Abstract
Localized upregulation of Type I IFN was previously implicated in development of Borrelia burgdorferi induced arthritis in C3H mice, and was remarkable due to its absence in the mildly arthritic C57BL/6 (B6) mice. Independently, forward genetics analysis identified a quantitative trait locus (QTL) on Chr4, termed Bbaa1 that regulates Lyme arthritis severity and includes the 15 Type I IFN genes. Involvement of Bbaa1 in arthritis development was confirmed in B6 mice congenic for the C3H allele of Bbaa1 (B6.C3-Bbaa1), which developed more severe Lyme arthritis and K/B×N model of rheumatoid arthritis (RA) than did parental B6 mice. Administration of a Type I IFN receptor blocking mAb reduced the severity of both Lyme arthritis and RA in B6.C3-Bbaa1 mice, formally linking genetic elements within Bbaa1 to pathological production of Type I IFN. Bone marrow derived macrophages (BMDM) from Bbaa1 congenic mice implicated this locus as a regulator of Type I IFN induction and downstream target gene expression. Bbaa1 mediated regulation of IFN inducible genes was upstream of IFN receptor dependent amplification, however, the overall magnitude of the response was dependent on autocrine/paracrine responses to IFNβ. Additionally, the Bbaa1 locus modulated the functional phenotype ascribed to BMDM: the B6 allele promoted expression of M2 markers while the C3H allele promoted induction of M1 responses. This report identifies a genetic locus physically and functionally linked to Type I IFN that contributes to the pathogenesis of both Lyme and rheumatoid arthritis.
INTRODUCTION
Lyme disease is caused by infection with the tick borne spirochete, Borrelia burgdorferi, and is associated with a spectrum of disease symptoms and severity (1, 2). A localized skin lesion at the site of the tick bite is found in 70% of infected individuals, and can progress to disseminated infection resulting in skin lesions, peripheral neuropathies, inflammation of the central nervous system, carditis, and accompanying fatigue (3). Arthritis occurs in 30–60% of patients, and is often identified by effusive swelling and tendonitis in the knee (4). The variability in clinical symptoms reflects a number of factors including invasive potential of the infecting genotype of B. burgdorferi and inherent variances in the host response to infection (5, 6). Patient studies have identified multiple components of the innate and adaptive immune response that contribute to host defense, disease resolution, and arthritis pathogenesis (7–14). Much of the analysis of the varied spectrum of disease in patients has focused on a comparison between those whose disease resolves readily following antibiotic treatment and those whose symptoms persist for months following treatment, and suggests that failure to suppress the inflammatory response and/or initiation of an autoimmune response are important in chronic disease (15, 16).
Barthold et al made the seminal observation that different inbred strains of mice consistently display a spectrum of disease severity following infection with B. burgdorferi, thus demonstrating host factors inherent to different strains of mice are major determinants of Lyme arthritis severity (6). Two mouse strains, C57BL/6 (B6) and C3H, display extremes of arthritis severity and have been used extensively as experimental models for study of disease. These studies have revealed involvement of numerous components of the inflammatory response to B. burgdorferi in the development and resolution of Lyme arthritis, similar to those implicated in human disease (17). Previously, using global gene expression analysis, we identified an IFN signature response in the joint tissue of B. burgdorferi-infected C3H mice that preceded the development of severe arthritis and was absent from mildly arthritic B6 mice (18). Blocking the Type I IFN receptor (INFAR1), either with a neutralizing monoclonal antibody (mAb) or by gene ablation, reduced the severity of arthritis in C3H mice, formally linking Type I IFN to Lyme arthritis (19, 20). Numerous investigators have studied the Type I IFN response to B. burgdorferi in murine and human myeloid cells, and identified pathogen receptor signaling pathways involved in this response (21–25). Pathological production of Type I IFN has also been implicated in a number of inflammatory conditions, suggesting a predisposition to IFN production could be a common contributor to inflammatory disease (26–29).
We have also employed forward genetic analysis to identify quantitative trait loci (QTL) controlling the difference in Lyme arthritis severity in B6 and C3H mice. This approach led to the identification of 23 B. burgdorferi associated loci (Bbaa) regulating responses to B. burgdorferi infection, and included six Bbaa regulating arthritis severity (30–32). We have recently validated the utility of this rigorous and unbiased approach with the positional cloning of beta-glucuronidase, Gusb, within Bbaa2 on Chr5, as a major determinant of Lyme arthritis severity in mice (33). Gusb also regulates the severity of rheumatoid arthritis (RA) in mice. In the current study we have extended our analysis of Bbaa1 on Chr4 and have identified the Type I IFN gene cluster (IFNβ and 14 IFNα genes, 88.5–88.7 Mbp) as positional candidates for Bbaa1 (34). The development of interval specific congenic lines (ISCL) on the B6 and C3H backgrounds allowed direct assessment of the contribution of Bbaa1 to Lyme arthritis and RA, and the dependence on production of Type I IFN. The studies reported here identify Bbaa1 as a regulator of Type I IFN during both Lyme arthritis and RA development, thus indicating a second example of QTL analysis of Lyme arthritis providing novel insight to other models of inflammatory arthritis. These findings suggest Type I IFN as a tractable target for therapeutic intervention in Lyme and rheumatoid arthritis, and other syndromes associated with IFN dysregulation.
MATERIALS AND METHODS
Mice
C3H/HeNCrl mice were obtained from Charles River Breeding laboratories. C57BL/6NCr (B6) mice from the National Cancer Institute (NCI) were maintained as a colony in our Animal Research Center. Reciprocal interval specific congenic lines (ISCL) for Bbaa1 on Chr4 were generated on both B6 and C3H backgrounds as described (32) and are indicated as B6.C3-Bbaa1 (9.32–94.97 Mbp) and C3.B6-Bbaa1 (3.58–150.8 Mbp) with introgressed region of Chr4 indicated in parentheses. B6-IFNAR1−/− mice were provided by Dr. Murali-Krisna Kaja University of Washington, Seattle, WA, and backcrossed to C3H to generate C3H-IFNAR1−/− mice as described (19). All mice were housed in the University of Utah Animal Research Center (Salt Lake City, UT) and followed protocols approved by the institutional review committee for the care and use of mice in biomedical research.
Bacterial cultures and infections, and assessment of arthritis severity
A low passage clonal derivative of Borrelia burgdorferi strain N40 was stored at −80°C and cultured 4–5 days in BSK prior to infection experiments. Mice were infected with 2×104 spirochetes by intradermal injection into the skin of the back (35). Ankle measurements were obtained using a metric caliper before and at 4 weeks of infection. Rear ankle joints were prepared for assessment of histopathology by removal of skin and fixation of the tissue in 10% neutral buffered formalin as described (19). Decalcified joints were embedded in paraffin, sectioned at 3μm, and stained with hematoxylin and eosin. Coded slides were scored from 0 to 5 for various aspects of disease, including severity and extent of the lesion, PMN leukocyte and mononuclear cell (e.g., monocyte, macrophage) infiltration, tendon sheath thickening (e.g., synoviocyte and fibroblast hyperplasia), and reactive/reparative responses (e.g., periosteal hyperplasia and new bone formation and remodeling), with 5 representing the most severe lesion, and 0 representing no lesion. Infection was confirmed in mice euthanized prior to 14 days post infection by culturing bladder tissue in BSK II media containing 6% rabbit serum, phosphomycin, and rifampicin. ELISA quantification of B. burgdorferi-specific IgM and IgG concentrations were used to confirm infection in mice euthanized at and after 14 days post infection as described (36).
In vivo blocking of Type I IFN receptor
The IFN receptor (IFNAR1) blocking mAb MAR1-5A3 or isotype control (Bio X Cell) was administered by a single intraperitoneal injection of 2.5 mg mAb the day before infection with B. burgdorferi or the administration of K/B×N serum, Figs 2, 9 (20, 37).
