Abstract
Regulatory T cells (Tregs) are essential for tolerance to self and environmental antigens, acting in part by downmodulating costimulatory molecules on the surface of dendritic cells (DCs) and altering naïve CD4 T cell-DC interactions. Here, we show that Tregs form stable conjugates with DCs before, but not after, they decrease surface expression of the costimulatory molecule CD80 on the DCs. We use supported planar bilayers to show that Tregs dramatically slow down, but maintain a highly polarized and motile phenotype after recognizing antigen in the absence of costimulation. These motile cells are characterized by distinct accumulations of LFA-1-ICAM-1 in the lamella and TCR-MHC in the uropod, consistent with a motile immunological synapse or ‘kinapse’. However, in the presence of high, but not low, concentrations of CD80, Tregs form stationary, symmetrical synapses. Using blocking antibodies, we show that, while CTLA-4 is required for CD80 downmodulation, CD28-CD80 interactions are critical for modulating Treg motility in the presence of antigen. Together, these results support the hypothesis that Tregs are tuned to alter their motility depending on costimulatory signals.
Introduction
Foxp3+ CD4 regulatory T cells (Tregs) are critical for controlling immune responses and promoting tolerance to autoantigens and commensal gut microflora (1, 2). The transcription factor Foxp3 is necessary for Treg development and function (3–5), and controls the expression of hundreds of genes, several of which, including Ctla4, are crucial for Treg development or function (2). Indeed, CTLA-4 is required for proper Treg functioning, as specific ablation in Foxp3+ cells results in severe autoimmunity (6). The costimulatory molecule CD28 is required for Treg development and survival (7, 8). In addition to thymus derived or natural Tregs (nTregs), Foxp3+ Tregs can be induced from peripheral CD4 T cells both in vitro and in vivo (9).
Tregs use a variety of processes, involving both contact-dependent and cytokine mediated mechanisms, to suppress immune reactions in vitro and in vivo (10, 11). Several reports have shown that Tregs can downmodulate expression of CD80 and CD86 on DCs (6, 12–15), a process that involves trans-endocytosis (16) and depends on PKC-η(17). Downmodulation of CD80/86 on DCs requires expression of CTLA-4 by Tregs, and occurs in an antigen and LFA-1-ICAM-1 dependent manner (14, 15). CD80 downmodulation is observed even when nTregs and DCs are cocultured in the presence of LPS, IFN-γ, or other potent maturing stimuli, indicating that CTLA-4-mediated regulation of DCs by nTregs is a powerful mechanism for altering DC function (15). In vivo imaging experiments have demonstrated that in the presence of antigen, Tregs prevent sustained naïve T cell-DC interactions (18, 19) and induce dysfunction in CTL in tumor tissue (20). Tregs were not observed directly interacting with naïve T cells or CTL in these experiments, and instead formed antigen-dependent, unstable tethering-interactions with DC coinciding with marked downregulation of CD80 and CD86 (20). In experiments with TCR transgenic Tregs and naïve CD4 T cells cocultured with peptide-pulsed splenic DCs, Tregs were highly motile, swarmed around antigen loaded DCs and outcompeted the naïve cells for space around the DCs (15). This swarming phenotype, possibly in conjunction with CTLA-4 mediated downmodulation of CD80/86, could explain the in vivo imaging results described above. Due to the importance of Tregs in modulating the surface phenotype of DCs and the sparseness of studies examining the IS formed by Tregs, the factors governing Treg-DC interactions are of interest.
Here, we test the hypothesis that costimulatory molecules on the surface of APCs are responsible for determining Treg motility and immunological synapse (IS) structure. Given that Tregs are capable of downmodulating CD80 molecules on the surface of DCs, and that CD28-CD80 interactions can modulate synapse structure (21), we examined the role that CD80 plays in Treg-DC IS formation. We show that in vitro generated Tregs, like nTregs, are capable of downmodulating CD80 on the surface of DCs, and that alteration of the DC surface phenotype affects subsequent Treg-DC interactions. Using supported planar bilayers as artificial APCs, we demonstrate that Tregs slow down, but are still highly motile, when they recognize antigen in the absence of costimulation. However, the presence of high, but not low, levels of CD80 is sufficient to induce the formation of symmetrical, non-motile IS by Tregs. We suggest a model wherein highly motile Tregs are constantly scanning the surface of APCs, only stopping when the APC is activated and displaying high levels of costimulatory activity. This behavior may allow Tregs specific for self and environmental antigens to efficiently downmodulate costimulatory molecules on inappropriately activated cells, while ignoring resting cells displaying those antigens.
Materials and Methods
Animals
Heterozygous AD10 TCR transgenic mice on a B10.BR background, specific for pigeon cytochrome c 88–104 and reactive against moth cytochrome c 88–103 (MCC) (22), were provided by S. Hedrick (University of California at San Diego, La Jolla, CA) by way of P. Marrack (National Jewish Medical Center, Denver, CO). B6.Cg-Foxp3tm2Tch/J (Foxp3-GFP) mice were obtained from The Jackson Laboratory. Male AD10+ mice were bred to homozygous female Foxp3-GFP mice and the AD10+ progeny were used as a source of TCR transgenic GFP+ nTreg cells. All mice were housed in specific-pathogen free conditions and used in accordance with National Institutes of Health guidelines under an animal protocol approved by the Oregon Health & Science University Institutional Animal Care and Use Committee.
Antibodies
The antibodies used for flow cytometry were as follows: anti-CD4 Alexa Fluor 488 (GK1.5; Ebioscience), anti-CD4 PerCP (RM4–5; BioLegend), anti-CD152 PE (UC10-4B9; BioLegend), anti-Foxp3 Alexa Fluor 647 (150D; BioLegend), anti-CD11c FITC and PE (HL3; BD Bioscience), anti-CD25 PerCP (PC61; Biolegend) and anti-CD80 FITC and PerCP-Cy5.5 (16-10A1; BD Bioscience).
