Abstract
The need for dynamic, elastomeric polymeric biomaterials remains high, with few options with tunable control of mechanical properties, and environmental responses. Yet the diversity of these types of protein polymers pursued for biomaterials-related needs remains limited. Robust high-throughput synthesis and characterization methods will address the need to expand options for protein-polymers for a range of applications. To address this need, a combinatorial library approach with high throughput screening is used to select specific examples of dynamic protein silk-elastin-like polypeptides (SELPs) with unique stimuli responsive features, including tensile strength, and adhesion. Using this approach 64 different SELPs with different sequences and molecular weights are selected out of over 2,000 recombinant E. coli colonies. New understanding of sequence-function relationships with this family of proteins is gained through this combinatorial-screening approach and can provide a guide to future library designs. Further, this approach yields new families of SELPs to match specific material functions.
Keywords: silk-elastin-like proteins, high throughput screening, library construction, stimuli responses, physical properties
1. Introduction
Protein polymers provide a uniquely tunable family of functional biomaterials that can mimic natural protein structure and function or be designed de novo for new biomaterial needs. These polymers are fully degradable to meet the needs of biomaterials and regenerative medicine, and are tunable in terms of degradation rate and functional material properties.[1] The number of protein polymers in common use for biomaterials remains limited, yet with advances in genetic and protein engineering, genes can be constructed to encode protein polymers composed of natural or non-natural amino acid sequences,[2] offering a path towards new and useful functional protein-based biomaterials.
Conventional polymer research traditionally involves the preparation of polymer samples in a sequential or serial fashion for characterization. In contrast, combinatorial approaches allow rapid progression toward a functional material by exploiting parallel processing and appropriate screening tools. For example, a library of 112 polyarylates from 14 distinct tyrosine-derived diphenols and eight aliphatic diacids was generated and used to be screened by material properties.[3] A targeted library of 67 peptoids was prepared via solid-phase synthesis and screened to identify new gene delivery agents.[4] Recently, semi-automated, solution-phase parallel synthesis and evaluation of a library of 2,350 structurally diverse, degradable poly(β-amino esters) was designed and used for high-throughput screening to identify polymers that transfected cells with higher efficiency than poly(ethyleneimine) (PEI).[5] These prior studies demonstrated the potential of high-throughput combinatorial approaches to accelerate the discovery of new polymers.
However, aside from the few examples above, the utility of combinatorial approaches in biomaterials research has been limited and mostly focused towards gene delivery systems. Much of these limitations originate with the challenges presented in the biosynthesis and screening processes required to identify new functional materials. A new platform for the synthesis, high-throughput screening and functional evaluation of a diverse set of stimuli-responsive protein polymers was therefore sought, exploiting the existing starting base of silk and elastin peptide motifs for the building blocks. Sequence features of silk include functions as the hard (crystallizable) domain to provide physical crosslinking (GAGAGS) and elastin as the soft (amorphous) domain to provide dynamic features (GXGVP). Further variants and tunability in material features originates with sequence variants in position ‘X’ of the elastin domains, to address dynamic material functions responsive to specific environmental stimuli. Silk-elastin-like proteins (SELPs) combine the outstanding physical and biological properties of silk and elastin, and can be fabricated into a range of material properties,[6] thus providing a suitable protein combinatorial starting point for new functional materials discovery. These protein systems have also been previously utilized in biomaterials, nanodevices, biosensors, bioseparations, tissue engineering,[7–9] and targeted drug delivery for small molecule drugs, proteins and genes.[10–13] Curiously, despite the seminal early work on SELP related peptides where a range of stimuli-responsive features were demonstrated, the focus of subsequent materials studies with these systems has been primarily related to their temperature-dependent dynamic changes.[14, 15] Yet the sequence requirements and constraints that govern mechanical and stimulus-responsive properties remain largely unknown. Motivated by the gap in our fundamental understanding of the stimuli responsive properties of silk and elastin and the need for more elastic polymeric systems in many biomaterial applications, the combinatorial and high-throughput approach was developed to design, synthesize and screen silk elastin-like copolymers (SELPs) and study sequence constraints that govern mechanical and stimuli responsive properties. In the present study, the objective was to design, build and screen a library of SELP with a broader range of functional material properties. This goal was addressed by developing high throughput methods to select for specific material functions. Dynamic thermal and electrochemical functions were demonstrated along with mechanical and adhesive material properties.
