Abstract
Ocular immune privilege (IP) limits immune surveillance of intraocular tumors as certain immunogenic tumor cell lines (P815, E.G7-OVA) that are rejected when transplanted in the skin grow progressively when placed in the anterior chamber (a.c.) of the eye. As splenectomy (SPLNX) is known to terminate ocular IP, we characterized immune mechanisms responsible for rejection of intraocular tumors in SPLNX mice as a first step toward identifying how to restore tumoricidal activity within the eye. CD8+ T cells, IFNγ, and FasL, but not perforin, or TNFα were required for elimination of intraocular E.G7-OVA tumors that culminated in destruction of the eye (ocular phthisis). IFNγ and FasL did not target tumor cells directly as the majority of SPLNX IFNγR1−/− mice and Fas defective lpr mice failed to eliminate intraocular E.G7-OVA tumors that expressed Fas and IFNγR1. Bone marrow chimeras revealed that IFNγR1 and Fas expression on immune cells was most critical for rejection and SPLNX increased the frequency of activated macrophages (Mϕ) within intraocular tumors in an IFNγ-and-Fas/FasL-dependent manner suggesting an immune cell target of IFNγ and Fas. As depletion of Mϕs limited CD8 T cell-mediated rejection of intraocular tumors in SPLNX mice, our data support a model in which IFNγ-and-Fas/FasL-dependent activation of intratumoral Mϕs by CD8+ T cells promotes severe intraocular inflammation that indirectly eliminates intraocular tumors by inducing phthisis, and suggests that immunosuppressive mechanisms which maintain ocular IP interfere with the interaction between CD8+ T cells and Mϕs to limit immunosurveillance of intraocular tumors.
Keywords: tumor, eye, immune privilege, CD8, splenectomy
Introduction
Ocular “immune privilege” (IP) is exemplified by the observations that certain immunogenic tumor cell lines (P815 and E.G7-OVA), which are rejected by host immune responses when transplanted in the skin, grow progressively when placed into the anterior chamber (a.c.) of the eye (1). IP is not immune ignorance as several studies have shown that intraocular tumor growth primes systemic immune responses to tumor antigens (2–4). Rather, ocular immune responses are very tightly regulated to limit inflammation during pathogen removal so that certain intraocular tissues, which do not regenerate and are essential for vision, are not damaged (5, 6).
Ocular IP is maintained by anatomical and biochemical barriers to host immune responses along with the generation of systemic tolerance to antigens encountered within the eye (reviewed in (7)). Splenectomy (SPLNX) terminates ocular IP and promotes rejection of immunogenic tumors transplanted in the a.c. of the eye (8). However, the mechanism of tumor elimination has not been defined. Herein, we characterized the requirements for elimination of intraocular tumors in SPLNX mice as a first step toward identifying how to overcome IP and restore tumoricidal activity within intraocular tumors. We demonstrate that CD8+ T cells, macrophages (Mϕ), IFNγ and FasL but not perforin or TNFα were necessary for intraocular tumor elimination. Although tumors expressed IFNγR1 and Fas, IFNγ and FasL did not directly target tumors. Rather, IFNγ and Fas/FasL interactions were required for intratumoral Mϕ activation that was associated with severe ocular inflammation which indirectly eliminated intraocular tumors by inducing complete destruction of the eye (ocular phthisis). Our data suggest that immunosuppressive mechanisms that preserve ocular IP interfere with the complex interplay between CD8+ T cells and intratumoral Mϕs necessary to eliminate intraocular tumors.
Materials and Methods
Tumor Cell Lines
P815, provided by Dr. Judith A. Kapp (University of Alabama, Birmingham) in 2006 and E.G7-OVA (ATCC® CRL-2113™), obtained from ATCC (Manassas, VA) in 2008 were utilized. E.G7-OVA were transduced to express firefly luciferase (Luc-E.G7) using ViraPower™ (Invitrogen, Carlsbad, CA). Luc-E.G7 cultures initiated from frozen stocks were cultured monthly with 1.0 mg/ml G418 sulfate to maintain expression of OVA and used in experimentation for no longer than three months at which time a new Luc.E.G7 culture was initiated. Luc-E.G7 cultures were authenticated every three months in comparison to ATCC® CRL-2113™ by evaluating bioluminescence and sensitivity to lysis by OVA-specific CD8+ OT-I CTL. P815 was authenticated by IDEXX RADIL(Columbia, MO) in 2014 by direct comparison to P815 obtained from ATCC (ATCC® TIB-64™) in 2014 (Supplemental-Table I).
Mice
Male and female mouse strains greater than seven weeks of age from Jackson Laboratories (Bar Harbor, ME), detailed in Supplemental Table II, were used. OT-I mice (9), were crossed with gld or IFNγR1−/− mice to generate OT-I mice deficient in FasL or IFNγR1. All experimentation was approved by the IACUC at the University of Pittsburgh.
Splenectomy
Mice were anesthetized with a solution of ketamine (100 mg/kg) and xylazine (10 mg/kg). The skin above the spleen was prepared for surgery by depilation and contact sterilization. Sterile scissors were used to open the skin and fascia to visualize the spleen. The spleen was gently held with sterile forceps, splenic vessels cauterized, and the spleen removed. The incision site was closed with sterile surgical staples (Roboz, Gaithersburg, MD) that were removed 10–14 days post-surgery.
Ocular Injections and Intraocular tumor growth measurements
Tumor cell lines (104 cells) were injected into the a.c. of the eye as previously described (4). P815 tumor growth was monitored by visual inspection (Supplemental-Fig.1), and scored as either progressive or phthisical at least 17 days after tumor challenge. A very significant correlation was observed between bioluminescence measurements and Luc-E.G7 numbers in vitro (Supplemental-Fig.1C). Hence, sequential growth of Luc-E.G7 in the a.c. of the eye of live mice was monitored by bioluminescent imaging (BLI) using an IVIS imager (Caliper Life Sciences, Hopkinton, MA) following sedation of mice with isoflurane and within fifteen minutes after intraperitoneal injection of 6 mg D-luciferin salt (Gold Biotechnology, St. Louis, MO) (Supplemental-Fig.1D). Background bioluminescence was defined at 104 photons/sec (Supplemental-Fig.1E). Rejection of Luc-E.G7 tumors was scored in individual mice as a two-log decrease in tumor bioluminescence that was maintained for at least two successive measurements.
In vivo depletion of immune cell subsets and Fas/FasL neutralization
To selectively remove CD4+ or CD8+ T cells, mice were given intraperitoneal injections of anti-CD4 (clone GK1.4) or anti-CD8 (clone 2.43) antibodies from BioXCell (West Lebanon, NH). Antibody treatment (0.2 – 0.4 mg) began three days before or seven days after ocular tumor injections and continued every 3–4 days thereafter (0.1 mg injections). Depletion was greater than 96% as determined by flow cytometric analysis of peripheral blood (data not shown). Macrophages were depleted by subconjunctival (scon.) injections (10 µl) or, scon and intravenous (i.v.) injections (100–200 µl) as indicated. Neutralization of Fas/FasL interactions was accomplished by 0.1 mg intraperitoneal (i.p.) injections of Ultra-LEAF™anti-mouse CD178(FasL) antibodies (BioLegend, San Diego, CA) that were given before tumor challenge and every three to four days thereafter. Equivalent injections of Hamster IgG (BioXCell) were given to control for antibody injection.
Flow cytometric Analysis
15–16 days after tumor challenge, single-cell suspensions of whole tumor-bearing eyes were prepared as previously described (10), Fc receptors blocked and then stained with combinations of fluorescently conjugated antibodies from BD Pharmingen to the following cell surface molecules: CD45, CD11b, Thy 1.2, GR-1, and/or F4/80 in FACS buffer (PBS + 2% fetal bovine serum). Cells were then washed and fixed in Cytofix/Cytoperm (BD Pharmingen) and in some experiments incubated with PE-conjugated-anti-CD68, or polyclonal rabbit anti-mouse NOS2 antibody (BD Pharmingen) in Perm/Wash buffer (BD Pharmingen). Cells treated with polyclonal anti-mouse NOS2 were then stained with Alexafluor 546 anti-rabbit IgG (R&D systems). Events were collected using a FACSDiva flow cytometer (Becton Dickinson, San Jose, CA) and analyzed using FACSDiva (Becton Dickinson) and Flow Jo (Treestar, Ashland, OR) software.
