Abstract
Cancer patients frequently suffer from fatigue, a complex syndrome associated with loss of muscle mass, weakness, and depressed mood. Cancer-related fatigue (CRF) can be present at the time of diagnosis, during treatment, and persists for years after treatment. CRF negatively influences quality of life, limits functional independence, and is associated with decreased survival in patients with incurable disease. Currently there are no effective treatments to reduce CRF. The aim of this study was to use a mouse model of tumor growth and discriminate between two main components of fatigue: loss of muscle mass/function and altered mood/motivation. Here we show that tumor growth increased fatigue- and depressive-like behaviors, and reduced body and muscle mass. Decreased voluntary wheel running activity (VWRA) and increased depressive-like behavior in the forced swim and sucrose preference tests were evident in tumor-bearing mice within the first two weeks of tumor growth and preceded the loss of body and muscle mass. At three weeks, tumor-bearing mice had reduced grip strength but this was not associated with altered expression of myosin isoforms or impaired contractile properties of muscles. These increases in fatigue and depressive-like behaviors were paralleled by increased expression of IL-1β mRNA in the cortex and hippocampus. Minocycline administration reduced tumor-induced expression of IL-1β in the brain, reduced depressive-like behavior, and improved grip strength without altering muscle mass. Taken together, these results indicate that neuroinflammation and depressed mood, rather than muscle wasting, contribute to decreased voluntary activity and precede major changes in muscle contractile properties with tumor growth.
Keywords: fatigue, depression, neuroinflammation, cancer, minocycline, cytokines
1. Introduction
Fatigue is the most common symptom reported by cancer patients before and during treatment, and can continue for years after completion of treatment (Bower and Lamkin, 2013; Husson et al., 2013; Minton et al., 2012). It often co-occurs with depression, (Bower et al., 2011; Kim et al., 2012; Pertl et al., 2013) and reduces quality of life (Vissers et al., 2013). However, the cause of cancer-related fatigue (CRF) is unknown (Berger et al., 2012) and there are no effective treatments (Bower and Lamkin, 2013).
Mounting evidence indicates that CRF and depressed mood are associated with elevated serum levels of pro-inflammatory mediators, including C-reactive protein (Pertl et al., 2013) and cytokines such as tumor necrosis factor-alpha (TNFα), interleukin (IL)-1β and IL-6 (Saligan and Kim, 2012; Wood and Weymann, 2013). These cytokines are likely produced by the tumor and host tissues in response to tumor growth or anti-tumor treatments (Wang et al., 2012). Pro-inflammatory cytokines increase expression of biomarkers of autophagy and the ubiquitin-proteasome pathway in skeletal muscle which reduce muscle mass (Fearon et al., 2012; Sandri, 2013; Toledo et al., 2011). The loss of muscle mass, or sarcopenia, can be seen even before cancer treatment (Baracos et al., 2010; Cao et al., 2010) and likely explains patient complaints of exhaustion associated with physical activity and muscle weakness (Hofman et al., 2007).
Systemic increases in pro-inflammatory mediators mount a complex response that is not limited to the periphery. The central nervous system (CNS) interprets inflammatory responses that originate in the periphery. Microglia, innate immune cells of the CNS, contribute to the propagation of inflammatory cytokines and secondary messengers throughout the CNS (Wood and Weymann, 2013). Increases in brain IL-1β are linked to both muscle atrophy (Braun et al., 2011) and depressed mood (Haroon et al., 2012). Recent evidence from rodent models indicates that inflammatory cytokines within the CNS are associated with symptoms of fatigue, such as decreased voluntary wheel running activity (Carmichael et al., 2006). Although a link between inflammation and fatigue in cancer patients has been suggested (Bower, 2007), no clear connection between CNS inflammation and CRF has been reported.
The aim of this study was to discriminate between loss of muscle mass and depressed mood in a mouse model of CRF. Fatigue was modeled as reduced voluntary wheel running activity (VWRA) (Novak et al., 2012; Wood et al., 2006; Zombeck et al., 2013) and weakness was modeled as reduced forelimb grip strength (Murphy et al., 2012). Depressed mood was modeled using the sucrose preference (Lamkin et al., 2011) and forced swim tests (Pyter et al., 2009). We show that depressive-like behavior and brain cytokine expression were increased and VWRA was decreased in tumor-bearing mice prior to the loss of muscle mass and decrease in grip strength. Decreased grip strength, however, was not associated with reduced contractile properties of skeletal muscle. Administration of minocycline to tumor-bearing mice reduced inflammatory cytokine expression in the brain, reduced depressive-like behavior, and increased grip strength with no effects on muscle mass. These data indicate that grip strength may reflect motivation or mood as much as muscle strength. Overall, decreased physical activity and depressive-like behaviors are mediated by pro-inflammatory cytokine expression in the brain of tumor-bearing mice.
2. Materials and methods
2.1 Mice
Adult female BALB/c x DBA/2 F1 (CD2F1) adult (10 weeks) mice weighing 20–22 g were obtained from Charles River Laboratories. Female mice were used because we and others have shown that tumor-bearing females maintain their food intake and lose a smaller percent of body mass than male mice (Cosper and Leinwand, 2011) and male mice often gnaw and bite at the tumor site (Yang et al., 2014). Mice were housed 1–3 per cage and maintained at 25°C under a 12 h light cycle with ad libitum access to water and rodent chow. All procedures were performed in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by The Ohio State University Institutional Animal Care and Use Committee.
2.2 Mouse model of tumor-growth
The colon26 adenocarcinoma (colon26) cell line was maintained in culture and prepared for injection as previously described (Xu et al., 2011). Mice were injected subcutaneously between the scapulae with 5×105 cells in 0.2 ml of PBS or PBS alone. This tumor cell line secretes IL-6 and TNF-α (Graves et al., 2006) and does not metastasize when injected subcutaneously (Okayama et al., 2009). Tumor growth is usually palpable by day 7 and mice become moribund by day 24 of tumor-growth. In the present study, all data collection was completed by day 21 of tumor growth. Body mass and food and water intake were monitored three times a week for the first 2 weeks, and daily during the 3rd week. Behavioral data were collected in the range of 1 week (7 day), 2 weeks (12–14 day) and 3 weeks (19–21 day) following tumor cell inoculation. Except as noted below, mice were euthanized by inhalation of CO2 gas and blood was withdrawn by cardiac puncture. Hindlimb muscles were dissected, weighed, and snap frozen in liquid nitrogen until biochemical analyses; tumor mass was removed and weighed; the brain was quickly dissected and hippocampus and cortex brain tissue were snap frozen in liquid nitrogen.
2.3 Oral minocycline administration
Mice were housed 3 per cage for the minocycline study. Mice were provided bottles of water or water supplemented with 1mg/ml minocycline for a dose of 100 mg/kg/day (Sigma, St. Louis) starting one day after PBS or tumor cell injection. Water bottles were changed every other day throughout the study. There were no differences in total fluid intake between any of the experimental groups (Control-minocyline, Control-vehicle, Tumor-minocycline, and Tumor-Vehicle) (data not shown).
2.4 Grip strength measurements
Forelimb grip strength was determined as previously described (Murphy et al., 2012). In brief, each mouse was allowed to grasp a platform with both forelimbs and was pulled by the tail until it released itself from the platform (Columbus Instruments, model 1027DSM). Peak force measurements (N) were recorded in five trials and the average was calculated. Because smaller mice have smaller grip strength, peak force was also normalized to body mass of the animal.
