Abstract
Spinach leaf discs accumulated betaine when exposed to a mannitol solution of −20 bars. The accumulation was 12 micromoles per gram original fresh weight in a 24-hour period.
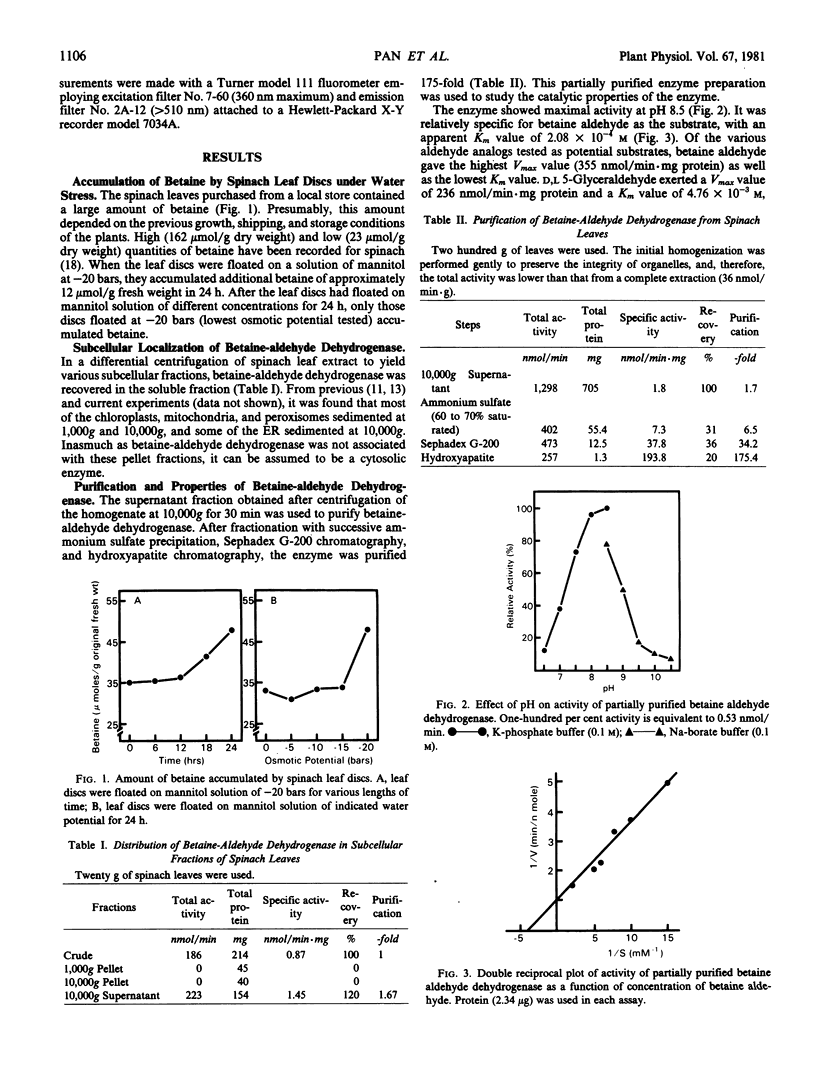
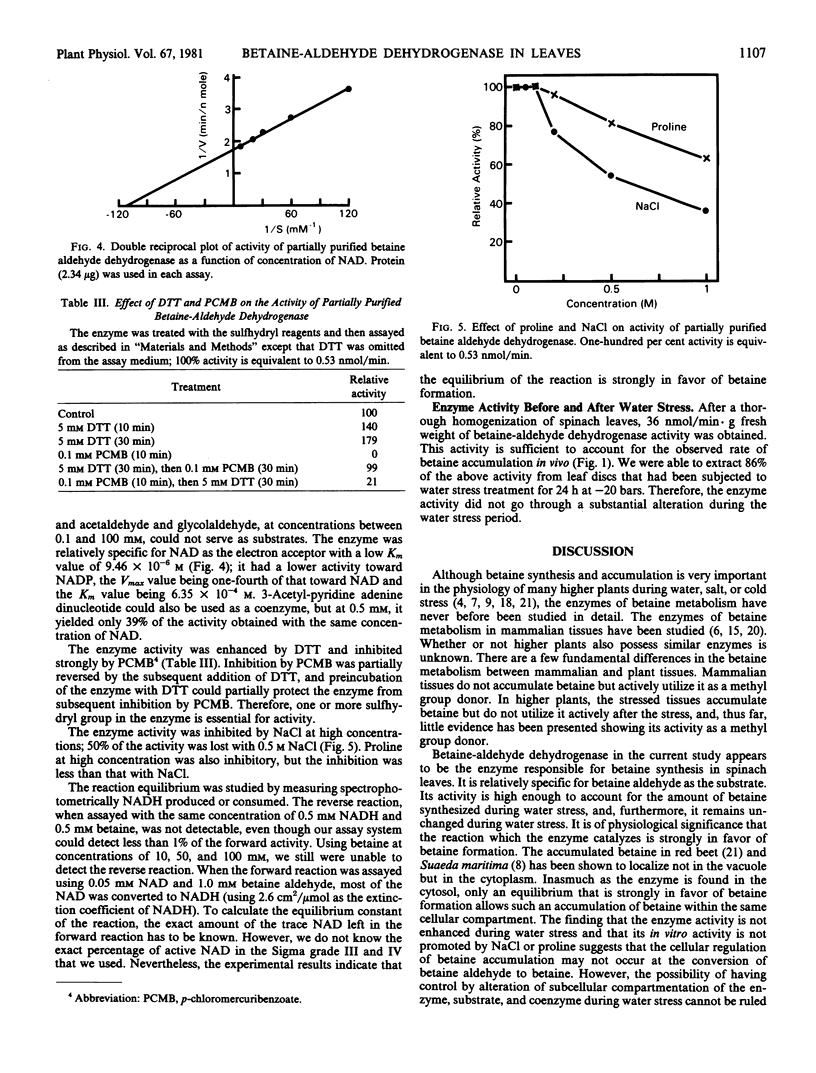
Betaine-aldehyde dehydrogenase (EC 1.2.1.8) was assayed in various subcellular fractions prepared from spinach leaves, and it was found only in the soluble fraction. This cytosolic enzyme was purified 175-fold, and its properties were studied. The enzyme was relatively specific for betaine aldehyde as the substrate with an apparent Km value of 2.08 × 10−4 molar. It also exerted activity on other aldehyde analogs tested, but with lower Vmax and higher Km values. The enzyme was relatively specific for nicotinamide adenine dinucleotide as the coenzyme, having an apparent Km value of 9.46 × 10−6 molar; lower activities were observed when nicotinamide adenine dinucleotide phosphate or 3-acetyl pyridine adenine dinucleotide were tested as electron acceptors. The activity was enhanced by dithiothreitol and inhibited by p-chloromercuribenzoate, and the inhibition by p-chloromercuribenzoate was partially reversed by the subsequent addition of dithiothreitol. The activity was inhibited by high concentrations of NaCl and, to a lesser extent, proline. The equilibrium of the enzymic reaction was strongly in favor of betaine formation.
The in vitro activity of the enzyme under optimal assay conditions was high enough to account for the amount of betaine accumulated under water stress conditions. The enzyme activity was the same in unstressed leaves and in leaves that had been water stressed for 24 hours.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DELWICHE C. C., BREGOFF H. M. Pathway of betaine and choline synthesis in Beta vulgaris. J Biol Chem. 1958 Aug;233(2):430–433. [PubMed] [Google Scholar]