Generation of Bone Marrow Chimeric Mice
Mice were irradiated (10 Gy) in a Cs source irradiator (Nordion, Ottawa, ON, Canada) and then injected intravenously (i.v.) with bone marrow (4.5 × 106 cells) isolated from femurs, and tibias of B6, lpr, or IFNγR1−/− mice. Experimentation was performed 8 weeks later after reconstitution of the immune system.
Gene Array Analysis and RT-PCR
15–16 days after ocular tumor challenge eyes were removed, homogenized in RLT buffer (from RNeasy kit, Qiagen, Valencia, CA) in a Tenbroeck™ frosted glass tissue grinder, then stored at −80°C until isolation of total RNA using Qiashredder and RNeasy kits (Qiagen). cDNA was synthesized using a High Capacity cDNA Reverse Transcription Kit and quantitative real-time PCR was performed using a StepOne Plus instrument with commercially available TaqMan® primer probe sets (Applied Biosystems, Foster City, CA). Pyruvate decarboxylase (Pcx) was used as the normalizing (housekeeping) gene. Extracted RNA (~500 ng) was also processed using a 3’ IVT Express Kit to yield amplified RNA (~20 µg) which was hybridized to M430 2.0 microarrays; the microarrays were scanned using a GeneChip 3000 Array scanner (Affymetrix Inc. Santa Clara, CA). Raw data were processed using Affymetrix GCOS v.1.4 software with default settings, then exported to Microsoft Excel. The ratio (mean SPLNX / mean control) was calculated for each microarray panel, with candidate panels having ratios ≥ 1.5 or ≤ 0.67. Valid candidates showed detectable (i.e., called Present by GCOS) transcript in both samples of the higher-expressing group, and no overlap of values between groups. The data set is available from the NCBI GEO database www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE57101.
Adoptive Transfer of OT-I T cells
In some experiments naïve CD8+ T cells were isolated from spleens of OT-I, OT-I X gld, or OT-I X IFNγR1−/− mice using mouse CD8 T-cell enrichment kits (Stem Cell Technologies, Vancouver, BC) and then injected i.v. via tail vein injection (3.0 × 106 cells). To generate OT-I T-cell effectors, splenocytes from OT-I mice were stimulated in vitro for 3 days with 0.1 µg/ml SIINFEKL peptide, and dead cells were removed by centrifugation over Fico/Lite LM (mouse) (Atlanta, Biologicals, Atlanta, GA.) OT-I T-cell effectors (1.0–3.0 × 106 cells) were injected i.v.
Statistical Analysis
Prism (Graph Pad, La Jolla, CA) software was used.
Results
SPLNX promotes CD8 T cell-mediated rejection of intraocular tumors
Preliminary studies showed that P815 tumors which normally grew progressively when transplanted in the a.c. of the eye were rejected in SPLNX mice by a process that culminated in ocular phthisis (8). To characterize the requirements for elimination of intraocular tumors, we monitored intraocular tumor growth after injection of 104 P815 tumor cells (H-2d) into the a.c. of SPLNX Balb/C J mice or Balb/C J mice that were not surgically manipulated (hereafter referred to as controls). The majority of SPLNX mice rejected P815 tumors with phthisis beginning 10–14 days post tumor challenge whereas progressive intraocular tumor growth was observed in controls (Table I) confirming previous observations (8). This delayed immune response suggested the contribution of adaptive immunity. Therefore, intraocular tumor growth was monitored in SPLNX mice that received depleting anti-CD4 or anti-CD8 antibodies. Removal of CD4+ T cells before (Table I, Exp.2) or at seven days after tumor challenge (Table I, Exp.4) did not influence the rejection of intraocular tumors in SPLNX mice. In contrast, SPLNX mice depleted of CD8+ T cells (Table I, Exp.3 & Exp.4) did not reject intraocular tumors.
Table I.
Splenectomy promotes CD8 T cell mediated rejection of intraocular P815 mastocytomas in Balb/C mice
| No. mice with tumor / Total No. of mice (Percent with tumor) |
||||
|---|---|---|---|---|
| Exp. | Surgery | Antibody Tx. | ||
| 1 | None | None | 5/8(63%) | |
| Splenectomy | None | 0/7(0%) | p=0.0256 | |
| 2 | Splenectomy | RatIgG | 0/4(0%) | |
| Splenectomy | Anti-CD4 | 1/4(25%) | ns | |
| 3 | None | None | 6/7(86%) | |
| Splenectomy | Rat IgG | 0/8(0%) | p=0.0014 | |
| Splenectomy | Anti-CD8 | 8/8(100%) | ns | |
| 4 | None | Rat IgG | 6/6(100%) | |
| Splenectomy | Rat IgG | 0/6(0%) | p=0.0022 | |
| None | Anti-CD4 | 5/5(100%) | ||
| Splenectomy | Anti-CD4 | 0/5(0%) | p=0.0079 | |
| None | Anti-CD8 | 5/5(100%) | ||
| Splenectomy | Anti-CD8 | 4/5(80%) | ns |
Balb/C J mice were used in these experiments and 104 P815 tumor cells were injected in the a.c. of the eye. In experiments(Exp.) #2 and #3 antibody treatments began one day before tumor challenge and continued every 3 to four days thereafter. In Exp. #4 antibody treatments began seven days after tumor challenge. Splenectomized mice were compared to control mice by Fisher’s exact test. p values are shown.
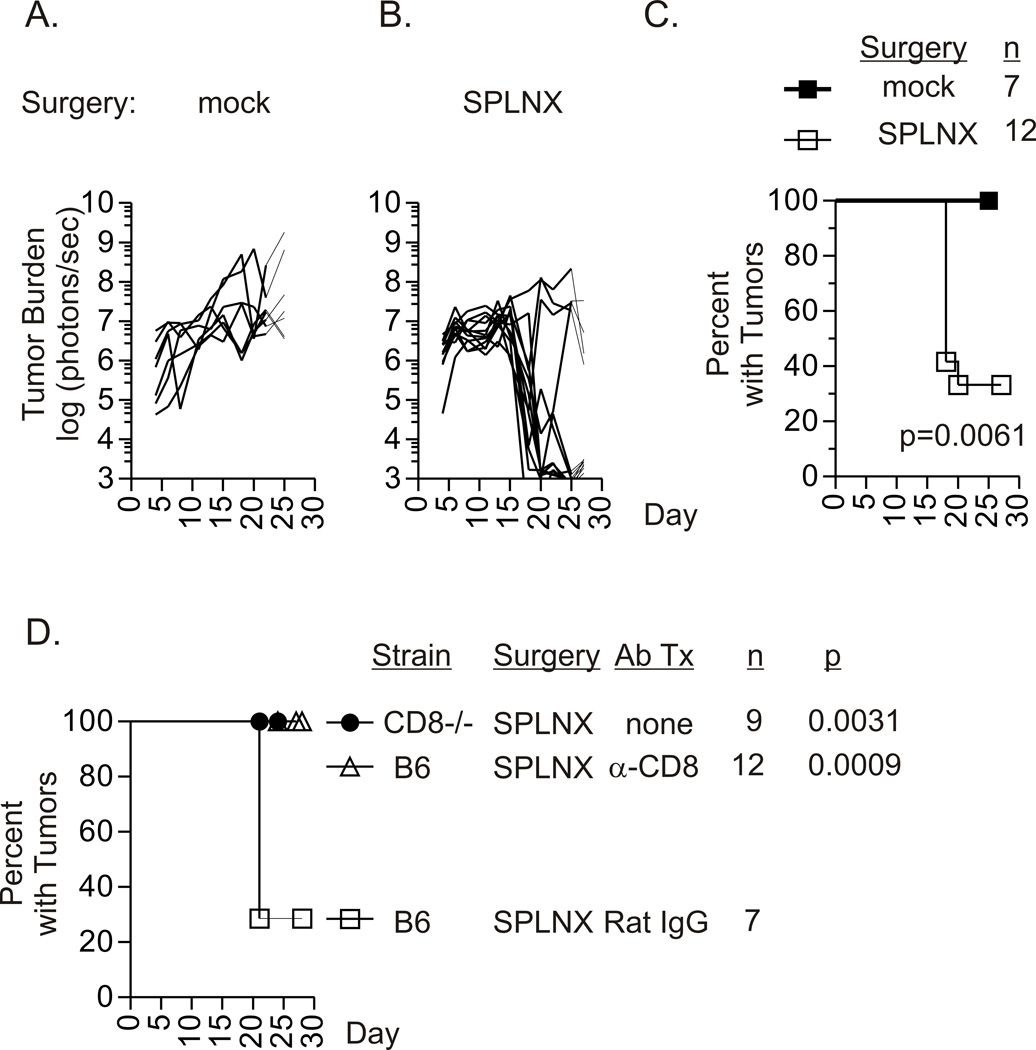
Intraocular growth of transplanted bioluminescent Luc-E.G7 tumors (H-2b) that expressed OVA as a defined tumor antigen (11) was also monitored by BLI in SPLNX and control C57Bl/6 J (B6) mice that received a mock surgery (Fig.1A–C). During the first fourteen days after tumor challenge, equivalent tumor growth was observed (Fig.1A & 1B). However, by day 21 the majority of SPLNX mice eliminated these established intraocular tumors (Fig.1B & 1C) resulting in ocular phthisis. The rejection of intraocular Luc-E.G7 tumors required CD8+ T cells as progressive intraocular tumor growth was observed in SPLNX mice deficient in CD8+ T cells (CD8−/− mice) and in SPLNX B6 mice given depleting anti-CD8 antibodies (Fig.1D) but not in SPLNX B6 mice given Rat IgG as a control for antibody administration. Combined with our equivalent observations in the P815/Balb/C J intraocular tumor model (Table I), these data identified a common requirement for CD8+ T cells in phthisical rejection of intraocular tumors in SPLNX mice.