2. Results and Discussion
2.1 Construction of Dynamic Protein SELP Library
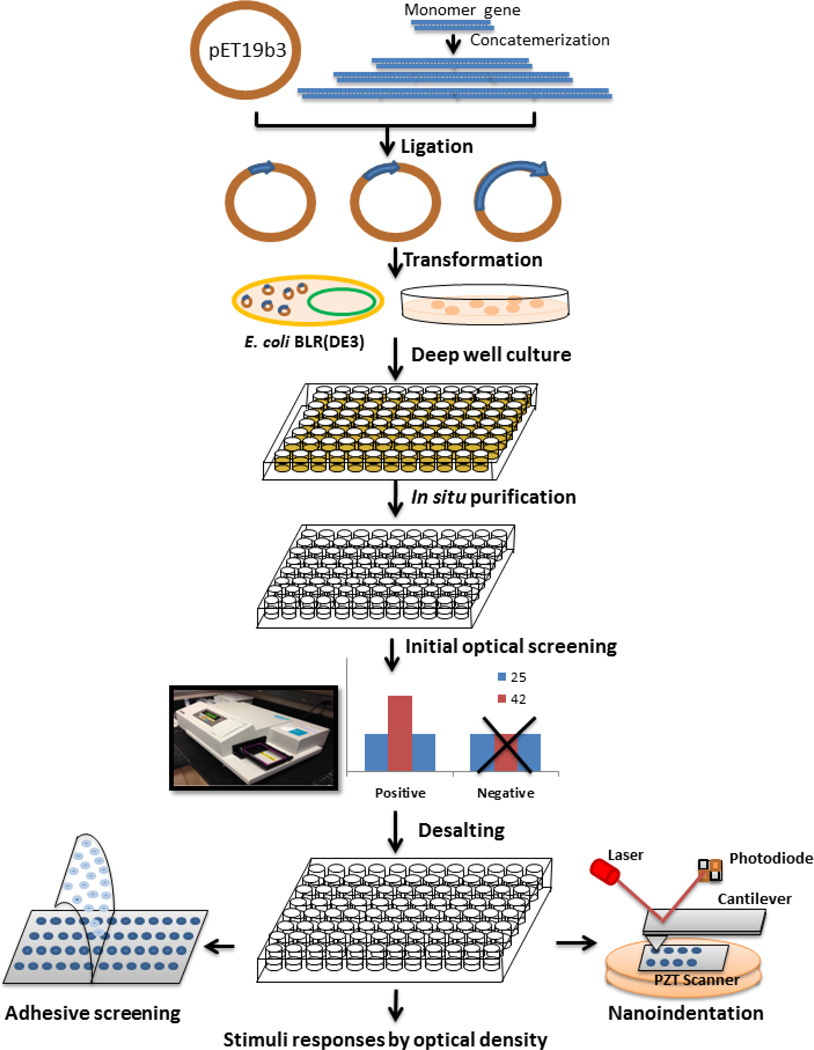
The silk and elastin peptide motifs are shown in Figure 1, with the features of the silk (GAGAGS) and elastin domains (GXGVP), combined with variants in position ‘X’ of the elastin domain to achieve the dynamic functions via the specific stimuli. Twelve monomeric genes (Table S1) were chemically synthesized followed by multimerization of each monomer gene achieved through a “concatemerization” strategy employing the restriction enzyme site BanII (Figure 2).[16] The seamless cloning strategy avoids the introduction of extra amino acid residues at the junctions between the monomers to preserve chemical sequence designs. Expression plasmids were then generated, encoding a library of SELPs with silk to elastin ratios at 1:4 and 1:2. Different amino acids (G, F, K, E, C, Y, RGYSLG, I) were employed in the second amino acid position of the fifth elastin block in each monomer to generate the diversity in protein polymer responses for screening. This X position permits elastin domains to display reversible conformational transitions in response to various stimuli, including pH, temperature, redox and electric, among other triggers. This versatility of responses to different stimuli, coupled with the tunability of these responses based on chemical composition of the elastin sequence, provides a robust and diverse set of protein polymers to build new families of dynamic protein polymers.
Figure 1.
Responses to different stimuli of SELPs-based dynamic protein polymers
Figure 2.
