Abstract
Post-traumatic stress disorder (PTSD) and other anxiety disorders stemming from dysregulated fear memory are problematic and costly. Understanding the molecular mechanisms that contribute to the formation and maintenance of these persistent fear associations is critical to developing treatments for PTSD. Epigenetic mechanisms, which control gene expression to produce long-lasting changes in cellular function, may support the formation of fear memory underlying PTSD. Here, we address the role of epigenetic mechanisms in the formation, storage, updating, and extinction of fear memories and discuss methods of targeting these epigenetic mechanisms to reduce the initial formation of fear memory or to enhance its extinction. Epigenetic mechanisms may provide a novel target for pharmaceutical and other treatments to reduce aversive memory contributing to PTSD.
Keywords: Epigenetics, Fear Conditioning, Consolidation, Extinction, Updating, PTSD
Fear Memory as a Model for PTSD
Understanding how the brain converts temporary sensory stimuli into persistent memory has been a fundamental focus of neuroscience research for the past few decades [1]. One important question is how such temporary changes in the environment can be encoded in a relatively persistent manner by the cell to produce long-lasting memory, such as memory for a fearful event. Identifying the molecular mechanisms of fear memory formation is particularly important in light of the prevalence of post-traumatic stress disorder (PTSD), a debilitating condition characterized by inappropriate fear generalization to safe contexts and stimuli, and other anxiety disorders such as phobias and panic disorders, which together affect nearly 18.1% of adults in the United States [2] and cost an estimated $42.3 billion each year [3]. Learning to avoid cues that signal danger is important to minimize injury, but excessive or persistent responding to nonthreatening stimuli (as occurs in PTSD), can also cause harm.
In rodents, PTSD and general anxiety disorders can be modeled with Pavlovian fear conditioning (see Glossary), a learning task in which an initially neutral conditional stimulus (CS), like a tone or context, is paired with a naturally aversive unconditional stimulus (UCS), usually a footshock (Figure 1A) [4]. Epigenetic mechanisms have recently been implicated in various forms of memory, including fear memory [5–9] and may represent one important way that transient cell signaling following a brief learning event can produce lasting changes in cellular function and, accordingly, enduring changes in behavior [10].
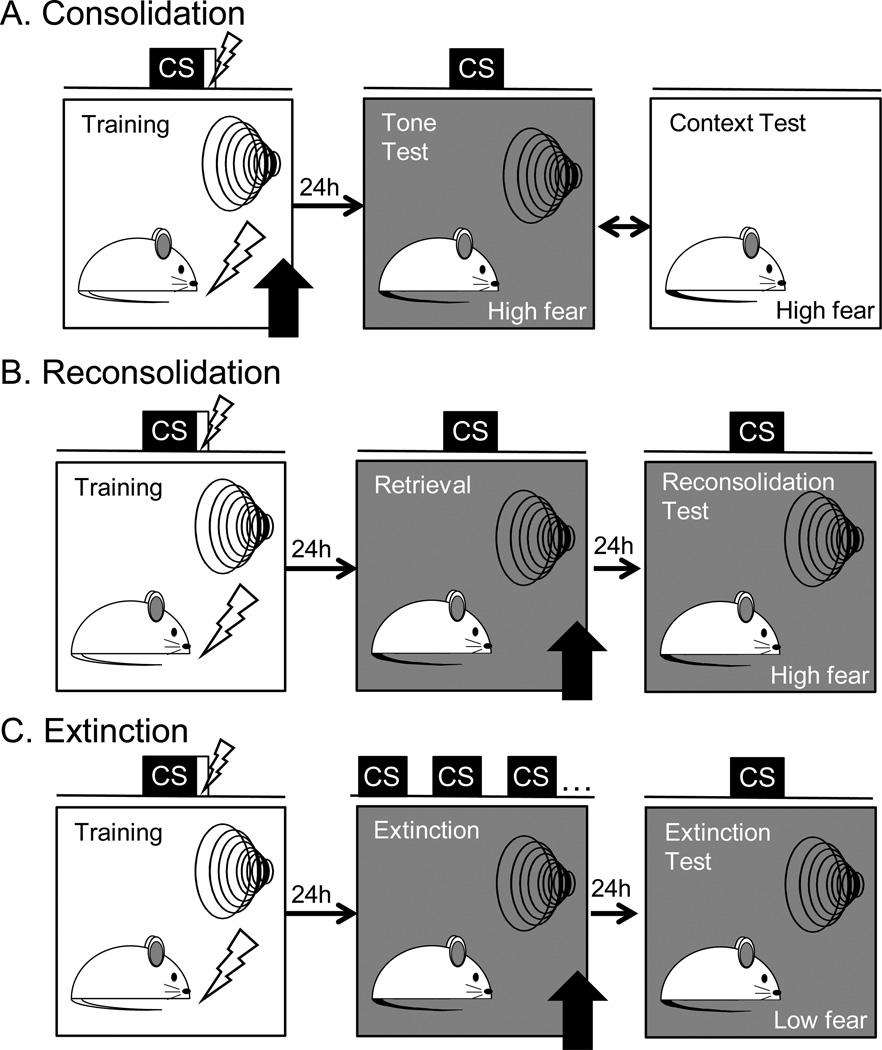
Figure 1.
Fear conditioning, reconsolidation, and extinction procedures. A) Typical procedure for studying consolidation. Animals are trained with a neutral conditional stimulus (CS) that is paired with an aversive unconditional stimulus (UCS). Pictured, a tone CS is paired with a footshock UCS. Consolidation is usually tested by manipulating gene expression following training (arrow). 24h after training, tone fear is independently tested in a novel context (gray background) and context fear is assessed by returning the animal to the training chamber. Freezing is measured as an index of fear. B) Reconsolidation procedure. Usually, the tone CS is presented a single time in a novel context and gene expression is manipulated after the retrieval session. Fear to the CS is tested the following day. C) Extinction procedure, in which CS is repeatedly presented without the UCS. If extinction is properly acquired, the animal should show low tone freezing the following day at test. Arrows indicate appropriate time to perform manipulations. Lightning blot indicates UCS presentation.
Epigenetic mechanisms can be defined as changes in gene expression that occur through alterations in chromatin structure, rather than changes in DNA sequence [11]. A range of epigenetic mechanisms have been implicated in long-term memory formation, including, but not limited to, histone acetylation [6], phosphorylation [12], and methylation [5], DNA methylation [13], and nucleosome remodeling [8]. These learning-related epigenetic changes could change the state of the cell long after the learning event, so that the resulting behavior is long-lasting and robust. For fear memory, this means that epigenetic changes may drive the persistent behaviors associated with PTSD, including re-experiencing the event, avoiding cues that trigger memories of the trauma, and continuous hyperarousal [4]. Here, we review the evidence that epigenetic mechanisms are involved in acquiring, storing, updating, and extinguishing fear memory.
Fear Conditioning Circuitry
The central circuitry underlying fear conditioning has been revealed through the past two decades of research (Figure 2). The amygdala is generally recognized as a critical site of associative convergence between the initially neutral tone (or context) and the shock [14, 15] although some argue that the amygdala strictly modulates memory storage in other brain regions [16]. Composed of several functionally distinct nuclei that interact during fear learning, the amygdala itself is a relatively complex circuit (for a detailed review of amygdala microcircuitry, see [17]). Learning that the training context also predicts the shock requires the participation of the dorsal hippocampus and medial prefrontal cortex in addition to the amygdala. The hippocampus is believed to compile the distinct elements of the training chamber (for example, the lighting, shape, color, and texture of the environment) into a single configural representation of the context [18]. This representation can then be processed by the amygdala where associative convergence with UCS information occurs, as with a discrete auditory CS [14]. Although the role of the medial prefrontal cortex (mPFC) in fear acquisition is less clear, the prelimbic portion of the mPFC seems to play a role in contextual and higher-order learning [19, 20]. Disrupting either the dorsal hippocampus or prelimbic mPFC around the time of training selectively impairs the context-shock association without affecting fear to the auditory CS [19, 21]. Within the context of this well-characterized circuit, detailed questions about the cellular and molecular components of fear memory can be addressed.