Figure 1. Splenectomy promotes CD8 T cell-mediated rejection of intraocular Luc-E.G7 tumors.
Intraocular Luc-E.G7 (H-2b) tumor growth monitored by BLI in C57Bl/6 (B6) mice that were subjected to a mock surgical procedure (A.) or were splenectomized (SPLNX, B.). Each line represents measurements from an individual mouse within each treatment group that was used to generate survival curves shown in (C.) The p value indicates comparison of groups by a log rank test. Percent of SPLNX CD8−/− H-2b mice, SPLNX B6 or SPLNX B6 mice that were given depleting anti-CD8 antibodies or Rat IgG with intraocular Luc-E.G7 tumors (D.). (A. – C.) is a single experiment reproduced in subsequent figures. Data in (D.) are combined from five independent experiments. p values in (D.) indicate comparison to the SPLNX Rat IgG treated group by a log rank test. n=number of mice per group.
Rejection of intraocular tumors requires FasL and IFNγ
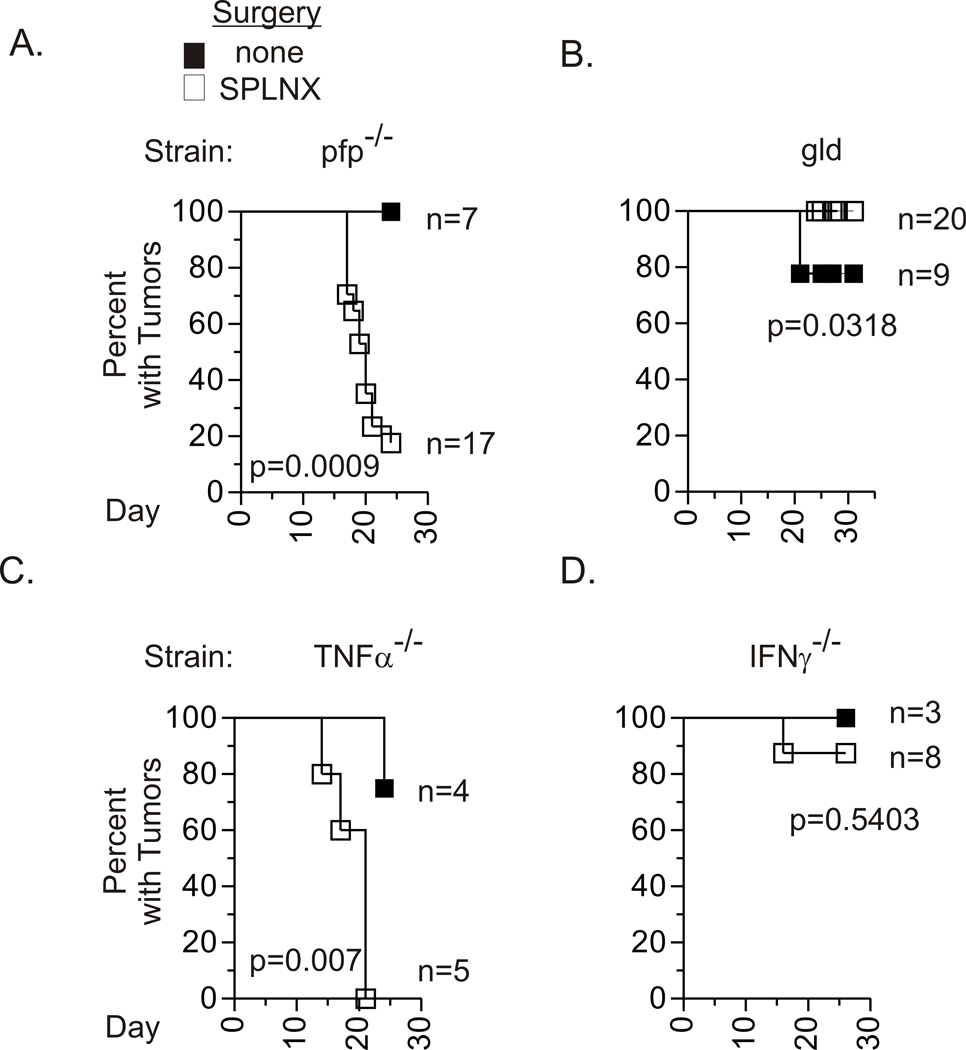
CD8+ T cells can directly eliminate tumors by perforin-dependent cytolytic activity, FasL-induced apoptosis or via production of tumoricidal cytokines including TNFα and IFNγ (12). Therefore, to determine the contribution of these molecules in the control of intraocular tumors, Luc.EG7 intraocular tumor growth was monitored in SPLNX and control mice with genetic deficiencies in perforin (pfp−/−), FasL (gld), TNFα (TNFα−/−), or IFNγ (IFNγ−/−). The majority of controls in these immunodeficient strains showed progressive intraocular tumor growth (Fig.2). SPLNX pfp−/− mice rejected established intraocular Luc-E.G7 which indicated that lytic activity of CD8+ T cells was not required to eliminate these intraocular tumors. Similarly, P815 tumors that grew progressively in pfp−/− control mice on a Balb/C ByJ background were rejected when pfp−/− mice were SPLNX prior to tumor challenge (Supplemental-Table III). Hence, perforin was dispensable for rejection of intraocular tumors. TNFα was also not required for rejection of Luc-EG7 tumors in SPLNX mice (Fig.2C). However, FasL and IFNγ were necessary as intraocular Luc-E.G7 tumors grew progressively in SPLNX gld and IFNγ−/− mice (Fig.2B & 2D). A greater frequency of SPLNX Balb/C ByJ mice given anti-FasL antibodies showed progressive intraocular P815 tumor growth in comparison to SPLNX mice given control antibodies (Supplemental-Table III, Exp.3), which indicated that Fas/FasL interactions also contributed to elimination of P815 tumors in SPLNX Balb/C J mice.
Figure 2. FasL and IFNγ are required for intraocular tumor regression.
Percent of mice with intraocular Luc-E.G7 tumors in SPLNX and control pfp−/− (A.), FasL defective gld (B.), TNFα−/− (C.) and IFNγ−/− (D.) H-2b mice. n= number of mice per group. Data shown are pooled from three independent experiments in A., four experiments in B., and two experiments in D. C. represents one experiment. p values indicate comparison of groups within individual panels by a log rank test.