Dendritic cell culture
Bone marrow derived dendritic cells (BMDCs) were cultured as described (23). Briefly, bone marrow cells were cultured for nine days in bacteriological Petri dishes in complete RPMI 1640 media supplemented with GM-CSF supernatant (final conc.: 20 ng/mL). On day 9 of culture, immature DCs were plated in LabTek II eight-well chambers (#1.5) (Nunc) or 6 well plates in fresh media with 20 ng/mL GM-CSF and 1 µg/mL LPS.
In vitro Treg polarization
CD4+ cells and B cells were purified from AD10 and B10.BR spleen cell suspensions respectively using EasySep immunomagnetic negative selection (Stemcell technologies). 5 × 106 B cells and 2.5 × 106 CD4+ cells were cultured in 1 mL complete RPMI 1640 media in 6 well plates with 2.5 µM MCC peptide, 20 ng/mL TGF-β, 100 U/mL IL-2 and 10 nM all trans retinoic acid. On days 2 and 3, 1 mL of media supplemented with 100 U/mL IL-2 was added to the cultures. To confirm that T cells were polarized to an Treg phenotype, cells were fixed, permeabilized and stained for CD4, CTLA-4 and Foxp3. Fixation and permeabilization reagents were from BioLegend. Cells were used on day 4.
CD80 downmodulation assay
1.6 × 105 day 9 BMDCs were seeded onto 12 well plates and treated LPS as described above. One day later, the indicated numbers of day 4 Tregs were added per well in the presence or absence of 2.5 µm MCC. In some experiments, Fab fragments of anti-CD28 (E18; gift from Thomas Hünig)(24) or anti-CTLA-4 (UC10-4F10-11; Bio-X-Cell) were added at 100 µg/mL. The Fab fragments were generated with a Pierce Fab Preparation Kit (Thermo Scientific). As a control, Tregs were not added to some wells. 24 hrs later, the cells were harvested and stained for 30 min on ice for CD4, CD11c and CD80. All antibodies were used at 1:200. EDTA (1 µM) was included in the FACS buffer to discourage continued interactions. Samples were collected on a BD FACSCalibur with CellQuest software and analyzed with FlowJo (Tree Star, Inc.).
Imaging Treg-DC interactions
3 × 104 day 9 BMDCs were seeded onto coverslips in 8 well chambers and treated with LPS as described above. One day later, 1.5 × 105 CFSE-loaded, day 4 Tregs were added to the wells. Imaging commenced as soon as Tregs were added to the wells. Differential interference contrast (DIC) and fluorescent images were obtained every minute for 2 hrs. All imaging was conducted at 37°C with 5% CO2. Widefield imaging was performed with an Applied Precision DeltaVision system using an Olympus 20X/0.75 NA Plan Apo objective. This system included an Applied Precision chassis with a motorized XYZ stage, Weatherstation environmental chamber, Olympus IX71 inverted fluorescent microscope, Xenon lamp and CoolSnap HQ2 camera. The DeltaVision SoftWorx software package was used for image acquisition.
Supported planar bilayer experiments
GPI-linked forms of Oregon Green 488 labeled I–Ek (200 molecules/µm2) and Cy5-labeled ICAM-1 (300 molecules/µm2) were incorporated into dioleoylphosphatidylcholine bilayers exactly as described (25, 26). For some experiments, GPI-linked CD80 was incorporated into bilayers at 20, 40 or 200 µm2 (27). These bilayers were supported on a coverslip in a Bioptechs flow cell, and were loaded with 100 µM MCC or hemoglobin (Hb) peptide (GKKVITAFNEGLK) in a PBS/citrate buffer (pH 4.5) for 24 hr at 37°C (25). 107 T cells in 1 ml HBS buffer with 1% bovine serum albumin were injected onto bilayers at 37°C. In some experiments, anti-CD28 and/or anti-CTLA-4 Fab fragments were added to the T cells at 50 or 100 ug/mL 10 min prior to injection onto the bilayers. Images were acquired every minute for 30–60 min on the DeltaVision system using an Olympus 60X/1.42 NA Plan Apo objective.
Imaris 6.3 (Bitplane) was used to track cells interacting with the bilayer. To ensure that only cells productively interacting with the bilayer were analyzed, Cy5 fluorescence (ICAM-1 accumulation) was tracked automatically with the Spots tool in Surpass mode. In situations where the ICAM-1 signal was ambiguous, DIC images were examined to determine if the cell was flattened against the bilayer. DIC images were used to track cells interacting with bilayers loaded with irrelevant peptide, as there was only sparse ICAM-1 accumulation under these conditions.
Scoring T cell-bilayer interactions
Cells were scored as non-motile if they moved one cell diameter or less during the entire imaging session. Cells were considered to have reformed an IS if they stopped forward progress, lost their uropod and ICAM-1 arc and remained non-motile for at least 10 min. Cells were considered to have broken symmetry if they were non-motile for 10 min or more before gaining motility and moving more than one cell diameter. The vast majority of cells were symmetrical when first contacting the bilayer, but this state was transient (much less than 10 min) and cells that subsequently became motile were not considered to have broken symmetry. Cells were scored as having a uropod anchor if the uropod of a motile cell was attached to the same place for at least 10 min, causing the cell to pivot.
Results
Tregs transition from stable to motile contacts with dendritic cells, concomitant with downmodulation of costimulatory molecules
We set out to study the IS formed between Tregs and APCs. In order to generate a population of antigen-specific cells for use in imaging experiments, Tregs were induced in vitro by culturing purified CD4 T cells from AD10 TCR-transgenic mice with naïve B cells in the presence of MCC peptide, TGF-β, IL-2 and all trans retinoic acid, as previously described (28). After four days of culture under Treg conditions, the cells were almost completely polarized to a Treg phenotype with high levels of Foxp3 and CTLA-4 (Fig. 1A). Day 4 Tregs were loaded with CFSE, introduced to peptide-loaded BMDCs and imaged over time.