2.5 Voluntary wheel running activity
Fatigue-like behavior was determined using voluntary wheel running activity as previously described (Zombeck et al. 2013). Mice were singly housed for studies in which voluntary wheel running activity (VWRA) was determined. Mice were acclimated to a four inch running wheel in the cage for one week, and baseline measures (week 0) of VWRA were recorded overnight prior to injection with tumor cells or PBS. Wheels were again placed in the home cages of all mice overnight on days 7 (week 1), 14 (week 2) and 19 (week 3) of tumor growth and the total number of turns was digitally recorded (Columbus Instruments, model 0297-004M).
2.6 Home cage locomotor activity
Mice were maintained in their home cage with a floor area of 26 × 20 cm, and activity was video recorded for 3 minutes. On the video records, cages were divided into 6 identical virtual rectangles and the number of line crossings was determined.
2.7 Depressive-like behavior
Depressive-like behavior was determined using resignation in the forced swim test (FST) and anhedonia in the sucrose preference test, as described previously (Godbout et al., 2008; Henry et al., 2008). In the FST, mice were placed in an inescapable cylinder (diameter 16 cm, height 30 cm) containing 15 cm of water and behavior was recorded for five min. The latency to become immobile and the duration of immobility were determined. For the sucrose preference test, mice were provided two solutions: water or water supplemented with 2% sucrose. Mice were fluid- and food-deprived for 2 h prior to testing. At the start of the dark phase of the photoperiod, plain water and the sucrose water were both placed in each home cage overnight (15 h). At the end of each testing period, the fluid content was measured and the percent of sucrose preference was determined.
2.8 In vitro Muscle Contractile Properties and Fatigue Resistance
Contractile properties of freshly isolated hindlimb muscles were determined, as described in detail previously (Bicer et al., 2009). In brief, mice were euthanized with CO2 inhalation and one soleus muscle and one EDL muscle were removed and placed in oxygenated Ringer’s solution. One muscle was maintained in oxygenated Ringer’s solution in a petri dish at room temperature while contractile measurements were made on the other muscle. The order in which the soleus and EDL were studied was alternated between experiments and it was previously determined that this did not impact the results (Bicer et al., 2009). All of the measurements were made at room temperature (21.2–23.8°C). The muscle was mounted horizontally in an in vitro apparatus (model 801C, Aurora Scientific, Aurora, Ontario, Canada) and was continuously superfused with oxygenated Ringer’s solution (137 mM NaCl, 5 mM KCl, 13 mM NaHCO3, 1.8 mM KH2PO4, 2 mM CaCl2, 11 mM glucose, 1 mM MgSO4, and 0.025 mM tubocurarine chloride). One tendon of the muscle was glued to a wire hook and the other tendon was similarly attached to a force transducer (model 300B, Aurora Scientific). Care was taken to ensure that none of the glue contacted the muscle fibers. The hook and transducer were mounted on three-way positioners. The muscle was set at optimal length by adjusting the position of the hook or transducer, until maximal twitch force was generated. The muscle was then stimulated to elicit five twitches and the peak twitch force, time to peak force, and time from peak force to one-half relaxation during each twitch were recorded. Next, the tetanic force-frequency curve was established using stimulus trains. The lowest stimulation frequency that yielded the maximal peak tetanic force in the force-frequency measurements was used to measure peak tetanic force and the time from the last stimulus to one-half relaxation. Five tetani, spaced 2 minutes apart, were studied in each muscle and the means were calculated. Maximal twitch and tetanic forces were also normalized to muscle cross-sectional area which was estimated from muscle mass and length measurements (Bicer et al., 2009). After a five minute rest, fatigue resistance was measured by stimulating the muscle with trains at 1 Hz consisting of pulses (pulse duration 0.1 ms) at 70 Hz for 400 ms. A fatigue index was quantitated as the peak force during the train at 2 minutes divided by the peak force during the initial train.
2.9 In situ contractile properties
Control and tumor-bearing mice were anesthetized with urethane and placed in a supine position on an in situ apparatus (model 809B, Aurora Scientific). The sciatic nerve was exposed above the knee joint and the uninsulated end of a stainless steel wire electrode (Biomed Wire AS 631, Cooner Wire Co.) was placed in contact with the nerve. The other end of the wire was connected to a stimulator (model 701C, Aurora Scientific) which was gated with another stimulator (Grass Technologies, model S48). The knee was clamped and the skin overlying the tibialis anterior was cut. The distal tendon of the muscle was glued to a wire which was hooked to the arm of a force transducer (model S100-5N, Strain Measurement Devices, Wallingford CT) that was connected to a custom-built power supply/amplifier. The muscle was set to the length at which the greatest peak twitch force was generated. The stimulation and recording protocols were identical to the protocol used to determine in vitro contractile properties. Muscle cross-sectional area was determined as in the in vitro muscle measurements. Core temperature of the mouse was maintained with a heating pad.
2.10 Gel electrophoresis
The preparation of samples and of gels for the analysis of myosin heavy chain (MHC) and light chain (MLC) isoform composition was identical to that described previously (Bicer et al., 2009). Briefly, MHC isoforms were run on 8% acrylamide gels with 30% glycerol. MLC isoforms were analyzed on 12% acrylamide (no glycerol). The gels were silver-stained and the relative amounts of fast- and slow-type MHC isoforms in each sample were determined relative to total MHC in the same sample.
2.11 RNA isolation and RT-PCR analysis
RNA was isolated from hippocampus and cortex brain sections using the Tri-Reagent protocol (Sigma) and reverse transcribed to cDNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Quantitative PCR was performed using the Applied Biosystems Assay-on-Demand Gene Expression protocol. In brief, experimental cDNA was amplified with an ABI PRISM 7300-sequence detection system (Applied Biosystems) by real-time PCR and normalized based on reference cDNA (GAPDH). Data were analyzed with the comparative threshold cycle method.
2.12 Immunohistochemistry and digital image analyses
Mice were deeply anesthetized by CO2 inhalation and transcardially perfused with sterile PBS followed by 4% formaldehyde. Brains were post-fixed in 4% formaldehyde for 24 h and cryoprotected in 20% sucrose for 48 h. Preserved brains were frozen using dry-ice cooled isopentane and sectioned (25 μm) using a Microm HM550 cryostat. Iba-1 staining was performed as previously described (Wohleb et al., 2011). In brief, free-floating sections were blocked and then incubated with rabbit anti-mouse Iba-1 antibody (Wako Chemicals) overnight at 4°C. Sections were washed with PBS and incubated with a fluorochrome-conjugated secondary antibody (Alexa Flour 594). Fluorescent images were visualized using an epifluorescent Leica DM5000B microscope and were captured using a Leica DFC300 FX camera and imaging software. Quantitation was assessed using digital image analysis (Donnelly et al., 2009) in the hippocampus (12 representative images) and prefrontal cortex (6 representative images) at 20× magnification. Threshold staining was determined using NIH ImageJ software. Results are reported as the average percent area for Iba-1+ staining.
2.13 IL-6 ELISA
IL-6 was determined from plasma using the BD OptEIA Mouse IL-6 ELISA, according to the manufacturer’s instructions (BD Biosciences). Absorbance was read at 450 nm using a Synergy HT Plate Reader (Bio-tek instruments). The assay was sensitive to 10 ng/ml IL-6 and intra-assay coefficients of variation were less than 10%.