Library construction and high throughput screening of dynamic protein polymers SELPs
2.2 High Throughput Library Screening
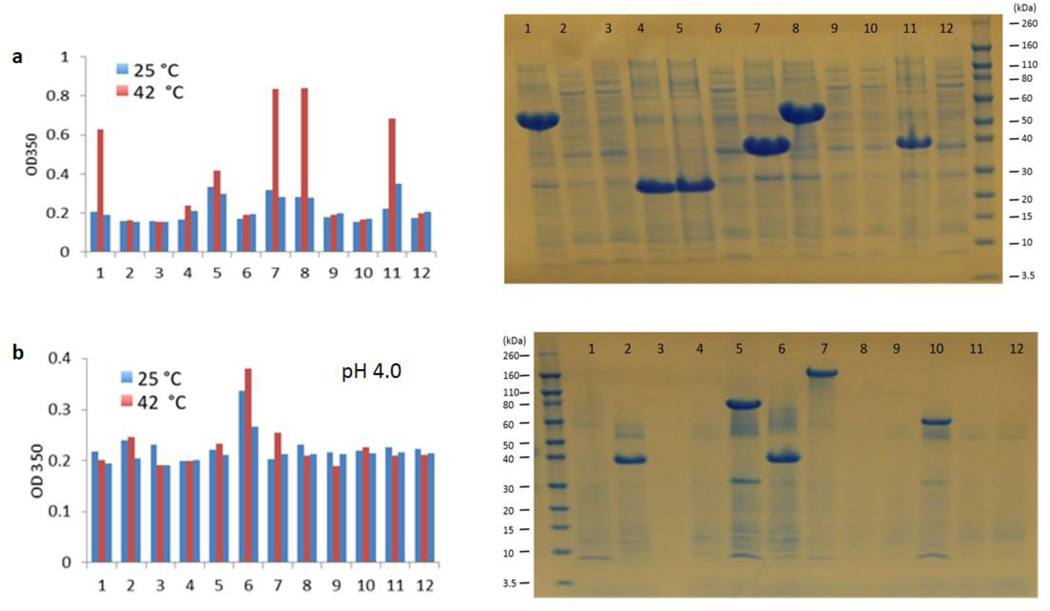
After ligation of the expression vector pET19b3 with SELP genes and transformation of the resulting plasmids into the host cell E. coli BLR(DE3), the colonies containing plasmids carrying SELP genes in vary lengths or empty plasmids were obtained in one plate. The protein polymer library was expressed in 96 well plates and purified in situ through inverse temperature transition cycling. After purification, the proteins dissolved in water or pH buffer were initially screened by optical response, with the OD of protein solutions recorded at 350 nm as a function of temperature. Reversibility of the transitions was examined first at 25°C, then at 42°C, and finally at 25°C again. Figure 3 shows two examples of high throughput screening. One row from a 96 well plate of the S2E8F library screened by thermal response (Figure 3a), and one row from a 96 well plate of the S2E8E library screened by thermal and pH responses (Figure 3b). As confirmed by SDS-PAGE, all the colonies with positive thermal responses had SELPs present but with different molecular weights.
Figure 3.
Initial high throughput screening of physical triggered responses of dynamic SELP library. a. Temperature-induced responses of library of S2E8F. b. pH-induced responses of S2E8E.
After the initial screening of over 2000 colonies, 64 SELPs of 12 different monomers responding to different stimuli were selected, with a molecular weight range from ~20 kDa to ~130 kDa (Table S1). These dynamic proteins were then further selected for specific functions by screening for optical, mechanical or adhesive responses (Figure 2).
2.3 Characterization of the Different Stimuli Responsive Protein Polymers
In order to explore the differences in the transition temperatures with different amino acids in the “X” position for the S2E8X series, S2E8C 12 mer, S2E8E 13 mer, S2E8F 11mer, S2E8K 10 mer, and S2E8Y 12mer, with similar molecular weights, were selected for assessment of thermal and ionic responses (Figures 4a1 and 4a2). For the S4E8X series, S4E8C 11mer, S4E8E 10 mer, S4E8G 11 mer, S4E8I 10 mer, and S4E8Y 11mer were selected (Figures 4b1 and 4b2). When 1 mg/ml S2E8X proteins were dissolved in water, S2E8C, S2E8F, and S2E8Y showed a clear increase in OD350 when the temperature was increased, while the OD350 of S2E8E and S2E8K did not show significant increase when the temperature was increased (Figure 4a1). After the addition of 0.5 N NaCl into the S2E8X protein solution, all five proteins showed a clearer trend of increased OD350 when the temperature was increased (Figure 4a2). For the S4E8X series, when 1 mg/ml proteins were dissolved in water, there was no clear increase in OD350 for all five proteins when the temperature was increased (Figure 4b1). However, when 0.5 N NaCl was added into the protein solution, S4E8G showed an increase in OD350 at 50°C, and S4E8Y showed an increase at 42°C (Figure 4b2). Therefore, the more hydrophobic the amino acid in the “X” position, the clearer the thermal response of the SELPs. This trend is similar to that observed in elastin like polymers.[14, 15] By comparison of S2E8X and S4E8X series (Figures 4a and 4b), the results suggested the higher the elastin to silk ratio in the polymer, the clearer trend of the thermal response SELPs had. Therefore, the high silk to elastin ratio diminishes the dynamic role of the elastin in the polymer. High ionic strength increases the thermal response of the SELPs. When NaCl was added into the protein solution, the transition temperature (Tt) of all SELPs decreased, which has also been observed in elastin based polymers.[17, 18]
Figure 4.