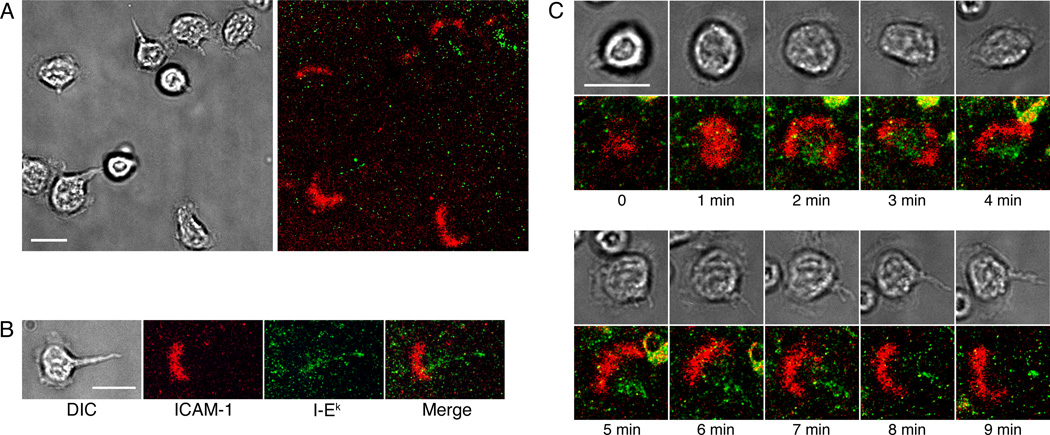
Figure 1. Tregs modify DC phenotype, concomitant with a change in the stability of Treg-DC interactions.
(A) TCR transgenic CD4 T cells were cultured under Treg-polarizing conditions and expressed high levels of FoxP3 and CTLA-4 at day 4 post-stimulation. Naïve B10.BR CD4 T cells are shown for comparison. (B) Tregs were labeled with CFSE and introduced to BMDCs loaded with MCC peptide. The majority of Treg-DC interactions were stable. For further examples of stable Treg-DC contacts, see Movie 1. (C) 24 hrs after addition, the Tregs were highly motile and swarmed around BMDCs. (D) Fresh, CFSE-labeled Tregs were added to BMDCs 24 hrs after the addition of unlabeled Tregs. These freshly added Tregs had a polarized phenotype and crawled along the surface of BMDCs. Scale bars represent 10 µm. The still images shown in Fig. 1D are from Movie 2. (E–H) Tregs were incubated with BMDCs in the presence or absence of MCC peptide, and the BMDCs were assayed at 24 hrs for surface levels of CD80. The T cell:DC ratios were 1:1 (E), 5:1 (F) or 10:1 (G and H). Fab fragments of anti-CTLA-4 (G) or anti-CD28 (H) were included in the cultures at 100 µg/mL. Results are representative of three (B–D) or six (E–H) independent experiments.
The initial interactions between Tregs and BMDCs were very stable, with the vast majority of Tregs flattening against BMDCs and maintaining a rounded, unpolarized shape for the duration of the experiment (Fig. 1B and Movie 1). However, after 24 hours of co-culture, the Tregs became highly motile and formed large, swarming clusters around the BMDCs (Fig. 1C). To further investigate this change in motility, we added fresh Tregs labeled with CFSE to the Treg-BMDC co-cultures and observed their behavior. Surprisingly, the freshly added Tregs also displayed a motile, swarming phenotype. As shown in Figure 1D and Movie 2, the freshly added Tregs adopted a polarized shape and crawled along the surface of the BMDCs. The tendency of freshly added cells to form motile, rather than stable, contacts indicates a change in the BMDCs rather than a change in the Tregs following 24 hours of culture together.
Natural Tregs have been shown to cluster around DCs after a 12-hour incubation, and this phenotype was associated with downmodulation of CD80 and CD86 on the DC surface (15). To determine if changes in the surface phenotype of the DCs could be responsible for the altered motility of Tregs after a 24 hour co-culture, we conducted assays to measure downmodulation of CD80. Incubating Tregs with BMDCs resulted in antigen-specific downmodulation of CD80 at both 1:1 and 5:1 Treg:BMDC ratios (Fig. 1E and F). In agreement with published work (15), Fab fragments of an anti-CTLA-4 antibody completely inhibited CD80 downmodulation, even at a 10:1 Treg:BMDC ratio (Fig. 1G). Treatment with Fab fragments of an anti-CD28 antibody known to efficiently block CD28-CD80 interactions (24) resulted in a decrease in the efficiency of CD80 downmodulation, but the effect was relatively minor compared to anti-CTLA-4 treatment (Fig. 1H).
CD80 modulates immunological synapse formation in Tregs
While qualitatively informative, the Treg-DC interactions were not conducive to precise measurements of Treg motility because the DC in these experiments were often highly motile. In order to carefully measure the interactions between Tregs and APC in two dimensions, we used supported planar bilayers containing GPI-linked, fluorescently labeled ICAM-1 and MHC class II (I-Ek) loaded with MCC peptide (25).
Day 4 Tregs were injected onto the supported planar bilayers and examined for the distribution of peptide-MHC (pMHC)-TCR and ICAM-1-LFA-1 interactions at the Treg-APC interface. Although the cells fluxed calcium immediately upon touching the bilayers (data not shown), we did not observe symmetrical IS, with well-defined supra-molecular activation clusters (SMACs), in agreement with a recent report (Fig. 2A) (29). Instead, the Tregs interacting with the bilayer had the polarized morphology of motile cells, with a well-defined uropod. An arc of ICAM-1 was located in the mid-cell region (Fig. 2B), as previously described for motile T cells (30). The strongest accumulation of pMHC-TCR was in the uropod of the crawling cells (Fig. 2B), consistent with a phenotype seen for CD8 T cell blasts (31). As shown in Figure 2C, the Tregs broke radial symmetry and formed motile synapses almost immediately upon interacting with the bilayers. Motile IS, or ‘kinapses’, have been previously reported in naïve T cells in vitro (32) and in vivo (33).