IFNγR1 and Fas expression on hematopoietic cells are critical for intraocular tumor rejection
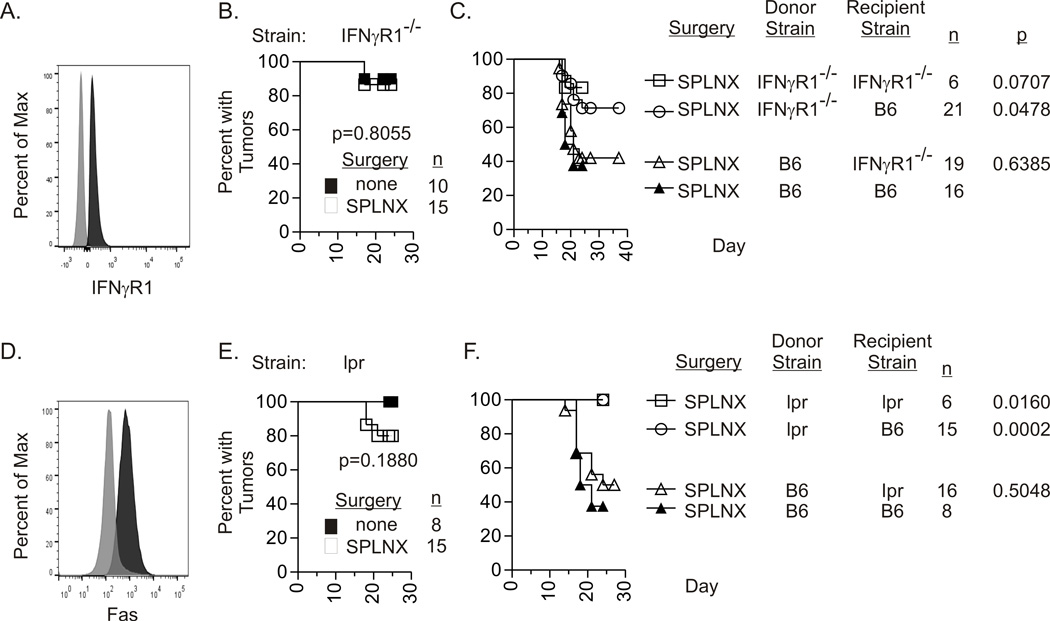
Flow cytometric analysis indicated that Luc-E.G7 tumors expressed both IFNγR1 (Fig.3A) and Fas (Fig.3D) suggesting that IFNγ and FasL expressed by CD8+ T cells could target tumor cells directly. However, 3-day culture of Luc-E.G7 or P815 tumors with IFNγ at concentrations ranging from 12.5–5000 units/ml did not affect tumor viability and had only a modest effect on growth (Supplemental-Fig.2). In addition, EL-4 cells, the parental cell line of Luc.E.G7 are resistant to FasL-induced apoptosis due to overexpression of cellular FLICE inhibitory protein (c-FLIP) (13). Hence, we tested the hypothesis that IFNγ and FasL acted on non-tumor (stromal) cells within the tumor microenvironment to eliminate intraocular tumors by monitoring intraocular Luc-E.G7 growth in SPLNX and control IFNγR1−/− and Fas defective lpr mice. Luc-E.G7 tumors grew progressively in the majority of controls of both strains. Despite tumor expression of IFNγR1 and Fas, the majority of SPLNX IFNγR1−/− (Fig.3B) and SPLNX lpr (Fig.3E) failed to eliminate intraocular Luc-E.G7 tumors. Similarly, progressive intraocular P815 tumor growth was observed in SPLNX and control IFNγR1−/− on a Balb/C ByJ background (Supplemental-Table III, Exp.2). These data indicated that IFNγ and FasL indirectly eliminated intraocular tumors by targeting stromal cells within the tumor microenvironment.
Figure 3. IFNγR1 and Fas expression on hematopoietic cells are required for intraocular tumor regression.
Cell surface expression of IFNγR1 (A.) or Fas (D.) on cultured Luc.EG7 tumor cells. Black histograms represent antibody specific staining and grey histograms represent background fluorescence after staining with isotype control antibodies. Percent of mice with intraocular Luc-E.G7 tumors in SPLNX and control IFNγR1−/− (B.) or Fas defective lpr (E.) H-2b mice, and in indicated bone marrow chimeric H-2b mice (C., F.). n= number of mice per group. Two or more independent experiments were combined in data presented in B. – F. p values in (B.) and (E.) are comparisons between treatment groups by log rank tests. p values in C. and F. are comparisons to SPLNX B6 recipients of B6 bone marrow by log rank tests.
Tumor stroma comprised hematopoietic and nonhematopoietic cells (14). To determine the critical cell populations that expressed IFNγR1 and FasL, bone marrow chimeras were generated with selective deficiencies in IFNγR1 or Fas in hematopoietic cells, nonhematopoietic cells or both cell populations. IFNγR1 expression on hematopoietic cells was critical for intraocular tumor rejection in SPLNX mice as a lesser frequency of B6 recipients of IFNγR1−/− bone marrow rejected Luc-EG7 intraocular tumors in comparison to B6 recipients of B6 bone marrow (Fig.3C). Indeed, the percent of B6 recipients of IFNγR1−/− bone marrow was statistically comparable to IFNγR1−/− recipients of IFNγR1−/− bone marrow. SPLNX IFNγR1−/− recipients of B6 bone marrow rejected intraocular Luc.EG7 tumors confirming the necessity for IFNγR1 on hematopoietic cells. Fas expression on hematopoietic cells was also required for intraocular tumor elimination as all SPLNX B6 recipients of lpr bone marrow demonstrated progressive intraocular Luc-EG7 tumor growth (Fig.3F) whereas SPLNX lpr recipients of B6 bone marrow rejected intraocular Luc-EG7 tumors (Fig.3F).
IFNγR1 and FasL on CD8+ T cells are not required for intraocular tumor elimination
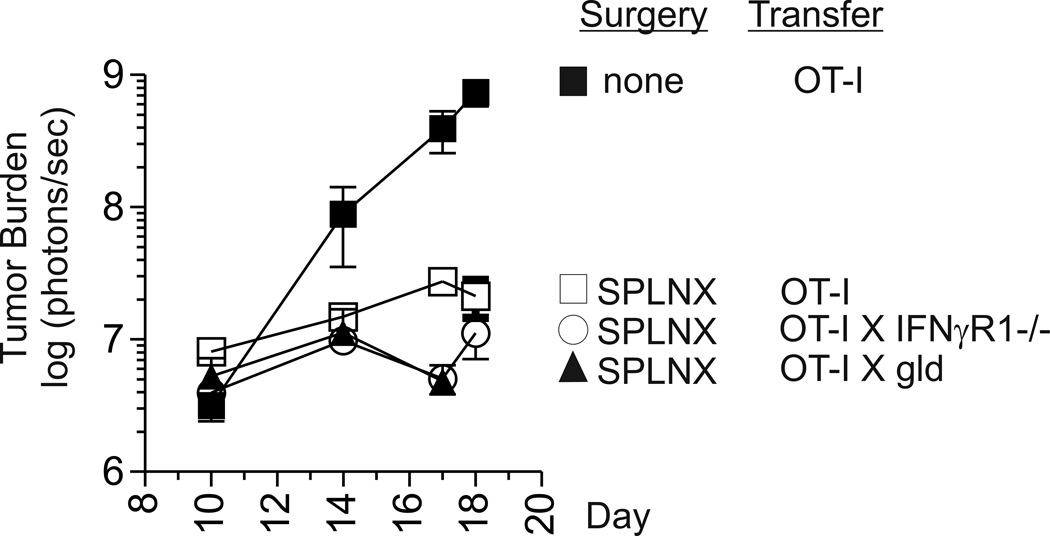
One explanation for impaired intraocular tumor rejection in SPLNX IFNγR1−/−, gld, and lpr mice is that priming of tumor-specific CD8+ T Cells is impaired in these strains. Therefore, to address the functionality of tumor specific CD8+ T cells in tumor-bearing mice, naïve OVA-specific CD8+ OT-I T cells were transferred into CD8 deficient mice that were SPLNX or were not surgically manipulated prior to challenge with Luc-E.G7 tumors in the a.c. Intraocular Luc-EG7 tumor burden was significantly lower in OT-I transferred SPLNX CD8−/− mice than in control CD8−/− mice that were not surgically manipulated although they received an equivalent number of OT-I T cells (Fig.4) which indicated that intraocular tumor growth in SPLNX but not control mice induced tumoricidal activity in transferred OT-I T cells. To determine whether IFNγR1 or FasL deficiency in CD8+ T cells abrogated tumoricidal activity, OT-I mice were bred onto an IFNγR1−/− or gld (H-2b) background. SPLNX CD8 deficient mice that received naïve OT-I X IFNγR1−/− or OT-I X gld CD8+ T cells prior to Luc-E.G7 challenge also controlled intraocular tumor growth indicating that deficiency in IFNγR1 or FasL on CD8+ T cells did not impair CD8 tumoricidal activity.
Figure 4. CD8 T cell expression of IFNγR1 or FasL is dispensable for tumoricidal activity.
Intraocular Luc-E.G7 tumor burden in SPLNX or control CD8−/− H-2b mice that received naïve OT-I, OT-I X IFNγR1, or OT-I X gld CD8+ T cells prior to intraocular tumor challenge. This experiment was performed twice with similar results.