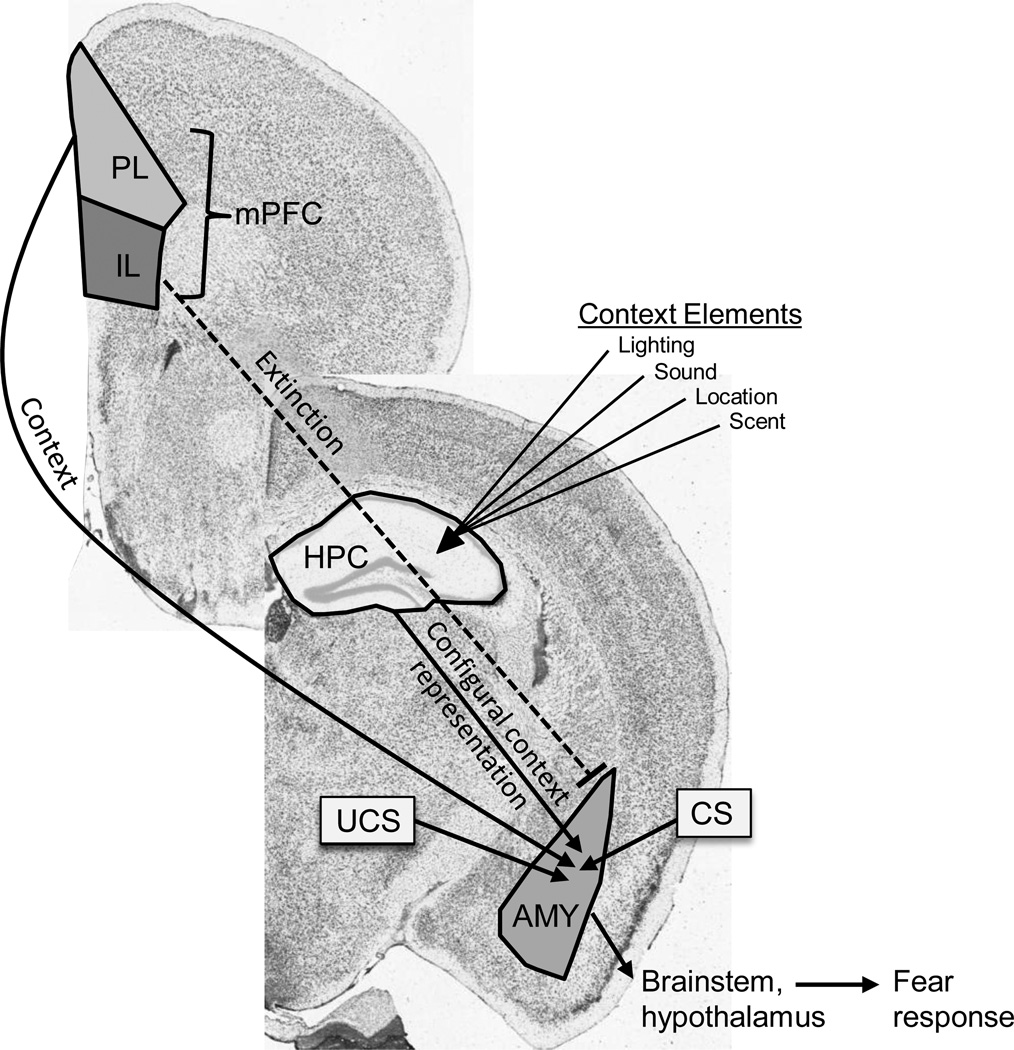
Figure 2.
Basic fear conditioning circuit. The amygdala (AMY) is the site of associative convergence between the tone or context CS and the footshock UCS. Output from the amygdala drives the fear response, including freezing. Individual context elements are formed into a configural “context” representation in the hippocampus (HPC) before being projected to the amygdala. The prelimbic mPFC (PL) also drives context fear during learning. During extinction, the infralimbic mPFC (IL) blocks amygdala output to block fear output. (Figures adapted from Allen Brain Atlas)[99].
Epigenetic Mechanisms that Directly Modulate Chromatin Structure
Long-term memory is stabilized through a process called consolidation [1], which converts labile short-term memory into a robust, durable long-term memory (Figure 1A). A hallmark of the consolidation process is the requirement for de novo gene expression; blocking either transcription or translation in the amygdala impairs long-term fear memory (tested at 24h after learning) without affecting short-term retention (usually ~1h after acquisition) [e.g. 22]. Several intracellular signaling cascades both up- and downstream of gene expression have been shown to be critical for synaptic plasticity and successful memory formation in the amygdala [23, 24], but it is unclear how these signaling cascades integrate into the coordinated program of gene expression required to produce synapse-specific, long-lasting alterations required for successful long-term memory.
Epigenetic mechanisms are particularly well-suited to provide the type of precise, bidirectional regulation of gene expression and cellular function required for memory formation and long-lasting changes in behavior. For transcription to occur, the transcriptional machinery needs to gain access to the DNA template, which is condensed into chromatin. Chromatin is the protein assembly that organizes and compacts DNA into the nucleus of each cell. Chromatin structure can be altered in specific ways to open or restrict access to DNA, thereby facilitating or impairing the expression of specific genes in response to environmental stimuli [10]. This process of altering chromatin structure to control gene expression without changing the DNA sequence itself is known as epigenetics [6, 11]. When a learning event occurs, epigenetic mechanisms likely turn off genes that restrict memory while simultaneously enable expression of memory-promoting genes to establish long-lasting changes in cell function required for long-term memory.
The basic unit of chromatin, the nucleosome, is a histone octamer wrapped by approximately 147 base pairs of DNA. Each histone octamer is composed of four pairs of histone proteins (H2A, H2B, H3, and H4), each with its own amino-terminal tail. These tails are extremely important to the dynamic nature of chromatin; histone tail modifications can either restrict or promote access to the DNA [6, 12, 25]. Histone tails can be modified by the removal or addition of a number of chemical modifications, including acetylation, phosphorylation, and methylation [12]. The most commonly studied histone modification is acetylation, in which an acetyl group is added to the lysine residue of a histone tail. Histone acetylation, carried out by enzymes called histone acetyltransferases (HATs), reduces the interaction between the negatively charged DNA phosphate backbone and the positively charged lysine residues, relaxing chromatin structure and thus promoting transcription. Enzymes that remove acetyl groups, called histone deacetylases (HDACs), induce a repressive chromatin structure that correlates with transcriptional silencing. Histone tail phosphorylation is also associated with transcriptional activation [26], but this modification is less well-studied and is understood far less completely than histone acetylation. Methylation of histones is a relatively complex modification that can either promote or repress transcription depending on the site of methylation and the number of methyl groups transferred to the histone tail (For review, see [5]). The combinatorial complexity of histone modifications generates immense information for the coordinate regulation of gene expression to carry out specific cell functions.
Beyond the histone, chromatin can also be altered by direct DNA modification. Methylation of the DNA itself can modulate chromatin, as enzymes called DNA methyl transferases (DNMTs) trigger the binding of a methyl group onto the DNA, usually on cytosine residues positioned next to guanine nucleotides (CpG) [7, 27]. DNA methylation generally suppresses transcription by blocking the binding of the transcriptional machinery to the DNA and by recruiting transcriptional repressors [For review, see 28], although there are exceptions in which DNA methylation promotes transcription [29, 30]. DNA methylation may therefore provide some of the transcriptional repression required to silence genes that inhibit memory formation [31].
Finally, nucleosome remodeling, an epigenetic mechanism that has been largely overlooked in neuroscience until recently, has also been implicated in learning and memory processes (for review, see [8]). Nucleosome remodeling refers to the addition, removal, or shifting of nucleosomes along the DNA in an ATP-dependent manner to control access to different expression control elements of a given gene [8, 32]. The exact mechanisms by which nucleosomes remodeling occurs is still poorly understood. In any case, nucleosome remodeling and the epigenetic mechanisms briefly mentioned above have key functions in regulating gene expression during the consolidation phase of memory formation.
The fear associations that contribute to PTSD and other anxiety disorders are particularly persistent and intense. The molecular and cellular mechanisms that support these memories must therefore be similarly robust and long-lasting to produce these persistent changes in behavior. As epigenetic mechanisms alter cell function in a stable manner, they are logical candidates for providing the type of long-lasting cellular memory that could give rise to fear-based anxiety disorders. Understanding the role that epigenetic mechanisms play in fear memory is therefore essential to develop treatments to prevent the formation of excessive fear memory and also to reduce the aversive nature of these associations once they are formed. They may even be able to identify aspects of susceptibility or resistance to PTSD in the future.
Epigenetic Mechanisms of Fear Memory Consolidation
The first phase of long-term memory formation is consolidation, as described above (Figure 1A) [1]. During consolidation, learning first catalyzes a number of post-translational modifications on existing proteins, activating multiple signaling cascades to produce short-term memory that lasts for an hour or two after training. Without de novo transcription and translation, however, the memory will be rapidly lost [1, 33], suggesting that new gene expression is critically important to convert transient short-term memory into persistent long-term fear memory.
The role of epigenetic mechanisms in memory consolidation have only recently been examined. Epigenetic mechanisms should play a role in converting transient short-term fear memory into persistent and robust long-term memory via the epigenetic regulation of gene expression. By rearranging chromatin, epigenetic mechanisms can shift which gene products are available for expression following learning [6, 8, 27], dictating which associations reach the threshold to be consolidated into lasting long-term memory. It is possible that epigenetic mechanisms contribute to the formation of the excessively strong and persistent fear memories underlying anxiety disorders by encouraging the overproduction of memory-promoting gene products in response to a frightening event. If this is the case, susceptible individuals might benefit from treatments that limit epigenetic responding to environmental cues to effectively raise the threshold at which transient information is consolidated into long-term memory.
Histone Acetylation in Fear Memory Consolidation
Numerous epigenetic mechanisms have been implicated in the consolidation of fear memory, including, but not limited to, histone modifications (acetylation, methylation, and phosphorylation), DNA methylation, and nucleosome remodeling (see Tables 1 and 3). Fear conditioning triggers epigenetic changes that work in concert to simultaneously promote the transcription of memory-enhancing genes and inhibit the expression of memory-restricting genes [7]. Histone acetylation is the most widely studied epigenetic mechanism in fear consolidation and is subsequently the best characterized. Nonspecific HAT inhibitors (drugs that block histone acetylation) delivered systemically generally disrupt fear consolidation [34] whereas HDAC inhibitors (which prevent histone deacetylation) usually enhance fear consolidation (Table 1) [35–42]. In general, blocking histone acetylation is detrimental to memory formation whereas enhancing acetylation promotes the formation of memory.
Table 1.