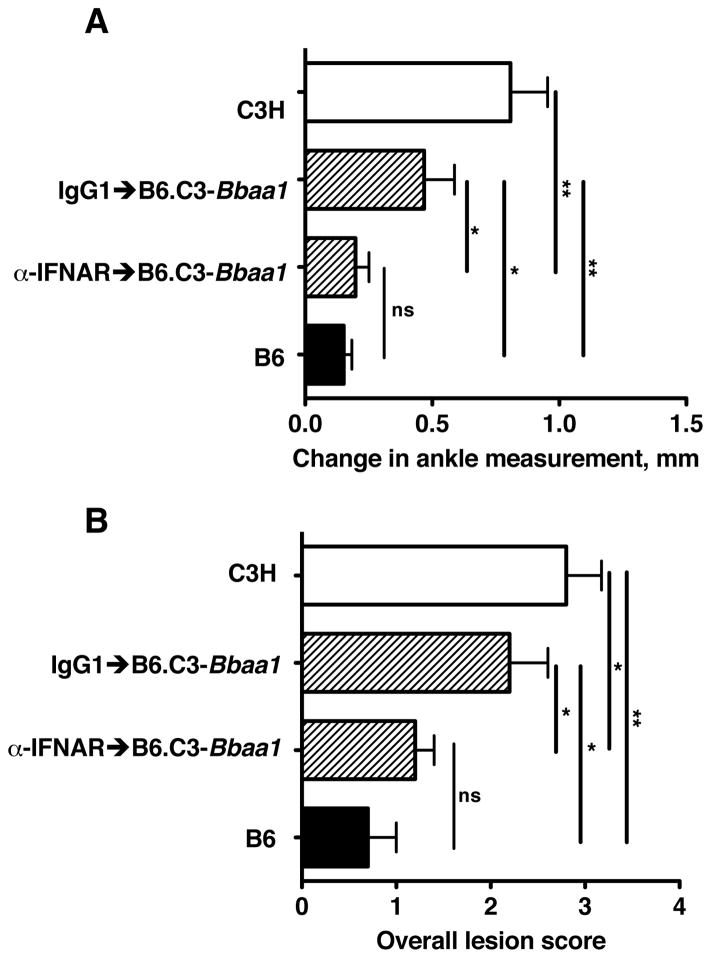
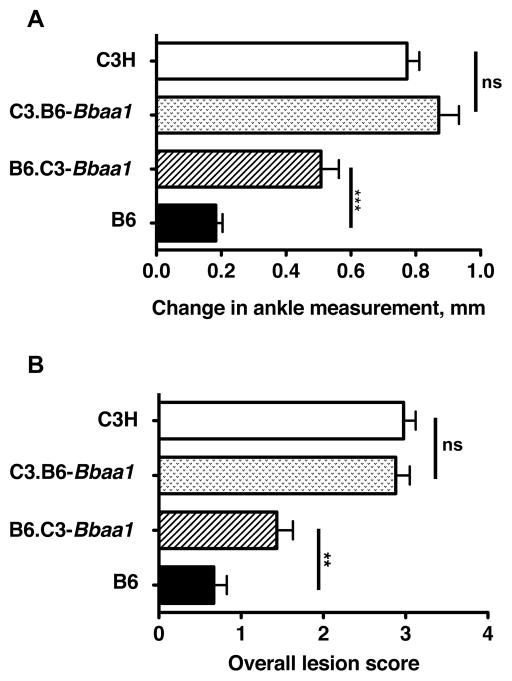
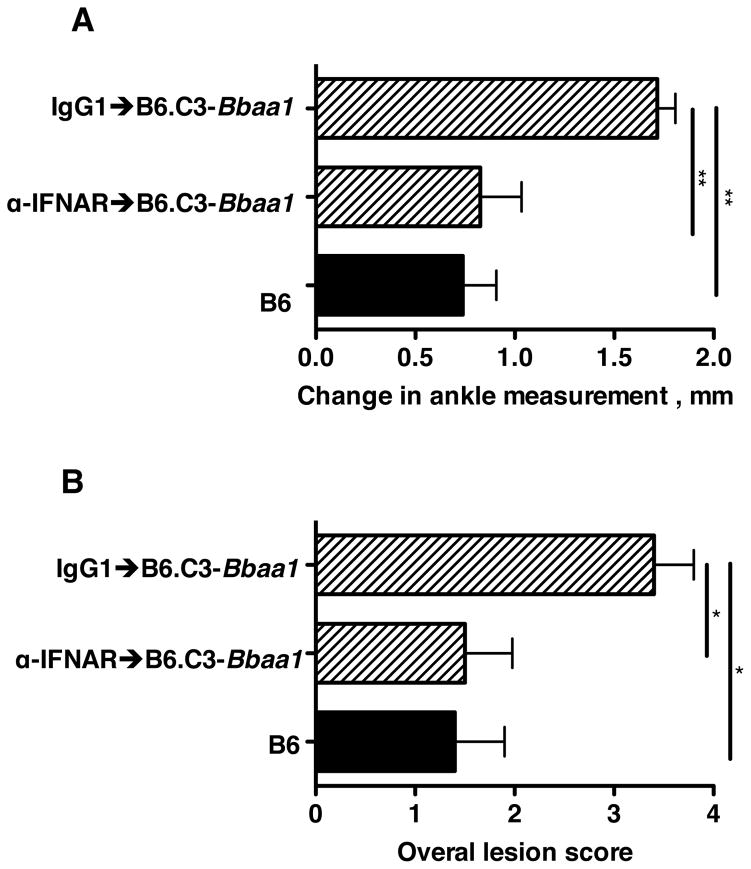
Figure 2. mAb blocking of Type I IFN signaling prevents the Bbaa1 dependent increase in arthritis in B. burgdorferi infected mice.
B6.C3-Bbaa1 mice were treated with 2.5 mg MAR1-5A3 blocking mAb or isotype control the day before infection. Congenic and parental B6 and C3H mice were infected with B. burgdorferi and arthritis severity analyzed at 4 wks. of infection, shown for (A) Change in ankle measurement and (B) Overall lesion score. Statistical significance was determined by Student’s t-test for ankle swelling while the Mann Whitney U test was used for Overall lesion. n=5 per group.
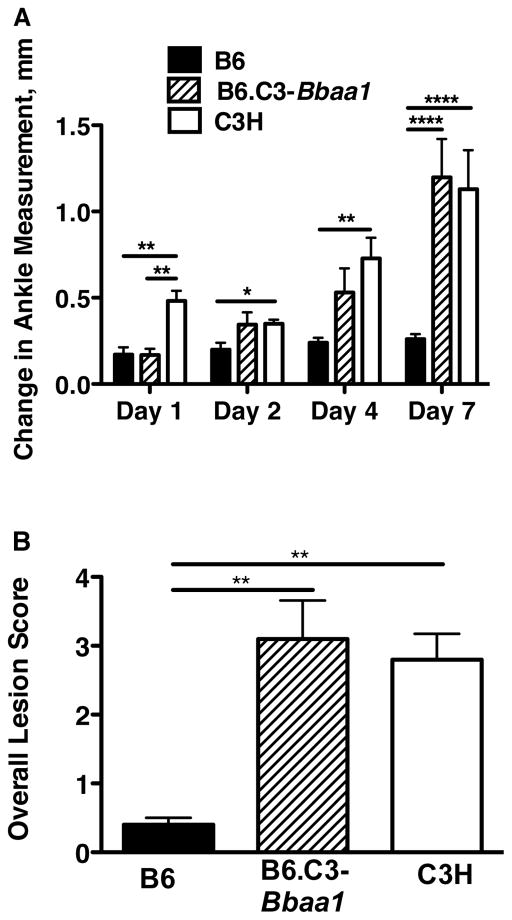
Figure 9. Impact of Bbaa1 on severity of K/B×N serum transfer arthritis.
B6, B6.C3-Bbaa1, and C3H mice were treated with 100 μl K/B×N serum on Days 0 and 2, and arthritis was assessed by change in ankle measurement on Days 1–7 (A) and by histopathological scoring for overall lesion score on Day 7 (B), as described in Materials and Methods. Statistical significance was determined by Student’s t-test for ankle swelling and Mann Whitney U test Overall lesion. n=5
K/B×N serum transfer model of rheumatoid arthritis
K/B×N serum was collected from KRN×NOD offspring at the peak of spontaneous arthritis (9 wks.), as described(38). K/B×N serum (100μl) was administered by intraperitoneal injection on days 0 and 2 (33, 39). Rear ankle measurements were made prior to serum transfer on Day 0, and on Days 1, 2, 4, and 7. At day 7, joints were removed for histopathological assessment of arthritis, as described for Lyme arthritis.
Isolation of RNA and quantitative RT-PCR
For all experiments examining gene expression in joint tissue, mice were killed, and the skin was removed from the tibiotarsal joints. Ankle joints were excised, immersed in RNA Later (Qiagen) and stored at −80°C. Total RNA was recovered from homogenized tissue using Trizol reagent (Invitrogen) (19). RNA recovered from tissue and cells was reverse-transcribed and transcripts were quantified using a Roche LC-480 according to our previously described protocols (32). Primer sequences used in this study for β–actin, Igtp, Iigp, Stat1, Nos2, Arg (18), Cxcl9, Cxcl10, Oasl2, Tyki (20) Gbp2 (25), Tnfα, Ifnβ (40), IL-10 (41), Mrc1 (42) can be found in the indicated citations. Primers for Stat2 were: Stat2 forward (5′-GCTTCCTCTATCCCCGAATC -3′) and reverse (5′-ATCAATGGCAACTCCTGGTC -3′).
Cell culture
Bone marrow derived macrophages (BMDM) were isolated from the femurs and tibias of mice that were cultured for 7 days in L929-cell conditioned media as a source of macrophage colony stimulating factor, as previously described (43). Macrophage cultures were plated in 12 well dishes at a density of 7.5 × 105/ml in media containing the serum replacement Nutridoma (Roche) and stimulated with live B. burgdorferi cN40 (7.4 × 106/ml), sonicated B. burgdorferi (5μg/ml) (40), or Poly (dI:dC) (10 ng/ml, Sigma). Macrophage cultures were stimulated at 37°C, 5% CO2 and harvested either at 1, 3, or 6 hours for RNA extraction, as indicated. IFNβ (PBL laboratories) was added at the indicated concentrations.
Phagocytosis Assay
Peritoneal macrophages were harvested 4 days after intraperitoneal administration of 3 ml of 3% sterile thioglycolate. Macrophages were collected with ice-cold PBS, and red blood cells lysed with ACK lysis buffer. Cells in RPMI-10%FBS were plated at 5×105/well in 12-well plate and allowed to adhere overnight, when non-adherent cells were removed by washing. B. burgdorferi N40 expressing GFP were added to the macrophages in RPMU.B (75% RPMI+10%FBS+24%BSKII) at a 50:1 ratio(44) (45). Plates were centrifuged at 500×g for 5 minutes and incubated for 1 or 2 hours at 37°C, conditions previously shown to capture midway and maximal phagocytosis (46). Wells were washed to remove unassociated bacteria. Cells from one set of replicate wells were collected with a cell scraper and represented total cell associated bacteria. A second set of replicate cells were incubated with 0.25% trypsin in RPMI for 7 minutes at 37°C to release extracellular bacteria from the macrophages prior to collecting, and are referred to as trypsin-resistant (47). Cells collected in both manners were washed 3 times in cold PBS, suspended in flow buffer, and analyzed using a BD LSRII flow cytometer. Baseline fluorescence was determined for cells not receiving GFP B. burgdorferi in each treatment group.