Figure 2. Tregs form immunological kinapses on supported planar bilayers containing pMHC and ICAM-1.
(A) Representative field of Tregs interacting with bilayers containing pMHC and ICAM-1. DIC (left) and fluorescence (right) images with pMHC (green) and ICAM-1 (red) are shown. (B) Representative image of a motile Treg with ICAM-1 accumulated in the mid-body of the cell and pMHC accumulation in the uropod. (C) Representative time-lapse images of the initial Treg-bilayer contact showing pMHC (green) and ICAM-1 (red). Tregs displayed a polarized phenotype rapidly upon contact with the bilayer. Scale bars represent 10 µm. Images are representative of seven independent experiments.
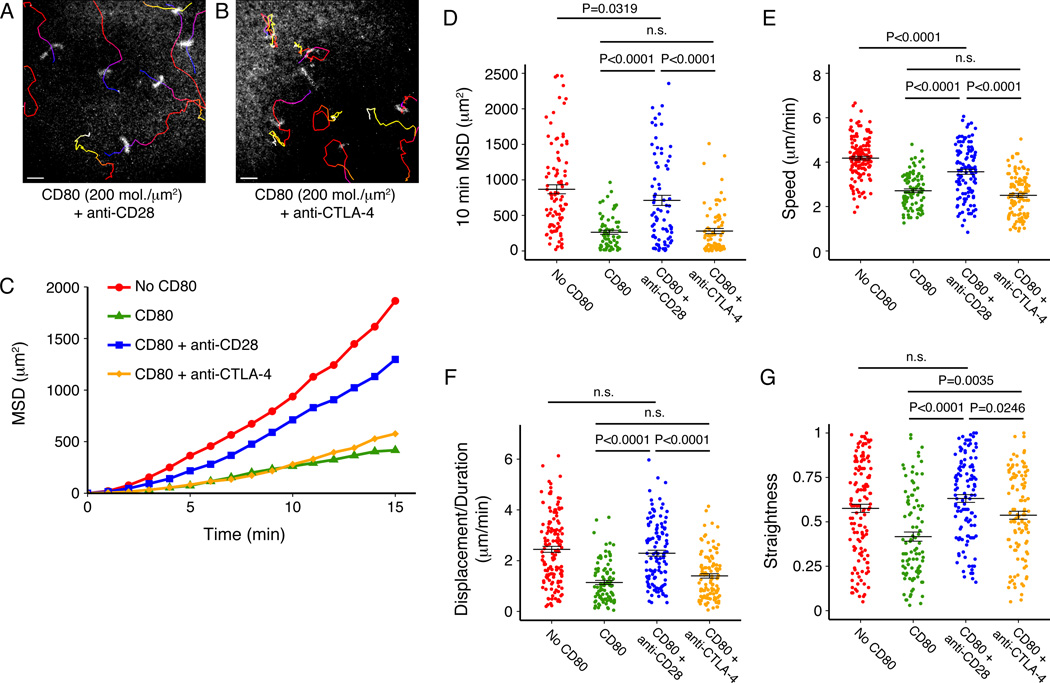
Our examination of Treg-DC interactions suggested that the level of costimulatory molecules on the APC surface could modulate Treg behavior. Therefore, we examined IS and kinapse formation in the presence of 0, 40 and 200 molecules/µm2 of GPI-linked CD80 in the bilayers. In the presence of low levels of CD80 (40 molecules/µm2), the Tregs predominantly formed motile kinapses, similar to the phenotype observed in the absence of CD80 (Fig. 3A and Movie 3 and Fig. 3B and Movie 4). However, when Tregs were introduced to bilayers containing 200 molecules/µm2 CD80, their movement was drastically reduced with some of the Treg cells forming stable IS (Fig. 3C and Movie 5). Analysis of the motility of Tregs on bilayers containing varying amounts of CD80 showed that high levels of CD80 significantly decreased the mean square displacement (MSD) of Tregs over time (Fig. 3D and 3E). As shown in Figure 3F, the average speed of Tregs was significantly reduced in the presence of high levels of CD80. Since changes in the centroid of relatively non-motile cells can inflate speed measurements, we also measured the total displacement of each cell and divided by track duration. This measurement yielded values close to zero for non-motile cells and showed that Tregs are dramatically less motile when introduced to bilayers with high levels of CD80 (Fig. 3G). We also noted a decrease in the straightness of Treg tracks in the presence of high levels of CD80 (Fig. 3H).
Figure 3. High concentrations of CD80 reduce the motility of Tregs.
Tregs were introduced to bilayers containing pMHC, ICAM-1 and 0 (A), 40 (B) or 200 (C) molecules/µm2 of CD80. Time-coded tracks (blue to white from the beginning to the end of the experiment) are overlaid on ICAM-1 images. Scale bars represent 10 µm. The tracks shown are representative of four (A), two (B) or six (C) independent experiments. Movies 3, 4 and 5 are time-lapse videos of Tregs interacting with bilayers containing 0, 40 and 200 molecules/µm2 of CD80 respectively. (D) Mean square displacement (MSD) over time is plotted for three densities of CD80, and the MSD at 10 min is shown (E). The average speed (F), displacement/duration (G) and straightness (track displacement/length) (H) of Treg tracks on bilayers with 0, 40 or 200 molecules/µm2 of CD80 are shown. Results (D–H) are from one representative experiment of three with n=38, 52 and 41 for 0, 40 and 200 molecules/µm2 of CD80 respectively. Error bars represent SEM. One-way ANOVAs followed by Tukey’s HSD tests were used to determine P values. P>0.05 was considered not significant (n.s.). Scale bars represent 10 µm.