Behavioral studies manipulating epigenetic mechanisms during consolidation
| Epigenetic Mechanism |
Structure | Manipulation | Type of FC | Effect | Citation |
|---|---|---|---|---|---|
| HAT | Global/Systemic | (+/−) CBP KO | Auditory | Impaired auditory & context fear | [104] |
| CBP (+/−) CBP truncation | Auditory | Impaired auditory (but not context) fear | [105] | ||
| CBPkix/kix mutation (blocks CREB-binding domain) | Auditory or Context-only | Impaired context (but not auditory) fear | [108] | ||
| HAT activity block (garcinol) | Auditory | Impaired auditory fear | [34] | ||
| Forebrain Neurons | CaMKII-driven CBP KO | Context-only | Impaired context fear | [119] | |
| CaMKII-tet-driven CBP mutation | Auditory | No effect | [100] | ||
| CaMKII-driven CBPΔ1 mutation | Auditory or Context-only | Impaired context (but not auditory) fear | [102] | ||
| CaMKII-tet-driven p300Δ1 mutation | Auditory or Context-only | Impaired context (but not auditory) fear | [103] | ||
| Amygdala | HAT activity block (garcinol) | Auditory | Impaired auditory fear | [34] | |
| P300/CBP activity block (c646) | Auditory | Impaired auditory fear | [45] | ||
| Hippocampus | AAV-Cre-induced CBPflox/flox mutation | Context-only | Impaired context fear | [107] | |
| Prelimbic mPFC | PCAF activity block (H3-CoA-20-Tat) | Auditory | Enhanced auditory fear | [91] | |
| HDAC | Global/Systemic | HDAC activity block (SAHA or NaBut) | Context-only | Enhanced context fear | [37–40] |
| HDAC activity block (NaBut) | Auditory | Enhanced auditory & context fear | [35] | ||
| HDAC activity block (NaBut) | Auditory | Enhanced context (but not auditory) fear | [41] | ||
| HDAC activity block (TSA) | Context-only | Impaired context fear | [119] | ||
| HDAC activity block (VPA) | Auditory | Enhanced auditory fear | [42] | ||
| Throughout Brain | Genetic HDAC2 overexpression | Auditory or Context-only | Impaired auditory & context fear | [37] | |
| HDAC2 KO | Auditory or Context-only | Enhanced auditory & context fear | [37] | ||
| Nestin-Cre-induced SIRT1Δflox mutant | Auditory or Context-only | Impaired auditory & context fear | [111] | ||
| HDAC activity block (NaBut or TSA) | Context-only | Enhanced context fear | [38] | ||
| Amygdala | HDAC activity block (TSA) | Visual or Auditory | Enhanced visual or auditory fear | [44, 46] | |
| Hippocampus | HDAC activity block (TSA) | Auditory or Context-only | Enhanced context (but not auditory) fear | [36] | |
| AAV-synapsin1-driven HDAC1 overexpression | Context-only | No effect | [92] | ||
| HDAC/DNMT | Hippocampus | HDAC & DNMT block (NaBut & 5-AZA) | Context-only | NaB rescues 5-AZA impairment | [47] |
| DNMT | Forebrain Neurons | CaMKII-Cre-induced Dnmt1 & Dnmt2 flox DKO | Context-only | Impaired context fear | [55] |
| Amygdala | DNMT activity block (5-AZA or RG108) | Auditory | Impaired auditory fear | [44, 56] | |
| Hippocampus | DNMT activity block (5-AZA, zebularine, or RG108) | Context-only | Impaired context fear | [31, 47, 49] | |
| HKM | Forebrain Neurons | CaMKII-Cre-driven KM2B flox KO (prevents H3K4me) | Context-only | Impaired context fear | [53] |
| Entorhinal Cortex | G9a/GLP block (prevents H3K9me2; BIX01294) | Auditory or Context-only | Enhanced auditory & context fear | [52] | |
| Hippocampus | G9a/GLP block (BIX01294) | Context-only | Impaired context fear | [39] | |
| Histone methylation block (+/−) eed or MII KO | Context-only | Impaired context fear | [39] | ||
| NRC | Global | (+/−) Baf53b KO (brain-specific subunit of NRC) | Auditory or Context-only | Impaired context (but not auditory) fear | [59] |
| Forebrain neurons | CaMKII-driven BAF53b mutation | Auditory or Context-only | Impaired context (but not auditory) fear | [59] |
Abbreviations: ACQ: acquisition; DNMT: DNA methyltransferase; HAT: Histone acetyltransferase; HDAC; Histone deacetylase; HKM: Histone lysine methylation; KO: Knockout; NRC: Nucleosome remodeling complex; Drugs shown in parentheses
Table 3.
Epigenetic changes during each phase of memory
| Memory Phase | Mechanism | Structure | Modification | Effect | Timepoint | Type of FC | Citation |
|---|---|---|---|---|---|---|---|
| Consolidation | HAT | Amygdala | H3 acetylation | Increased | 90m post-ACQ (not 30 or 60m) | Auditory | [34, 44, 45] |
| HAT activity | Increased | 10m to 6h post-ACQ | Auditory | [46] | |||
| Hippocampus | H3 acetylation | Increased | 1h post-ACQ | Context | [35, 40, 47, 48] | ||
| H3 acetylation at Homer1 promoter | Increased | 2h post-ACQ | Auditory | [41] | |||
| H3 acetylation & phosphoacetylation | Increased | 2h post-ACQ | Context | [49] | |||
| DNMT | Amygdala | DNMT3A protein expression | Increased | 90m post-ACQ (not 30 or 60m) | Auditory | [44] | |
| Hippocampus | Methylation of Bdnf promoter exon VI | Increased | 2h post-ACQ | Context | [49] | ||
| Methylation of Bdnf promoter I & IV | Decreased | 2h post-ACQ | Context | [49] | |||
| DNMT3A & 3B mRNA expression | Increased | 30m post-ACQ | Context | [31] | |||
| PP1 methylation | Increased | 1h (but not 24h) post-ACQ | Context | [31] | |||
| Reln methylation | Decreased | 1h (but not 24h) post-ACQ | Context | [31] | |||
| mPFC | Reln methylation | Increased | 1h post-ACQ | Context | [64] | ||
| Zif268 methylation | Decreased | 1h post-ACQ | Context | [64] | |||
| HPO4 | Hippocampus | H3 phosphorylation | Increased | 1h post-ACQ | Auditory | [35] | |
| H3 phosphorylation & acetylation | Increased | 1h post-ACQ | Context | [48] | |||
| HKM | Amygdala | H3K9me at Homer1 promoter | Decreased | 2h post-ACQ | Auditory | [41] | |
| Hippocampus | H3K4me3 | Increased | 1h (but not 24h) post-ACQ | Context | [39, 52] | ||
| H3K4me3 at Bdnf promoter 1 | Increased | 30m post-ACQ | Context | [39] | |||
| H3K4me3 at Zif268 promoter | Increased | 30m post-ACQ | Context | [39] | |||
| H3K9me2 | Increased | 1h post-ACQ | Context | [39] | |||
| H3K9me2 at Comt promoter | Increased | 1h post-ACQ | Context | [52] | |||
| H3K9me2 at Zif268, cFos, BDNF IV promoters | Decreased | 1h post-ACQ | Context | [52] | |||
| Entorhinal Ctx | H3K4me3 | Increased | 1h post-ACQ | Context | [52] | ||
| H3K9me2 at cFos, BDNF IV promoters | Increased | 1h post-ACQ | Context | [52] | |||
| H3K9me2 | Increased | 1h (but not 24h) post-ACQ | Context | [52] | |||
| Storage | HAT | mPFC | H3 acetylation at Bdnf promoter 1&4 | Increased | 1d post-ACQ (2h after novel context | Auditory | [88] |
| DNMT | dmPFC | Reln methylation | Increased | 1d & 7d (but not 30d) post-ACQ | Context | [64] | |
| Z if 268 methylation | Decreased | 1d, 7d, 30d post-ACQ | Context | [64] | |||
| CaN methylation | Increased | 1d, 7d, 30d post-ACQ | Context | [64] | |||
| HKM | Hippocampus | H3K9me2 | Decreased | 1d post-ACQ | Context | [39, 52] | |
| Entorhinal Ctx | H3K4me3 | Decreased | 1d post-ACQ | Context | [52] | ||
| Reconsolidation | HAT | Amygdala | H3 acetylation | Increased | 90m (not 60m or 120m) post-retrieval | Auditory | [34, 45, 78] |
| Hippocampus | HDAC2 bound to cFos promoter | Decreased | 1h post-retrieval of recent memory | Context | [77] | ||
| H3 phosphorylation and acetylation | Increased | 1h post-retrieval | Context | [76] | |||
| H3 phosphoacetylation and acetylation at Zif268 | Increased | 1h post-retrieval | Context | [76] | |||
| H3 phosphorylation and acetylation at IKBα | Increased | 1h post-retrieval | Context | [76] | |||
| AcH3K9/14 binding to cFos promoter | Decreased | 1h post-retrieval of remote memory | Context | [77] | |||
| Extinction | HAT | Hippocampus | H3K9 acetylation at cFos promoter | Decreased | 1h post-EXT | Context | [92] |
| HDAC1 binding at cFos promoter | Increased | 1h post-EXT | Context | [92] | |||
| IL mPFC | CBP expression | Decreased | 2h post-EXT | Auditory | [91] | ||
| mPFC | p300 expression | Decreased | 2h post-EXT | Auditory | [91] | ||
| PCAF expression | Increased | 2h post-EXT | Auditory | [91] | |||
| HDAC2 expression | Decreased | 2h post-EXT | Auditory | [91] | |||
| H3 acetylation at Bdnf promoter 1 | Decreased | 2h post-EXT | Auditory | [88] | |||
| H3 acetylation at Bdnf promoter 4 | Increased | 2h post-EXT | Auditory | [88] | |||
| H4 acetylation at Bdnf promoter 4 | Increased | 2h post-EXT | Auditory | [88] | |||
| DNMT | IL mPFC | MECP2 expression | Increased | 2h post-EXT | Auditory | [91] | |
| H3R2Me2s | Increased | 2h post-EXT | Auditory | [93] | |||
| HKM | Hippocampus | H3K9me3 at cFos promoter | Increased | 1h post-EXT | Context | [92] | |
| IL mPFC | H3K9me2 expression | Decreased | 2h post-EXT | Auditory | [91] |
Abbreviations: ACQ: Acquisition; Ctx: Context; DNMT: DNA methyltransferase or methylation-related process; EXT: Extinction; HAT: Histone acetyltransferase; HKM: Histone lysine methylation; HPO4: histone phosphorylation; mPFC: Medial prefrontal cortex; dmPFC: dorsomedial prefrontal cortex; IL: infralimbic
In fear conditioning, the amygdala is required to form the CS-UCS association whereas the hippocampus is specifically involved in learning the contextual information [14, 43]. Accordingly, manipulating histone acetylation directly in the amygdala affects auditory fear memory [34, 44–46] whereas hippocampus-specific manipulations impair or enhance context fear [36]. For example, infusing an HDAC inhibitor (which enhances histone acetylation) directly into the amygdala enhances auditory fear memory [44] while infusing the same drug in the hippocampus enhances context fear memory without affecting auditory fear [36]. Indeed, histone acetylation increases in the amygdala [34, 44–46] and hippocampus [35, 40, 41, 47–49] following fear conditioning (Table 3). In the amygdala, HAT activity rapidly increases following fear conditioning [46] followed shortly by acetylation of histone H3 [34, 44, 45]. In the hippocampus, histone acetylation also increases following fear conditioning [47–49], presumably to encode the context-shock association. In line with this, histone H3 acetylation has been observed to increase one hour after either context-only or auditory fear conditioning [35, 40, 47–49].