Data and statistical analyses
All graphical data represent the mean ± SEM. Statistical analysis was performed using Prism 5.0c software. Multiple-sample data sets were analyzed by one-way ANOVA with appropriate post-hoc test as indicated: Bonferroni (Figs. 3,4,7,9, Table 1). Two-sample data sets were analyzed by Student’s t-test (Figs. 1,2,6,8). Categorical data for histopathology were assessed by the Mann Whitney U test (Figs. 1,2,8,9) or Kruskal-Wallis test with Dunn’s multiple comparison (Fig 9). Statistical significance (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
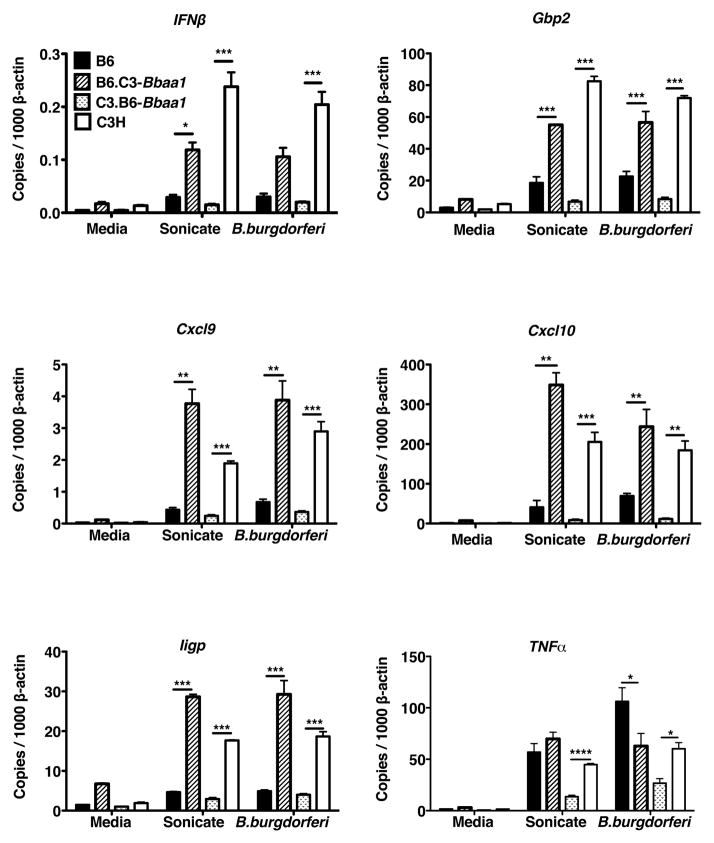
Figure 3. Bone marrow derived macrophages (BMDM) reveal Bbaa1 regulates the magnitude of the IFN response to B. burgdorferi.
RT-PCR analysis of transcripts in BMDM from B6, C3H, B6.C3-Bba1 and C3.B6-Bbaa1 mice treated with media, sonicated B. burgdorferi (sonicate) or living B. burgdorferi (B. burgdorferi) for 6 hours. Transcript levels for Ifnβ, Gbp2, Cxcl9, Cxcl10, Iigp, and Tnfα were normalized to β-actin. Data are averages ± SE, with trends representative of 5 separate experiments. Significance determined by ANOVA, with Bonferroni post-hoc comparison, shown for ISCL with appropriate background parental strain. Differences between B6 and C3H for Ifnβ, Gbp2, Cxcl9, Cxcl10, and Iigp were also significant (not shown).
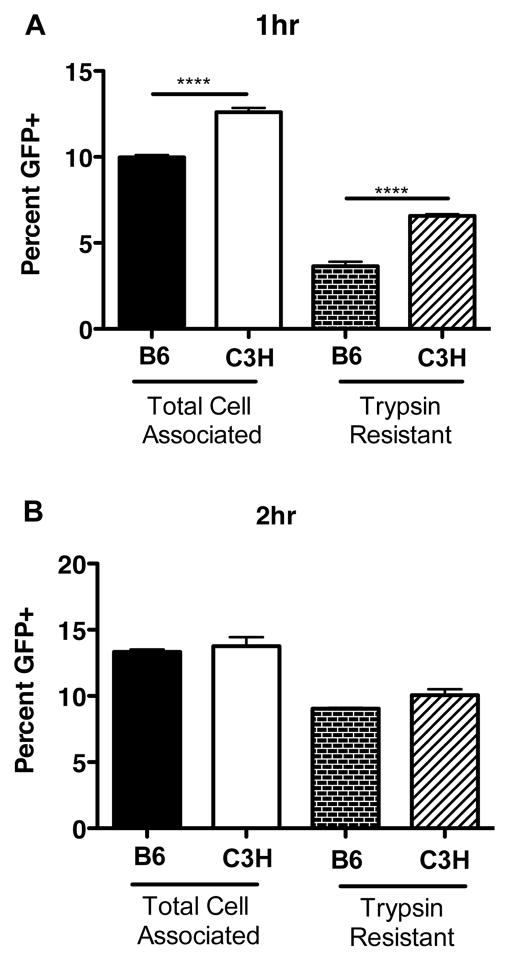
Figure 4. Phagocytic capacity of macrophages from B6 and C3H mice.
Peritoneal macrophages (5 × 105) were incubated with GFP-B. burgdorferi for 1 (A) and 2(B) hours at 37°C. Cells collected in the absence of trypsin (Total Cell Associated) or following incubation with trypsin (Trypsin Resistant) to remove extracellular bacteria. The percentage of macrophages with associated bacteria was determined by flow cytometry, as compared with cells not receiving bacteria. Data are averages ± SE, and are representative of 2 separate experiments. Significance determined by ANOVA, with Bonferroni post-hoc comparison; for B6 vs. C3H in each treatment group.
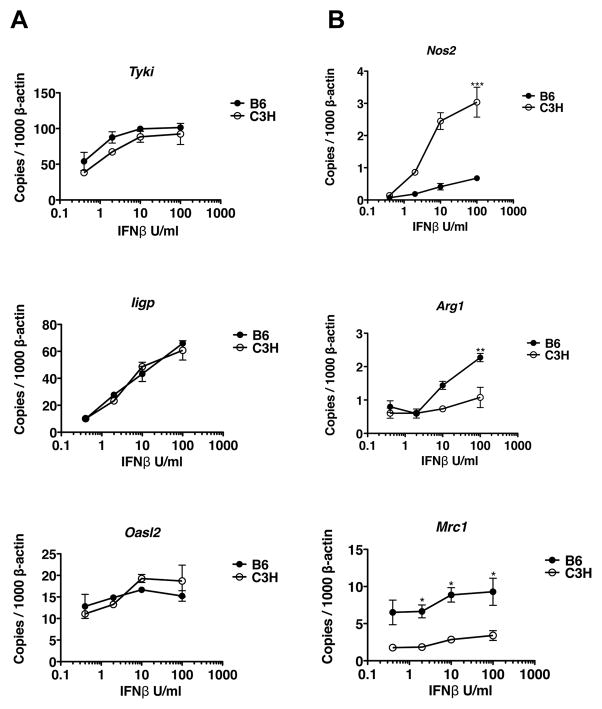
Figure 7. Responses of BMDM to increasing doses of IFNβ.
BMDM from B6 and C3H mice were treated with 0.4–100 Units/ml of IFNβ, and transcriptional responses measured at 3 hours. Transcript levels of Tyki, Nos2, Iigp, Arg1, Oasl2, and Mrc1 were normalized to β-actin. Data points indicate mean ± SE. Statistical significance between mouse strains were determined by Student’s t-test, as are indicated for Nos2 and Arg1.
Table 1.
Upregulation of IFN-inducible genes in joint tissue of B. burgdorferi infected B6.C3-Bbaa1 mice at 1 week of infection.
| Gbp2 | Igtp | Cxcl10 | Iigp | Cxcl9 | |
|---|---|---|---|---|---|
| C57BL/6 | 1.21 ± 0.12ab | 0.92 ± 0.06 | 1.58 ± 0.30 | 1.44 ± 0.09 | 1.27 ± 0.11 |
| B6.C3-Bbaa1 | 2.28 ± 0.29c | 1.44 ± 0.15 | 4.93 ± 0.92 | 3.04 ± 0.36 | 4.33 ± 0.78 |
| C3H/HeN | 5.06 ± 0.36 | 8.48 ± 1.32 | 9.86 ± 1.71 | 9.38 ± 0.74 | 3.8 ± 0.48 |
Transcript levels were determined in samples collected at 1 wk. p.i., normalized to β-actin, and reported as fold-change relative to uninfected controls.
mean ± standard error.
Bold indicates values in B6.C3-Bbaa1 mice significantly greater than in B6 mice.
Figure 1. Interval specific congenic mice reveal contribution of C3H allele of Bbaa1 to Lyme arthritis severity.
B6, C3H, B6.C3-Bbaa1, and C3.B6-Bbaa1 mice were infected with 2 × 104 B. burgdorferi and arthritis was assessed at 4 wks. of infection, as described in Materials and Methods, and shown for (A) Change in ankle measurement and (B) Overall lesion score. Statistical significance of differences between ISCL and background parental strain were determined by Student’s t-test for ankle swelling and Mann Whitney U test for Overall lesion. All categories were negative for uninfected mice, injected with BSK media, and are not shown in the figure. Groups consisted of approximately equal numbers of male and female mice, with n=22 B6, 20 C3H, 23 B6.C3-Bbaa1, and 30 C3.B6-Bbaa1.
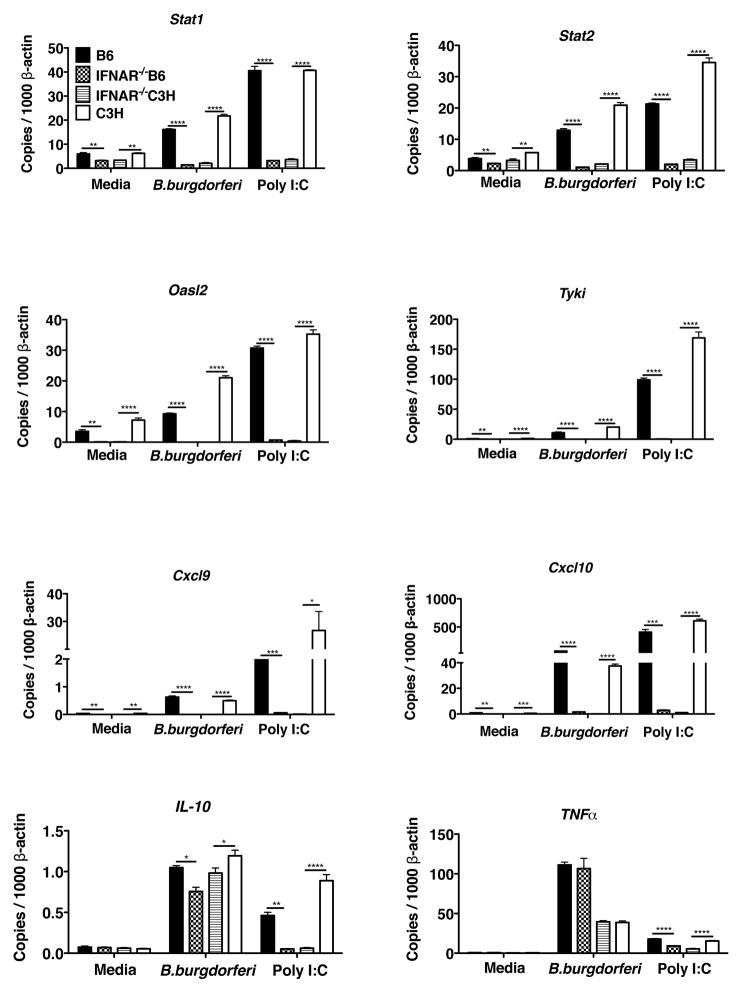
Figure 6. Bbaa1 regulation of IFN profile is dependent on IFNAR feed forward.