2.14 Statistical Analysis
Data were subjected to a Shapiro-Wilk test using Statistical Analysis Systems (SAS) software (Cary, NC). Observations greater than three interquartile ranges from the first and third quartile were considered outliers and were excluded in the subsequent analyses. To determine significant main effects and interactions between main factors, data were analyzed using one-, two-, or three-way ANOVA using the General Linear Model procedures of SAS. Differences between group means were evaluated with the t-test using the Least-Significant Difference procedure of SAS. All data are expressed as treatment means ± standard error of the mean (SEM).
3. Results
3.1 Tumor growth was associated with muscle loss and muscle weakness
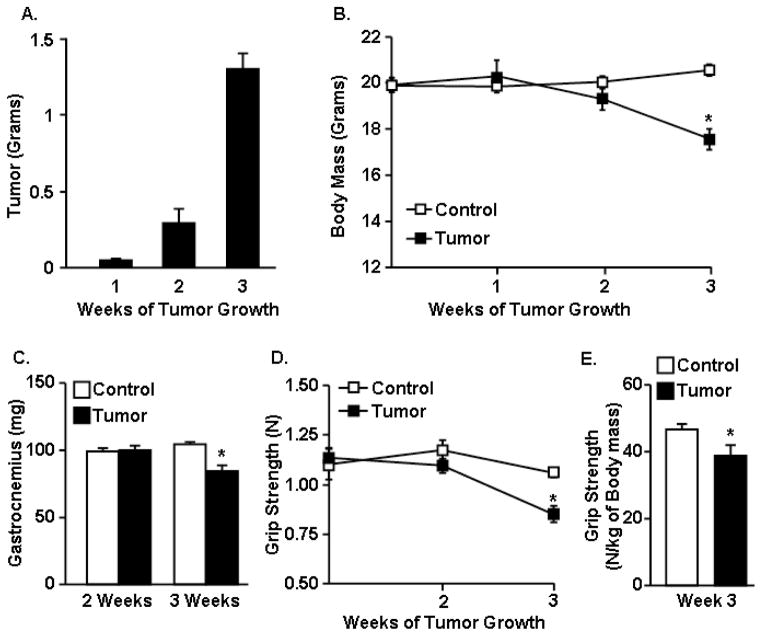
To begin to understand the effects of tumor growth on muscle function and mood, mice were inoculated subcutaneously between the scapulae with C26 adenocarcinoma cells. Tumor mass was 0.3 g by 2 weeks and reached an average of 1.3 g (5% of body mass) by 3 weeks (F1,24=38.22, p<0.0001, Fig. 1A). Body mass of tumor-bearing mice was not different from healthy controls at 1 and 2 weeks, but was reduced by 3 weeks (F2,30=3.51, p<0.05, Fig. 1B). There was no decline in muscle mass within 2 weeks of tumor growth; however, there was a significant loss of gastrocnemius muscle mass by three weeks of tumor growth (F1,26=13.17, p<0.02, Fig. 1C). Tumor growth also reduced the mass of tibialis cranialis, soleus, and biceps brachii muscles (p<0.05 for each, data not shown).
Figure 1. Tumor growth was associated with muscle mass loss and muscle weakness.
Mice were inoculated s.c. with PBS (Control) or PBS with C26 adenocarcinoma cells (Tumor). A) Tumor mass, B) body mass, and C) gastrocnemius muscle mass was determined 1, 2, and 3 weeks after inoculation (n=6). D) Grip strength (Newtons) was determined at baseline (day 0) and then again at 2 and 3 weeks (n=8). E) The normalized grip strength (Newtons of grip strength to kilograms of body mass) was also determined at the 3 week endpoint (n=8). Data are expressed as mean ± SEM. Means with * are different from control mice (p<0.05).
Next, the extent to which loss of muscle mass contributed to muscle weakness was determined. In this experiment, forelimb grip strength was determined prior to tumor cell inoculation (week 0) and at weeks 2 and 3 of tumor growth. Tumor growth decreased grip strength (F1,66=4.72, p<0.03) in a time-dependent manner (F1,66=3.08, p<0.05) (Fig. 1D). Grip strength of tumor-bearing mice was similar to controls at baseline and 2 weeks after tumor cell inoculation but was decreased compared to controls by 3 weeks (p<0.003, Fig. 1D). Similar data were obtained when grip strength was normalized to body mass (F1,16=8.84, p<0.02, Fig. 1E).
3.2 Effects of tumor growth on contractile properties of skeletal muscle
To better understand the decline in grip strength in tumor-bearing mice, we examined contractile properties of the soleus and EDL from tumor-bearing mice in vitro at 1, 2, and 3 weeks after tumor cell inoculation and from control mice at 4 weeks after saline injection. Absolute twitch force (i.e., not normalized with muscle cross-sectional area) in the soleus was not affected by tumor burden (Table 1). In contrast, absolute tetanic force in the soleus was significantly reduced after two and three weeks of tumor burden (F1,24=5.468, p<0.007). Absolute twitch force in the EDL was significantly lower at one, two and three weeks of tumor burden (F1,24=4.852, p<0.011), as was absolute tetanic force at one and three weeks (F1,24=3.185, p<0.04, Table 2). Peak twitch and tetanic forces normalized to cross sectional area of the muscle (specific force) were not different from control animals at weeks 1, 2 or 3 of tumor growth in either the soleus (Table 1) or EDL (Table 2). In the soleus, the time to peak twitch force and the time to one-half relaxation were significantly longer in tumor-bearing mice, beginning at one week after inoculation (p<0.05). The time to one-half relaxation of the tetanus was, however, unaltered by tumor growth. In the EDL, relaxation during the tetanus was not affected by tumor growth. Moreover, fatigue resistance was not altered in either muscle at weeks 1, 2, or 3 of tumor growth.
Table 1.
Soleus in vitro contractile properties.
| Parameters | Control | Tumor Week 1 |
Tumor Week 2 |
Tumor Week 3 |
|---|---|---|---|---|
| Twitch | ||||
| Time to peak (ms) | 52 ± 4 | 90 ± 7* | 81 ± 5* | 79 ± 1* |
| Time to relax 50% (ms) | 82 ± 10 | 259 ± 41* | 206 ± 28* | 167 ± 33* |
| Absolute force (mN) | 52 ± 3 | 51 ± 3 | 46 ± 3 | 45 ± 4 |
| Specific force (kN/m2) | 86 ± 5 | 95 ± 4 | 95 ± 6 | 94 ± 7 |
| Tetanus | ||||
| Time to relax 50% (ms) | 151 ± 5 | 178 ± 11 | 161 ± 8 | 198 ± 20 |
| Absolute force (mN) | 206 ± 14 | 180 ± 6 | 158 ± 11* | 153 ± 9* |
| Specific force (kN/m2) | 339 ± 20 | 336 ± 13 | 324 ± 22 | 316 ± 19 |
| Fatigue (2 min /initial force) | 0.53 ± 0.03 | 0.42 ± 0.03 | 0.47 ± 0.02 | 0.50 ± 0.06 |
|
| ||||
| Number of mice | 6 | 6 | 6 | 6 |
Mice were inoculated s.c. with PBS (Control) or PBS with C26 adenocarcinoma cells (Tumor). Control and tumor mice were sacrificed at 1, 2 or 3 weeks after tumor cell inoculation and contractile properties of soleus skeletal muscle were determined in vitro (n=6). Values are mean ± sem. Means with * are different from control mice (p<0.05).