Recent research has begun to characterize the roles of individual HATs and HDACs in fear memory consolidation (Box 1). Importantly, HDACs appear to block subthreshold or irrelevant learning events from forming long-term memory [50]. For example, in an object recognition memory task, a 3-minute training session is not sufficient to produce long-term memory in wildtype mice [51]. If this subthreshold training occurs in the presence of systemic HDAC inhibition, however, mice show robust long-term memory the following day [51], suggesting that HDAC inhibition allows this subthreshold learning event to produce long-term memory. One compelling idea is that individuals who are susceptible to PTSD may have a lower threshold for HDAC inhibition, so exposure to a traumatic event could trigger excessive HDAC inhibition, in turn producing a much stronger and more persistent memory for the event. This could explain how exposure to a traumatic event could produce a “normal” fear memory in one individual and an extremely robust and lasting maladaptive memory in another person who is prone to excessive HDAC inhibition.
Box 1. Specific HATs and HDACs involved in fear consolidation.
Work has begun to characterize the role of specific HATs and HDACs in fear consolidation. For HATs, cyclic AMP-responsive element (CREB)-binding protein (CBP) and E1A binding protein (p300) both play a role in fear memory consolidation [45, 91, 100–107]. Disrupting CBP or p300 genetically throughout the brain often only impairs context fear, leaving auditory fear intact [102, 103, 108] (but see [104] and [105]), suggesting that these HATs may play a specific role in hippocampus-dependent context fear. Indeed, localized knockout of CBP in the hippocampus impairs context fear consolidation [107], indicating that CBP HAT activity is critical for hippocampus-dependent context fear. Direct infusion of a p300/CBP inhibitor into the amygdala also disrupts auditory fear consolidation [45], however, suggesting that CBP and p300 are involved in amygdala-dependent fear consolidation. Global knockout of CBP may therefore trigger compensatory mechanisms in the amygdala that are not activated when p300/CBP activity is transiently impaired in the amygdala following learning.
Individual HDACs have also been characterized in fear conditioning. HDAC activity has been proposed to work as a “molecular brake pad” to prevent irrelevant or subthreshold learning events from forming long-term memories [25, 109]. Class I HDACs may be particularly important in this process, as “general” memory-enhancing HDAC inhibitors, like sodium butyrate (NaBut) and valproic acid (VPA) actually only inhibit Class I HDACs, without affecting Class IIa, IIb, or Class III HDACs [110]. Similarly, suberoylanilide hydroxamic acid (SAHA), only blocks Class I HDACs and the Class IIb HDAC6 [110]. Of these class I HDACs, which include HDACs 1, 2, 3, and 8, only HDACs 1 and 2 have been characterized in fear learning [25]. Genetic HDAC1 overexpression has no effect on fear memory [37, 92], but globally overexpressing HDAC2 impairs memory consolidation for both context and auditory fear (Table 1) [37]. HDAC2 elimination, on the other hand, enhances fear memory [37]. HDAC2 may therefore normally suppress the formation of both hippocampus- and amygdala-dependent fear memory. HDAC3, another class I HDAC, has been shown to regulate hippocampus-dependent memory formation in a similar manner [50], although whether fear memory formation requires HDAC3 specifically has not yet been tested. Finally, SIRT1, a Class III HDAC, has also been implicated in fear consolidation. Blocking SIRT1 activity throughout the brain impairs both auditory and contextual fear conditioning [111]. HDACs (both Class I and non-Class I) therefore appear to regulate fear memory consolidation by preventing subthreshold events from forming long-term memories.
Other Histone Modifications in Fear Consolidation: Phosphorylation and Methylation
Although much of the research has concentrated on histone acetylation, other epigenetic modifications have been demonstrated to be important for fear memory consolidation, as well. Most notably, histone phosphorylation and histone lysine methylation are dynamically regulated following fear learning. Phosphorylation of histone H3 at serine 10, which correlates with gene activation [26], increases following learning in the hippocampus [35, 48, 49]. H3 phosphorylation therefore appears to promote fear memory formation.
Histone lysine methylation can either activate or repress transcription, depending on the residue being modified and number of methyl groups transferred to the histone tail (see ref [5]). Two methylation marks have been studied most extensively: tri-methylation of histone H3 lysine 4 (H3K4me3), which is generally permissive to transcription, and dimethylation of histone H3 lysine 9 (H3K9me2), which represses transcription [5, 39, 52]. Fear conditioning dynamically regulates both of these marks (Table 3). The permissive mark (H3K4me3) is initially increased in the hippocampus and entorhinal cortex (a major input to the hippocampus) following context fear conditioning [39, 52]. H3K9me2 is also increased in the hippocampus and entorhinal cortex one hour after fear conditioning [39, 52], suggesting that methylation of H3 might simultaneously promote and inhibit gene expression. As these observed increases in H3K4me3 and H3K9me2 are global, rather than gene-specific, these histone methylation marks probably target different genes after learning. Indeed, gene-specific approaches, primarily chromatin immunoprecipitation (ChIP) followed by qPCR, have found that H3K4me3 and H3K9me2 are increased at different gene promoters following fear conditioning. For example, the memory-promoting genes Zif268 and Bdnf have increased H3K4me3 and decreased H3K9me2 following fear conditioning [39, 52]. On the other hand, H3K9me2 is increased at the Comt promoter after fear learning [52]. A balance between permissive and restrictive histone methylation marks might therefore be required to produce appropriate gene expression following fear learning. Importantly, blocking either methylation mark in the hippocampus [39, 52] or throughout the brain [53] before training impairs the consolidation of fear conditioning, suggesting that both H3K9me2 and H3K4me3 are required to form fear memory.
This precise balance of histone methylation fits with the idea that individual epigenetic marks recruit proteins that bind specific acetylation or methylation marks on the chromatin, creating combinatorial protein complexes for transcriptional regulation. Combinations of epigenetic marks, including histone di- and tri-methylation at distinct lysine residues, could provide a molecular signature to produce complicated downstream effects that change the fate of the cell and promote long-lasting memory. Indeed, epigenetic modifications are thought to create a signal integration platform that integrates information from our interactions with the environment and our experience with the ultimate output of gene expression [54].
Non-histone Epigenetic Modifications in Fear Consolidation: DNA Methylation and Nucleosome Remodeling
Beyond histone modifications, chromatin can also be altered through DNA methylation and nucleosome remodeling, both of which have recently been shown to play a role in fear memory consolidation. DNA methylation generally inhibits gene expression by preventing transcription factors from binding to promoter regions [28]. Surprisingly, although DNA methylation restricts transcription, blocking this process impairs, rather then enhances fear learning. Expression of DNA methyltransferases (DNMTs; the enzymes responsible for adding methyl groups to the DNA) increases in the hippocampus [31] and amygdala [44] following fear conditioning. Further, blocking DNMT activity either genetically throughout the forebrain [55] or pharmacologically in the amygdala [44, 56] or hippocampus [47] impairs consolidation. Although one might expect increased DNA methylation to correlate with poor memory formation much in the way that HDAC expression blocks memory formation, a closer look reveals that it really comes down to which genes are being regulated as one might predict. DNA methylation appears to increase at promoter regions for genes that impede memory formation, such as PP1 and decrease at genes that enhance memory formation, like reelin and Zif268 [47]. Therefore, although global DNMT expression may increase, it is the increase and decrease of methylation at specific genes that reveals how long-term memory may be achieved. Additionally, demethylation may play an equally important role in fear memory. Preventing the oxidation of 5-methylcytosine to 5-hydroxymethylcytosine by overexpressing the enzyme responsible for this conversion (Tet1) impairs the formation of context fear memory [57]. Thus, it is important to consider site-specific methylation patterns as well as the numerous methylation and demethylation mechanisms currently being discovered [58].
