et al. The European Medicines Agency review of vemurafenib (Zelboraf(R)) for the treatment of adult patients with BRAF V600 mutation-positive unresectable or metastatic melanoma: summary of the scientific assessment of the Committee for Medicinal Products for Human Use. Eur J Cancer. 2013;49:1654–61. doi: 10.1016/j.ejca.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 31.McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323–32. doi: 10.1016/S1470-2045(14)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao M, Tian F, Mariadason JM, Tsao CC, Lemos R, Jr., Dayyani F, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:657–67. doi: 10.1158/1078-0432.CCR-11-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rushworth LK, Hindley AD, O’Neill E, Kolch W. Regulation and role of Raf-1/B-Raf heterodimerization. Molecular and cellular biology. 2006;26:2262–72. doi: 10.1128/MCB.26.6.2262-2272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 35.Gibney GT, Messina JL, Fedorenko IV, Sondak VK, Smalley KS. Paradoxical oncogenesis--the long-term effects of BRAF inhibition in melanoma. Nat Rev Clin Oncol. 2013;10:390–9. doi: 10.1038/nrclinonc.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flaherty KT. Dividing and conquering: controlling advanced melanoma by targeting oncogene-defined subsets. Clinical & experimental metastasis. 2012;29:841–6. doi: 10.1007/s10585-012-9488-y. [DOI] [PubMed] [Google Scholar]
- 37.Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 39.Gilmartin AG, Bleam MR, Groy A, Moss KG, Minthorn EA, Kulkarni SG, et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:989–1000. doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- 40.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. The New England journal of medicine. 2012;367:107–14. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 41.Sharkey MS, Lizee G, Gonzales MI, Patel S, Topalian SL. CD4(+) T-cell recognition of mutated B-RAF in melanoma patients harboring the V599E mutation. Cancer research. 2004;64:1595–9. doi: 10.1158/0008-5472.can-03-3231. [DOI] [PubMed] [Google Scholar]
- 42.Andersen MH, Fensterle J, Ugurel S, Reker S, Houben R, Guldberg P, et al. Immunogenicity of constitutively active V599EBRaf. Cancer research. 2004;64:5456–60. doi: 10.1158/0008-5472.CAN-04-0937. [DOI] [PubMed] [Google Scholar]
- 43.Somasundaram R, Swoboda R, Caputo L, Otvos L, Weber B, Volpe P, et al. Human leukocyte antigen-A2-restricted CTL responses to mutated BRAF peptides in melanoma patients. Cancer research. 2006;66:3287–93. doi: 10.1158/0008-5472.CAN-05-1932. [DOI] [PubMed] [Google Scholar]
- 44.Donia M, Fagone P, Nicoletti F, Andersen RS, Hogdall E, Straten PT, et al. BRAF inhibition improves tumor recognition by the immune system: Potential implications for combinatorial therapies against melanoma involving adoptive T-cell transfer. Oncoimmunology. 2012;1:1476–83. doi: 10.4161/onci.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. The Journal of experimental medicine. 2006;203:1651–6. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khalili JS, Liu S, Rodriguez-Cruz TG, Whittington M, Wardell S, Liu C, et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:5329–40. doi: 10.1158/1078-0432.CCR-12-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sapkota B, Hill CE, Pollack BP. Vemurafenib enhances MHC induction in BRAF homozygous melanoma cells. Oncoimmunology. 2013;2:e22890. doi: 10.4161/onci.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Comin-Anduix B, Chodon T, Sazegar H, Matsunaga D, Mock S, Jalil J, et al. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:6040–8. doi: 10.1158/1078-0432.CCR-10-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vosganian GS, Bos R, Sherman LA. Immunologic effects of an orally available BRAFV600E inhibitor in BRAF wild-type murine models. J Immunother. 2012;35:473–7. doi: 10.1097/CJI.0b013e3182618209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer research. 2010;70:5213–9. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 51.Hong DS, Vence L, Falchook G, Radvanyi LG, Liu C, Goodman V, et al. BRAF(V600) inhibitor GSK2118436 targeted inhibition of mutant BRAF in cancer patients does not impair overall immune competency. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:2326–35. doi: 10.1158/1078-0432.CCR-11-2515. [DOI] [PubMed] [Google Scholar]