Table 2.
EDL in vitro contractile properties.
| Parameters | Control | Tumor Week 1 |
Tumor Week 2 |
Tumor Week 3 |
|---|---|---|---|---|
| Twitch | ||||
| Time to peak (ms) | 26 ± 1 | 21 ± 1* | 21 ± 1* | 29 ± 2 |
| Time to relax 50% (ms) | 41 ± 4 | 31 ± 2 | 23 ± 1* | 45 ± 5 |
| Absolute force (mN) | 124 ± 5 | 93 ± 3* | 98 ± 7* | 91 ± 11* |
| Specific force (kN/m2) | 139 ± 5 | 125 ± 6 | 129 ± 8 | 137 ± 13 |
| Tetanus | ||||
| Time to relax 50% (ms) | 58 ± 3 | 55 ± 2 | 52 ± 1 | 64 ± 4 |
| Absolute force (mN) | 326 ± 14 | 251 ± 9* | 288 ± 21 | 249 ± 29* |
| Specific force (kN/m2) | 366 ± 17 | 336 ± 16 | 380 ± 23 | 373 ± 36 |
| Fatigue (2 min/initial force) | 0.09 ± 0.02 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.10 ± 0.01 |
|
| ||||
| Number of mice | 6 | 6 | 6 | 6 |
Mice were inoculated s.c. with PBS (Control) or PBS with C26 adenocarcinoma cells (Tumor). Control and tumor mice were sacrificed at 1, 2 or 3 weeks after tumor cell inoculation and contractile properties of Extensor digitorum longus (EDL) skeletal muscle were determined in vitro (n=6). Values are mean ± sem. Means with * are different from control mice (p<0.05).
Given the absence of significant effects of tumor growth on normalized muscle force generation in vitro, in situ contractile analyses were performed on the tibialis anterior muscle at three weeks of tumor growth. Absolute peak twitch and tetanic forces were lower in the tumor-bearing mice, compared to the control group (F1,12=8.863, p<0.01, Table3). However, the forces normalized to cross sectional area, kinetics of contraction and relaxation, and fatigue resistance were not different between control mice and tumor-bearing mice (Table 3).
Table 3.
Tibialis in situ contractile properties.
| Parameters | Control | Tumor |
|---|---|---|
| Twitch | ||
| Time to peak (ms) | 30 ± 2 | 27 ± 1 |
| Time to relax 50% (ms) | 30 ± 3 | 27 ± 2 |
| Time to relax 90% (ms) | 66 ± 7 | 57 ± 4 |
| Absolute force (mN) | 417 ± 15 | 353 ± 16* |
| Specific force (kN/m2) | 120 ± 4 | 141 ± 4 |
| Tetanus | ||
| Time to relax 50% (ms) | 56 ± 4 | 50 ± 4 |
| Time to relax 90% (ms) | 96 ± 9 | 88 ± 8 |
| Absolute force (mN) | 1127 ± 65 | 859 ± 48* |
| Specific force (kN/m2) | 323 ± 17 | 329 ± 18 |
| Fatigue (2 min force/initial force) | 0.22 ± 0.04 | 0.29 ± 0.08 |
|
| ||
| Number of mice | 6 | 6 |
Mice were inoculated s.c. with PBS (Control) or PBS with C26 adenocarcinoma cells (Tumor). In situ contraction analyses of the tibialis were determined 3 weeks after tumor cell inoculation (n=6). Values are mean ± sem. Means with * are different from control mice (p<0.05).
Myosin heavy chain (MHC) and myosin light chain (MLC) are major determinants of contractile properties in skeletal muscle. Therefore, we evaluated the MHC and MLC isoform composition of the soleus and EDL in control and tumor-bearing mice on SDS gels. There were no differences detected in MHC or MLC isoform expression in either the soleus or EDL between control and tumor-bearing mice at three weeks of tumor growth (Fig. 1S). Overall, these data indicate that while tumor growth decreased skeletal muscle mass and reduced absolute grip strength over time, myosin composition and normalized contractile forces of skeletal muscles were not altered by tumor growth.
3.3 Tumor growth increased fatigue- and depressive-like behavior
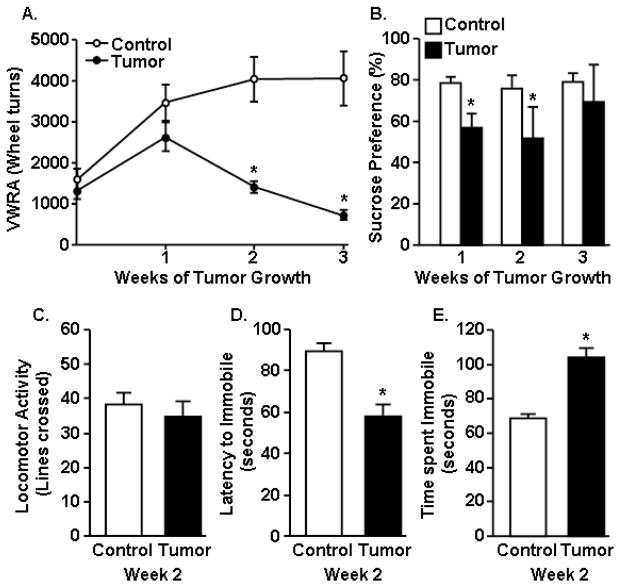
The lack of effect of tumor growth on normalized contractile force of muscle suggests that tumor-associated changes in mood might decrease motivation of the tumor-bearing mice to engage in the grip strength test. To test this hypothesis, mice were evaluated for fatigue and depressive-like behaviors. In the first experiment, activity was assessed in control and tumor-bearing mice using VWRA before tumor cell inoculation and again 1, 2, and 3 weeks later. VWRA progressively declined in the tumor-bearing mice (F1,16=15.23, p<0.001) (Fig. 2A). VWRA was decreased by week 2 (p<0.001) of tumor growth, prior to loss of muscle mass, and was further decreased by week 3 (p<0.001) compared to controls.
Figure 2. Tumor growth increased fatigue- and depressive-like behavior.
A) Voluntary wheel running activity (VWRA) was determined overnight at baseline and again at 1, 2, and 3 weeks after tumor cell inoculation. B) Sucrose preference was determined 1, 2, and 3 weeks after tumor cell inoculation. C) Home cage activity was determined 2 weeks after tumor inoculation. Control and tumor mice were exposed to the forced swim test (FST) at 2 weeks and D) latency to become immobile and E) total time immobile were determined (n=6). Bars represent the mean ± SEM. Means with * are different from control (p<0.05).
To better understand the influence of tumor growth on mood, anhedonia (sucrose preference) was determined in control and tumor-bearing mice before tumor cell inoculation and again 1, 2 and 3 weeks of tumor growth. At baseline, all mice preferred drinking sucrose, i.e. over 80% of total fluid consumed (data not shown). Moreover, control mice maintained a preference for sucrose at each time point (Fig. 2B). In tumor-bearing mice, however, sucrose preference was decreased (F1,100=4.09, p<0.004) to 56% by 1 week (p<0.006) and 52% by 2 weeks (p<0.05) of tumor growth (Fig. 2B). This decrease in sucrose preference was no longer present by week three of tumor growth.