Intraocular tumor regression in SPLNX mice is associated with IFNγ and Fas/FasL-dependent Mϕ activation and markers of ocular inflammation
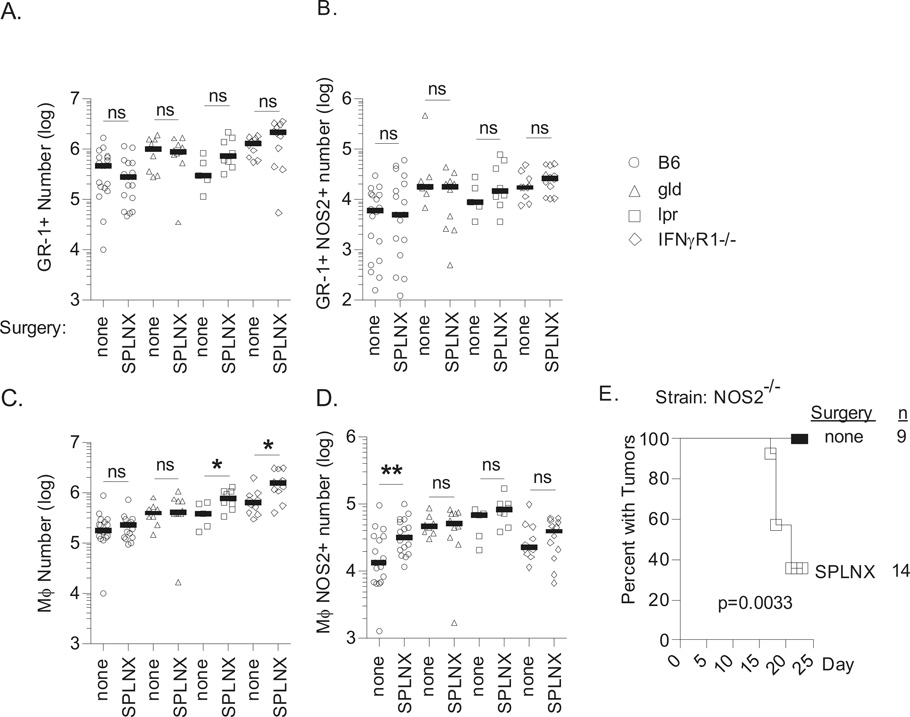
Previously we and others demonstrated that rejection of established E.G7 skin tumors required IFNγ expression by tumor-specific CD8+ T cells to activate tumoricidal nitric oxide (NO) production by intratumoral Mϕs (10, 15, 16). NO is synthesized in activated Mϕs by the enzyme NO synthase 2 (NOS2) (17). Therefore, to determine whether a similar mechanism was involved in rejection of intraocular tumors in SPLNX mice, we compared the frequency of NOS2+ Mϕs by flow cytometry in day 16 Luc-EG7 intraocular tumors of SPLNX and control B6, gld, lpr and IFNγR1−/− mice. This time point, immediately prior to Luc-EG7 intraocular tumor regression in SPLNX B6 mice, was chosen as tumor burden measured by BLI was statistically comparable in both treatment groups. Some intraocular tumors in SPLNX mice did, however, show lower tumor burden indicating the beginning of tumor regression.
In all strains of mice tested and regardless of surgical treatment, intraocular Luc-EG7 tumors were infiltrated by CD45+ CD11b+ myeloid cells composed of GR-1+ and GR-1− cells (Supplemental-Fig.3). GR-1− cells also expressed F4/80 and CD68 indicating that they were Mϕs (Supplemental-Fig.3). The number of GR-1+ cells was statistically equivalent between SPLNX and control mice of each strain although a trend of lower or higher GR-1+ cell numbers was observed in SPLNX B6 and SPLNX lpr mice, respectively (Fig.5A). The percentage of NOS2+ GR-1+ cells in intraocular tumors was very low <5% (Supplemental-Fig.3B) and their numbers were equivalent between SPLNX and control mice in all strains tested (Fig.5B). Mϕ numbers were comparable between SPLNX and control B6 or gld mice whereas the number of Mϕs increased in SPLNX lpr and SPLNX IFNγR1−/− in comparison to their controls (Fig.5C). A statistically significant 2.4-fold increase in the number of NOS2+ Mϕs was observed in SPLNX B6 mice in comparison to control B6 mice whereas no differences in NOS2+ Mϕs were observed between SPLNX and control gld, lpr or IFNγR1−/− mice (Fig.5D). These data indicated that both IFNγ and Fas/FasL interactions were required for Mϕ activation and that increased Mϕ expression of NOS2 correlated with intraocular tumor regression.
Figure 5. Intraocular Luc-E.G7 tumor rejection in SPLNX H-2b mice is associated with IFNγ-and-Fas/FasL-dependent activation of intratumoral macrophages.
Number of GR-1+ (A.), GR-1+ NOS2+ (B.) GR-1− Mϕs (C.) and Mϕs NOS2+ (D.) within whole intraocular tumor bearing eyes of indicated (H-2b) mouse strains 16 days following challenge with Luc-E.G7 in the a.c. of the eye. Data from B6, gld, and IFNγR1−/− mice were pooled from two independent experiments. lpr data is from a single experiment. Each symbol represents measurements from an individual intraocular tumor bearing eye and bars indicate the median. SPLNX mice were compared to mice that were not surgically manipulated (none) within mouse strains by two-tailed student’s t-tests or Mann-Witney tests depending on the normality of data determined by a D’Agostino & Pearson omnibus normality test. *=p<0.05, **=p<0.01, ns = not statistically significant p>0.05. (E.) Percent of NOS2−/− H-2b mice that were SPLNX or not surgically manipulated with intraocular Luc-E.G7 tumors. p value indicates comparison of treatment groups by a log rank test.
To determine whether NO production was required for elimination of intraocular tumors in SPLNX mice, Luc-E.G7 tumor growth was monitored in SPLNX and control NOS2 deficient B6 mice. Luc-E.G7 grew progressively in all control NOS2−/− mice whereas the majority of SPLNX NOS2−/− mice rejected intraocular Luc-E.G7 tumors (Fig.5F). P815 tumors were also rejected in SPLNX NOS2−/− mice on a Balb/C background (Supplementary-Table III, Exp.4) which indicated that NO production was dispensable for intraocular tumor elimination in SPLNX mice. Nevertheless, NOS2 expression remained a very sensitive marker of Mϕ activation.
To evaluate additional genes associated with intraocular tumor rejection, oligonucleotide microarrays were used to identify gene expression differences between whole tumor-bearing eyes (d15) from control or SPLNX mice (2 mice per group). Relative to controls, SPLNX mice showed increased (> 1.5-fold) expression of 1,488 genes and decreased expression of 523. Canonical pathways analysis (IPA) parsed 397 of these genes into 88 significantly enriched pathways (Supplemental-Table IV), of which the most significant were inflammation pathways. As IFNγ mRNA increased in intraocular Luc-E.G7 tumors of SPLNX mice, a group of ~600 IFN-stimulated genes was manually assembled from the NCBI Gene database, the Interferome.org database, and Schoggins and colleagues (18); the microarrays surveyed 408 of these genes. In whole tumor-bearing eyes from SPLNX mice 127 IFNγ-stimulated genes (31.1%) showed increased expression whereas only 9 (2.2%) showed decreased expression.
Only 4 of the 88 canonical pathways identified (Cell Cycle Control of Chromosomal Replication, Estrogen-mediated S-phase entry, Role of CHK Proteins in Cell Cycle Checkpoint Control, and Role of BRCA1 in DNA Damage Response) contained more genes showing decreased than increased expression. A net decrease in cell cycle activity and cell proliferation in the tumor-bearing eyes of SPLNX mice was confirmed by examining 192 cell cycle (19) genes present on the microarrays: 40 (20.8%) were downregulated while only 4 (< 1%) were upregulated. Of note was Ki67, a marker of cell proliferation, with a valid decrease to 71% of control tumor-bearing eyes which was consistent with the lower tumor burden seen in some SPLNX mice at this time.
The expression of inflammatory genes in tumor-bearing eyes of SPLNX and control mice (d16) was confirmed by RT-PCR (Supplemental-Fig.4). FasL, CXCL2, NOS2, and Tbet expression was significantly greater in tumor-bearing eyes of SPLNX mice. IFNγ expression was also greater in tumors of SPLNX mice but the increase was not statistically significant. Fas expression was comparable between SPLNX and control tumors. Combined, these data indicated that rejection of intraocular tumors in SPLNX mice was associated with increased expression of genes associated with ocular inflammation.