Dynamic protein SELPs respond to different stimuli. a1. S2E8X series respond to thermal changes. a2. S2E8X series respond to ionic strength and thermal changes. b1. S4E8X series respond to thermal changes. b2. S4E8X series respond to ionic strength and thermal changes. c. S2E8E 16 mer responds to different pHs. d. S2E8C 12 mer responds to redox. e. S2E8RGYDLG responds to phosphorylation. f. Electrochemical responses of six representative SELPs that contain either positively charged lysine residues or negatively charged glutamic acid residues along with water and PBS as the negative controls. pI = isoelectric point.
To study specific functional features, the pH response of the SELPs was assessed. The S2E8E 11 mer was dissolved in different pH buffers at 1 mg/ml, and the OD at 350nm was measured as a function of temperature (Figure 4c). When S2E8E was dissolved in lower pH buffer (pH3 and 4), the trend showed an increase in OD350 as the temperature increased. A similar trend was also observed for other S2E8E proteins with different molecular weights (Figure S3). This result was due to the lowering of pH causing a decrease of ionization of the glutamic acid at position X, which resulted in elevated hydrophobicity of the proteins.[19, 20]
The OD of oxidized and reduced S2E8C 12 mer (1mg/ml) was recorded at 350 nm at different temperature (Figure 4d). S2E8C 12 mer was oxidized by 3% H2O2, or reduced by reducing buffer for 12 h. The phase transition of reduced S2E8C was observed at 70°C, while there was no obvious phase transition for oxidized S2E8C. The oxidized S2E8C is cross-linked by disulfide bonds, while the reduced S2E8C disassociates. The folding of oxidized S2E8C at higher temperature may be impeded by such crosslinking, so the inverse temperature transition was not observed.
The protein S2E8RGYDLG 10 mer contains a recognition site (RGYDLG) for protein kinase (PKA) at the “X” position. The OD350 of unphosphorylated S2E8RGYDLG (1mg/ml) increased starting at 42°C. However, after treatment by PKA, the phosphorylated S2E8RGYDLG (1mg/ml) no longer showed an increase in OD350 until 60°C (Figure 4e). Therefore, the transition temperature of S2E8GYDKG was increased after phosphorylation. This is due to the addition of a phosphate group increased the hydrophilicity of the proteins.[21]
Electrogelation is a reversible sol-gel behavior that we have observed with regenerated B. mori silk when a low DC current is passed through an aqueous solution of the protein.[22, 23] For detection of electrochemical responses of SELPs, six representative proteins that contained either positively charged lysine residues or negatively charged glutamic acid residues were selected to construct the SELP library (S2E8K-6mer & -10mer, S2E8E-12mer & -31mer, S4E8E-10mer) (Figure 4f). In responding to an electric field the charged SELPs migrated toward the electrode carrying the opposite charge. During this migration, the SELPs formed micron size hydrogel particle suspensions as local pH matched the isoelectric point due to the electro-diffusion of H+ or OH− ions generated by the electrolysis of water. The optical density at 800 nm of the SELPs was measured before and after applying a constant voltage of 10 V across the parallel electrodes using a DC power supply a small DC current through the solutions for 5 seconds, denoted as OD800, on and OD800, off, respectively. A high ratio of OD800, on/OD800, off, as observed in S2E8E-12mer (20 mg/mL), S2E8E-31mer (12 mg/mL) and S4E8E-10mer (25 mg/mL), indicates an increase in light scattering by the electrogelled suspensions and therefore significant electrochemical response. The results suggested negatively charged SELPs with Glu in “X” position had clearer electrochemical response than the positively charged counterparts with Lys in “X” position, and lower PI and higher molecular weights can promote the electrochemical response.