Although the fraction of cells that maintained symmetrical, non-motile interactions with the planar bilayers for the entirety of a 45–60 min experiment was 3-fold higher in the presence of 200 molecules/µm2 CD80, this result did not quite meet the standard for statistical significance (Fig. 4A). However, significantly fewer cells were motile for the entire experiment in the presence of high levels of CD80 (Fig. 4B). These results indicate that in addition to slowing the motility of Tregs, high levels of CD80 also induced stable IS formation. As shown in Figure 2C, Tregs that formed kinapses broke radial symmetry almost immediately upon contacting the bilayers. However, we observed that a fraction of the cells regained symmetry and formed a stable IS during the course of the experiment (Fig 4C). The percentage of Tregs displaying this behavior was dramatically increased in the presence of high levels of CD80 (Fig. 4D).
Figure 4. Treg-APC interactions have an altered phenotype in the presence of CD80.
The percentage of Tregs that were either non-motile (A) or continuously motile (B) over the entire length of the experiment is shown. (C) An example of a cell transitioning from a motile phenotype to a stable IS. DIC (top) and fluorescence (bottom) images with pMHC (green) and ICAM-1 (red) are shown. (D) The percentage of cells that reformed IS over the length of an experiment. (E) An example of a cell attached to the bilayer via its uropod. The cell’s track is overlaid on DIC images. (F) The percentage of cells that were anchored by their uropods over the course of an experiment. Data in A, B, D and F show the mean of the fraction of cells displaying a given phenotype from two (40 molecules/µm2 CD80) or three (0 and 200 molecules/µm2 CD80) independent experiments with n=115, 87 and 129 for 0, 40 and 200 molecules/µm2 of CD80 respectively. Error bars represent SEM. One-way ANOVAs followed by Tukey’s HSD tests were used to determine P values. P>0.05 was considered not significant (n.s.). Scale bars represent 10 µm.
In the absence of CD80, the vast majority of Tregs moved freely across the bilayer, but the addition of CD80 caused some motile cells to become anchored to the substrate via their uropods, frustrating the forward motion of the cells and causing them to turn in circles (Fig. 4E). This behavior was seen at both concentrations of CD80, but was especially prominent when high levels were used (Fig. 4F).
It has been shown that TCR-transgenic mice contain a population of nTregs that are most likely derived from cells expressing endogenous TCR α-chains in addition to transgenic α- and β-chains (34). Indeed, we found that a fraction of naïve CD4+ cells from AD10 mice expressed FoxP3 (Fig. 5A). In order to confirm that our findings were generalizable to nTregs, we crossed Foxp3-GFP mice to our AD10 TCR transgenic mice, and introduced purified CD4 cells to bilayers (Fig. 5B and 5C). Unlike activated Tregs, a significant fraction of the nTregs were sessile regardless of CD80 concentration (Fig. 5E), possibly because these cells are relatively metabolically inactive compared to in vitro generated Tregs. Nevertheless, the presence on CD80 in the bilayers resulted in a significant decrease in motility (Fig. 5D–F). Interestingly, naïve non-Tregs were much less motile than Tregs in the absence of CD80. These results demonstrate that nTregs behave similarly to in vitro induced Tregs in the presence and absence of CD80.
Figure 5. CD80 reduces the motility of nTregs on supported planar bilayers.
(A) Naïve CD4 cells from B10.BR or AD10 mice were assayed for the expression of CD25 and FoxP3. (B and C) Naïve AD10 mice CD4 cells were purified from the spleens of AD10 X Foxp3-GFP mice and introduced to supported planar bilayers containing pMHC, ICAM-1 and 0 (B) or 200 (C) molecules/µm2 CD80. Time-coded tracks (blue to white from the beginning to the end of the experiment) are overlaid on ICAM-1 images. The movements of both nTregs (GFP+) and naïve CD4 cells (GFP) were followed over time, but only the nTreg tracks are shown here. Scale bars represent 10 µm. (D) Mean square displacement (MSD) over time is plotted for nTregs and naïve CD4 cells on bilayers lacking CD80 and on bilayers with 200 molecules/µm2 CD80. The MSD at 10 min and the fraction of cells with an MSD greater than 500 um2 are shown (E and F). The results are pooled from three experiments with n=48, 40, 73 and 77 for nTreg/No CD80, nTreg/CD80 200 molecules/µm2, naïve/No CD80 and naïve/CD80 200 molecules/µm2 respectively. Error bars represent SEM (E) or 95% confidence interval (F). P values were determined with a one-way ANOVA followed by Tukey’s HSD test (E) or Fisher’s exact test (F). Scale bars represent 10 µm.
Treg motility decreases dramatically in the presence of antigen
Tregs interacting with bilayers accumulated pMHC-TCR in the contact zone (Fig. 2B) and fluxed intracellular calcium (data not shown), but were almost always continuously motile over the entire imaging experiment (Fig. 4B). This led us to ask whether there were any changes in Treg motility upon antigen recognition, or if motility was only altered in the presence of high levels of CD80. Therefore, we introduced AD10+ Tregs to bilayers containing ICAM-1 and MHC loaded with either cognate peptide (MCC) or an irrelevant peptide (Hb). As shown in Figure 6A, in the absence of cognate antigen, Tregs moved rapidly across the bilayers with only transient accumulations of ICAM-1, and at no time were pMHC-TCR clusters apparent. Analysis of the tracks made by Tregs in the absence of antigen showed that Tregs scanning the surface of an APC lacking cognate antigen move much faster and straighter than Tregs that have recognized antigen (Fig 6B–F). Thus, Tregs are highly motile when scanning the surface with ICAM and MHC without specific antigen, slow considerably in the presence of antigen, and slow even more, often to the point of stopping, in the presence of antigen and high levels of costimulation.