Mϕs are required for elimination of intraocular Luc-E.G7 tumors in SPLNX mice
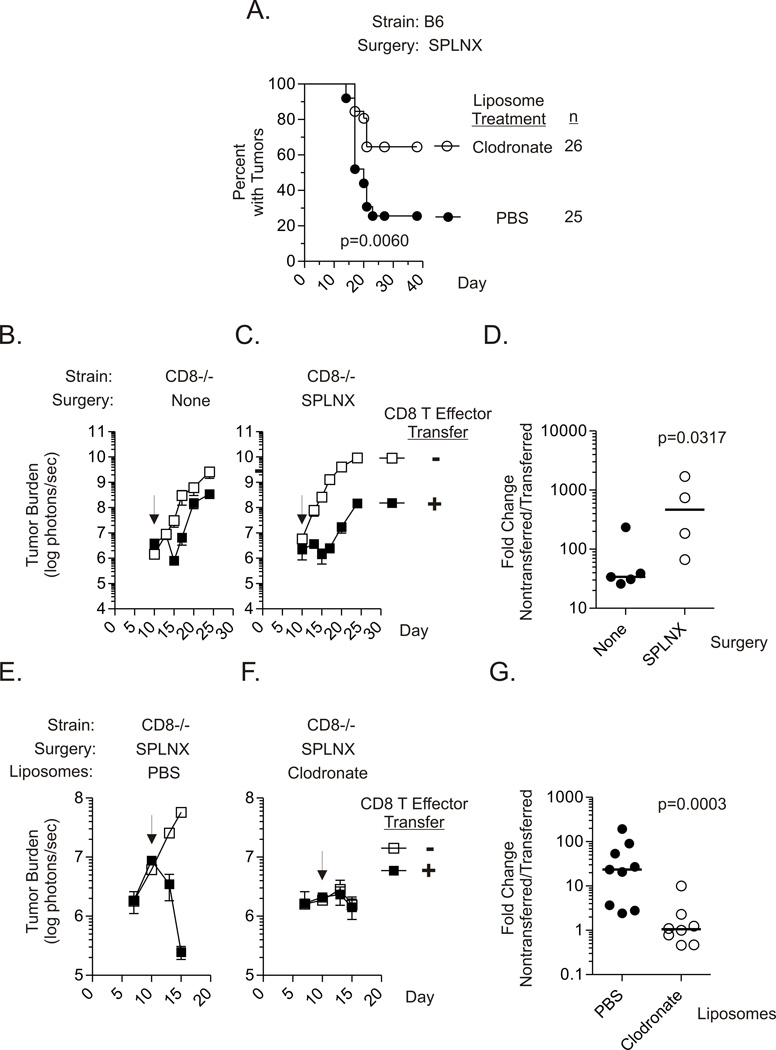
To determine the contribution of Mϕs in the elimination of Luc-E.G7 intraocular tumors in SPLNX B6 mice, clodronate liposomes were utilized to deplete Mϕs in vivo according to established protocols (20). In comparison to SPLNX mice that received PBS liposomes, a significantly greater percentage of SPLNX mice given clodronate liposomes showed progressive intraocular tumor growth (Fig.6A).
Figure 6. Mϕs are required for elimination of ocular Luc-E.G7 tumors in SPLNX mice.
(A.) Percent of SPLNX B6 mice, treated by scon. injections with clodronate or PBS liposomes with intraocular Luc-E.G7 tumors. Liposome injections were initiated three days before tumor challenge and continued every 3–4 days thereafter. p value indicates comparison of treatment groups by a log rank test. Intraocular Luc-E.G7 tumor burden measured by BLI in control (B.) or SPLNX (C.) CD8−/− H-2b mice that received on day 10 (arrow) post-tumor challenge in vitro primed CD8 T effector OT-I cells or were left untreated. (D.) Summary of fold-change in intraocular Luc-E.G7 tumor burden between nontransferred and CD8 T effector transferred control or SPLNX CD8−/− mice. Fold-change calculated 15 days after tumor challenge for each transferred mouse as (mean tumor burden of nontransferred mice within group) / tumor burden of individual transferred mice. Each symbol represents measurements from an individual CD8 T effector transferred mouse. p value indicates comparison of treatment groups by a one-tailed Mann Whitney test. Intraocular Luc-E.G7 tumor burden in SPLNX CD8−/− H-2b mice, treated with PBS (E.) or clodronate (F.) liposomes, that received on day 10 post-tumor challenge (arrow) in vitro primed CD8 T effector OT-I cells. (G.) Summary of fold-change in intraocular Luc-E.G7 tumor burden between nontransferred and CD8 T effector transferred SPLNX CD8−/− mice treated with PBS or clodronate liposomes as calculated in (D.). Data were pooled from two independent experiments. p value indicates comparison of treatment groups by a one-tailed Mann Whitney test. In addition to scon. liposome treatments administered as in (A.), mice were also given i.v. liposome treatments on day 3 and 7 in experimentation shown in E. – F.
To further evaluate the role of Mϕs in the elimination of intraocular tumors, we developed a system in which in vitro primed tumor-specific OT-I CD8+ T-cell effectors controlled growth of established intraocular Luc-E.G7 tumors in SPLNX CD8−/− mice. As shown in Fig.6B & 6C, Luc-E.G7 tumors grew progressively in control and SPLNX CD8−/− mice reproducing our previous observations. Control (Fig.6B) and SPLNX (Fig.6C) CD8−/− mice that were given OT-I CD8 T effectors ten days after tumor challenge in the a.c. of the eye both showed reduced tumor burden in comparison to that in non-transferred mice. However, the magnitude of tumor regression (fold-change in tumor burden between non-transferred and transferred mice) was significantly greater in SPLNX mice than in controls five days after transfer (d15). Hence, despite an equivalent T-cell transfer, the tumoricidal activity of transferred CD8+ T effectors was much greater in SPLNX mice.
To determine whether the interaction between CD8+ T effectors and Mϕs contributed to the observed increased tumoricidal activity in OT-I effector transferred SPLNX CD8−/− mice, SPLNX CD8−/− mice were given PBS or clodronate liposome treatments prior to CD8+ T effector cell transfer given ten days after intraocular Luc-E.G7 tumor challenge. It is important to note that day 10 tumor burden was lower in mice give clodronate liposomes and this was a consistent observation in multiple experiments. CD8 T effector transfer at day 10 caused a significant reduction in tumor burden in SPLNX CD8−/− mice treated with PBS liposomes (Fig.6E). In contrast, the same effector T cell transfer did not cause tumor regression in SPLNX CD8−/− mice treated with clodronate liposomes (Fig.6F) despite lower tumor burden at the time of transfer. These data confirmed the critical role of Mϕs at the effector phase of CD8 T cell-mediated elimination of intraocular tumors in SPLNX mice.
Discussion
Niederkorn, Streilein and Shadduck reported over 30 years ago that immune responses directed against tumors transplanted in the a.c. of the eye were different from those induced by transplantation of the same tumors in the skin (21). For example, P815 tumors that were rejected when placed in the skin of Balb/C mice induced robust DTH and CTL responses directed against minor alloantigens expressed by tumors (2). In contrast, P815 tumors transplanted in the a.c. of the eye grew progressively, did not induce DTH responses but interestingly, demonstrated tumor-specific CTL responses equivalent to those observed in mice that rejected P815 in the skin (2). The term anterior chamber-associated immune deviation (ACAID) was used to describe this unique immune response.
SPLNX terminated ACAID as DTH responses were restored in mice injected with P815 in the a.c. and these intraocular tumors were eliminated (8). The logical interpretation of these data, that CD4 T cell-dependent DTH responses mediated intraocular tumor rejection in SPLNX mice, was further supported by the destructive nature of tumor elimination which caused fulminant inflammation that culminated in ocular phthisis (8) In contrast, CD8 T cell-mediated rejection of other intraocular tumors left the eye intact (22). However, we definitively demonstrate that CD4+ T-cell responses were not required for rejection of P815 tumors in SPLNX Balb/C J mice. Rather, CD8+ T cells were necessary. These data are consistent with another study describing a P815 variant, P91, which was spontaneously rejected in the a.c. of non SPLNX DBA/2 mice (23, 24). In these reports, the administration of depleting anti-CD4 antibodies abrogated DTH responses to P91 antigens measured in the skin but failed to prevent phthisical rejection of intraocular tumors that demonstrated histopathologic features of a DTH response in the eye (23, 24). In contrast, progressive intraocular P91 tumor growth was observed in mice given depleting anti-CD8 antibodies although DTH responses in the skin were manifested (23, 24). Hence, CD8+ T cells are also capable of inducing destructive inflammatory responses resembling DTH responses within the eye that eliminate intraocular tumors by inducing ocular phthisis.