RT-PCR of transcript induction in BMDM from B6, C3H, B6-IFNAR1−/−, and C3H-IFNAR1−/− mice incubated with media, B. burgdorferi, or poly I:C for 6 hours. Transcript levels for Stat1, Stat2, Oasl2, Nos2. Tyki, Cxcl9, Cxcl10, IL-10, and Tnfα were normalized to β-actin. Significance determined by ANOVA, with Bonferroni post-hoc comparison; shown for B6 vs. B6-IFNAR1−/− and C3H vs. C3H-IFNAR1−/−.
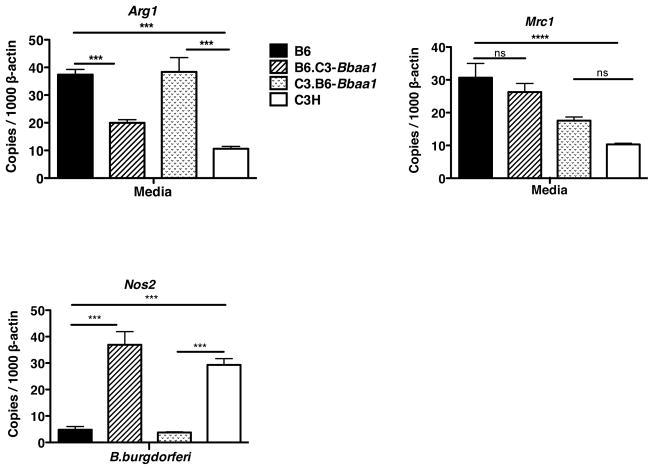
Figure 8. Bbaa1 regulated levels of expression of M1 and M2 markers in resting and activated macrophages.
BMDM from B6, C3H, B6.C3-Bbaa1 and C3.B6-Bbaa1 mice were assessed for M2 markers Arg1 and Mrc1 prior to addition of stimulant. Expression of the M1 marker Nos2 was assessed at 6 hours of stimulation with B. burgdorferi. All transcripts were normalized to β-actin. Shown are mean ± SE for one experiment, representative of 2 separate experiments. Significance determined by ANOVA, with Bonferroni post-hoc comparison; shown for ISCL with appropriate background parental strain and for B6 vs. C3H.
RESULTS
The C3H allele of Bbaa1 confers increased arthritis severity when transferred to B6 mice
Our previous studies revealed a major, differential contribution of Type I IFN to the development of Lyme arthritis in C3H mice, which could be overcome by blocking the Type I IFN receptor signaling with neutralizing mAb or by gene ablation (19, 20). We also noted that Bbaa1, a QTL controlling the severity of Lyme arthritis on Chr4 encompassed a cluster of 15 Type I IFN genes, prompting a more mechanistic analysis of the role of Bbaa1 as a regulator of Lyme arthritis severity (30, 32).
To assess the impact of Bbaa1 on Lyme arthritis severity, reciprocal ISCL were developed in which Bbaa1 was introgressed onto each background. B6 mice possessing C3H Bbaa1 (B6.C3-Bbaa1) displayed more severe Lyme arthritis than B6 mice at four weeks of infection with B. burgdorferi, as assessed by ankle swelling and histopathology (Fig. 1. A, B). Importantly, arthritis in B6.C3-Bbaa1 mice was intermediate between that of the B6 and C3H parental strains, consistent with the presence of multiple other QTL that were not transferred to the B6 background in the Bbaa1 congenic. Notably, the reciprocal transfer of the B6 allele of Bbaa1 onto the C3H background (C3.B6-Bbaa1) did not result in a reduction in B. burgdorferi-induced arthritis severity when compared with the infected C3H parent. This may reflect the presence of allelic loci outside of Bbaa1 that mask its impact, and is consistent with the identification of Bbaa1 with the (B6×C3)F2 intercross, but not with the (B6×C3)F1 crossed to either B6 or C3H parent (30).
Effect of blocking Type I IFN receptor on Lyme arthritis in B6.C3-Bbaa1 mice
The results from Fig 1 demonstrated that Bbaa1 regulates arthritis severity on the B6 background. Although the B6.C3-Bbaa1 congenic interval is large (9.32–94.97 Mbp) and encodes numerous genes, it nevertheless encompasses the IFNα/β cluster (88.5–88.7 Mbp) that encode Type I IFNs implicated in Lyme arthritis (19, 20, 34). All IFNα and IFNβ proteins signal through the IFNAR receptor, comprised of IFNAR1 and IFNAR2 chains (48, 49). To determine if the greater arthritis in B6.C3-Bbaa1 mice relative to B6 mice was dependent on Type I IFN, B6.C3-Bbaa1 mice were treated with a blocking mAb to IFNAR1 component of the Type I IFN receptor (MAR1-5A3) one day prior to infection (20). B6.C3-Bbaa1 mice treated with isotype control mAb developed more severe Lyme arthritis than B6 mice as assessed by ankle measurement and lesion scoring (Fig. 2), similar to the results of Fig. 1. Administration of the receptor blocking mAb reduced arthritis severity to levels indistinguishable from wild type B6 (Fig. 2). Thus, the increased arthritis severity of B6.C3-Bbaa1 mice is a function of Type I IFN responses dependent on feed forward amplification through the IFNAR1 receptor, and identify the IFNαβ cluster as a positional candidate for Bbaa1.
Magnitude of the Type I IFN response in joint tissue is partially regulated by Bbaa1
As previously reported, joint tissue of B. burgdorferi infected C3H mice revealed a strong IFN signature at 1 week of infection that was absent in B6 mice (18). Tissue from B6.C3-Bbaa1 also displayed an elevation in several of the transcripts previously characterized as upregulated only in C3H mice, Table I. Interestingly, the magnitude of transcriptional upregulation of the relatively long-lived chemokines Cxcl9 and Cxcl10 approached the magnitude seen in C3H joint tissue, Table I. As these and other chemokines directly participate in recruitment of inflammatory cells to the joint, their upregulation is highly relevant to arthritis (50, 51). These results are consistent with the combined contribution of Bbaa1 and additional Bbaa to the complete IFN transcriptional profile seen C3H mice. Importantly, the biological significance of the portion of the IFN response regulated by Bbaa1 was demonstrated by the impact of receptor blocking mAb on arthritis development in infected B6.C3-Bbaa1 mice (Fig. 2).
Bbaa1 dependent regulation of Type I IFN activation revealed in BMDMs
Previously, ex vivo analysis of cells from the joint tissue of uninfected C3H mice identified myeloid cells as the likely initiators of B. burgdorferi-induced IFN production (19). Myeloid cells are present at relatively low levels in the joint tissue and difficult to recover, therefore bone marrow derived macrophages (BMDM) were used as a pure population to assess the impact of the Bbaa1 locus on IFN induction and signature response, further utilizing the Bbaa1 ISCL. BMDM were generated from 6–8 wk old B6, C3H, and the two Bbaa1 ISCL by culture of bone marrow cells for 6 days in the presence of M-CSF. Transcriptional responses of BMDM were analyzed at 6 hours following addition of living or sonicated B. burgdorferi or media control. As reported previously, macrophages from C3H mice displayed a higher level of transcriptional induction of IFNβ and numerous IFN-inducible transcripts than did macrophages from B6 mice (20, 25, 32). B6.C3-Bbaa1 macrophages displayed elevated transcriptional responses, similar to those seen in C3H macrophages and consistent with Bbaa1 regulation of the IFN responses (Fig. 3). The IFN transcriptional response of C3.B6-Bbaa1 macrophages was greatly reduced relative to C3H macrophages, consistent with a reduced induction of Type I IFN when the B6-Bbaa1 allele was present. Sonicated B. burgdorferi elicited responses similar to living B. burgdorferi, indicating that ligands only present in viable organisms were not responsible for the differential magnitude.
This finding supports a major regulatory role for C3-Bbaa1 in regulating Type I IFN responses in B6.C3-Bbaa1 myeloid lineage cells, which translates to the arthritis exacerbating effect also seen in B6.C3-Bbaa1. The reciprocal situation is somewhat confounding, in that the suppressive effect of B6-Bbaa1 on IFN responses was readily demonstrated in highly purified BMDM from C3.B6-Bbaa1 mice (Fig. 3), but did not translate to reduced severity of Lyme arthritis (Fig 1). This suggests that distinct pathways play a major role in arthritis development in C3H mice, and may overwhelm the impact of myeloid produced Type I IFN in the complex context of the joint tissue. Consistent with this is the less stringent control of expression of the NF-κB dependent cytokine TNFα, a component of an important distinct signaling pathway (Fig 3).
One interpretation of the findings of Fig 3 was that Bbaa1 encoded a sensor for B. burgdorferi that was responsible for the range in magnitude of the IFN response. Alternatively, the effect of Bbaa1 could be dependent on a component of phagocytic recognition or internalization necessary for degradation/trafficking of B. burgdorferi that has been linked to macrophage initiation of IFN responses to this organism (21, 52, 53). Peritoneal macrophages from B6 and C3H mice were used to assess the impact of Bbaa1 on the uptake of GFP-expressing B. burgdorferi at 1 and 2 hours incubation, times previously determined to capture intermediate and maximal phagocytosis (46). Trypsin treatment was used to release adherent extracellular bacteria and allow estimation of those that had been incorporated into an intracellular compartment. Macrophages from C3H mice displayed somewhat greater association and phagocytosis of B. burgdorferi than those from B6 mice at 1 hour, but by 2 hours both cell-associated and trypsin-resistant bacteria were equivalent, arguing that the dramatic difference in magnitude of the IFN response at 6 hours was not reflective of major differences in phagocytic capacity, Fig 4.