Figure 6. Tregs migrate rapidly in the absence of cognate antigen.
Tregs were introduced to bilayers containing ICAM-1 and I–Ek loaded with either MCC or irrelevant (Hb) peptides. (A) Representative time-coded tracks (blue to white from the beginning to the end of the experiment) of Tregs migrating on bilayers containing MHC loaded with Hb peptide overlaid on an ICAM-1 image are shown. Scale bar represent 10 µm. (B) Mean square displacement (MSD) over time is plotted for Tregs interacting with bilayers loaded with MCC or Hb peptide, and the MSD at 10 min is shown (C). The straightness (D), speed (E) and average displacement/duration (F) of Treg tracks on bilayers with MCC or Hb are shown. Results (B–F) are from one representative experiment of two with n=62 and 84 for MCC and Hb respectively. Error bars represent SEM. P values were determined with two-tailed Student’s t tests. Scale bars represent 10µm.
CD28-CD80 interactions control Treg motility
To dissect the mechanism by which CD80 modulates Treg motility, we incubated cells with Fab antibodies against CD28 or CTLA-4 and introduced them to bilayers containing pMHC, ICAM-1 and high levels of CD80. As shown in Figure 7A, blockade of CD28 resulted in highly motile Tregs in the presence of CD80. In contrast, many of the Tregs treated with anti-CTLA-4 formed relatively non-motile contacts with the bilayer or had their uropods anchored (Fig. 7B). We analyzed the tracks of Tregs loaded onto bilayers without CD80 or with CD80 and either anti-CD28, anti-CTLA-4 or no antibody. Tregs treated with anti-CD28 were much more motile than cells in the no antibody control condition and were almost as motile as cells loaded onto bilayers lacking CD80 (Fig. 7C–G). Treatment with anti-CTLA-4 did not have an appreciable effect on motility by most measures, as 10 min MSD, displacement/duration and speed were not different from the no antibody control (Fig. 7C–E). Treatment with anti-CTLA-4 did result in somewhat straighter tracks, although the significance of this finding is unclear (Fig. 7G). Together, these data show that CD28-CD80 interactions control the motility of Tregs.
Figure 7. CD28-CD80 interactions are responsible for modulating Treg motility.
Tregs were introduced to bilayers containing pMHC, ICAM-1 and 200 molecules/µm2 of CD80 in the presence of anti-CTLA-4 (A) or anti-CD28 (B) Fab antibodies. Time-coded tracks (blue to white from the beginning to the end of the experiment) are overlaid on ICAM-1 images. Scale bars represent 10 µm. The tracks shown are representative of two (A) or three (B) independent experiments. (C) Mean square displacement (MSD) over time is plotted for Tregs on bilayers lacking CD80 and on bilayers with 200 molecules/µm2 CD80 with or without anti-CTLA-4 and anti-CD28 Fab antibodies, and the MSD at 10 min is shown (D). The average speed (E), average displacement/duration (F) and straightness (track displacement/length) (G) of Treg tracks on bilayers with or without CD80 and Fab antibodies are shown. Results (C–G) are from one representative experiment of three with n=135, 92, 113 and 109 for No CD80, CD80, CD80 + anti-CD28 and CD80 + anti-CTLA-4 respectively. Error bars represent SEM. One-way ANOVAs followed by Tukey’s HSD tests were used to determine P values. P>0.05 was considered not significant (n.s.). Scale bars represent 10µm.
Discussion
Here we propose a model wherein Treg-DC interactions are modulated by relative levels of costimulatory molecules. In the absence of antigen recognition, Tregs rapidly migrate over the surface of the APC, scanning for their cognate antigen. Upon antigen recognition, Tregs form either relatively slow moving kinapses or non-motile IS depending on the levels of costimulatory molecules. In the presence of high levels of costimulation, symmetrical IS are favored. After levels of costimulatory molecules are reduced, kinapse formation is favored and the Treg starts migrating again. Thus, by alternating between kinapses and stable IS, Tregs could efficiently contact and modulate levels of costimulatory molecules or otherwise suppress inappropriately activated DCs.
Our results are consistent with a recent paper showing that nTregs interacting with supported planar bilayers containing 90 molecules/µm2 CD80 form unstable IS, but because CD80 was not tested at higher concentrations in that report, the profound effect that CD80 has on Treg motility was not discovered (29). In contrast to our study, human Tregs were observed to form hyperstable IS compared to T effector cells (35). This discrepancy could be due to a species difference, but is probably best explained by differences in the TCR stimulus provided to the Tregs. Zanin-Zhorov et al. introduced T cells to supported planar bilayers containing anti-CD3ɛ. It is likely that the presence of such a strong and qualitatively different TCR stimulus obviates the need for CD80 to induce stopping of Tregs, masking the differences in motility we noted when using saturating concentrations of a physiological TCR ligand. However, it must be noted that CD25− non-Treg displayed rapid symmetry breaking and motility in the same conditions, demonstrating that anti-CD3 does not generate a durable stop signal for all human T cells.