2.4 Screening of Adhesive Properties of SELPs
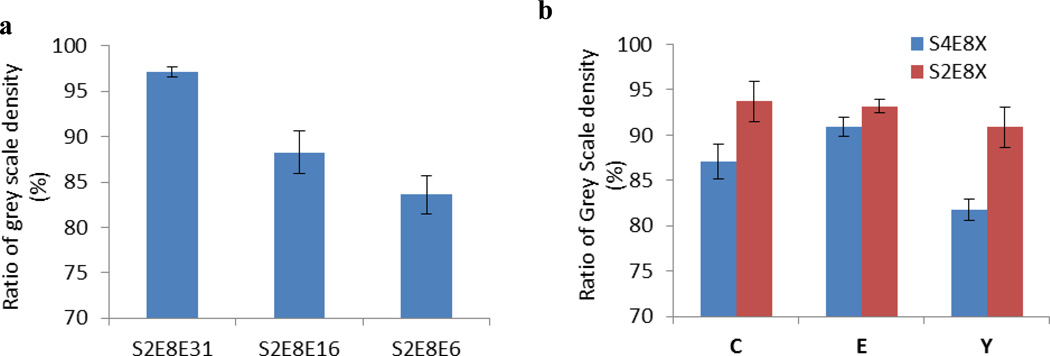
Adhesion to substrates is important for coatings. By screening adhesive properties of SELPs, SELPs for adhesive coating on different surfaces can be determined. One microliter 1 mg/ ml SELP solutions were spotted onto glass slides, air-dried, and assessed for adhesive properties by adhesive tape peel test.[24] The S2E8E 31 mer, S2E8E 16 mer, and S2E8E 6mer with different molecular weights were tested. The S2E8E with higher molecular weight had stronger adhesion to the glass slides (Figure 5a). The adhesive properties of the C (cysteine) series, E (Glutamic acid) series, and Y (Tyrosine) series of SELPs with similar molecular weights but with different silk to elastin ratios were also evaluated (Figure 5b). The results showed high elastin percentage increased the adhesive properties, and the amino acid at position "X" affected the adhesion for S4E8X. However, no significant differences were observed for S2E8X series when changing the amino acid at position "X" (Figure 5b & Figure S4). Adhesion was stronger with the higher molecular weight SELPs and higher elastin to silk ratios (Figure 5), which suggested the elasticity and adhesion of SELPs had a positive correlation.
Figure 5.
Adhesive tests for dynamic protein SELPs. Y axis shows the ratio of the grey scale densities of SELP spots after peeling tests against the ones before peeling. a. Adhesive tests for S2E8E series with different molecular weights. b. Adhesive tests of SELPs with different silk to elastin ratio.
2.5 Mechanical Properties
Mechanical properties of SELPs were studied using nano-indentation by AFM (Figure 6). A typical AFM image of an SELP film is shown in Figure 6a, and a deflection vs. probe displacement curve is shown in Figure 6b. To convert the raw data of cantilever deflection vs. probe displacement into force–distance relations, the deflection sensitivity was calibrated by performing a measurement on the silicon substrate, and the spring constant of the AFM tip was updated by the thermal tune method. By fitting the force-distance curves with the Hertz model, [25–27] the elastic moduli of the SELP films were obtained. The results showed that (1) all SELP films had elastic moduli in the range from 2 to 15 MPa (Figure 6c), which was comparable to the values reported for other synthetic silk-elastin-like or elastin-like polypeptides,[27, 28] as well as native elastin;[27, 29] (2) the Young's modulus was directly proportional to the density of cross-links, and therefore increased with molecular weight; and (3) the addition of the silk domain reduced the elastic modulus of the SELPs.
Figure 6.
High throughput screening of mechanical properties by nano-indentation: a. AFM 3-D height image of SELP film, exemplified by S2E8E 6mer (5um X 5um); b. Deflection vs. probe displacement curve of SELP, exemplified by S2E8F11mer; c. Elastic modulus screening of SELPs by AFM nano-indentation.
3. Conclusions
Protein polymers are a uniquely tunable family of functional biomaterials, yet the diversity of current protein polymers as biomaterials remains limited. This limitation is due to tedious and low-yielding cloning and biosynthesis, as well as the limited screening tools to exploit their unique properties in a high throughput manner. Robust, high-throughput synthesis and characterization methods are needed to expand the reservoir of protein-polymers for different applications, matched more selectively to required functions. In the present study, an efficient repetitive gene cloning method was developed and used for the construction of a dynamic silk-elastin library. A set of proteins for each stimulus was screened and identified to better understand sequence-function relationships and to help guide future library designs. The approach also allows us to scale up to achieve sufficient protein yields for use as source materials for screening, which can inform a wide range of research needs. This study is the first time that an SELP library was construced and screened where different amino acids at the “X” position were included along with systematic comparisons of dynamic properties. We first experimentally demonstrate that these SELPs shared similar environmental responses with ELPs and we were then able to build correlations between the sequences and structures of SELPs and their functional adhesive or mechanical properties. These results will be useful to guide future library designs with specific targeted properties, as well as to identify suitable proteins, thus clones, to scale up for more detailed exploration of material properties, which relate to cell substrates, stem cell research and related needs.[30] This combinational method can be expanded for library construction of other fibrous protein motifs (e.g., collagens, keratins, actins) and recognition motifs (e.g., mineral, cell, binding domains) to continue to build on this platform and provide a discovery tool to guide many directions related to needs in material properties.