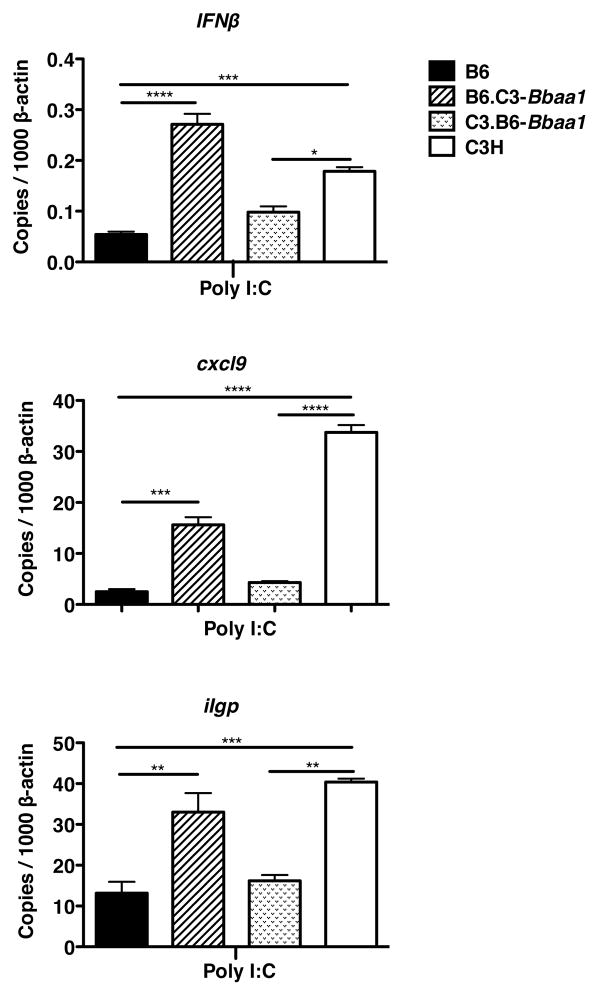
To test the generality of Bbaa1 alleles on IFN induction the TLR3 ligand poly I:C, a surrogate for double stranded viral RNA, was assessed. In fact, the relative induction of IFNβ and downstream transcripts in B6, C3H, and Bbaa1 congenic macrophages revealed a pattern for poly I:C similar to that observed with B. burgdorferi (Fig. 5). This argues that the impact of alleles of Bbaa1 on IFN dysregulation is not limited to a particular bacterial sensor or to a bacterial specific trafficking pathway. Rather, the hyperinduction of Type I IFN associated with the C3H allele of Bbaa1 is an inherent property of this locus, and is not unique to the responses to B. burgdorferi and Lyme arthritis.
Figure 5. Bbaa1 regulates BMDM responses to poly I:C.
RT-PCR analysis of transcript induction in BMDM from B6, C3H, B6-C3.Bba1 and C3.B6-Bbaa1 mice treated with poly I:C for 6 hours. Transcript levels for Ifnβ, Gbp2, and Iigp were normalized to β-actin. Data are averages ± SE, with trends representative of 2 separate experiments. Significance determined by ANOVA, with Bonferroni post-hoc comparison; shown for ISCL with appropriate background parental strain and for B6 vs. C3H.
Are macrophages from C3H mice “primed” for the Type I IFN response?
The heightened IFN signature response of C3H and B6.C3-Bbaa1 macrophages suggested that the C3H allele of Bbaa1 promotes transcriptional priming of macrophage responses to B. burgdorferi or other stimuli. To interrogate the initial response to B. burgdorferi, dissociated from the receptor dependent feed forward stage, BMDM from C3H and B6 mice deficient in the IFNAR1 gene were treated with B. burgdorferi or poly I:C and assessed for elevation in transcripts. None of the IFN inducible transcripts were elevated in resting BMDM from IFNAR1−/− mice on either background, and none of the IFN inducible transcripts were upregulated following treatment with B. burgdorferi or poly I:C (Fig. 6). Thus responses to B. burgdorferi were dependent on autocrine release of Type I IFN and amplification through the Type I IFN receptor. This was true even for very early transcriptional responses reported to be independent of feed forward engagement in the response to certain intracellular bacterial pathogens (Stat1, Stat2, Tyki) (54) (Fig. 6). These transcripts were also not detectable at 1 hour of stimulation (not shown). Thus, the manifestation of hyperactivation of Type I IFN is dependent on a functioning IFN receptor. B. burgdorferi induction of TNFα and IL-10 was not influenced by the absence of IFNAR1, consistent with the known dependence of these cytokines on NF-κB mediated responses to the spirochete, and confirming the fidelity of NF-κB pathway in IFNAR1−/− BMDM. Interestingly, poly I:C induction of IL-10 was dependent on IFNAR1 signaling, while poly I:C induction of TNFα was too low to determine the impact of receptor deletion (Fig. 6).
We considered the possibility that BMDM from C3H mice and B6.C3-Bbaa1 mice were poised to respond to Type I IFN by expressing a higher resting level of the IFN receptor associated signal transducers and activators of transcription family members Stat1 and Stat2, relative to B6 and C3.B6-Bbaa1 BMDM (55). In fact, resting levels of Stat1 and Stat2 transcripts were similar in all four genotypes of BMDM, therefore, not supporting a C3H-Bbaa1 dependent poised state (Fig. 6 and not shown). The resting levels of the transcription factor IRF3, upstream of IFN initiation, were also similar in all four BMDM genotypes as were the extremely low levels of the inducible transcription factor IRF7 (not shown) (56, 57). Additional experiments also failed to reveal increased presence of STAT1 protein or phosphorylated STAT1 in resting C3H or B6.C3-Bbaa1 BMDM, (not shown).
The results of Fig 6 demonstrated that Bbaa1 regulation of IFN inducible transcripts by B. burgdorferi and poly I:C was only observable in the later stage of induction, following autocrine/paracrine activities of Type I IFN. To determine the impact of Bbaa1 allele on this amplification stage of the IFN response, BMDM from C3H and B6 mice were treated with increasing concentrations of IFNβ and transcripts were analyzed. By assessing the response to IFNβ, any possible differences in uptake and sensing of B. burgdorferi were eliminated. BMDM from B6 and C3H mice revealed virtually identical magnitude of induction of numerous IFN signature genes at 3 hours of treatment (Fig. 7A). Importantly, dose response curves revealed similar thresholds and maximal responses to IFNβ by both mouse strains. This is consistent with similar ligand affinity and activation state of the Type I IFN receptor expressed by B6 and C3H macrophages, and independence from Bbaa1 regulation at this stage of the response.
Transcripts known to be associated with the well-characterized macrophage effector states M1 and M2 were also assessed in B6 and C3H treated with IFNβ (58). Transcripts for the M1 associated enzyme inducible nitric oxide synthase (Nos2) were more highly upregulated in response to IFNβ in C3H than B6 macrophages (Fig 7B). In contrast, transcripts for the alternatively activated M2 macrophage marker, arginase (Arg1) were more highly induced in BMDM from B6 than C3H mice (Fig 7B) (59). Levels of a second M2 macrophage marker, Mrc1, trended higher in B6 than C3H macrophages. These findings suggest the interesting hypothesis that Bbaa1 impacts the functional phenotype of macrophages by modulating downstream signaling pathways. As M1 macrophages are considered to be pro-inflammatory while M2 macrophages are associated with wound repair and modulation of inflammation, this difference could be highly relevant to the inflammatory state of the B. burgdorferi-infected joint (60).
Does Bbaa1 regulate expression of M1 and M2 markers in macrophages?
BMDM generated by culturing with M-CSF are considered to possess characteristics associated with M2 phenotype macrophages, functionally associated with wound repair and restriction of inflammation (59). In fact, we previously identified Arg1 as transcriptionally upregulated in joint tissue of B. burgdorferi infected B6 mice but not C3H mice, suggesting correlation of Arg1 expression with the reduced inflammation and arthritis seen in B6 mice (18). To assess the intriguing possibility that Bbaa1 alleles influence the functional phenotype of macrophages, freshly derived and fully differentiated BMDM from B6, C3H, B6.C3-Bbaa1, and C3.B6-Bbaa1 mice were assessed for Arg1 and Mrc1. Remarkably, resting macrophages from B6 mice expressed higher levels of Arg1 than did macrophages from C3H mice, and this distinction was dependent on Bbaa1, as indicated with macrophages from Bbaa1 congenic mice (Fig. 8). In contrast, although the level of Mrc1 was greater in B6 than C3H macrophages, this difference was not regulated by Bbaa1 (Fig. 8). Transcripts for the M1 marker Nos2 were not expressed in resting BMDM (not shown), but were rapidly upregulated in response to B. burgdorferi (Fig. 8). Induction of Nos2 was regulated by Bbaa1, as levels were higher in BMDM from C3H and B6.C3-Bbaa1 mice than from B6 and C3.B6-Bbaa1 mice (Fig 8). This finding is consistent with the hypothesis that inherent patterns of gene expression, regulated by Bbaa1 alleles, direct the functional phenotype of macrophages as classically (M1) vs. alternatively (M2) poised. Importantly, Nos2 was previously found to be dispensable for arthritis development, therefore, its expression should be considered an indicator of macrophage phenotype rather than a mediator of disease (61).
Impact of Bbaa1 on the K/B×N serum transfer model of rheumatoid arthritis
QTL analysis for Lyme disease severity that recently allowed identification of Gusb as a major regulator of Lyme arthritis also revealed a similar regulatory potential for the K/B×N serum transfer model of RA (33). This prompted us to consider the possibility that Bbaa1 might also regulate RA. Additionally, an IFN signature has recently been described in a subgroup of RA patients who fail to respond to TNF blocking therapies, suggesting an existing patient population with dysregulated Type I IFN (62). The K/B×N serum transfer model has been used extensively to assess factors that contribute to the effector stage of arthritis as it bypasses the requirement for permissive MHC alleles (39). K/B×N serum contains an autoantibody to glucose-6-phosphate isomerase, which is widely expressed and particularly accessible on bone within the joints, and the severity of this arthritis can be regulated by limiting quantities of administered K/B×N serum. To test the impact of Bbaa1 a submaximal dose (100μl) of K/B×N serum was given to B6 and B6.C3-Bbaa1 mice. C3H mice were included as a in this experiment as their response to K/B×N serum has not previously been reported. Measurement of the rear ankle joints indicated more severe arthritis in C3H than B6 mice as early as 1 day following the first dose of K/B×N serum, and much greater severity by Day 7 (Fig. 9A). By Day 7, ankle swelling in B6.C3-Bbaa1 mice was much greater than seen in B6 mice, and indistinguishable from C3H (Fig. 9A). Histopathological assessment of the overall lesion score was determined at Day 7, and confirmed severe lesions in C3H and B6.C3-Bbaa1 mice and very mild disease in B6 mice (Fig. 9B). Thus, Bbaa1 is a robust regulator of RA severity.