How does signaling through CD28 alter Treg motility? Crosslinking CD28 results in Lck-mediated phosphorylation of the YMNM motif and recruitment of PI3K and Grb2 (36). The activity of PI3K leads to activation of Akt, while Grb2 recruits the guanine nucleotide exchange factor Vav1, resulting in activation of the small GTPases Rac1 and Cdc42, which in turn activate WAVE2 and WASp respectively, ultimately leading to Arp2/3 mediated actin remodeling (37). Additionally, a proline-rich domain of CD28 is known to recruit several kinases downstream of the TCR, including Lck and PKC-θ (36). We hypothesize that Tregs have a relatively higher threshold for activation than conventional T cells, which allows them to monitor their environment without being inappropriately activated, even in the face of ubiquitously expressed, low affinity cognate antigens. Under this scenario, Tregs would require a robust signal through CD28 before transitioning from motile kinapses to stable synapses. In support of a model where CD28 signaling through PI3K is critical for Treg function, it has been shown that Tregs expressing an inactive form of the PI3K catalytic subunit, p110δ, are poor suppressors and fail to prevent inflammation in a colitis model (38). Strong CD28 crosslinking may simply augment TCR-induced signaling and/or cytoskeletal remodeling, or could provide a unique signal. A similar phenomenon has recently been observed in thymocytes, in which CD28-dependent actin remodeling was required for maximal activation after TCR triggering (39).
An annular ring of CTLA-4 surrounding the cSMAC characterizes the IS formed by induced Tregs interacting with bilayers containing CD80 (40). This dense accumulation of CTLA-4 may increase the efficiency of costimulatory molecule downmodulation. Thus, the reduced motility and increased IS formation we observed in the presence of high levels of costimulation may serve two purposes: 1) increase the dwell time of a Treg on a particular activated APC and 2) enhance the efficiency of downmodulation. The IS functions as a platform for the polarized secretion of effector molecules towards the APC (41, 42). Therefore, it is possible that additional Treg functions, including antigen-specific delivery of perforin (43, 44) or IL-10 (42), may require the formation of a stable IS.
CTLA-4 ligation has been reported to increase motility of T cells both in vitro and in vivo (45–47). Because CTLA-4 has a much higher affinity for CD80 than CD28 (48), it follows that high levels of CD80 are needed to trigger the CD28-mediated stop signal we see in our experiments. We suggest that the abundant expression of CTLA-4 on Tregs tunes these cells to be highly motile unless they recognize antigen in the context of large amounts of costimulation. Conventional CD4 T cells, unlike self-reactive Tregs that are constantly seeing antigen, are able to efficiently scan APCs without this mechanism. In disagreement with our model, a recent report showed that activated CD4+CD25+ cells from CD28−/− mice did not migrate differently in lymph node slices from CD4+CD25+ cells from CD28+/+ mice (49). The apparent disparity between these results and ours could be explained by the fact that the absence of CD28 has a profound effect on the development and homeostasis of Tregs (8). In the absence of CD28, the small number of surviving peripheral Tregs may have compensatory mechanisms for activation and motility.
Mice that have CD28 specifically ablated in Foxp3+ cells develop severe multi-organ autoimmunity (50). We suggest that efficient downmodulation of costimulatory molecules on activated APCs presenting self-antigen may be a CD28-dependent function that is missing in these mice. This hypothesis leads to the prediction that blocking CD28 will result in less efficient downmodulation and we did see a modest decrease in downmodulation in the presence of anti-CD28 (Fig. 1H). Alternatively, CD28-dependent arrest and symmetrical synapse formation may be required for other Treg suppressive functions, as mentioned above.
Mice deficient in the actin regulatory protein WASp develop chronic colitis, and Tregs from these mice are poor suppressors both in vitro and in vivo (51–53). WASp is also required for T cells to maintain IS symmetry (30). Although defective migration to lymphoid tissue likely plays a role in the phenotype described for WASp deficient Tregs, defective IS formation may also be important. Tregs incapable of IS formation could also be deficient in CD80/86 downmodulation. Thus, constant surveillance and modulation of DCs (via IS dependent downmodulation of costimulatory molecules) may be required to prevent autoimmune disease. Consistent with this idea, Tang et al. have demonstrated that nTregs from BDC2.5 TCR Tg mice (which have TCRs specific for an antigen associated with pancreatic β-cells) are motile in the pancreatic LN when transferred into NOD mice. However, when the same nTregs are transferred into NOD.CD28−/− mice lacking Tregs, they are non-motile (19). Given the results presented here, it is likely that the DCs in the Treg-deficient animals had relatively high levels of CD80/86 compared to animals containing endogenous Tregs, thereby altering the behavior of the transferred Tregs. Higher levels of costimulatory molecules on DCs in the pancreatic LN of these already diabetes-prone animals could partially explain why NOD mice lacking Tregs develop diabetes more quickly than WT mice (54).
To maintain tolerance and immune homeostasis, Tregs need to monitor the activation state of all the DC in the body. Our finding that antigen can slow Treg migration may help explain how Tregs accumulate where their antigens are displayed. High CD80/CD86 levels then alter Treg behavior further to enable antigen-specific delivery of suppressive signals. The functional consequences of regulation of Treg motility and synapse formation by CD28 will be a fertile field of investigation in future experiments.
Supplementary Material
Acknowledgments
We thank members of the Parker laboratory for helpful discussions, Thomas Hünig for the anti-CD28 monoclonal antibody (Clone E18) and V. K. Thomas for the purified GPI anchored CD80.
Footnotes
This work was supported by National Institutes of Health grants R01 AI050823 (D.C.P.), R01 AI092080 (D.C.P.) and R01 AI043542 (M.L.D.) and a Wellcome Trust Principal Research Fellowship (M.L.D.). T.J.T. was supported by T32 AI007472 and T32 AI078903 from the National Institute for Allergy and Infectious Diseases.