To further examine the effects of tumor growth on mood in tumor-bearing mice, depressive-like behavior (resignation) was determined at 2 weeks using the forced swim test (FST). This time point represents a time when sucrose preference and VWRA were decreased in tumor-bearing mice, but body mass and muscle mass were not different from controls. Because these evaluations of mood/motivation can be confounded by differences in locomotion, home cage locomotor activity was determined prior to the FST. There was no difference in home cage activity between control and tumor-bearing mice at 2 weeks of tumor growth (Fig. 2C). Nonetheless, tumor-bearing mice were immobile faster (F1,12=7.05, p<0.02, Fig. 2D) and spent more time immobile in the FST compared to controls (F1,12= 43.18, p<0.001, Fig. 2E). Overall, these data indicate that tumor growth was associated with increased depressive-like behavior at the same time as VWRA was reduced in tumor-bearing mice.
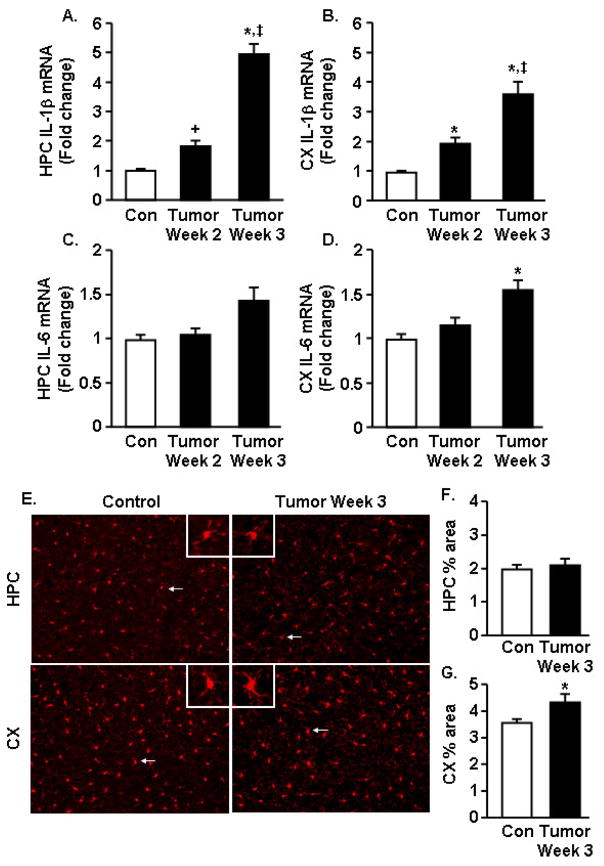
3.4 Increased IL-1β and IL-6 mRNA expression in the brain of tumor-bearing mice
Our data indicate that tumor growth increased depressive-like behavior and decreased VWRA prior to reducing grip strength and muscle mass in the tumor-bearing mice. Because depressed mood is associated with increased levels of inflammatory cytokines within the brain, IL-1β, IL-6, and TNFα mRNA expression was determined at 2 and 3 weeks in the cortex (CX) and hippocampus (HPC) of control and tumor-bearing mice. Tumor growth increased IL-1β mRNA expression in the HPC (F1,44=24.17, p<0.0001) (Fig. 3A). For example, IL-1β mRNA was increased by 2 weeks after tumor cell inoculation (p<0.03) and was further increased by 3 weeks (p<0.004). A similar pattern for IL-1β mRNA expression was evident in the CX of tumor-bearing mice (F1,37=10.31, p<0.003, Fig. 3B). Tumor growth also tended to increase IL-6 mRNA in the HPC (F1,44=3.3, p=0.08) (Fig. 3C) and CX (F1,34=4.15, p<0.05) (Fig. 3D) by 3 weeks, but not by 2 weeks. TNFα mRNA expression was not increased in tumor-bearing mice at either time point (data not shown).
Figure 3. Increased IL-1β and IL-6 mRNA expression in the brain of tumor-bearing mice.
IL-1β mRNA expression was determined in the A) hippocampus (HPC) and B) cortex (CX) 2 and 3 weeks after tumor cell injection. IL-6 mRNA was also determined in the C) HPC and D) CX (n=6). E) Representative images of Iba-1 labeling of microglia in the HPC and CX collected 3 weeks after tumor cell inoculation. Inset includes enlarged image of Iba-1+ cell indicated by white arrow. Proportional area for Iba-1 labeling in the F) HPC and G) CX (n=6). Data are expressed as mean ± SEM. Means with * are different from control mice (p<0.05) and means with ‡ are different from Tumor-Week 2 (p<0.05).
Next, microglial activated morphology was determined in the HPC and CX of control and tumor-bearing mice at 3 weeks. Representative images of Iba-1 positive microglia from the HPC and CX are shown in Fig. 3E. There was no difference in Iba-1 immunoreactivity in the HPC between control mice and tumor mice (top panels). In the CX, however, tumor-bearing mice had increased Iba-1 immunoreactivity. Microglia from the brain of tumor mice had larger cell bodies with thicker and more condensed processes (Fig. 3E). Proportional analysis of Iba-1 staining (Wohleb et al., 2011) confirmed that tumors increased Iba-1 immunoreactivity in the CX (F1,12=4.65, p=0.05, Fig. 3F). Taken together, these data indicate tumor growth was associated with increased pro-inflammatory cytokine expression in the hippocampus and cortex and increased Iba-1 immunoreactivity.
3.5 Minocycline attenuated depressive-like behavior, neuroinflammation, and restored grip strength in tumor-bearing mice
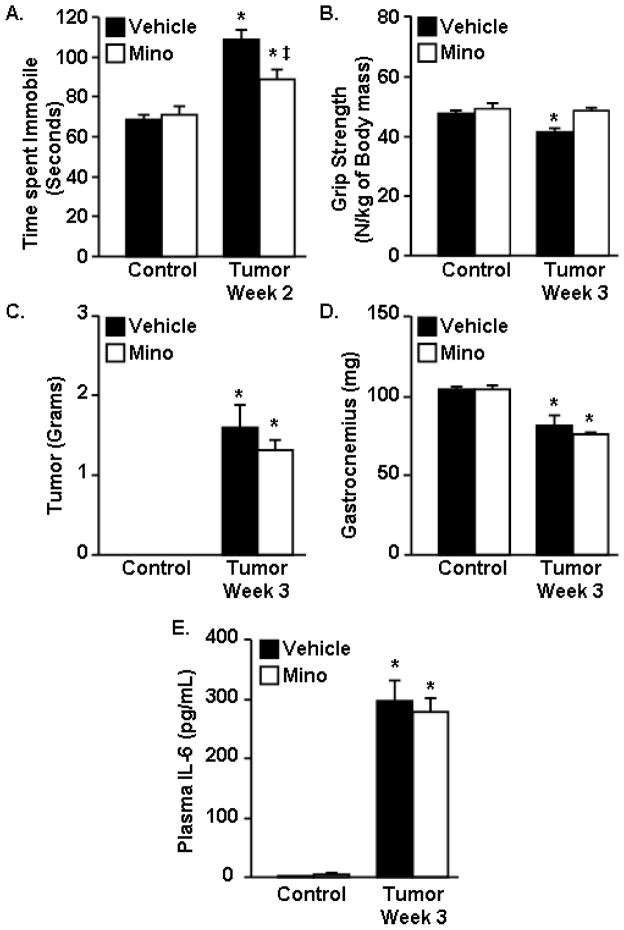
Reduced VWRA, increased depressive-like behavior, and increased brain expression of IL-1β were evident by two weeks of tumor growth and preceded the reduction in muscle mass and grip strength at three weeks. Therefore, minocycline, an anti-inflammatory agent and purported microglial inhibitor (Nikodemova et al., 2006), was used to determine the extent to which elevated IL-1β and IL-6 expression in the CNS contributes to altered behavior in tumor-bearing mice. For this experiment, mice were administered minocycline in their drinking water from day 1 after tumor cell inoculation to the completion of the study.