To determine if the effect of Bbaa1 was mediated through Type I IFN production and feed forward, the B6.C3-Bbaa1 mice were treated with IFNAR1 blocking mAb, as in the Lyme arthritis experiment in Fig 2. B6.C3-Bbaa1 ISCL received a single dose of MAR1-5A3 or isotype control the day before the first administration of K/B×N serum. Blocking the Type I IFN receptor in B6.C3-Bbaa1 resulted in a reduction in arthritis severity to the level seen in B6 mice, implicating Type I IFN in this model of RA (Fig. 10A). Histopathological analysis also revealed greatly reduced lesion scores in receptor blocked B6.C3-Bbaa1 mice, (Fig. 10B).
Figure 10. Enhanced rheumatoid arthritis in B6.C3-Bbaa1 mice is dependent on Type I IFN.
B6.C3-Bbaa1 mice were treated with 2.5 mg MAR1-5A3 blocking mAb or isotype control the day before administration of K/B×N serum. B6.C3-Bbaa1 and B6 mice received 100 μl of K/B×N serum on Days 0 and 2. Arthritis was assessed by change in ankle measurement on Days 1–7 (A) and by histopathological scoring for Overall lesion score on Day 7 (B), as described in Materials and Methods. Statistical significance was determined by ANOVA, with Bonferroni post-hoc comparison for ankle measurements, and Kruskal-Wallis test with Dunn’s multiple comparison for Overall lesion. n=5.
DISCUSSION
In this study forward genetic analysis of Lyme arthritis severity has implicated the IFNαβ gene cluster within Bbaa1 as a candidate regulator of Lyme arthritis: B. burgdorferi infection of the B6.C3-Bbaa1 ISCL resulted in more severe ankle swelling and arthritic lesions than seen in parental B6 mice (Fig. 1). A second major finding was that Bbaa1 also regulated RA, as demonstrated in B6.C3-Bbaa1 mice treated with K/B×N serum (Fig. 9). In both arthritis models the heightened severity in B6.C3-Bbaa1 mice was reduced by treatment with an IFN receptor blocking mAb (Figs. 2,10). Thus, the exacerbated Lyme and rheumatoid arthritis in B6.C3-Bbaa1 mice is dependent on Type I IFN production and feed forward amplification, directly establishing a shared pathological process dependent on localized production of Type I IFN.
This is the second example of forward genetics identification of a Lyme arthritis QTL that also influences RA. Gusb was positionally cloned as a major regulator of Lyme arthritis severity within Bbaa2 on Chr5, and the disease exacerbating Gusb allele was also found to increase the severity of the K/B×N model of RA (33). The identification of Gusb provided an unexpected correlation of disease severity with reduced lysosomal enzyme activity and the subsequent accumulation of undigested glycosaminoglycans in joint tissue of both B. burgdorferi infected and autoantibody treated arthritis. Thus, both infection-triggered and antibody-induced arthritis share a second pathway for exacerbated disease.
In the case of Bbaa1, the involvement of Type I IFN in Lyme arthritis in C3H mice had previously been established through ablation of the IFNAR1 gene and by blocking IFNAR function with mAb (19, 20). Type I IFNs have also been identified in patients with various manifestations of Lyme disease, including skin lesions and cognitive deficits (63, 64), and have been implicated in B lymphocyte accumulation in the lymph nodes of infected mice (65). Taken in context with these previous findings, the current genetic identification of Bbaa1 implicates the cluster encoding the IFNαβ genes as a candidate regulator of Lyme arthritis. Importantly, disruption of IFNAR signaling had no effect on control of B. burgdorferi in joint or other tissue, indicating Type I IFN is selectively involved in pathological arthritis development and, therefore, could serve as a target for therapeutic intervention in patients (19, 20). The linkage of Bbaa1 to RA was somewhat surprising as others have reported that exogenous administration of human IFNα reduces the severity of RA in a murine model of collagen antibody induced arthritis (66). However, our findings are consistent with several published patient studies that have characterized a Type I IFN signature in synovial and blood cells from a cohort of patients who fail to respond to TNF targeting biologicals (62, 67). Therefore, the B6.C3-Bbaa1 mouse could provide an important new animal model for this RA patient group. Dysregulation of Type I IFN has also been associated with the pathological development of systemic lupus erythematosus, Sjogrens syndrome, scleroderma, Type I diabetes (29, 68–72), and in serious side effects from therapeutic use of IFNα/β in patients with hepatitis C virus and multiple sclerosis (26, 28). Thus, the identification of a genetic predisposition to exaggerated IFN responses could ultimately impact additional pathological conditions.
The identification of a genetic predisposition to Type I IFN hyperactivation in Lyme and rheumatoid arthritis emphasizes that entirely distinct initiators can trigger common pathways of arthritis exacerbation. It further validates the unbiased approach of forward genetics in identification of biologically relevant pathological pathways. Interestingly, neither Gusb nor Type I IFN have been identified as candidates for RA in numerous other murine QTL analyses, possibly reflecting the exclusion of C3H mice in collagen-induced arthritis studies due to lack of required MHC susceptibility allele (73). GWAS studies of patients with RA and juvenile idiopathic arthritis have identified polymorphisms in genes involved in the IFN signaling cascade such as Irf5, Irf8, Tyk2 and IL-21, although, the Type I IFN gene cluster itself has not been identified (74, 75). Thus, the B6.C3-Bbaa1 mouse provides a new experimental model to assess genetic predisposition to both microbial and autoimmune induced Type I IFN.
In considering the Type I IFN cluster as a candidate for Lyme arthritis and RA, IFNβ is the most obvious candidate gene as it is expressed at higher levels than any of the IFNα genes in response to B. burgdorferi in joint tissue and macrophages (20, 76). Additionally, the affinity of the IFNAR for IFNβ protein is much higher than for any of the IFNα proteins, making it a dominant contributor to the receptor dependent induction of downstream effectors (77, 78). However, support for IFNβ as a candidate gene is undermined by the complete absence of SNPs distinguishing C3H and B6 IFNβ genes, including the coding sequences and 3000 bps of flanking sequence that encompasses the well-characterized promoter/enhancer region (34, 79). Interestingly, there are numerous SNPs within and immediately adjacent to the IFNα genes. SNPs in IFNα1, IFNα2, IFNα4, IFNα6, IFNα11, and IFNαb result in altered protein sequences that potentially influence function while other SNPs are positioned within putative regulatory regions and could influence promoter strength or RNA stability. There are also transcribed pseudogenes within this 200,000 bp region whose regulatory functions have not been investigated. Additionally, there are a large number of non-IFN genes within Bbaa1 that could act to influence the level of IFNαβ transcription, possibly through assembly of transcriptional complexes or through altered chromatin accessibility. Future studies will require development of advanced recombinant ISCL in order to identify and critically test candidate genes.
The development of Bbaa1 ISCL provides a powerful tool to assess the regulation of Type I IFN induction. Several laboratories have characterized a Type I IFN response to B. burgdorferi RNA in human mononuclear cells that is dependent on TLR7 or TLR8 and MyD88, while others have characterized MyD88 independent responses to B. burgdorferi RNA and other components in murine BMDM (21–25, 52). Together, these findings present a complex picture of the Type I IFN response in human and murine cells, involving multiple ligands that vary with particular cell types. Our findings with macrophages from Bbaa1 ISCL indicate a dominant effect of this locus on the magnitude of IFN response to B. burgdorferi ligands and to the viral mimic poly I:C (Figs. 3–6). Thus, Bbaa1 appears to function as a global rheostat of Type I IFN responses, regulating the initial production of Type I IFN and the ultimate magnitude of the autocrine/paracrine amplified response (Fig 7). These findings clearly translate to the increased arthritis seen in B. burgdorferi infected B6.C3-Bbaa1 mice, indicating relevance to disease pathogenesis in the context of the B6 background (Fig. 1).
Unexpectedly, BMDM from Bbaa1 congenic mice also revealed differential expression of classic markers of the M1/M2 phenotype in BMDM (Figs.7,8). It has recently been suggested that low or “tonic” levels of Type I IFN actually prime macrophages for an M1 response, consistent with the hyperactivation of Type I IFN in BMDM from C3H and B6.C3-Bbaa1 mice (80, 81). In fact, our previous global gene expression analysis identified Arg1 as strongly upregulated in joint tissue of B. burgdorferi infected B6 but not C3H mice, also consistent with a modulatory role of Arg1 in B6 responses and possible suppression of the Type I IFN response (18). Taken together, these findings lead to the hypothesis that Type I IFN exacerbates arthritis development in C3H mice by promoting a pro-arthritic M1 macrophage response in the joint tissue, while the M2 response inherent to B6 mice modulates localized inflammation and prevents severe arthritis. This is an unexpected finding with clear implications for understanding and counteracting inflammatory responses involved in arthritic lesion formation, but distinct from host defense. It also poses the intriguing possibility that Type I IFN promotes M1 macrophage development in vivo, impacting the severity of Lyme arthritis and RA. Thus, the continued development of advanced Bbaa1 congenic lines will provide an opportunity for identification of genetic elements linked to Type I IFN expression and their involvement in pathological conditions extending beyond Lyme arthritis.
Acknowledgments
We thank members of the Weis laboratories for helpful discussions during the course of these studies, and acknowledge the technical assistance of James E. Brewster.
Abbreviations
- Bbaa
Borrelia burgdorferi arthritis associated locus
- ISCL
interval specific congenic lines
- QTL
Quantitative Trait Loci
Footnotes
Disclosure:
The authors have no financial conflicts.