Abbreviations used in this article: BMDC, bone marrow derived dendritic cell; DC, dendritic cell; Hb, hemoglobin peptide; IS, immunological synapse; MCC, moth cytochrome c peptide; pMHC, peptide-MHC; Treg, regulatory T cell
References
- 1.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions. Annu Rev Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4(+)CD25(+) regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 4.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 5.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 6.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 7.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 8.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 9.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 13.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 14.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong KF, Fu G, Zhang Y, Yokosuka T, Casas J, Canonigo-Balancio AJ, Becart S, Kim G, Yates JR, 3rd, Kronenberg M, Saito T, Gascoigne NR, Altman A. Protein kinase C-eta controls CTLA-4-mediated regulatory T cell function. Nat Immunol. 2014;15:465–472. doi: 10.1038/ni.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer CA, Kim EY, Marangoni F, Carrizosa E, Claudio NM, Mempel TR. Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. J Clin Invest. 2014;124:2425–2440. doi: 10.1172/JCI66375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wetzel SA, McKeithan TW, Parker DC. Live cell dynamics and the role of costimulation in immunological synapse formation. J Immunol. 2002;169:6092–6101. doi: 10.4049/jimmunol.169.11.6092. [DOI] [PubMed] [Google Scholar]
- 22.Kaye J, Vasquez NJ, Hedrick SM. Involvement of the same region of the T cell antigen receptor in thymic selection and foreign peptide recognition. J Immunol. 1992;148:3342–3353. [PubMed] [Google Scholar]
- 23.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 24.Beyersdorf N, Ding X, Blank G, Dennehy KM, Kerkau T, Hunig T. Protection from graft-versus-host disease with a novel B7 binding site-specific mouse anti-mouse CD28 monoclonal antibody. Blood. 2008;112:4328–4336. doi: 10.1182/blood-2008-03-146662. [DOI] [PubMed] [Google Scholar]
- 25.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 26.Thauland TJ, Koguchi Y, Wetzel SA, Dustin ML, Parker DC. Th1 and Th2 cells from morphologically distinct immunological synapses. J Immunol. 2008;181:393–399. doi: 10.4049/jimmunol.181.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bromley SK, Iaboni A, Davis SJ, Whitty A, Green JM, Shaw AS, Weiss A, Dustin ML. The immunological synapse and CD28-CD80 interactions. Nat Immunol. 2001;2:1159–1166. doi: 10.1038/ni737. [DOI] [PubMed] [Google Scholar]
- 28.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomiyama T, Ueda Y, Katakai T, Kondo N, Okazaki K, Kinashi T. Antigen-specific suppression and immunological synapse formation by regulatory T cells require the mst1 kinase. PLoS One. 2013;8:e73874. doi: 10.1371/journal.pone.0073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, Cameron TO, Thomas VK, Varma R, Wiggins CH, Sheetz MP, Littman DR, Dustin ML. Opposing effects of PKCθ and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 31.Beemiller P, Jacobelli J, Krummel MF. Integration of the movement of signaling microclusters with cellular motility in immunological synapses. Nat Immunol. 2012;13:787–795. doi: 10.1038/ni.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunzer M, Schafer A, Borgmann S, Grabbe S, Zanker KS, Brocker EB, Kampgen E, Friedl P. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–332. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 33.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 34.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 35.Zanin-Zhorov A, Ding Y, Kumari S, Attur M, Hippen KL, Brown M, Blazar BR, Abramson SB, Lafaille JJ, Dustin ML. Protein kinase C-{theta} mediates negative feedback on regulatory T cell function. Science. 2010;328:372–376. doi: 10.1126/science.1186068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 38.Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, Leung E, Rowan WC, Sancho S, Walker LS, Vanhaesebroeck B, Okkenhaug K. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 39.Tan YX, Manz BN, Freedman TS, Zhang C, Shokat KM, Weiss A. Inhibition of the kinase Csk in thymocytes reveals a requirement for actin remodeling in the initiation of full TCR signaling. Nat Immunol. 2014;15:186–194. doi: 10.1038/ni.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokosuka T, Kobayashi W, Takamatsu M, Sakata-Sogawa K, Zeng H, Hashimoto-Tane A, Yagita H, Tokunaga M, Saito T. Spatiotemporal basis of ctla-4 costimulatory molecule-mediated negative regulation of T cell activation. Immunity. 2010;33:326–339. doi: 10.1016/j.immuni.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Griffiths GM, Tsun A, Stinchcombe JC. The immunological synapse: a focal point for endocytosis and exocytosis. J Cell Biol. 2010;189:399–406. doi: 10.1083/jcb.201002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 43.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, Pace L, Valet F, Kissenpfennig A, Sparwasser T, Malissen B, Fetler L, Amigorena S. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32:266–278. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 46.Schneider H, Smith X, Liu H, Bismuth G, Rudd CE. CTLA-4 disrupts ZAP70 microcluster formation with reduced T cell/APC dwell times and calcium mobilization. Eur J Immunol. 2008;38:40–47. doi: 10.1002/eji.200737423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, Liu M, Formenti SC, Dustin ML, Demaria S. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest. 2012;122:3718–3730. doi: 10.1172/JCI61931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, Stuart DI, van der Merwe PA, Davis SJ. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 49.Lu Y, Schneider H, Rudd CE. Murine regulatory T cells differ from conventional T cells in resisting the CTLA-4 reversal of TCR stop-signal. Blood. 2012;120:4560–4570. doi: 10.1182/blood-2012-04-421420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest. 2013;123:580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maillard MH, Cotta-de-Almeida V, Takeshima F, Nguyen DD, Michetti P, Nagler C, Bhan AK, Snapper SB. The Wiskott-Aldrich syndrome protein is required for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells. J Exp Med. 2007;204:381–391. doi: 10.1084/jem.20061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marangoni F, Trifari S, Scaramuzza S, Panaroni C, Martino S, Notarangelo LD, Baz Z, Metin A, Cattaneo F, Villa A, Aiuti A, Battaglia M, Roncarolo MG, Dupre L. WASP regulates suppressor activity of human and murine CD4(+)CD25(+)FOXP3(+) natural regulatory T cells. J Exp Med. 2007;204:369–380. doi: 10.1084/jem.20061334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu CH, Hagemann TL, Kwan SP, Ferrini R, Davidson L, Bhan AK, Alt FW. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 54.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.