4. Experimental Section
Construction of SELP Protein Polymer Library
The tailor-made expression vector, pET-19b3, was constructed for the expression of silk-elastin-like protein polymers under the T7 promoter.[16] DNA sequences were designed to encode the silk-elastin-like sequences: S2E8X [(GAGAGS)2(GVGVP)4(GXGVP)(GVGVP)3] and S4E8X [(GAGAGS)4(GVGVP)4(GXGVP)(GVGVP)3], where X was Tyr, Glu, Gly, Cys, Lys, Ile, Phe, or RGYSLG. The monomer oligonucleotide sequences were cloned into the EcoRV site of the vector pUC57 from GenScript (Piscataway, NJ). The BanII restriction sites were designed to flank each of the monomer oligonucleotide sequences. The monomer DNA sequences were liberated by digesting the pUC57 derivatives with BanII (New England Biolabs, Beverly, MA), isolated by preparative gel electrophoresis, and purified using the QIAquick Gel Extraction kit (Qiagen, Valencia, CA). The purified monomer DNA was then self-ligated with T4 DNA ligase (New England Biolabs, Beverly, MA) for 8 h at 16°C to yield DNA multimers. Next, the BanII and alkaline phosphatase-treated pET-19b3 plasmid was added to the reaction mixture and incubated for an additional 16 h. The ligation mixture was then used to transform electrocompetent cells of E. coli BLR(DE3). The transformants contained recombinant plasmids that carried repetitive genes of varying lengths.
Expression and Purification
The transformants were selected randomly and inoculated in 96 well plates containing 200 µl of Luria-Bertani medium for overnight culture in a shaking incubator at 250 rpm, 37 °C. A 100 µl seed culture was transferred to 96 well plates containing 1 ml of Luria-Bertani medium. Cells were induced with 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) when the optical density at 600 nm reached approximately 0.7. At 6 h after induction cells were harvested by centrifugation at 3,700 rpm for 30 min at 4°C. The supernatant was decanted, and the cell pellets were resuspended in 300 µl B-PER Protein Extraction Reagents (Thermo Scientific, Rockford, IL) for 30 min at 37°C followed with the addition of 75 µl of 5 M NaCl. The solution was centrifuged at 3,700 rpm for 30 min at 40°C. The resulting precipitants were resuspended in 50 µl water or pH buffers for 30 min at 4°C. The soluble solution was transferred to a new 96 well plate for screening. The purity of the proteins was monitored via SDS-PAGE, and the molecular weights of the proteins were determined by MALDI-TOF (Bruker Corporation, Billerica, MA). Protein concentrations were measured by a Pierce BCA Protein Assay kit (Thermo Scientific, Rockford, IL).
Thermal Responses
Optical screening was performed by SpectraMax M2 multi-mode microplate reader (Molecular Devices, Sunnyvale, CA). The purified proteins were solubilized in water or pH buffers for the specific screen. The absorbance of aqueous protein solutions was recorded at 350 nm as a function of temperature. Reversibility of thermal transitions was first examined at 25°C, then at 42°C, and finally at 25°C again. The presence of target proteins in samples with a positive response to temperature was confirmed by SDS-PAGE, and then they were desalted by Zeba 96-well Spin Desalting Plates (Thermo Scientific, Rockford, IL).
For further detection of thermal responses, samples were solubilized in water or other buffers. For example, proteins were solubilized in different pH buffer solutions to screen pH responsive polymers or solubilized in oxidized (0.3% H2O2) or reduced buffer (Invitrogen, Carlsbad, CA) for oxidation/reduction responsive polymers. For phosphorylation of S2E8RGYSLG, purified protein was incubated in phosphorylation buffer (50mM Tris, 10 mM MgCl2, pH7.5) with 5 units of protein kinase A and 20 mM ATP at 30°C for 24 h. [31] The solutions were then incubated at 25°C, 37°C, 42°C, 50°C, 60°C, and 70°C. The OD of protein solutions at different temperatures was detected at 350 nm by microplate reader.