In our study, CD8+ T cells, Mϕs and IFNγ were critical for phthisical elimination of intraocular Luc-E.G7 tumors. These data suggest that SPLNX restored the DTH response (25) within intraocular tumors and support a model in which CD8+ T cells expressed IFNγ to activate Mϕs which induced intraocular inflammation. In further support of that interpretation, gene array analysis indicated increased expression of IFNγ, IFNγ inducible genes, and several other inflammatory genes in intraocular Luc-E.G7 tumor-bearing eyes of SPLNX mice.
IFNγ and Mϕs were also necessary for elimination of another immunogenic tumor cell line, Ad5E1, transplanted in the a.c. (20, 26–29) However, there are several differences between elimination of intraocular Ad5E1 and intraocular P815 or Luc-E.G7 that bear noting. Rejection of intraocular Ad5E1 tumors was spontaneous and did not require SPLNX. In addition, Ad5E1 tumor rejection did not induce ocular phthisis, and required CD4+ but not CD8+ T cells (26–28). The cellular target of IFNγ was also different between the experimental models. IFNγ targeted Ad5E1 tumors directly as intraocular Ad5E1 tumors were rejected in IFNγR1−/− mice (28) and in vitro addition of IFNγ induced Ad5E1 apoptosis via increased expression of TRAIL (27). As Ad5E1 is MHC Class II negative, these data suggest that cross-presentation of Ad5E1 tumor antigens to CD4+ T cells by intratumoral Mϕs promoted T cell expression of IFNγ that induced tumor cell apoptosis via TRAIL interactions. IFNγ also appeared to limit tumor vascularization by inducing Ad5E1 expression of anti-angiogenic chemokines (28). In contrast, rejection of P815 and Luc-E.G7 in SPLNX mice required that IFNγ-targeted immune cells, including Mϕs, within the tumor.
The extent of Mϕ activation may determine whether intraocular tumors are eliminated in a sight preserving or blinding (phthisical) fashion. For example, Coursey and coworkers recently generated Ad5E1 variants that were rejected in a nonphthisical (Clone 4) or phthisical (Clone 2.1) manner by a process that required Mϕs and IFNγ (30). Phthisical rejection was dictated by tumor sensitivity to TNFα, as Clone 2.1 tumors were rejected without phthisis in TNFα deficient mice whereas TNF receptor 1 deficient mice rejected Clone 2.1 tumors in a phthisical manner. These data suggested that a second signal released from necrotic tumor cells in combination with IFNγ induced stronger Mϕ activation thereby causing greater intraocular inflammation that eliminated intraocular tumors by destroying the eye.
Phthisical rejection of intraocular Luc.EG7 or P815 tumors in SPLNX mice was also associated with Mϕ activation that required two signals (IFNγ and Fas/FasL interactions) presumably delivered by CD8+ T cells that were also indispensable. Interestingly, FasL expression by CD8+ T cells was not necessary for regression of Luc-E.G7 intraocular tumors. However, Mϕs (31, 32) and CD8+ T cells express both Fas and FasL. Therefore, Fas expression by CD8+ T cells could engage FasL on tumor-associated Mϕs to induce their activation. Although Fas/FasL interactions have traditionally been considered pro-apoptotic, accumulating evidence indicates that Fas/FasL interactions induce Mϕ activation (31, 32) (33, 34) without apoptosis induction which supports this model.
We do not fully understand how splenectomy restored tumoricidal activity of CD8+ T cells and Mϕs within the eye. One potential explanation is that SPLNX influences tumor-specific CD8+ T cell numbers within intraocular tumors. Consistent with that notion, Boonman and coworkers demonstrated that another tumor cell line (Ad5E1 plus EJ-ras) transplanted in the a.c. of the eye formed progressively growing tumors in 40% of B6 mice whereas the remaining mice spontaneously eliminated tumors by a CD8 T cell-dependent process that culminated in phthisis (35, 36). Although tumor growth in both progressor and regressor mice induced expansion of tumor-specific CD8+ T cells in draining lymph nodes, only regressor mice showed systemic dissemination of these effector CD8+ T cells. Hence, these data could suggest that SPLNX restored tumor-specific CD8 T cell migration into intraocular Luc-E.G7 tumors.
It is also well appreciated that intraocular tumor growth induces the generation of tumor antigen-specific CD8+ Tregs that mediate suppression of DTH responses to tumor antigens (7). SPLNX prevents the generation of these CD8+ Tregs suggesting that the splenic microenvironment is critical for their generation (37). Therefore, it is possible that a splenic CD8+ Treg infiltrates intraocular tumors to limit Mϕ activation by CD8+ T-cell effectors. Based on the data presented herein a mechanism of CD8 Treg immunosuppression could involve inhibiting IFNγ production by CD8 T-cell effectors or interfering with Fas/FasL interactions between CD8+ T cells and Mϕs within the tumor microenvironment. We favor the later mechanism, as the reduction in IFNγ mRNA in control vs. SPLNX mice was modest, and we previously demonstrated that primed CD8+ CTLs that infiltrated established intraocular tumors were not impaired in IFNγ production (10). Gregory and coworkers (38, 39) demonstrated that tumors engineered to express a membrane only form of FasL were spontaneously rejected when placed in the a.c. of the eye whereas tumors that expressed both membrane and soluble FasL formed progressively growing intraocular tumors that metastasized. Hence, a CD8 Treg that expressed soluble FasL could limit intraocular inflammation by binding CD8 T cell expressed Fas thereby preventing Mϕ activation.
The role of Mϕs in tumor growth is complex as we demonstrate that intraocular Luc-E.G7 tumors were smaller in B6 mice depleted of Mϕs by clodronate liposome injections (Fig. 6). These data suggest that Mϕs contributed to intraocular tumor growth which is consistent with the observations of Ly and coworkers (40). Therefore, tumor regression, which also required Mϕs, may be due to conversion of intratumoral Mϕs from a protumor to an antitumor phenotype. However, it is important to note that direct tumoricidal activity by Mϕs may not be required to eliminate intraocular tumors as phthisis can result from destruction of the ciliary body of the eye (36). Therefore, SPLNX may remove an immunosuppressive mechanism that normally protects the ciliary body from destructive inflammatory mediators expressed by Mϕs.
In conclusion, SPLNX promotes indirect elimination of intraocular tumors by CD8+ T cells that is associated with IFNγ and Fas/FasL-dependent activation of intratumoral Mϕs. Therefore, mechanisms that maintain ocular IP may interfere with the interaction between CD8+ T cells and Mϕs to limit immunosurveillance of intraocular tumors.
Supplementary Material
Acknowledgements
The author’s thank Nancy Zurowski for excellent technical assistance in flow cytometry, Robert Hendricks, Walter Storkus, and Pawel Kalinski for critical review of this manuscript, Richard Bilonick for statistical analysis, Joseph Brown and Jessamee Mellon (University of Texas Southwestern Medical School) for preparation of liposomes, and Rachel Sikorski for transduction of E.G7-OVA to express firefly luciferase. We regret to announce Rachel’s untimely death and dedicate this manuscript to her memory.
Financial Support: This work was supported by National Institute of Health Grants R01 EY018355 (to K.C.M), P30-EY08098, and P30-CA047904, The Eye and Ear Foundation of Pittsburgh, and by an unrestricted grant from Research to Prevent Blindness Inc.