References
- 1.Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–152. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 3.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Lyme Disease Data US, 1996–2010. 2010 http://www.cdc.gov/lyme/stats/index.html.
- 5.Dykhuizen DE, Brisson D, Sandigursky S, Wormser GP, Nowakowski J, Nadelman RB, Schwartz I. The propensity of different Borrelia burgdorferi sensu stricto genotypes to cause disseminated infections in humans. Am J Trop Med Hyg. 2008;78:806–810. [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold SW, Beck DS, Hansen GM, Terwilliger GA, Moody KD. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 7.Steere AC, Dwyer E, Winchester R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N Engl J Med. 1990;323:219–223. doi: 10.1056/NEJM199007263230402. [DOI] [PubMed] [Google Scholar]
- 8.Drouin EE, Seward RJ, Strle K, McHugh G, Katchar K, Londono D, Yao C, Costello CE, Steere AC. A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease. Arthritis Rheum. 2013 doi: 10.1002/art.37732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroder NW, Diterich I, Zinke A, Eckert J, Draing C, von Baehr V, Hassler D, Priem S, Hahn K, Michelsen KS, Hartung T, Burmester GR, Gobel UB, Hermann C, Schumann RR. Heterozygous Arg753Gln polymorphism of human TLR-2 impairs immune activation by Borrelia burgdorferi and protects from late stage Lyme disease. J Immunol. 2005;175:2534–2540. doi: 10.4049/jimmunol.175.4.2534. [DOI] [PubMed] [Google Scholar]
- 10.Oosting M, Ter Hofstede H, Sturm P, Adema GJ, Kullberg BJ, van der Meer JW, Netea MG, Joosten LA. TLR1/TLR2 heterodimers play an important role in the recognition of Borrelia spirochetes. PLoS One. 2011;6:e25998. doi: 10.1371/journal.pone.0025998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oosting M, ter Hofstede H, van de Veerdonk FL, Sturm P, Kullberg BJ, van der Meer JW, Netea MG, Joosten LA. Role of interleukin-23 (IL-23) receptor signaling for IL-17 responses in human Lyme disease. Infect Immun. 2011;79:4681–4687. doi: 10.1128/IAI.05242-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strle K, Shin JJ, Glickstein LJ, Steere AC. Association of a toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2012;64:1497–1507. doi: 10.1002/art.34383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, Medzhitov R, Fikrig E, Flavell RA. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002;8:878–884. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- 14.Kang I, Barthold SW, Persing DH, Bockenstedt LK. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–3111. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steere AC, Klitz W, Drouin EE, Falk BA, Kwok WW, Nepom GT, Baxter-Lowe LA. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med. 2006;203:961–971. doi: 10.1084/jem.20052471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–3086. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 17.Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crandall H, Dunn DM, Ma Y, Wooten RM, Zachary JF, Weis JH, Weiss RB, Weis JJ. Gene expression profiling reveals unique pathways associated with differential severity of Lyme arthritis. J Immunol. 2006;177:7930–7942. doi: 10.4049/jimmunol.177.11.7930. [DOI] [PubMed] [Google Scholar]
- 19.Lochhead RB, Sonderegger FL, Ma Y, Brewster JE, Cornwall D, Maylor-Hagen H, Miller JC, Zachary JF, Weis JH, Weis JJ. Endothelial cells and fibroblasts amplify the arthritogenic type I IFN response in murine Lyme disease and are major sources of chemokines in Borrelia burgdorferi-infected joint tissue. J Immunol. 2012;189:2488–2501. doi: 10.4049/jimmunol.1201095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JC, Ma Y, Bian J, Sheehan KC, Zachary JF, Weis JH, Schreiber RD, Weis JJ. A critical role for type I IFN in arthritis development following Borrelia burgdorferi infection of mice. J Immunol. 2008;181:8492–8503. doi: 10.4049/jimmunol.181.12.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cervantes JL, Dunham-Ems SM, La Vake CJ, Petzke MM, Sahay B, Sellati TJ, Radolf JD, Salazar JC. Phagosomal signaling by Borrelia burgdorferi in human monocytes involves Toll-like receptor (TLR) 2 and TLR8 cooperativity and TLR8-mediated induction of IFN-beta. Proc Natl Acad Sci U S A. 2011;108:3683–3688. doi: 10.1073/pnas.1013776108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petzke MM, Brooks A, Krupna MA, Mordue D, Schwartz I. Recognition of Borrelia burgdorferi, the Lyme disease spirochete, by TLR7 and TLR9 induces a type I IFN response by human immune cells. J Immunol. 2009;183:5279–5292. doi: 10.4049/jimmunol.0901390. [DOI] [PubMed] [Google Scholar]
- 23.Cervantes JL, La Vake CJ, Weinerman B, Luu S, O’Connell C, Verardi PH, Salazar JC. Human TLR8 is activated upon recognition of Borrelia burgdorferi RNA in the phagosome of human monocytes. J Leukoc Biol. 2013;94:1231–1241. doi: 10.1189/jlb.0413206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love AC, Schwartz I, Petzke MM. Borrelia burgdorferi RNA Induces Type I and III Interferons via Toll-Like Receptor 7 and Contributes to Production of NF-kappaB-Dependent Cytokines. Infect Immun. 2014;82:2405–2416. doi: 10.1128/IAI.01617-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JC, Maylor-Hagen H, Ma Y, Weis JH, Weis JJ. The Lyme disease spirochete Borrelia burgdorferi utilizes multiple ligands, including RNA, for interferon regulatory factor 3-dependent induction of type I interferon-responsive genes. Infect Immun. 2010;78:3144–3153. doi: 10.1128/IAI.01070-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 27.Chaussabel D, Allman W, Mejias A, Chung W, Bennett L, Ramilo O, Pascual V, Palucka AK, Banchereau J. Analysis of significance patterns identifies ubiquitous and disease-specific gene-expression signatures in patient peripheral blood leukocytes. Ann N Y Acad Sci. 2005;1062:146–154. doi: 10.1196/annals.1358.017. [DOI] [PubMed] [Google Scholar]
- 28.Wilson LE, Widman D, Dikman SH, Gorevic PD. Autoimmune disease complicating antiviral therapy for hepatitis C virus infection. Semin Arthritis Rheum. 2002;32:163–173. doi: 10.1053/sarh.2002.37277. [DOI] [PubMed] [Google Scholar]
- 29.Higgs BW, Liu Z, White B, Zhu W, White WI, Morehouse C, Brohawn P, Kiener PA, Richman L, Fiorentino D, Greenberg SA, Jallal B, Yao Y. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann Rheum Dis. 2011;70:2029–2036. doi: 10.1136/ard.2011.150326. [DOI] [PubMed] [Google Scholar]
- 30.Weis JJ, McCracken BA, Ma Y, Fairbairn D, Roper RJ, Morrison TB, Weis JH, Zachary JF, Doerge RW, Teuscher C. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J Immunol. 1999;162:948–956. [PubMed] [Google Scholar]
- 31.Roper RJ, Weis JJ, McCracken BA, Green CB, Ma Y, Weber KS, Fairbairn D, Butterfield RJ, Potter MR, Zachary JF, Doerge RW, Teuscher C. Genetic control of susceptibility to experimental Lyme arthritis is polygenic and exhibits consistent linkage to multiple loci on chromosome 5 in four independent mouse crosses. Genes Immun. 2001;2:388–397. doi: 10.1038/sj.gene.6363801. [DOI] [PubMed] [Google Scholar]
- 32.Ma Y, Miller JC, Crandall H, Larsen ET, Dunn DM, Weiss RB, Subramanian M, Weis JH, Zachary JF, Teuscher C, Weis JJ. Interval-specific congenic lines reveal quantitative trait Loci with penetrant lyme arthritis phenotypes on chromosomes 5, 11, and 12. Infect Immun. 2009;77:3302–3311. doi: 10.1128/IAI.00396-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bramwell KK, Ma Y, Weis JH, Chen X, Zachary JF, Teuscher C, Weis JJ. Lysosomal beta-glucuronidase regulates Lyme and rheumatoid arthritis severity. J Clin Invest. 2014;124:311–320. doi: 10.1172/JCI72339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardy MP, Owczarek CM, Jermiin LS, Ejdeback M, Hertzog PJ. Characterization of the type I interferon locus and identification of novel genes. Genomics. 2004;84:331–345. doi: 10.1016/j.ygeno.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Barthold SW, Persing DH, Armstrong AL, Peeples RA. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 36.Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol. 2002;168(1):348–355. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- 37.Sheehan KC, Lai KS, Dunn GP, Bruce AT, Diamond MS, Heutel JD, Dungo-Arthur C, Carrero JA, White JM, Hertzog PJ, Schreiber RD. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J Interferon Cytokine Res. 2006;26:804–819. doi: 10.1089/jir.2006.26.804. [DOI] [PubMed] [Google Scholar]
- 38.Monach PA, Mathis D, Benoist C. The K/BxN arthritis model. Curr Protoc Immunol. 2008;Chapter 15(Unit 15):22. doi: 10.1002/0471142735.im1522s81. [DOI] [PubMed] [Google Scholar]
- 39.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, Benoist C, Mathis D. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 40.Ma Y, Seiler KP, Tai K, Yang L, Woods M, Weis JJ. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect Immun. 1994;62:3663–3671. doi: 10.1128/iai.62.9.3663-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown JP, Zachary JF, Teuscher C, Weis JJ, Wooten RM. Dual Role of Interleukin-10 in Murine Lyme Disease: Regulation of Arthritis Severity and Host Defense. Infect Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menzies FM, Henriquez FL, Alexander J, Roberts CW. Sequential expression of macrophage anti-microbial/inflammatory and wound healing markers following innate, alternative and classical activation. Clin Exp Immunol. 2010;160:369–379. doi: 10.1111/j.1365-2249.2009.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meerpohl HG, Lohmann-Matthes ML, Fischer H. Studies on the activation of mouse bone marrow-derived macrophages by the macrophage cytotoxicity factor (MCF) Eur J Immunol. 1976;6:213–217. doi: 10.1002/eji.1830060313. [DOI] [PubMed] [Google Scholar]