Recent work also suggests that nucleosome remodeling may play a role in fear memory consolidation [59]. In this epigenetic mechanism, ATP-dependent nucleosome remodeling complexes shift, insert, remove, or exchange nucleosomes along the DNA, thereby changing which genes are accessible to the transcription machinery. The only nucleosome remodeling complex known to be brain-specific is nBAF, which contains a neuron-specific subunit, BAF53b [8, 60]. Recently, Vogel-Ciernia and colleagues created genetic mutants of BAF53b to test whether this subunit of the nBAF nucleosome remodeling complex plays a role in memory consolidation [59]. Both a heterozygous Baf53b knockout and a more specific deletion of the Baf53b hydrophobic domain (creating a dominant negative mutant protein) impaired fear memory consolidation for contextual, but not auditory fear conditioning. This indicates that hippocampus-dependent memory may require nucleosome remodeling through the nBAF complex whereas amygdala-dependent memory may not require nBAF-mediated nucleosome remodeling. Interestingly, both the hippocampus-dependent object location memory task and the hippocampus-independent object recognition memory task require intact BAF53b [59], suggesting that some hippocampus-independent tasks were affected by BAF53b deletion. Although these results suggest that nBAF-dependent nucleosome remodeling in the amygdala is not necessary for successful fear memory formation, it remains to be seen whether deletion of Baf53b in the amygdala more precisely during the consolidation period would affect fear memory formation. It also remains to be determined exactly how nucleosomes are being remodeled by nBAF during regulation of gene expression during memory consolidation. For a comprehensive discussion of neuron-specific chromatin remodeling, the reader is referred to a recent review [8].
Epigenetic Mechanisms in Fear Memory Storage: DNA and Histone Methylation
After consolidation is complete, a memory must be maintained. Fear memory storage requires many of the same structures as the consolidation process, particularly the amygdala and hippocampus. Lesions of the amygdala impair fear memory at both recent and remote time points after conditioning, disrupting fear even 16 months after conditioning, nearly the entire adult lifespan of a rat [61]. Interestingly, the hippocampus is only temporarily required for fear memory storage; lesioning the hippocampus a few days after fear conditioning will disrupt contextual fear, but lesions given a month or more after learning have no effect on established context fear memory [21]. It seems that during the first month after learning, hippocampus-dependent memories (e.g. context fear) are “transferred” from the hippocampus to a more permanent storage site in the dorsomedial prefrontal cortex (dmPFC, including the anterior cingulate and prelimbic cortices) [62]. Indeed, inactivating the anterior cingulate cortex at “remote” time points 30d after acquisition impairs context fear memory, suggesting the memory has been transferred to this region for long-term storage [63]. Treating PTSD that is caused by remote memories may therefore require targeting therapeutics to cortical regions to weaken the storage of these aversive associations.
Although this work is in the early stages, some evidence does exist to suggest that epigenetic changes occur in the hippocampus and cortex to promote the storage of context fear memory (Table 3). DNA and histone methylation are both regulated following fear conditioning at time points that are well outside of the consolidation window [39, 52, 64]. Methylation may provide a relatively stable mark that could perpetually alter the state of the cell long after the initial formation of memory. DNA methylation is both self-perpetuating and capable of self-regeneration [7], making it a good candidate for maintaining long-term molecular memory in a cell. Methylation changes triggered by learning are preserved in the cell, as maintenance DNMTs recognize when a single strand of DNA is methylated and methylate the complementary strand to match [7, 28]. Thus, even when methyl marks are degraded over time as the proteins are turned over, maintenance DNMTs can replenish and maintain methylation at specific residues. This persistence makes methylation capable of maintaining changes in the state of a cell long after the environmental signal that triggered those changes has faded [7, 27].
Consistent with this, it was recently shown that changes in DNA methylation persist at memory-related genes long after the consolidation process is complete. Work by Miller et al. (2010) showed that DNA methylation levels persistently change at specific promoter regions for up to a month after training in the dorsomedial prefrontal cortex. Specifically, they observed increased methylation at the promoter for calcineurin, a gene that normally suppresses memory formation [65], beginning 1d after memory formation and lasting at least one month after acquisition [64]. Methylation decreased at the memory-promoting gene Zif268 in the dmPFC at this time point, however, suggesting that long-term changes in methylation may promote memory storage by bidirectionally regulating gene expression to enhance expression of memory-promoting genes and blocking memory-suppressing genes. Blocking this persistent methylation in the dmPFC with three successive infusions of a DNMT inhibitor also impaired the retrieval of remote memory, suggesting DNA methylation in this cortical region is critical for successful remote storage of context fear memory [64].
Fear conditioning also produces persistent changes in histone methylation that may be required for long-term memory storage. The repressive histone mark H3K9me3, which is initially increased in the hippocampus after fear conditioning, is decreased in the hippocampus and entorhinal cortex 24h after acquisition [39, 52]. Although it is unclear whether blocking this delayed decrease in histone methylation would impair the long-term storage of context fear, it does indicate that changes in histone methylation dynamically change over time following fear learning, ultimately resulting in a sustained decrease that may promote increased gene expression after consolidation is complete. Whether this decrease in H3K9me3 would persist at more remote time points in either the hippocampus or medial prefrontal cortex has not yet been tested.
Although this preliminary research is promising, much more work is needed to fully understand the role of epigenetic changes in fear memory storage. For example, it is unclear whether epigenetic marks besides methylation show lasting increases that persist beyond the consolidation window, either in the hippocampus or medial prefrontal cortex. It is tempting to speculate that changes in histone acetylation might contribute to the long-term storage of fear memory, as blocking histone deacetylation during learning is known to produce memory for spatial information that is more persistent than memory acquired under normal circumstances [50, 51]. To date, there is little evidence to suggest that changes in histone acetylation persist beyond the consolidation window in fear conditioning, however. Finally, it is unknown whether epigenetic changes in the amygdala are also required to store long-term fear memory. Methylation in the amygdala is known to increase shortly after fear conditioning [44], but it is unknown whether these methylation changes persist beyond the consolidation window.
Epigenetic Mechanisms and Updating Fear Memory
Memory is not permanently stored in a fixed state, but instead can be updated as new information is learned. Understanding how memories change in the face of new information is particularly important for treating anxiety disorders; if an aversive memory can be updated so that it no longer evokes fear, it should no longer be problematic. Although stored memories are relatively stable and resistant to disruption, the presentation of a reminder cue will trigger a period of reconsolidation, during which the memory is again susceptible to amnesic agents [22, 66, 67]. Recent work has shown that this reconsolidation process allows existing memory to incorporate new information [68–70]. It was historically assumed that recall of the memory alone is sufficient to trigger reconsolidation [66], but recent studies have shown that new information may be a key requirement for the reconsolidation process. Specifically, when the reminder or “retrieval” trial is identical to what was used in training (including identical presentation of the context and shock cues), the memory is not rendered labile [68, 70]. When new information is presented, however, the memory destabilizes [70–72], presumably allowing it to update before restabilizing. The restabilization process requires protein synthesis [68], suggesting that transcription and translation are necessary for neurons to make stable changes in plasticity to encode new information as part of the original memory in a persistent fashion. Epigenetic mechanisms that can be manipulated to enhance these mechanisms could promote successful memory updating to reduce the fearful component of aversive associations.
In fear conditioning, memory updating is usually studied using reconsolidation procedures (Figure 1B). Following training, the animal is placed in a novel context and given a retrieval trial, generally a single presentation of the auditory CS. Notably, during retrieval, the contextual cues are novel and the CS is not followed by the shock, important changes that trigger updating of the existing memory. During the period immediately after this updating session, the memory is labile for approximately six hours [66, 73]. Blocking any mechanism that impairs the restabilization process (such as protein synthesis) will prevent the memory from being properly placed back into storage and the original memory will be disrupted [66], or access to that memory will be impaired at least temporarily [74]. For example, disrupting protein synthesis in the hippocampus or amygdala following reconsolidation generally impairs memory for context and auditory fear conditioning, respectively, when tested the following day [66, 67, 75].
Although only a few studies have investigated the epigenetic mechanisms involved in fear reconsolidation (see Tables 2 and 3), the evidence to date suggests that there is a high degree of overlap between the role of epigenetic mechanisms in consolidation and reconsolidation. As in the initial consolidation of fear memory, histone H3 acetylation increases in the hippocampus during context fear reconsolidation and in the amygdala for auditory fear reconsolidation [34, 45, 76, 77], although H3 acetylation was not observed to increase following remote (30-day-old) memory retrieval [77]. Context memory retrieval also triggers the phosphorylation of histone H3 in the hippocampus [76], suggesting that multiple histone modifications occur following exposure to updated information. Blocking HAT activity systemically or directly in the amygdala following the retrieval session impairs the reconsolidation of auditory fear, so that the original fear memory is disrupted [34, 45]. Blocking HDAC activity, on the other hand, enhances reconsolidation, so that freezing to the auditory CS is enhanced [42, 78]. Histone acetylation therefore appears to play a similar role in reconsolidation and consolidation; blocking acetylation with HAT inhibitors impairs both processes and increasing acetylation with HDAC inhibitors produces an enhancement.
Table 2.