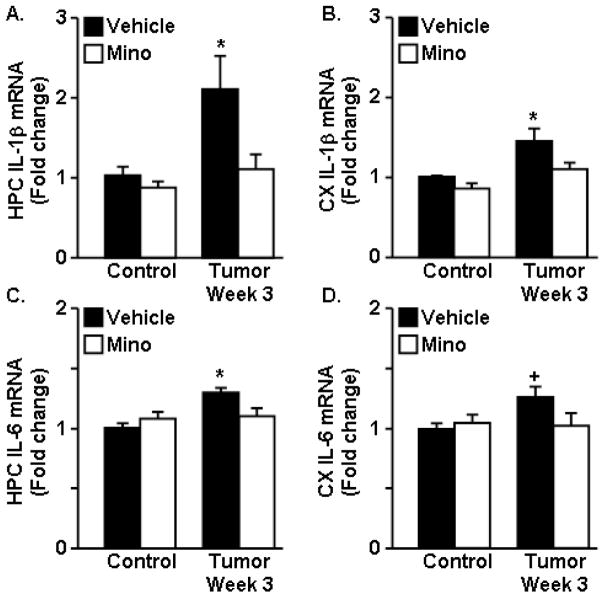
Depressive-like behavior was determined at 2 weeks of tumor growth using the FST. Consistent with our earlier finding (Fig. 2E), tumor-bearing mice had an increased total time immobile in the FST (F1,46=53.83, p<0.001) (Fig. 4A). Tumor-bearing mice treated with minocycline, however, had decreased total time immobile compared to tumor-bearing mice given water (F(1,46)=7.65, p<0.01) (Fig. 4A). At 3 weeks of tumor growth, absolute (data not shown) and normalized grip strength were decreased in tumor-bearing mice (F1,22=5.88, p<0.03) (Fig. 4B). Administration of minocycline restored the normalized grip strength to the same levels as control mice (F1,22=3.75, p=0.07) (Fig. 4B). After the completion of this test, mice were euthanized and tumor mass, gastrocnemius muscle mass, plasma IL-6 levels and brain IL-1β and IL-6 mRNA expression were determined. As expected, minocycline intervention had no significant effect on tumor mass (Fig. 4C) or gastrocnemius muscle mass (Fig. 4D). Plasma IL-6 was increased in tumor-bearing mice (F1,23=37.04, p<0.001) but was not changed by minocycline administration (Fig. 4E). Consistent with previous results (Fig. 3), expression of IL-1β and IL-6 mRNA were increased in the cortex and hippocampus of tumor-bearing mice at 3 weeks of tumor growth (Fig. 5A–D). Minocycline intervention, however, blocked tumor associated increases in IL-1β and IL-6 mRNA in the hippocampus and cortex (F1,46=5.18, p<0.03, for each). In conclusion, minocycline intervention had no effect on tumor size or muscle mass, but ameliorated the tumor-associated effects on neuroinflammation, depressive-like behavior, and grip strength normalized to body mass.
Figure 4. Minocycline attenuated depressive-like behavior and restored grip strength in tumor-bearing mice.
Control and Tumor-bearing mice were administered vehicle or minocycline (Mino) in the drinking water starting one day after tumor cell injection. A) Control and Tumor mice were exposed to the forced swim test at 2 weeks and total time immobile was determined. B) Grip strength was assessed and normalized to body mass at the 3 week endpoint. C) Tumor mass, D) muscle mass, and E) plasma IL-6 levels were determined at 3 weeks. Means with * are different from control mice (p<0.05), means with ‡ are different from Tumor-Vehicle mice (p<0.05) (n=8–12).
Figure 5. Minocycline attenuated neuroinflammation in tumor-bearing mice.
Control and Tumor-bearing mice were administered vehicle or minocycline (Mino) in the drinking water. A–D) IL-1β and IL-6 mRNA expression was determined in the HPC and CX at 3 weeks. Data are expressed as mean ± SEM. Means with * are different from control mice (p<0.05), means with + tend to be different from control mice (p=0.07) (n=8–12).
4. Discussion
Fatigue is a common and distressing symptom reported by cancer patients before, during, and after cancer treatments. Patients with CRF often describe muscle weakness and reduced effort tolerance which have a negative effect on quality of life and functional status (Hofman et al., 2007). Muscle wasting and depressive symptoms are prevalent in patients with CRF and are associated with higher mortality rates in cancer patients with persistent or incurable disease (Mols et al., 2013). Therefore, treatments to reduce fatigue and depression in cancer patients are needed to increase quality of life and perhaps prolong survival. In this study, a mouse model of CRF was used to discriminate between loss of muscle mass and altered mood in the onset of fatigue behaviors. Here we report that tumor-bearing mice demonstrate behaviors of fatigue (decreased VWRA) and depressed mood (resignation and anhedonia) prior to the onset of muscle fatigue, as determined from in vitro measurements. In addition, the decreases in VWRA and grip strength in tumor-bearing mice were not associated with decreases in contractile properties of skeletal muscle. Depressive-like behavior in tumor-bearing mice was associated with increased expression of IL-1β and IL-6 in the cortex and hippocampus, brain regions associated with mood and motor activity. Treatment of the tumor-bearing mice with minocycline, an anti-inflammatory agent and purported microglial inhibitor, did not affect tumor growth or muscle mass. However, minocyline reduced tumor-induced depressive-like behavior and brain cytokine expression and improved grip strength in tumor-bearing mice. Overall, these data support the hypothesis that tumor induced cytokine-dependent changes in mood play a larger role in behaviors of CRF compared to loss of skeletal muscle mass.
Consistent with other studies, tumor growth was associated with decreased body mass and muscle mass (Acharyya et al., 2004; Xu et al., 2011). Moreover, reduced muscle mass was associated with reduced grip strength by three weeks of tumor growth. Although absolute force generating ability was reduced and was proportional to differences or decreases in muscle cross-sectional area, fundamental contractile properties of the EDL and soleus in vitro, or the tibialis anterior in situ were not altered by tumor burden (Tables 1–3) (Murphy et al., 2013). The lack of differences in the in situ contractile properties of muscles of control and tumor-bearing mice, other than absolute force generation, indicates that the neuromotor innervation was not significantly impaired by tumor progression. These data are consistent with a clinical study showing that absolute strength of the quadriceps muscle in cancer patients was decreased, but was identical to control subjects when normalized to muscle cross sectional area (Weber et al., 2009). Others have reported a decrease in muscle force generation, normalized with cross-sectional area, in association with cancer cachexia (Roberts et al., 2013; Toth et al., 2013). Roberts et al. (2013) used a more severe tumor load (same number of tumor cells per injection, but injected bilaterally in the flank region) and reported a decrease in specific force that was not observed in the present study. The difference between the previous and current studies suggests that the greater tumor load caused a decrease in specific force through a mechanism that was independent of the loss of muscle mass. Murphy and co-workers (2012 and 2013) also report that specific force generation is not affected by tumor burden, using the same model as in the present study. Our results demonstrate that motor/behavioral deficits can precede a reduction in specific force generation or muscle fatigue.