- 44.Lazarus JJ, Kay MA, McCarter AL, Wooten RM. Viable Borrelia burgdorferi enhances interleukin-10 production and suppresses activation of murine macrophages. Infect Immun. 2008;76:1153–1162. doi: 10.1128/IAI.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carroll JA, Stewart PE, Rosa P, Elias AF, Garon CF. An enhanced GFP reporter system to monitor gene expression in Borrelia burgdorferi. Microbiology. 2003;149:1819–1828. doi: 10.1099/mic.0.26165-0. [DOI] [PubMed] [Google Scholar]
- 46.Lochhead RB, Ma Y, Zachary JF, Baltimore D, Zhao JL, Weis JH, O’Connell RM, Weis JJ. MicroRNA-146a provides feedback regulation of lyme arthritis but not carditis during infection with Borrelia burgdorferi. PLoS Pathog. 2014;10:e1004212. doi: 10.1371/journal.ppat.1004212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Y, Sturrock A, Weis JJ. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect Immun. 1991;59:671–678. doi: 10.1128/iai.59.2.671-678.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pestka S. The interferons: 50 years after their discovery, there is much more to learn. J Biol Chem. 2007;282:20047–20051. doi: 10.1074/jbc.R700004200. [DOI] [PubMed] [Google Scholar]
- 49.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 50.Brown CR, V, Blaho A, Loiacono CM. Susceptibility to experimental Lyme arthritis correlates with KC and monocyte chemoattractant protein-1 production in joints and requires neutrophil recruitment via CXCR2. J Immunol. 2003;171:893–901. doi: 10.4049/jimmunol.171.2.893. [DOI] [PubMed] [Google Scholar]
- 51.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petnicki-Ocwieja T, Chung E, Acosta DI, Ramos LT, Shin OS, Ghosh S, Kobzik L, Li X, Hu LT. TRIF mediates Toll-like receptor 2-dependent inflammatory responses to Borrelia burgdorferi. Infect Immun. 2013;81:402–410. doi: 10.1128/IAI.00890-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shin OS, Isberg RR, Akira S, Uematsu S, Behera AK, Hu LT. Distinct roles for MyD88 and Toll-like receptors 2,5, and 9 in phagocytosis of Borrelia burgdorferi and cytokine induction. Infect Immun. 2008;76:2341–2351. doi: 10.1128/IAI.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herskovits AA, Auerbuch V, Portnoy DA. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog. 2007;3:e51. doi: 10.1371/journal.ppat.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takaoka A, Yanai H. Interferon signalling network in innate defence. Cell Microbiol. 2006;8:907–922. doi: 10.1111/j.1462-5822.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 56.Stockinger S, Reutterer B, Schaljo B, Schellack C, Brunner S, Materna T, Yamamoto M, Akira S, Taniguchi T, Murray PJ, Muller M, Decker T. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J Immunol. 2004;173:7416–7425. doi: 10.4049/jimmunol.173.12.7416. [DOI] [PubMed] [Google Scholar]
- 57.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 58.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raes G, Van den Bergh R, De Baetselier P, Ghassabeh GH, Scotton C, Locati M, Mantovani A, Sozzani S. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J Immunol. 2005;174:6561. doi: 10.4049/jimmunol.174.11.6561. author reply 6561–6562. [DOI] [PubMed] [Google Scholar]
- 60.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 61.Brown CR, Reiner SL. Development of lyme arthritis in mice deficient in inducible nitric oxide synthase. J Infect Dis. 1999;179:1573–1576. doi: 10.1086/314774. [DOI] [PubMed] [Google Scholar]
- 62.van der Pouw Kraan TC, Wijbrandts CA, van Baarsen LG, Voskuyl AE, Rustenburg F, Baggen JM, Ibrahim SM, Fero M, Dijkmans BA, Tak PP, Verweij CL. Rheumatoid arthritis subtypes identified by genomic profiling of peripheral blood cells: assignment of a type I interferon signature in a subpopulation of patients. Ann Rheum Dis. 2007;66:1008–1014. doi: 10.1136/ard.2006.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salazar JC, Pope CD, Sellati TJ, Feder HM, Jr, Kiely TG, Dardick KR, Buckman RL, Moore MW, Caimano MJ, Pope JG, Krause PJ, Radolf JD. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J Immunol. 2003;171:2660–2670. doi: 10.4049/jimmunol.171.5.2660. [DOI] [PubMed] [Google Scholar]
- 64.Jacek E, Fallon BA, Chandra A, Crow MK, Wormser GP, Alaedini A. Increased IFNalpha activity and differential antibody response in patients with a history of Lyme disease and persistent cognitive deficits. J Neuroimmunol. 2013;255:85–91. doi: 10.1016/j.jneuroim.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hastey CJ, Ochoa J, Olsen KJ, Barthold SW, Baumgarth N. MyD88- and TRIF-independent induction of type I interferon drives naive B cell accumulation but not loss of lymph node architecture in Lyme disease. Infect Immun. 2014;82:1548–1558. doi: 10.1128/IAI.00969-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yarilina A, DiCarlo E, Ivashkiv LB. Suppression of the effector phase of inflammatory arthritis by double-stranded RNA is mediated by type I IFNs. J Immunol. 2007;178:2204–2211. doi: 10.4049/jimmunol.178.4.2204. [DOI] [PubMed] [Google Scholar]
- 67.van Baarsen LG, Wijbrandts CA, Rustenburg F, Cantaert T, van der Pouw Kraan TC, Baeten DL, Dijkmans BA, Tak PP, Verweij CL. Regulation of IFN response gene activity during infliximab treatment in rheumatoid arthritis is associated with clinical response to treatment. Arthritis Res Ther. 2010;12:R11. doi: 10.1186/ar2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 2003;36:481–490. doi: 10.1080/08916930310001625952. [DOI] [PubMed] [Google Scholar]
- 69.Gill MA, Blanco P, Arce E, Pascual V, Banchereau J, Palucka AK. Blood dendritic cells and DC-poietins in systemic lupus erythematosus. Hum Immunol. 2002;63:1172–1180. doi: 10.1016/s0198-8859(02)00756-5. [DOI] [PubMed] [Google Scholar]
- 70.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black x New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174:2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 71.Emamian ES, Leon JM, Lessard CJ, Grandits M, Baechler EC, Gaffney PM, Segal B, Rhodus NL, Moser KL. Peripheral blood gene expression profiling in Sjogren’s syndrome. Genes Immun. 2009;10:285–296. doi: 10.1038/gene.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferreira RC, Guo H, Coulson RM, Smyth DJ, Pekalski ML, Burren OS, Cutler AJ, Doecke JD, Flint S, McKinney EF, Lyons PA, Smith KG, Achenbach P, Beyerlein A, Dunger DB, Clayton DG, Wicker LS, Todd JA, Bonifacio E, Wallace C, Ziegler AG. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes. 2014;63:2538–2550. doi: 10.2337/db13-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holmdahl R. Dissection of the genetic complexity of arthritis using animal models. Immunol Lett. 2006;103:86–91. doi: 10.1016/j.imlet.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 74.Eyre S, Bowes J, Diogo D, Lee A, Barton A, Martin P, Zhernakova A, Stahl E, Viatte S, McAllister K, Amos CI, Padyukov L, Toes RE, Huizinga TW, Wijmenga C, Trynka G, Franke L, Westra HJ, Alfredsson L, Hu X, Sandor C, de Bakker PI, Davila S, Khor CC, Heng KK, Andrews R, Edkins S, Hunt SE, Langford C, Symmons D, Concannon P, Onengut-Gumuscu S, Rich SS, Deloukas P, Gonzalez-Gay MA, Rodriguez-Rodriguez L, Arlsetig L, Martin J, Rantapaa-Dahlqvist S, Plenge RM, Raychaudhuri S, Klareskog L, Gregersen PK, Worthington J. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet. 2012;44:1336–1340. doi: 10.1038/ng.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hinks A, Cobb J, Marion MC, Prahalad S, Sudman M, Bowes J, Martin P, Comeau ME, Sajuthi S, Andrews R, Brown M, Chen WM, Concannon P, Deloukas P, Edkins S, Eyre S, Gaffney PM, Guthery SL, Guthridge JM, Hunt SE, James JA, Keddache M, Moser KL, Nigrovic PA, Onengut-Gumuscu S, Onslow ML, Rose CD, Rich SS, Steel KJ, Wakeland EK, Wallace CA, Wedderburn LR, Woo P, Bohnsack JF, Haas JP, Glass DN, Langefeld CD, Thomson W, Thompson SD. Dense genotyping of immune-related disease regions identifies 14 new susceptibility loci for juvenile idiopathic arthritis. Nat Genet. 2013;45:664–669. doi: 10.1038/ng.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller JC, Ma Y, Crandall H, Wang X, Weis JJ. Gene expression profiling provides insights into the pathways involved in inflammatory arthritis development: murine model of Lyme disease. Exp Mol Pathol. 2008;85:20–27. doi: 10.1016/j.yexmp.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Piehler J, Thomas C, Garcia KC, Schreiber G. Structural and dynamic determinants of type I interferon receptor assembly and their functional interpretation. Immunol Rev. 2012;250:317–334. doi: 10.1111/imr.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jaks E, Gavutis M, Uze G, Martal J, Piehler J. Differential receptor subunit affinities of type I interferons govern differential signal activation. J Mol Biol. 2007;366:525–539. doi: 10.1016/j.jmb.2006.11.053. [DOI] [PubMed] [Google Scholar]
- 79.Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, Furlotte NA, Eskin E, Nellaker C, Whitley H, Cleak J, Janowitz D, Hernandez-Pliego P, Edwards A, Belgard TG, Oliver PL, McIntyre RE, Bhomra A, Nicod J, Gan X, Yuan W, van der Weyden L, Steward CA, Bala S, Stalker J, Mott R, Durbin R, Jackson IJ, Czechanski A, Guerra-Assuncao JA, Donahue LR, Reinholdt LG, Payseur BA, Ponting CP, Birney E, Flint J, Adams DJ. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–294. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fleetwood AJ, Dinh H, Cook AD, Hertzog PJ, Hamilton JA. GM-CSF- and M-CSF-dependent macrophage phenotypes display differential dependence on type I interferon signaling. J Leukoc Biol. 2009;86:411–421. doi: 10.1189/jlb.1108702. [DOI] [PubMed] [Google Scholar]
- 81.Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36:166–174. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]