Behavioral studies manipulating epigenetic mechanisms during reconsolidation or extinction
| Memory Phase | Epigenetic Mechanism |
Structure | Manipulation | Type of FC | Effect | Citation |
|---|---|---|---|---|---|---|
| Reconsolidation | HAT | Systemic | HAT activity block (Garcinol) | Auditory | Impaired reconsolidation | [34] |
| Amygdala | HAT activity block (Garcinol) | Auditory | Impaired reconsolidation | [34] | ||
| p300/CBP HAT activity block (c646) | Auditory | Impaired reconsolidation | [45] | |||
| HDAC | Systemic | HDAC activity block (VPA) | Auditory | Enhanced reconsolidation in training ctx | [42] | |
| Amygdala | HDAC activity block (TSA) | Auditory | Enhanced reconsolidation | [78] | ||
| DNMT | Amygdala | DNMT activity block (5-AZA or RG108) | Auditory | Impaired reconsolidation | [45, 56] | |
| Anterior Cingulate | DNMT activity (5-AZA, zebularine, RG108) | Auditory | Impaired remote retrieval | [64] | ||
| Extinction | HAT | Infralimbic mPFC | PCAF activity block (H3-CoA-20-Tat) | Auditory | Impaired EXT | [91] |
| PCAF activity enhancer (SPV106) | Auditory | Enhanced EXT | [91] | |||
| P300 activity block (c646) | Auditory | Enhanced EXT | [120] | |||
| P300/CBP activity block (Lys-CoA-Tat) | Auditory | Enhanced EXT | [120] | |||
| HDAC | Systemic | HDAC activity block (NaBut) | Context-only | Enhanced EXT | [89, 90] | |
| HDAC activity block (VPA or NaBut) | Auditory | Enhanced EXT | [42, 88] | |||
| HDAC activity block (Cl-994) | Context-only | Enhanced EXT (in retrieval-EXT paradigm) | [77] | |||
| HDAC nitrosylation (L-NAME) | Context-only | Weakened EXT (in retrieval-EXT paradigm) | [77] | |||
| Hippocampus | HDAC activity block (NaBut or TSA) | Context-only | Enhanced EXT | [89, 90] | ||
| AAV-synapsin1-driven HDAC1 overexpression | Context-only | Enhanced EXT | [92] | |||
| HDAC1 block (HDAC1 siRNA) | Context-only | Impaired EXT | [92] | |||
| HDAC1 activity block (MS-275) | Context-only | Impaired EXT | [92] | |||
| Infralimbic mPFC | HDAC activity block (NaBut) | Context-only | Enhanced EXT | [89] | ||
| HDAC activity block (NaBut or TSA) | Context-only | Enhanced EXT | [89, 90] | |||
| AAV-synapsin1-driven HDAC1 overexpression | Context-only | Enhanced EXT | [92] | |||
| HDAC1 block (HDAC1 siRNA) | Context-only | Impaired EXT | [92] | |||
| HDAC1 activity block (MS-275) | Context-only | Impaired EXT | [92] |
Abbreviations: ACQ: acquisition; Ctx: context; DNMT: DNA methyltransferase EXT: extinction; HAT: Histone acetyltransferase; HDAC; Histone deacetylase; HKM: Histone lysine methylation; KO: Knockout; NRC: Nucleosome remodeling complex; Drugs shown in parentheses
Fear memory reconsolidation also requires DNA methylation in the amygdala, as pharmacologically inhibiting DNMT activity one hour after the update session impairs memory reconsolidation [56, 78]. At this point, it is unclear whether reconsolidation promotes DNA methylation at memory-suppressing genes like PP1, as occurs during consolidation, but this is a compelling possibility. While it is likely that other epigenetic mechanisms (such as histone methylation and nucleosome remodeling) are also required for successful memory reconsolidation, these mechanisms have not yet been tested and are ripe for future study.
Epigenetic Mechanisms in Fear Memory Extinction
PTSD and other anxiety disorders are commonly treated using exposure-based therapy, a form of extinction in which the individual is exposed to the frightening stimulus in the absence of an aversive outcome [79, 80]. As the person learns that the cue no longer predicts danger, his or her fear to that stimulus will gradually diminish. In rodents, extinction can be modeled by repeatedly presenting the CS in the absence of footshock (Figure 1C). Gradually, animals will learn that the CS no longer predicts an aversive outcome and will show reduced fear to that cue. Enhancing the molecular mechanisms responsible for extinction learning could therefore provide one route towards treating anxiety disorders.
Extinction is believed to primarily involve new learning instead of erasure of the original association. In other words, rather than simply causing “unlearning” of the relationship between the auditory cue and the shock, extinction learning creates a new memory (in which the tone no longer predicts shock) that competes with the original association. After extinction, the initial memory remains largely intact but inhibited. Evidence that the original fear memory persists comes from numerous studies that have observed renewed fear when the animal is re-exposed to the shock [81], presented with the tone in a new context [82], or tested after a rest period [83]. This leads to major issues when it comes to treating anxiety disorders; even after the aversive memory is fully extinguished in a clinical setting, fear responding often returns as the original memory persists and is revealed with the passage of time and exposure to unpredictable contexts and stimuli. Developing methods to enhance the strength and persistence of extinction so that it can out-compete the original association is critical to effectively treating fear-based disorders.
Extinction learning recruits much of the same neural circuitry as the initial consolidation of fear memory. The amygdala and hippocampus are both involved in extinction, as is the medial prefrontal cortex [84]. Unlike fear memory consolidation, which involves the dorsal portion of the prefrontal cortex, fear extinction recruits the ventral segment of the medial prefrontal cortex, called the infralimbic cortex (IL). The IL, which is not involved in the initial acquisition of fear memory, undergoes plasticity during extinction that is believed to inhibit the fear output generated by the amygdala [85]. Consistent with this, neurons in the IL project to a group of inhibitory interneurons in the intercalated cell layer of the amygdala that effectively shut off amygdala output to downstream brain regions to reduce the fear response [86]. Inactivating the IL prevents extinction memory formation [87], indicating that this region is crucially important for extinction. Targeting epigenetic mechanisms in the IL to improve the strength and persistence of extinction memories could therefore have major implications for the treatment of anxiety disorders.
Histone acetylation, histone methylation, and DNA methylation have all been implicated in the formation of extinction memory (Tables 2 and 3). Most of this work has focused on histone acetylation, which appears to promote extinction learning. Systemically blocking HDAC activity, for example, enhances extinction memory for both auditory [42, 88] and context [89, 90] fear. Inhibiting HDAC activity specifically in the hippocampus or infralimbic cortex similarly enhances extinction memory [89, 90] and HDAC2 expression decreases in the IL following extinction learning [91]. Although broad inhibition of HDAC activity in the hippocampus enhances extinction, specifically blocking HDAC1 impairs extinction [92]. This suggests that HDAC1 might play a unique role in facilitating extinction. With the exception of HDAC1, therefore, HDACs appear to negatively regulate extinction learning in much the same manner as they regulate fear memory consolidation. HAT activity, on the other hand, appears to promote extinction learning. Expression of the HAT PCAF is increased in the IL following extinction and blocking PCAF activity impairs extinction [91]. Further, histone acetylation is enriched at BDNF promoters in the IL following extinction [88], indicating that epigenetic mechanisms may promote the expression of plasticity-related genes following extinction. Demethylation may also be key to promoting successful extinction, as blocking the enzymes that promote oxidation of 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC), Tet1 and Tet3, impair fear extinction [93, 94]. Additionally, the accumulation of 5-hmC may promote a “primed” epigenetic state. Blocking the conversion of 5-mC to 5-hmC disrupts the symmetric dimethylation of H3 arginine 2 (H3R2Me2s) at the gephyrin locus after extinction [93]. As H3R2Me2s is known to play a key role in maintaining euchromatin [95], this mark may establish a “primed” epigenetic state after extinction to promote rapid future gene expression, although this is currently speculative.
Possibly the most important advance that epigenetics could make to the treatment of PTSD and anxiety disorders is to provide a novel target that could enhance the persistence of extinction memory [10]. As extinction learning is often not permanent, as described above, exposure-based therapies are limited in their long-term effectiveness, as the original fear memory often reappears. HDAC inhibitors are an ideal mechanism for promoting robust, persistent extinction memory that could out-compete the original fear association [10]. Indeed, when HDAC inhibitors are given systemically before or after extinction learning, extinction memory is enhanced [42, 89, 90, 96]. Whether extinction memories formed in the absence of normal HDAC activity are also resistant to the return of fear is currently unknown. In the field of addiction, however, it has already been shown that blocking general HDAC activity [97] or HDAC3 specifically [98] immediately after extinction produces extinction learning that is persistent and resists reinstatement. Although this has not yet been demonstrated for fear extinction, HDAC inhibitors provide an appealing therapeutic target for producing successful and enduring extinction for individuals with PTSD and other anxiety disorders. Using HDAC inhibitors in conjunction with behavioral therapy may promote persistent extinction (see Box 2).
Box 2. Epigenetic mechanisms in the reconsolidation-extinction paradigm.
Another method to promote the permanence of extinction learning is the reconsolidation-extinction paradigm, in which extinction conducted during the reconsolidation process is long-lasting and resistant to fear renewal. A single presentation of the threatening stimulus will trigger the reconsolidation process, as described above, which makes the original memory labile so that it can be updated. If extinction trials are conducted during this period of lability, the resultant extinction memory is more permanent in both rodents [73] and humans [112] than normal extinction memory. Importantly, this reconsolidation-extinction method does not persistently attenuate memory under all circumstances [113], so treatments that enhance this process could be very valuable. It was recently demonstrated that remote memory extinction (which does not permanently extinguish with the reconsolidation-extinction paradigm) was persistently attenuated when an HDAC inhibitor was given shortly after the retrieval trial [77]. This suggests that HDAC inhibition is one potential mechanism that could promote long-lasting extinction for memories that otherwise recover following extinction. Whether other epigenetic mechanisms, such as histone methylation, DNA methylation, or nucleosome remodeling can similarly be targeted to produce enduring extinction is currently unclear.