- 52.Hooijkaas A, Gadiot J, Morrow M, Stewart R, Schumacher T, Blank CU. Selective BRAF inhibition decreases tumor-resident lymphocyte frequencies in a mouse model of human melanoma. Oncoimmunology. 2012;1:609–17. doi: 10.4161/onci.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. The Journal of experimental medicine. 2013;210:1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. The Journal of experimental medicine. 2009;206:1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:1386–94. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 56.Ott PA, Henry T, Baranda SJ, Frleta D, Manches O, Bogunovic D, et al. Inhibition of both BRAF and MEK in BRAF(V600E) mutant melanoma restores compromised dendritic cell (DC) function while having differential direct effects on DC properties. Cancer immunology, immunotherapy: CII. 2013;62:811–22. doi: 10.1007/s00262-012-1389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schilling B, Sucker A, Griewank K, Zhao F, Weide B, Gorgens A, et al. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. International journal of cancer Journal international du cancer. 2013;133:1653–63. doi: 10.1002/ijc.28168. [DOI] [PubMed] [Google Scholar]
- 58.Ho PC, Meeth KM, Tsui YC, Srivastava B, Bosenberg MW, Kaech SM. Immune-Based Antitumor Effects of BRAF Inhibitors Rely on Signaling by CD40L and IFNgamma. Cancer research. 2014 doi: 10.1158/0008-5472.CAN-13-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knight DA, Ngiow SF, Li M, Parmenter T, Mok S, Cass A, et al. Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. The Journal of clinical investigation. 2013;123:1371–81. doi: 10.1172/JCI66236. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:598–609. doi: 10.1158/1078-0432.CCR-12-2731. [DOI] [PubMed] [Google Scholar]
- 61.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. The New England journal of medicine. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:1225–31. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koya RC, Mok S, Otte N, Blacketor KJ, Comin-Anduix B, Tumeh PC, et al. BRAF inhibitor vemurafenib improves the antitumor activity of adoptive cell immunotherapy. Cancer research. 2012;72:3928–37. doi: 10.1158/0008-5472.CAN-11-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:393–403. doi: 10.1158/1078-0432.CCR-12-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lemech C, Infante J, Arkenau HT. Combination molecularly targeted drug therapy in metastatic melanoma: progress to date. Drugs. 2013;73:767–77. doi: 10.1007/s40265-013-0049-8. [DOI] [PubMed] [Google Scholar]
- 66.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. The New England journal of medicine. 2013;368:1365–6. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 67.Cooper ZA, Frederick DT, Ahmed Z, Wargo JA. Combining checkpoint inhibitors and BRAF-targeted agents against metastatic melanoma. Oncoimmunology. 2013;2:e24320. doi: 10.4161/onci.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Altomonte M, Di Giacomo A, Queirolo P, Ascierto P, Spagnolo F, Bajetta E, et al. Clinical experience with ipilimumab 10 mg/kg in patients with melanoma treated at Italian centres as part of a European expanded access programme. J Exp Clin Cancer Res. 2013;32:82. doi: 10.1186/1756-9966-32-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ackerman A, Klein O, McDermott DF, Wang W, Ibrahim N, Lawrence DP, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014 doi: 10.1002/cncr.28620. doi: 10.1002/cncr.28620. [DOI] [PubMed] [Google Scholar]
- 70.Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14:e60–9. doi: 10.1016/S1470-2045(12)70539-9. [DOI] [PubMed] [Google Scholar]
- 71.Mann GJ, Pupo GM, Campain AE, Carter CD, Schramm SJ, Pianova S, et al. BRAF mutation, NRAS mutation, and the absence of an immune-related expressed gene profile predict poor outcome in patients with stage III melanoma. J Invest Dermatol. 2013;133:509–17. doi: 10.1038/jid.2012.283. [DOI] [PubMed] [Google Scholar]
- 72.Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:1239–46. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 73.Shi H, Hugo W, Kong X, Hong A, Koya RC, Moriceau G, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer discovery. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi H, Hong A, Kong X, Koya RC, Song C, Moriceau G, et al. A novel AKT1 mutant amplifies an adaptive melanoma response to BRAF inhibition. Cancer discovery. 2014;4:69–79. doi: 10.1158/2159-8290.CD-13-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jonsson G, Busch C, Knappskog S, Geisler J, Miletic H, Ringner M, et al. Gene expression profiling-based identification of molecular subtypes in stage IV melanomas with different clinical outcome. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:3356–67. doi: 10.1158/1078-0432.CCR-09-2509. [DOI] [PubMed] [Google Scholar]