- GLENN J. L., VANKO M. Choline and aldehyde oxidation by rat liver. Arch Biochem Biophys. 1959 May;82(1):145–152. doi: 10.1016/0003-9861(59)90099-2. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Nelsen C. E. Betaine Accumulation and [C]Formate Metabolism in Water-stressed Barley Leaves. Plant Physiol. 1978 Aug;62(2):305–312. doi: 10.1104/pp.62.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Scott N. A. Betaine Synthesis from Radioactive Precursors in Attached, Water-stressed Barley Leaves. Plant Physiol. 1980 Aug;66(2):342–348. doi: 10.1104/pp.66.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Isolation of microbodies from plant tissues. Plant Physiol. 1971 Nov;48(5):637–641. doi: 10.1104/pp.48.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Beevers H. Intracellular distribution of enzymes of the cytidine diphosphate choline pathway in castor bean endosperm. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2429–2432. doi: 10.1073/pnas.69.9.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHSCHILD H. A., BARRON E. S. G. The oxidation of betaine aldehyde by betaine aldehyde dehydrogenase. J Biol Chem. 1954 Aug;209(2):511–523. [PubMed] [Google Scholar]
- Wilken D. R., McMacken M. L., Rodriquez A. Choline and betaine aldehyde oxidation by rat liver mitochondria. Biochim Biophys Acta. 1970 Sep 1;216(2):305–317. doi: 10.1016/0005-2728(70)90222-7. [DOI] [PubMed] [Google Scholar]