Conclusions
Epigenetic mechanisms are therefore involved in every phase of fear memory, from the initial consolidation to extinction. These mechanisms, which produce relatively stable changes in cell function, may prove to be an ideal target for treating PTSD and other anxiety disorders, as they can be manipulated to diminish the strength of fear memory formation or make existing fear memory less aversive. HDAC inhibitors, for example, can enhance extinction learning [42, 89, 90, 96] and reconsolidation of fear memory [42, 78]. Updating or extinguishing fear memory in the presence of pharmacological HDAC inhibitors may therefore provide one route to reducing the aversive component of fear memory so that it is no longer maladaptive. Other epigenetic mechanisms, like histone or DNA methylation and nucleosome remodeling also play a role in the formation, updating, and extinction of fear memory, but less is known about the specific roles of these mechanisms. Future studies should focus on understanding how these mechanisms work in concert to promote memory formation and updating (Box 3). Appreciating the intricacies of the epigenetic system supporting memory formation will be critically important to developing precise, targeted treatments to prevent or reduce PTSD and other anxiety disorders.
Box 3. Outstanding questions.
What specific roles do individual epigenetic mechanisms play in each phase of fear memory? For example, why does HDAC1 overexpression facilitate fear extinction without affecting acquisition [92]? Further work should identify the functional significance of these epigenetic mechanisms that uniquely contribute to a given memory phase.
Do non-coding RNAs, like microRNAs, coordinate epigenetic processes? Recent evidence suggests that non-coding RNAs may control nucleosome positioning and alternative splicing (for review, see [114]), indicating they may influence downstream epigenetic processes. How these non-coding RNAs function during learning, especially in the context of nucleosome remodeling, is largely unclear.
How do these epigenetic mechanisms integrate to provide a coordinated pattern of gene expression following fear learning? No individual mechanism works in isolation, yet we have a very limited understanding of how these epigenetic processes interact.
Is there an epigenetic signature that characterizes a person as particularly susceptible/resistant to developing PTSD? For example, individuals with methylation at a single nucleotide polymorphism in the gene encoding the dopamine transporter (SLC6A3) show an increased PTSD risk [115]. On the other hand, hypermethylation of a serotonin transporter gene (SLCA4) appears to protect individuals from developing PTSD after repeated trauma exposure [116]. Could this epigenetic signature also be used to identify individuals who would benefit from treatments that manipulate epigenetic reactivity?
Which individual genes are regulated by each epigenetic mark? For example, what genes are normally blocked by HDAC3 in the absence of a sufficient learning event? Next-generation sequencing techniques, particularly RNA-seq and ChIP-seq, will be critical to providing information on the broad range of genes regulated by each epigenetic tag.
Is nucleosome remodeling involved in fear memory formation, reconsolidation, and extinction? Future studies should test whether disrupting nucleosome remodeling specifically in the hippocampus or amygdala affects fear memory formation. Additionally, it would be worthwhile to test whether nucleosome remodeling plays a role in memory updating or extinction.
How can epigenetic mechanisms be leveraged in humans to produce persistent extinction or to update memory so that it is less aversive? Recent work suggests that using HDAC inhibitors in conjunction with the retrieval-extinction paradigm may promote permanent extinction memory [77]. It remains to be seen whether this combination of behavioral therapy and HDAC inhibition will also work in a clinical setting to treat humans with PTSD.
Are the same epigenetic markers observed in other rodent models of PTSD, like the stress enhanced fear learning (SEFL) paradigm [117] or the predator-exposure model [118]? Extending epigenetics research to other fear paradigms will identify new targets for therapeutics and determine which mechanisms are consistent across PTSD models.
Going forward, it will be critically important to translate epigenetic mechanisms identified through rodent research to the human brain in order to develop effective treatments for PTSD. For example, comparing postmortem human brain tissue from individuals with PTSD to control tissue could provide valuable information about disease-related epigenetic marks. This data could also be used to determine whether epigenetic mechanisms are consistent across rodents and humans in analogous brain regions. Additionally, it will be important to identify peripheral epigenetic markers that can characterize individuals as particularly susceptible or resistant to developing PTSD. This information could potentially be used to prevent and treat PTSD in the most efficient way possible for susceptible individuals (Box 3).
Highlights.
We review the role of epigenetics in fear consolidation, updating, and extinction
For each memory phase, we document which epigenetic mechanisms are involved
We discuss the implications for treating PTSD and anxiety disorders
Acknowledgements
This work was supported by NIMH and NIDA grants (MH81004, MH101491, DA025922, DA036984, DA031989) to M.A. Wood and NIA T32 grant AG000096-31 to J.L.K.
Glossary
- Consolidation
the process of converting temporary short-term memory (lasting only a few hours) into persistent long-term memory that lasts at least 24h.
- DNMT
DNA methyltransferase, an enzyme that catalyzes binding of a methyl group onto the DNA.
- Epigenetics
changes in gene expression that occur through alterations in chromatin structure, rather than changes in DNA sequence.
- Extinction
the phenomenon in which responding to the conditional stimulus is reduced following repeated exposure to the cue in the absence of the unconditional stimulus. Extinction is widely believed to be learning of a CS-no UCS relationship rather than an erasure of the original CS-UCS memory.
- HAT
Histone acetyltransferase, an enzyme responsible for adding an acetyl group to histone tails.
- HDAC
Histone deacetylase, an enzyme responsible for removing acetyl groups from histone tails.
- Histone
Core of the nucleosome, consisting of 4 pairs of histone proteins. Each histone protein has an amino-terminal tail that can be modified (acetylated, phosphorylated, methylated, etc.) to restrict or promote access to the DNA wound around it.
- Memory Storage
the process of actively maintaining existing memory to prevent erosion over time as proteins and epigenetic markers are degraded.
- Nucleosome
Basic unit of chromatin, in which a histone octamer is wrapped by approximately 147 basepairs of DNA.
- Pavlovian fear conditioning
a learning paradigm in which an initially neutral conditional stimulus (CS) is paired with a naturally aversive unconditional stimulus (UCS). Most often, a tone or context CS is paired with a footshock UCS. Following training, the CS alone should evoke a fear response, indicating successful learning.
- Reconsolidation
the phenomenon in which existing memory becomes temporarily labile following a reminder cue. Recent work suggests reconsolidation makes existing memory malleable so that new information can be incorporated into the memory trace.
- Updating
the process of modifying existing memory to incorporate new, relevant information into the memory trace. Successful updating requires memory to undergo reconsolidation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg PE, et al. The economic burden of anxiety disorders in the 1990s. J. Clin. Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- 4.Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarome TJ, Lubin FD. Histone lysine methylation: critical regulator of memory and behavior. Rev. Neurosci. 2013;24:375–387. doi: 10.1515/revneuro-2013-0008. [DOI] [PubMed] [Google Scholar]
- 6.Barrett RM, Wood MA. Beyond transcription factors: the role of chromatin modifying enzymes in regulating transcription required for memory. Learn. Mem. 2008;15:460–467. doi: 10.1101/lm.917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zovkic IB, et al. Epigenetic regulation of memory formation and maintenance. Learn. Mem. 2013;20:61–74. doi: 10.1101/lm.026575.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel-Ciernia A, Wood MA. Neuron-specific chromatin remodeling: A missing link in epigenetic mechanisms underlying synaptic plasticity, memory, and intellectual disability disorders. Neuropharmacology. 2014;80C:18–27. doi: 10.1016/j.neuropharm.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White AO, Wood MA. Does stress remove the HDAC brakes for the formation and persistence of long-term memory? Neurobiol. Learn. Mem. 2013 doi: 10.1016/j.nlm.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lattal KM, Wood MA. Epigenetics and persistent memory: implications for reconsolidation and silent extinction beyond the zero. Nat. Neurosci. 2013;16:124–129. doi: 10.1038/nn.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allis CD, et al. Epigenetics. Cold Spring Harbor Laboratory Press; 2007. [Google Scholar]
- 12.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Zovkic IB, Sweatt JD. Epigenetic mechanisms in learned fear: implications for PTSD. Neuropsychopharmacology. 2013;38:77–93. doi: 10.1038/npp.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maren S. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 15.LeDoux J. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000 doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 16.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 17.Ehrlich I, et al. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 18.Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn. Mem. 2009;16:573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn. Mem. 2010;17:289–296. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilmartin MR, et al. Prefrontal activity links nonoverlapping events in memory. J. Neurosci. 2013;33:10910–10914. doi: 10.1523/JNEUROSCI.0144-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 22.Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J. Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansen JP, et al. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarome TJ, Helmstetter FJ. The ubiquitin-proteasome system as a critical regulator of synaptic plasticity and long-term memory formation. Neurobiol. Learn. Mem. 2013;105:107–116. doi: 10.1016/j.nlm.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogel-Ciernia A, Wood MA. Molecular brake pad hypothesis: pulling off the brakes for emotional memory. Rev. Neurosci. 2012;23:607–626. doi: 10.1515/revneuro-2012-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant PA. A tale of histone modifications. Genome biology. 2001;2 doi: 10.1186/gb-2001-2-4-reviews0003. REVIEWS0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miranda TB, Jones PA. DNA methylation: the nuts and bolts of repression. J. Cell. Physiol. 2007;213:384–390. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- 29.Weber M, et al. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat. Genet. 2007;39:457–466. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- 30.Chahrour M, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Hargreaves DC, Crabtree GR. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 2011;21:396–420. doi: 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maddox SA, et al. A naturally-occurring histone acetyltransferase inhibitor derived from Garcinia indica impairs newly acquired and reactivated fear memories. PloS one. 2013;8:e54463. doi: 10.1371/journal.pone.0054463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chwang WB, et al. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J. Neurosci. 2007;27:12732–12742. doi: 10.1523/JNEUROSCI.2522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vecsey CG, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J. Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guan JS, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer A, et al. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, et al. Histone methylation regulates memory formation. J. Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levenson JM, et al. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 41.Mahan AL, et al. Epigenetic modulation of Homer 1a transcription regulation in amygdala and hippocampus with pavlovian fear conditioning. J. Neurosci. 2012;32:4651–4659. doi: 10.1523/JNEUROSCI.3308-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn. Mem. 2008;15:39–45. doi: 10.1101/lm.801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 44.Monsey MS, et al. Epigenetic alterations are critical for fear memory consolidation and synaptic plasticity in the lateral amygdala. PloS one. 2011;6:e19958. doi: 10.1371/journal.pone.0019958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maddox SA, et al. p300/CBP histone acetyltransferase activity is required for newly acquired and reactivated fear memories in the lateral amygdala. Learn. Mem. 2013;20:109–119. doi: 10.1101/lm.029157.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeh S, et al. Acetylation of nuclear factor-κB in rat amygdala improves long-term but not short-term retention of fear memory. Mol. Pharmacol. 2004 doi: 10.1124/mol.65.5.1286. [DOI] [PubMed] [Google Scholar]