For detection of an electrochemical response, high throughput electroporation multi-well plates (BTX Harvard apparatus, USA) were custom modified based on our previous electrogelation experiments.[32] Different SELPs were added in each well where an electric field was later generated across the 4 mm apart parallel electrodes by applying a constant voltage (8 ~ 10 V) using a DC power supply (Agilent E3612A DC power supply, Agilent Technologies, Inc., Englewood, CO). The optical density at 800 nm was measured from each well before and after passing the DC current through the solutions for three to five seconds, denoted as OD800, on and OD800, off, respectively.
Adhesive Properties
One mg/ml of SELP protein solution was stained with Coomassie Brilliant Blue R-250 (Thermo Scientific, Rockford, IL) and then 1ul of the solution was spotted onto a glass slide. After drying at room temperature, Scotch packaging tapes (3M, St. Paul, MN) were firmly pressed onto the surface of the glass slides with even contact, and then the tape was peeled off from the slide slowly by hand (Figure 2). The images of the protein spots before and after peeling were recorded with a G:BOX Chemi system (SYNGENE, Frederick, MD), and the values of grey scale density before and after peeling were obtained using ImageJ.
Mechanical Properties
The purified SELP proteins were dissolved in water at a concentration of 2 mg/ml, and then cast on a clean silicon wafer to form films for AFM analysis. The SELPs films were imaged in tapping mode on a VEECO Dimension 3100 Atomic Force Microscope (Veeco Instruments Inc, Plainview, NY). To obtain mechanical profiles of the SELP films using high throughput methods, nano-indentation was performed in contact mode. The elastic modulus (Young’s modulus) for each sample was calculated by fitting the force-distance curves from the indentations with the Hertz model.[25–27] The protein networks obey rubber elasticity, therefore a Poisson ratio of 0.5 was used.[25] The spring constant of the AFM tips, in the range from 3.5 to 4 N/m was confirmed by the thermal tune method, and the half-opening angle of the cone was 20° according to the manufacturer’s specifications. For each sample, at least 5 indentation points in different regions were measured and the average elastic modulus and standard deviation were determined.
Statistics Analysis
All quantitative analysis were performed at least in triplicate. Results presented were based on the averages of data points and standard deviations as error bars. One way ANOVA was performed and multiple comparisons were made by Dunnett's t tests at a significance level of 0.05 (overall experiment-wise error rate).
Supplementary Material
Acknowledgment
This work is supported by NIH (P41 EB002520) and the Shanghai Pujiang Program (13PJ1404800). The program for professor of special appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning is also appreciated. The authors would like to thank Dr. David Wilbur and the Tufts Chemistry Department for allowing us to use the MALDI-TOF MS equipment.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Dr. Qin Wang, Department of Biomedical Engineering, Tufts University, 4 Colby Street, Medford, Massachusetts, 02155, United States
Xiaoxia Xia, State Key Laboratory of Microbial Metabolism, School of Life Sciences and Biotechnology, Shanghai Jiao Tong University, 800 Dong-Chuan Road, Shanghai, 200240, China
Dr. Wenwen Huang, Department of Biomedical Engineering, Tufts University, 4 Colby Street, Medford, Massachusetts, 02155, United States
Dr. Yinan Lin, Department of Biomedical Engineering, Tufts University, 4 Colby Street, Medford, Massachusetts, 02155, United States
Qiaobing Xu, Department of Biomedical Engineering, Tufts University, 4 Colby Street, Medford, Massachusetts, 02155, United States
David L. Kaplan, Department of Biomedical Engineering, Tufts University, 4 Colby Street, Medford, Massachusetts, 02155, United States.