Several previous reports indicate that the myosin heavy chain isoform composition of skeletal muscle, a major determinant of muscle contractile properties, changes with tumor burden (Diffee et al., 2002; Taskin et al., 2014), whereas others reported no change in myosin heavy isoform composition or myosin-based fiber type composition (Johns et al., 2014; Schmitt et al., 2007). Consistent with no change in specific force generation, significant alterations in physical activity were observed in tumor-bearing mice, compared to control mice, without any detectable change in myosin isoform expression.
Reduced VWRA is a widely used model of fatigue in response to immune challenge (Hopwood et al., 2009) or chemotherapy (Zombeck et al., 2013). A key finding in this study was that reduced VWRA and depressive-like behavior occurred in tumor-bearing mice prior to significant weight or muscle loss. The decrease in VWRA was not associated with reduced home cage activity. These findings indicate that tumor growth did not cause general malaise. Instead, tumor-bearing mice likely had reduced motivation to run, suggesting that depressed mood plays a role in CRF (Novak et al., 2012). In support of this idea, tumor-bearing mice showed reduced sucrose preference at one and two weeks after tumor cell inoculation, and increased time immobile in the FST at the two week time point. Other studies have shown that peripheral tumors induce depressive-like behavior concurrent with reduced body mass in rodents (Lamkin et al., 2011; Pyter et al., 2009; Yang et al., 2014). Our study, however, shows that depressive-like behavior occurs early in the course of tumor growth and precedes tumor induced weight loss or muscle wasting. Of note, the tumor-bearing mice no longer had a reduced preference for sucrose at the three week time point. This observation suggests that sucrose solution became the preferred calorie source for mice with significant tumor burden, though we observed no decease in solid food intake or increase in total fluid intake (data not shown).
CRF involves complex, interacting effects of tumor growth on multiple dimensions involved in the functional status of the cancer patient. Therefore, the underlying mechanisms of CRF are unknown but may be related to a heightened inflammatory state. In rodent models of inflammation and immune activation, elevated IL-1β expression in the brain has been associated with fatigue and depression (Carmichael et al., 2006; Godbout et al., 2008). In the current study, we provide several lines of evidence that increased pro-inflammatory cytokine expression in the brain influences fatigue and depressive-like behaviors in tumor-bearing mice. We observed increased IL-1β mRNA in the brain, reduced VWRA and depressive-like behavior in tumor-bearing mice with no decrease in home-cage locomotor activity. Furthermore, orally administered minocycline prevented tumor-induced increases in IL-1β and IL-6 mRNA expression in the cortex and hippocampus, decreased depressive-like behavior in the FST at two weeks and increased normalized grip strength at three weeks of tumor growth. The ability of minocycline to increase relative grip strength without increasing muscle mass indicates that there is a motivation component to grip strength testing. Minocycline treatment had no effect on plasma IL-6, muscle mass or tumor mass, suggesting that the behavioral effects of minocycline were related to decreases in neuroinflammation rather than delayed tumor growth or increased skeletal muscle mass. These results support our overall hypothesis that cytokine-dependent effects of tumor growth on neuroinflammation play a major role in the co-morbidity of fatigue and depression in cancer patients (Bower et al., 2011; Kim et al., 2012; Kirkova et al., 2011; Minton et al., 2012; Phillips and McAuley, 2013). In addition, others have shown that psycho-cognitive therapy is as effective as exercise therapy in reducing fatigue in cancer patients (Gielissen et al., 2006) which further supports an important role for the affective domain in CRF. Further study is needed to determine the potential of minocycline to reduce depression and attenuate fatigue in cancer patients.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01-NR-012618 to DOM, LEW, and PJR and by NIA grant R01-AG-033028 to JPG.
Footnotes
6. Financial disclosure
The authors of this manuscript declare that there are no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. The Journal of clinical investigation. 2004;114:370–378. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. The American journal of clinical nutrition. 2010;91:1133S–1137S. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118:2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- Bicer S, Reiser PJ, Ching S, Quan N. Induction of muscle weakness by local inflammation: an experimental animal model. Inflammation research. 2009;58:175–183. doi: 10.1007/s00011-008-8093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain, behavior, and immunity. 2007;21:863–871. doi: 10.1016/j.bbi.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of clinical oncology. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain, behavior, and immunity. 2013;30(Suppl):S48–57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun TP, Zhu X, Szumowski M, Scott GD, Grossberg AJ, Levasseur PR, Graham K, Khan S, Damaraju S, Colmers WF, et al. Central nervous system inflammation induces muscle atrophy via activation of the hypothalamic-pituitary-adrenal axis. The Journal of experimental medicine. 2011;208:2449–2463. doi: 10.1084/jem.20111020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao DX, Wu GH, Zhang B, Quan YJ, Wei J, Jin H, Jiang Y, Yang ZA. Resting energy expenditure and body composition in patients with newly detected cancer. Clin Nutr. 2010;29:72–77. doi: 10.1016/j.clnu.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Carmichael MD, Davis JM, Murphy EA, Brown AS, Carson JA, Mayer EP, Ghaffar A. Role of brain IL-1beta on fatigue after exercise-induced muscle damage. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291:R1344–1348. doi: 10.1152/ajpregu.00141.2006. [DOI] [PubMed] [Google Scholar]
- Cosper PF, Leinwand LA. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer research. 2011;71:1710–1720. doi: 10.1158/0008-5472.CAN-10-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffee GM, Kalfas K, Al-Majid S, McCarthy DO. Altered expression of skeletal muscle myosin isoforms in cancer cachexia. American journal of physiology Cell physiology. 2002;283:C1376–1382. doi: 10.1152/ajpcell.00154.2002. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Gensel JC, Ankeny DP, van Rooijen N, Popovich PG. An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J Neurosci Methods. 2009;181:36–44. doi: 10.1016/j.jneumeth.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell metabolism. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Gielissen MF, Verhagen S, Witjes F, Bleijenberg G. Effects of cognitive behavior therapy in severely fatigued disease-free cancer patients compared with patients waiting for cognitive behavior therapy: a randomized controlled trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4882–4887. doi: 10.1200/JCO.2006.06.8270. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, JOC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves E, Ramsay E, McCarthy DO. Inhibitors of COX activity preserve muscle mass in mice bearing the Lewis lung carcinoma, but not the B16 melanoma. Research in nursing & health. 2006;29:87–97. doi: 10.1002/nur.20114. [DOI] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. Journal of neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. The oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- Hopwood N, Maswanganyi T, Harden LM. Comparison of anorexia, lethargy, and fever induced by bacterial and viral mimetics in rats. Canadian journal of physiology and pharmacology. 2009;87:211–220. doi: 10.1139/y09-003. [DOI] [PubMed] [Google Scholar]