- 47.Miller CA, et al. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol. Learn. Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chwang WB, et al. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn. Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lubin FD, et al. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J. Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McQuown SC, et al. HDAC3 is a critical negative regulator of long-term memory formation. J. Neurosci. 2011;31:764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stefanko DP, et al. Modulation of long-term memory for object recognition via HDAC inhibition. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta-Agarwal S, et al. G9a/GLP histone lysine dimethyltransferase complex activity in the hippocampus and the entorhinal cortex is required for gene activation and silencing during memory consolidation. J. Neurosci. 2012;32:5440–5453. doi: 10.1523/JNEUROSCI.0147-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kerimoglu C, et al. Histone-methyltransferase MLL2 (KMT2B) is required for memory formation in mice. J. Neurosci. 2013;33:3452–3464. doi: 10.1523/JNEUROSCI.3356-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Badeaux AI, Shi Y. Emerging roles for chromatin as a signal integration and storage platform. Nature reviews. Molecular cell biology. 2013;14:211–224. doi: 10.1038/nrm3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng J, et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat. Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maddox SA, et al. DNA methyltransferase activity is required for memory-related neural plasticity in the lateral amygdala. Neurobiol. Learn. Mem. 2014;107:93–100. doi: 10.1016/j.nlm.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaas GA, et al. TET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation. Neuron. 2013;79:1086–1093. doi: 10.1016/j.neuron.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker-Andresen D, et al. Dynamic DNA methylation: a prime candidate for genomic metaplasticity and behavioral adaptation. Trends Neurosci. 2013;36:3–13. doi: 10.1016/j.tins.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Vogel-Ciernia A, et al. The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nat. Neurosci. 2013;16:552–561. doi: 10.1038/nn.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olave I, et al. Identification of a polymorphic, neuron-specific chromatin remodeling complex. Genes Dev. 2002;16:2509–2517. doi: 10.1101/gad.992102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gale GD, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J. Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frankland PW, Bontempi B. The organization of recent and remote memories. Nature reviews. Neuroscience. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 63.Frankland PW, et al. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 64.Miller CA, et al. Cortical DNA methylation maintains remote memory. Nat. Neurosci. 2010;13:664–666. doi: 10.1038/nn.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baumgartel K, et al. Control of the establishment of aversive memory by calcineurin and Zif268. Nat. Neurosci. 2008;11:572–578. doi: 10.1038/nn.2113. [DOI] [PubMed] [Google Scholar]
- 66.Nader K, et al. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 67.Parsons RG, et al. Long-term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. Eur. J. Neurosci. 2006;23:1853–1859. doi: 10.1111/j.1460-9568.2006.04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diaz-Mataix L, et al. Detection of a temporal error triggers reconsolidation of amygdala-dependent memories. Curr. Biol. 2013;23:467–472. doi: 10.1016/j.cub.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JL. Memory reconsolidation mediates the updating of hippocampal memory content. Frontiers in behavioral neuroscience. 2010;4:168. doi: 10.3389/fnbeh.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sevenster D, et al. Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Neurobiol. Learn. Mem. 2012;97:338–345. doi: 10.1016/j.nlm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 71.Lee S-H, et al. Science. Vol. 319. New York, N.Y.: 2008. Synaptic protein degradation underlies destabilization of retrieved fear memory; pp. 1253–1256. [DOI] [PubMed] [Google Scholar]
- 72.Lee JL. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat. Neurosci. 2008;11:1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- 73.Monfils MH, et al. Extinction-reconsolidation boundaries: Key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lattal KM, Abel T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4667–4672. doi: 10.1073/pnas.0306546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frankland PW, et al. Stability of recent and remote contextual fear memory. Learn. Mem. 2006;13:451–457. doi: 10.1101/lm.183406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lubin FD, Sweatt JD. The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron. 2007;55:942–957. doi: 10.1016/j.neuron.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graff J, et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell. 2014;156:261–276. doi: 10.1016/j.cell.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maddox SA, Schafe GE. Epigenetic alterations in the lateral amygdala are required for reconsolidation of a Pavlovian fear memory. Learn. Mem. 2011;18:579–593. doi: 10.1101/lm.2243411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barad M. Fear extinction in rodents: basic insight to clinical promise. Curr. Opin. Neurobiol. 2005;15:710–715. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 80.Parsons RG, Ressler KJ. Implications of memory modulation for post-traumatic stress and fear disorders. Nat. Neurosci. 2013;16:146–153. doi: 10.1038/nn.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J. Exp. Psychol. Anim. Behav. Process. 1975;1:88–96. [PubMed] [Google Scholar]
- 82.Bouton ME, King DA. Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J. Exp. Psychol. Anim. Behav. Process. 1983;9:248–265. [PubMed] [Google Scholar]
- 83.Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn. Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pare D, et al. New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 87.Sierra-Mercado D, et al. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bredy TW, et al. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn. Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stafford JM, et al. Increasing histone acetylation in the hippocampus-infralimbic network enhances fear extinction. Biol. Psychiatry. 2012;72:25–33. doi: 10.1016/j.biopsych.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lattal KM, et al. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav. Neurosci. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei W, et al. p300/CBP-associated factor selectively regulates the extinction of conditioned fear. J. Neurosci. 2012;32:11930–11941. doi: 10.1523/JNEUROSCI.0178-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bahari-Javan S, et al. HDAC1 regulates fear extinction in mice. J. Neurosci. 2012;32:5062–5073. doi: 10.1523/JNEUROSCI.0079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li X, et al. Neocortical Tet3-mediated accumulation of 5-hydroxymethylcytosine promotes rapid behavioral adaptation. Proc. Natl. Acad. Sci. U. S. A. 2014;111:7120–7125. doi: 10.1073/pnas.1318906111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rudenko A, et al. Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron. 2013;79:1109–1122. doi: 10.1016/j.neuron.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Migliori V, et al. Symmetric dimethylation of H3R2 is a newly identified histone mark that supports euchromatin maintenance. Nature structural & molecular biology. 2012;19:136–144. doi: 10.1038/nsmb.2209. [DOI] [PubMed] [Google Scholar]
- 96.Burgos-Robles A, et al. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 97.Malvaez M, et al. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol. Psychiatry. 2010;67:36–43. doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malvaez M, et al. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc. Natl. Acad. Sci. U. S. A. 2013;110:2647–2652. doi: 10.1073/pnas.1213364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allen Institute for Brain Science [Internet] Allen Mouse Brain Atlas. 2014 http://mouse.brain-map.org/
- 100.Korzus E, et al. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 102.Wood MA, et al. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn. Mem. 2005;12:111–119. doi: 10.1101/lm.86605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oliveira AM, et al. Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learn. Mem. 2007;14:564–572. doi: 10.1101/lm.656907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alarcon JM, et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 105.Oike Y, et al. Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum. Mol. Genet. 1999;8:387–396. doi: 10.1093/hmg/8.3.387. [DOI] [PubMed] [Google Scholar]
- 106.Wood M. Introduction to the special issue of Neurobiology of Learning and Memory on epigenetics and memory. Neurobiol. Learn. Mem. 2011;96:1. doi: 10.1016/j.nlm.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 107.Barrett RM, et al. Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacology. 2011;36:1545–1556. doi: 10.1038/npp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wood MA, et al. A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn. Mem. 2006;13:609–617. doi: 10.1101/lm.213906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McQuown SC, Wood MA. HDAC3 and the molecular brake pad hypothesis. Neurobiol. Learn. Mem. 2011;96:27–34. doi: 10.1016/j.nlm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kilgore M, et al. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2010;35:870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gao J, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Auber A, et al. Post-retrieval extinction as reconsolidation interference: methodological issues or boundary conditions? Psychopharmacology (Berl) 2013;226:631–647. doi: 10.1007/s00213-013-3004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bredy TW, et al. MicroRNA regulation of neural plasticity and memory. Neurobiol. Learn. Mem. 2011;96:89–94. doi: 10.1016/j.nlm.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 115.Chang SC, et al. Molecular variation at the SLC6A3 locus predicts lifetime risk of PTSD in the Detroit Neighborhood Health Study. PloS one. 2012;7:e39184. doi: 10.1371/journal.pone.0039184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koenen KC, et al. SLC6A4 methylation modifies the effect of the number of traumatic events on risk for posttraumatic stress disorder. Depress. Anxiety. 2011;28:639–647. doi: 10.1002/da.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rau V, et al. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 118.Zoladz PR, et al. Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress. 2008;11:259–281. doi: 10.1080/10253890701768613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen G, et al. CREB binding protein is required for both short-term and long-term memory formation. J. Neurosci. 2010;30:13066–13077. doi: 10.1523/JNEUROSCI.2378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marek R, et al. Paradoxical enhancement of fear extinction memory and synaptic plasticity by inhibition of the histone acetyltransferase p300. J. Neurosci. 2011;31:7486–7491. doi: 10.1523/JNEUROSCI.0133-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]