References
- 1.Chow D, Nunalee ML, Lim DW, Simnick AJ, Chilkoti A. Mater. Sci. Eng. R. Rep. 2008;62:125. doi: 10.1016/j.mser.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanford K, Kumar M. Curr. Opin. Biotechnol. 2005;16:416. doi: 10.1016/j.copbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Brocchini S, James K, Tangpasuthadol V, Kohn J. J. Am. Chem. Soc. 1997;119:4553. [Google Scholar]
- 4.Murphy JE, Uno T, Hamer JD, Cohen FE, Dwarki V, Zuckermann RN. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1517. doi: 10.1073/pnas.95.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson DG, Lynn DM, Langer R. Angew. Chem. 2003;115:3261. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2003;42:3153. [Google Scholar]
- 6.Qiu W, Teng W, Cappello J, Wu X. Biomacromolecules. 2009;10:602. doi: 10.1021/bm801296r. [DOI] [PubMed] [Google Scholar]
- 7.Stuart MAC, Huck WTS, Genzer J, Muller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Nat. Mater. 2010;9:101. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 8.Qiu W, Huang Y, Teng W, Cohn CM, Cappello J, Wu X. Biomacromolecules. 2010;11:3219. doi: 10.1021/bm100469w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daamen WF, Veerkamp JH, van Hest JCM, van Kuppevelt TH. Biomaterials. 2007;28:4378. doi: 10.1016/j.biomaterials.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Megeed Z, Cappello J, Ghandehari H. Adv. Drug Deliv. Rev. 2002;54:1075. doi: 10.1016/s0169-409x(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 11.Gustafson JA, Ghandehari H. Adv. Drug Deliv. Rev. 2010;62:1509. doi: 10.1016/j.addr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Numata K, Kaplan DL. Adv. Drug Deliv. Rev. 2010;62:1497. doi: 10.1016/j.addr.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haider M, Leung V, Ferrari F, Crissman J, Powell J, Cappello J, Ghandehari H. Mol. Pharm. 2005;2:139. doi: 10.1021/mp049906s. [DOI] [PubMed] [Google Scholar]
- 14.Urry DW. Int. J. Quantum Chem. 1988;34:235. [Google Scholar]
- 15.Urry DW. Prog. Biophys. Mol. Biol. 1992;57:23. doi: 10.1016/0079-6107(92)90003-o. [DOI] [PubMed] [Google Scholar]
- 16.Xia XX, Xu Q, Hu X, Qin G, Kaplan DL. Biomacromolecules. 2011;12:3844. doi: 10.1021/bm201165h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reguera J, Urry DW, Parker TM, McPherson DT, Rodríguez-Cabello JC. Biomacromolecules. 2007;8:354. doi: 10.1021/bm060936l. [DOI] [PubMed] [Google Scholar]
- 18.Park JE, Won JI. Biotechnol. Bioprocess Eng. 2009;14:662. doi: 10.1007/s12257-009-3012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girotti A, Reguera J, Arias FJ, Alonso M, Testera AM, Rodríguez-Cabello JC. Macromolecules. 2004;37:3396. [Google Scholar]
- 20.Nagarsekar A, Crissman J, Crissman M, Ferrari F, Cappello J, Ghandehari H. Biomacromolecules. 2003;4:602. doi: 10.1021/bm0201082. [DOI] [PubMed] [Google Scholar]
- 21.Pattanaik A, Gowda DC, Urry DW. Biochem. Biophys. Res. Commun. 1991;178:539. doi: 10.1016/0006-291x(91)90141-s. [DOI] [PubMed] [Google Scholar]
- 22.Kojic N, Panzer MJ, Leisk GG, Raja WK, Kojic M, Kaplan DL. Soft Matter. 2012;8:6897. doi: 10.1039/C2SM25783A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y, Xia X, Shang K, Elia R, Huang W, Cebe P, Leisk G, Omenetto F, Kaplan DL. Biomacromolecules. 2013;14:2629. doi: 10.1021/bm4004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffith JE, Qiu Y, Tombrello TA. Nucl. Instrum. Methods Phys. Res. 1982;198:607. [Google Scholar]
- 25.Hu X, Wang X, Rnjak J, Weiss AS, Kaplan DL. Biomaterials. 2010;31:8121. doi: 10.1016/j.biomaterials.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada K, Tsuboi Y, Itaya A. Thin Solid Films. 2003;440:208. [Google Scholar]
- 27.del Mercato LL, Maruccio G, Pompa PP, Bochicchio B, Tamburro AM, Cingolani R, Rinaldi R. Biomacromolecules. 2008;9:796. doi: 10.1021/bm7010104. [DOI] [PubMed] [Google Scholar]
- 28.Teng W, Cappello J, Wu X. Biomacromolecules. 2009;10:3028. doi: 10.1021/bm900651g. [DOI] [PubMed] [Google Scholar]
- 29.Aaron BB, Gosline JM. Biopolymers. 1981;20:1247. [Google Scholar]
- 30.Wang Y, Kim HJ, Vunjak-Novakovic G, Kaplan DL. Biomaterials. 2006;27:6064. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Winkler S, Wilson D, Kaplan DL. Biochemistry. 2000;39:12739. doi: 10.1021/bi001335w. [DOI] [PubMed] [Google Scholar]
- 32.Leisk GG, Lo TJ, Yucel T, Lu Q, Kaplan DL. Adv. Mater. 2010;22:711. doi: 10.1002/adma.200902643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.