- Husson O, Nieuwlaat WA, Oranje WA, Haak HR, van de Poll-Franse LV, Mols F. Fatigue Among Short- and Long-Term Thyroid Cancer Survivors: Results from the Population-Based PROFILES Registry. Thyroid : official journal of the American Thyroid Association. 2013;23:1247–1255. doi: 10.1089/thy.2013.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns N, Hatakeyama S, Stephens NA, Degen M, Degen S, Frieauff W, Lambert C, Ross JA, Roubenoff R, Glass DJ, et al. Clinical classification of cancer cachexia: phenotypic correlates in human skeletal muscle. PloS one. 2014;9:e83618. doi: 10.1371/journal.pone.0083618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Barsevick AM, Fang CY, Miaskowski C. Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer nursing. 2012;35:E1–E20. doi: 10.1097/NCC.0b013e318233a811. [DOI] [PubMed] [Google Scholar]
- Kirkova J, Aktas A, Walsh D, Davis MP. Cancer symptom clusters: clinical and research methodology. Journal of palliative medicine. 2011;14:1149–1166. doi: 10.1089/jpm.2010.0507. [DOI] [PubMed] [Google Scholar]
- Lamkin DM, Lutgendorf SK, Lubaroff D, Sood AK, Beltz TG, Johnson AK. Cancer induces inflammation and depressive-like behavior in the mouse: modulation by social housing. Brain, behavior, and immunity. 2011;25:555–564. doi: 10.1016/j.bbi.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton O, Alexander S, Stone PC. Identification of factors associated with cancer related fatigue syndrome in disease-free breast cancer patients after completing primary treatment. Breast cancer research and treatment. 2012;136:513–520. doi: 10.1007/s10549-012-2284-1. [DOI] [PubMed] [Google Scholar]
- Mols F, Husson O, Roukema JA, van de Poll-Franse LV. Depressive symptoms are a risk factor for all-cause mortality: results from a prospective population-based study among 3,080 cancer survivors from the PROFILES registry. Journal of cancer survivorship : research and practice. 2013;7:484–492. doi: 10.1007/s11764-013-0286-6. [DOI] [PubMed] [Google Scholar]
- Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Importance of functional and metabolic impairments in the characterization of the C-26 murine model of cancer cachexia. Disease models & mechanisms. 2012;5:533–545. doi: 10.1242/dmm.008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Inhibition of the renin-angiotensin system improves physiological outcomes in mice with mild or severe cancer cachexia. International journal of cancer. 2013;133:1234–1246. doi: 10.1002/ijc.28128. [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Duncan ID, Watters JJ. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IkappaBalpha degradation in a stimulus-specific manner in microglia. Journal of neurochemistry. 2006;96:314–323. doi: 10.1111/j.1471-4159.2005.03520.x. [DOI] [PubMed] [Google Scholar]
- Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neuroscience and biobehavioral reviews. 2012;36:1001–1014. doi: 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama T, Kokura S, Ishikawa T, Adachi S, Hattori T, Takagi T, Handa O, Naito Y, Yoshikawa T. Antitumor effect of pretreatment for colon cancer cells with hyperthermia plus geranylgeranylacetone in experimental metastasis models and a subcutaneous tumor model of colon cancer in mice. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2009;25:141–149. doi: 10.1080/02656730802631783. [DOI] [PubMed] [Google Scholar]
- Pertl MM, Hevey D, Boyle NT, Hughes MM, Collier S, O’Dwyer AM, Harkin A, Kennedy MJ, Connor TJ. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain, behavior, and immunity. 2013;34:108–119. doi: 10.1016/j.bbi.2013.07.177. [DOI] [PubMed] [Google Scholar]
- Phillips SM, McAuley E. Physical activity and fatigue in breast cancer survivors: a panel model examining the role of self-efficacy and depression. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22:773–781. doi: 10.1158/1055-9965.EPI-12-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Pineros V, Galang JA, McClintock MK, Prendergast BJ. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9069–9074. doi: 10.1073/pnas.0811949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BM, Frye GS, Ahn B, Ferreira LF, Judge AR. Cancer cachexia decreases specific force and accelerates fatigue in limb muscle. Biochemical and biophysical research communications. 2013;435:488–492. doi: 10.1016/j.bbrc.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saligan LN, Kim HS. A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain, behavior, and immunity. 2012;26:830–848. doi: 10.1016/j.bbi.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. The international journal of biochemistry & cell biology. 2013;45:2121–2129. doi: 10.1016/j.biocel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt TL, Martignoni ME, Bachmann J, Fechtner K, Friess H, Kinscherf R, Hildebrandt W. Activity of the Akt-dependent anabolic and catabolic pathways in muscle and liver samples in cancer-related cachexia. J Mol Med (Berl) 2007;85:647–654. doi: 10.1007/s00109-007-0177-2. [DOI] [PubMed] [Google Scholar]
- Taskin S, Stumpf VI, Bachmann J, Weber C, Martignoni ME, Friedrich O. Motor protein function in skeletal abdominal muscle of cachectic cancer patients. Journal of cellular and molecular medicine. 2014;18:69–79. doi: 10.1111/jcmm.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo M, Busquets S, Sirisi S, Serpe R, Orpi M, Coutinho J, Martinez R, Lopez-Soriano FJ, Argiles JM. Cancer cachexia: physical activity and muscle force in tumour-bearing rats. Oncology reports. 2011;25:189–193. [PubMed] [Google Scholar]
- Toth MJ, Miller MS, Callahan DM, Sweeny AP, Nunez I, Grunberg SM, Der-Torossian H, Couch ME, Dittus K. Molecular mechanisms underlying skeletal muscle weakness in human cancer: reduced myosin-actin cross-bridge formation and kinetics. J Appl Physiol (1985) 2013;114:858–868. doi: 10.1152/japplphysiol.01474.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers PA, Thong MS, Pouwer F, Zanders MM, Coebergh JW, van de Poll-Franse LV. The impact of comorbidity on Health-Related Quality of Life among cancer survivors: analyses of data from the PROFILES registry. Journal of cancer survivorship : research and practice. 2013 doi: 10.1007/s11764-013-0299-1. [DOI] [PubMed] [Google Scholar]
- Wang XS, Williams LA, Krishnan S, Liao Z, Liu P, Mao L, Shi Q, Mobley GM, Woodruff JF, Cleeland CS. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain, behavior, and immunity. 2012;26:699–705. doi: 10.1016/j.bbi.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MA, Krakowski-Roosen H, Schroder L, Kinscherf R, Krix M, Kopp-Schneider A, Essig M, Bachert P, Kauczor HU, Hildebrandt W. Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer-related cachexia. Acta Oncol. 2009;48:116–124. doi: 10.1080/02841860802130001. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. The Journal of neuroscience. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LJ, Nail LM, Gilster A, Winters KA, Elsea CR. Cancer chemotherapy-related symptoms: evidence to suggest a role for proinflammatory cytokines. Oncology nursing forum. 2006;33:535–542. doi: 10.1188/06.ONF.535-542. [DOI] [PubMed] [Google Scholar]
- Wood LJ, Weymann K. Inflammation and neural signaling: etiologic mechanisms of the cancer treatment-related symptom cluster. Current opinion in supportive and palliative care. 2013;7:54–59. doi: 10.1097/SPC.0b013e32835dabe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Crawford D, Hutchinson KR, Youtz DJ, Lucchesi PA, Velten M, McCarthy DO, Wold LE. Myocardial dysfunction in an animal model of cancer cachexia. Life sciences. 2011;88:406–410. doi: 10.1016/j.lfs.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Kim J, Kim JS, Kim SH, Kim JC, Kang MJ, Jung U, Shin T, Wang H, Moon C. Hippocampal dysfunctions in tumor-bearing mice. Brain, behavior, and immunity. 2014;36:147–155. doi: 10.1016/j.bbi.2013.10.022. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Fey EG, Lyng GD, Sonis ST. A clinically translatable mouse model for chemotherapy-related fatigue. Comparative medicine. 2013;63:491–497. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.