Footnotes
The authors declare no conflicts of interest
References
- 1.McKenna KC, Chen PW. Influence of immune privilege on ocular tumor development. Ocul Immunol Inflamm. 2010;18:80–90. doi: 10.3109/09273941003669950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niederkorn JY, Streilein JW. Alloantigens placed into the anterior chamber of the eye induce specific suppression of delayed-type hypersensitivity but normal cytotoxic T lymphocyte and helper T lymphocyte responses. J Immunol. 1983;131:2670–2674. [PubMed] [Google Scholar]
- 3.Ksander BR, Streilein JW. Analysis of cytotoxic T cell responses to intracameral allogeneic tumors. Invest Ophthalmol Vis Sci. 1989;30:323–329. [PubMed] [Google Scholar]
- 4.McKenna KC, Kapp JA. Accumulation of immunosuppressive CD11b+ myeloid cells correlates with the failure to prevent tumor growth in the anterior chamber of the eye. J Immunol. 2006;177:1599–1608. doi: 10.4049/jimmunol.177.3.1599. [DOI] [PubMed] [Google Scholar]
- 5.Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74:179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- 6.Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 7.McKenna KC, Previte DM. Influence of CD8+ T regulatory cells on intraocular tumor development. Front Immunol. 2012;3:303. doi: 10.3389/fimmu.2012.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med. 1981;153:1058–1067. doi: 10.1084/jem.153.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 10.Vicetti Miguel RD, Cherpes TL, Watson LJ, McKenna KC. CTL induction of tumoricidal nitric oxide production by intratumoral macrophages is critical for tumor elimination. J Immunol. 2010;185:6706–6718. doi: 10.4049/jimmunol.0903411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 12.Lee RK, Spielman J, Zhao DY, Olsen KJ, Podack ER. Perforin, Fas ligand, and tumor necrosis factor are the major cytotoxic molecules used by lymphokine-activated killer cells. J Immunol. 1996;157:1919–1925. [PubMed] [Google Scholar]
- 13.Kataoka T, Ito M, Budd RC, Tschopp J, Nagai K. Expression level of c-FLIP versus Fas determines susceptibility to Fas ligand-induced cell death in murine thymoma EL-4 cells. Exp Cell Res. 2002;273:256–264. doi: 10.1006/excr.2001.5438. [DOI] [PubMed] [Google Scholar]
- 14.Blankenstein T. The role of tumor stroma in the interaction between tumor and immune system. Curr Opin Immunol. 2005;17:180–186. doi: 10.1016/j.coi.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Hollenbaugh JA, Reome J, Dobrzanski M, Dutton RW. The rate of the CD8-dependent initial reduction in tumor volume is not limited by contact-dependent perforin, Fas ligand, or TNF-mediated cytolysis. J Immunol. 2004;173:1738–1743. doi: 10.4049/jimmunol.173.3.1738. [DOI] [PubMed] [Google Scholar]
- 16.Hollenbaugh JA, Dutton RW. IFN-gamma regulates donor CD8 T cell expansion, migration, and leads to apoptosis of cells of a solid tumor. J Immunol. 2006;177:3004–3011. doi: 10.4049/jimmunol.177.5.3004. [DOI] [PubMed] [Google Scholar]
- 17.McKenna KC, Beatty KM, Scherder RC, Li F, Liu H, Chen AF, et al. Ascorbate in aqueous humor augments nitric oxide production by macrophages. J Immunol. 2013;190:556–564. doi: 10.4049/jimmunol.1201754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizuno H, Nakanishi Y, Ishii N, Sarai A, Kitada K. A signature-based method for indexing cell cycle phase distribution from microarray profiles. BMC Genomics. 2009;10:137. doi: 10.1186/1471-2164-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boonman ZF, Schurmans LR, van Rooijen N, Melief CJ, Toes RE, Jager MJ. Macrophages are vital in spontaneous intraocular tumor eradication. Invest Ophthalmol Vis Sci. 2006;47:2959–2965. doi: 10.1167/iovs.05-1427. [DOI] [PubMed] [Google Scholar]
- 21.Niederkorn J, Streilein JW, Shadduck JA. Deviant immune responses to allogeneic tumors injected intracamerally and subcutaneously in mice. Invest Ophthalmol Vis Sci. 1981;20:355–363. [PubMed] [Google Scholar]
- 22.Knisely TL, Luckenbach MW, Fischer BJ, Niederkorn JY. Destructive and nondestructive patterns of immune rejection of syngeneic intraocular tumors. J Immunol. 1987;138:4515–4523. [PubMed] [Google Scholar]
- 23.Niederkorn JY, Meunier PC. Spontaneous immune rejection of intraocular tumors in mice. Invest Ophthalmol Vis Sci. 1985;26:877–884. [PubMed] [Google Scholar]
- 24.Niederkorn JY, Benson JL, Mayhew E. Efferent blockade of delayed-type hypersensitivity responses in the anterior chamber of the eye. Reg Immunol. 1990;3:349–354. [PubMed] [Google Scholar]
- 25.Kobayashi K, Kaneda K, Kasama T. Immunopathogenesis of delayed-type hypersensitivity. Microsc Res Tech. 2001;53:241–245. doi: 10.1002/jemt.1090. [DOI] [PubMed] [Google Scholar]
- 26.Schurmans LR, Diehl L, den Boer AT, Sutmuller RP, Boonman ZF, Medema JP, et al. Rejection of intraocular tumors by CD4(+) T cells without induction of phthisis. J Immunol. 2001;167:5832–5837. doi: 10.4049/jimmunol.167.10.5832. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Boonman ZF, Li HC, He Y, Jager MJ, Toes RE, et al. Role of TRAIL and IFN-gamma in CD4+ T cell-dependent tumor rejection in the anterior chamber of the eye. J Immunol. 2003;171:2789–2796. doi: 10.4049/jimmunol.171.6.2789. [DOI] [PubMed] [Google Scholar]
- 28.Dace DS, Chen PW, Alizadeh H, Niederkorn JY. Ocular immune privilege is circumvented by CD4+ T cells, leading to the rejection of intraocular tumors in an IFN-{gamma}-dependent manner. J Leukoc Biol. 2007;81:421–429. doi: 10.1189/jlb.0806489. [DOI] [PubMed] [Google Scholar]
- 29.Dace DS, Chen PW, Niederkorn JY. CD4+ T-cell-dependent tumour rejection in an immune-privileged environment requires macrophages. Immunology. 2008;123:367–377. doi: 10.1111/j.1365-2567.2007.02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coursey TG, Chen PW, Niederkorn JY. Abrogating TNF-alpha expression prevents bystander destruction of normal tissues during iNOS-mediated elimination of intraocular tumors. Cancer Res. 2011;71:2445–2454. doi: 10.1158/0008-5472.CAN-10-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SM, Kim EJ, Suk K, Lee WH. Stimulation of Fas (CD95) induces production of pro-inflammatory mediators through ERK/JNK-dependent activation of NF-kappaB in THP-1 cells. Cell Immunol. 2011;271:157–162. doi: 10.1016/j.cellimm.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Chakour R, Allenbach C, Desgranges F, Charmoy M, Mauel J, Garcia I, et al. A new function of the Fas-FasL pathway in macrophage activation. J Leukoc Biol. 2009;86:81–90. doi: 10.1189/jlb.1008590. [DOI] [PubMed] [Google Scholar]
- 33.Sato A, Nakashima H, Nakashima M, Ikarashi M, Nishiyama K, Kinoshita M, et al. Involvement of the TNF and FasL Produced by CD11b Kupffer Cells/Macrophages in CCl4-Induced Acute Hepatic Injury. PLoS One. 2014;9:e92515. doi: 10.1371/journal.pone.0092515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Ren J, Morgan S, Liu Z, Dou C, Liu B. Monocyte Chemoattractant Protein-1 (MCP-1) Regulates Macrophage Cytotoxicity in Abdominal Aortic Aneurysm. PLoS One. 2014;9:e92053. doi: 10.1371/journal.pone.0092053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boonman ZF, van Mierlo GJ, Fransen MF, Franken KL, Offringa R, Melief CJ, et al. Intraocular tumor antigen drains specifically to submandibular lymph nodes, resulting in an abortive cytotoxic T cell reaction. J Immunol. 2004;172:1567–1574. doi: 10.4049/jimmunol.172.3.1567. [DOI] [PubMed] [Google Scholar]
- 36.Boonman ZF, van Mierlo GJ, Fransen MF, de Keizer RJ, Jager MJ, Melief CJ, et al. Maintenance of immune tolerance depends on normal tissue homeostasis. J Immunol. 2005;175:4247–4254. doi: 10.4049/jimmunol.175.7.4247. [DOI] [PubMed] [Google Scholar]
- 37.Streilein JW, Niederkorn JY. Characterization of the suppressor cell(s) responsible for anterior chamber-associated immune deviation (ACAID) induced in BALB/c mice by P815 cells. J Immunol. 1985;134:1381–1387. [PubMed] [Google Scholar]
- 38.Gregory MS, Saff RR, Marshak-Rothstein A, Ksander BR. Control of ocular tumor growth and metastatic spread by soluble and membrane Fas ligand. Cancer Res. 2007;67:11951–11958. doi: 10.1158/0008-5472.CAN-07-0780. [DOI] [PubMed] [Google Scholar]
- 39.Gregory MS, Repp AC, Holhbaum AM, Saff RR, Marshak-Rothstein A, Ksander BR. Membrane Fas ligand activates innate immunity and terminates ocular immune privilege. J Immunol. 2002;169:2727–2735. doi: 10.4049/jimmunol.169.5.2727. [DOI] [PubMed] [Google Scholar]
- 40.Ly LV, Baghat A, Versluis M, Jordanova ES, Luyten GP, van Rooijen N, et al. In aged mice, outgrowth of intraocular melanoma depends on proangiogenic M2-type macrophages. J Immunol. 2010;185:3481–3488. doi: 10.4049/jimmunol.0903479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.