Abstract
Nitroxyl (HNO) donors have been shown to elicit a variety of pharmacological responses, ranging from tumoricidal effects to treatment of heart failure. Isopropylamine-based diazeniumdiolates have been shown to produce HNO on decomposition under physiological conditions. Herein, we report the synthesis and HNO release profiles of primary alicyclic amine-based diazeniumdiolates. These compounds extend the range of known diazeniumdiolate-based HNO donors. Acetoxymethyl ester-protected diazeniumdiolates were also synthesized to improve purification and cellular uptake. The acetoxymethyl derivative of cyclopentylamine diazeniumdiolate not only showed higher cytotoxicity toward cancer cells as compared to the parent anion but was also effective in combination with tamoxifen for targeting estrogen receptor α-negative breast cancer cells.
Keywords: Nitroxyl, Nitric oxide, Diazeniumdiolate, IPA/NO, Angeli’s salt, Tamoxifen
1. Introduction
Nitric oxide (NO) is a well-studied physiological signaling molecule with functions ranging from regulation of blood pressure, cellular defense against invading pathogens and neurotransmission to prevention of platelet aggregation [1–5]. Nitroxyl (HNO), the product of one-electron reduction of NO, has recently emerged as a promising pharmacological agent. Donors of HNO have been shown to enhance myocardial contractility [6,7], to induce vasodilation [8], and to provide protection against ischemia-reperfusion injury [9], through mechanisms distinct from those of NO [10]. HNO has also been used clinically in treatment of alcoholism [11]; the alcohol deterrent agent cyanamide undergoes metabolic activation to HNO, which then inhibits aldehyde dehydrogenase [12]. Recently, HNO has also been reported to inhibit tumor growth and angiogenesis [13].
Due to irreversible dimerization [14,15], HNO cannot be stored, necessitating use of donor compounds for production of HNO [16–18]. Angeli’s salt [19] (Na2N2O3; Scheme 1) has been used extensively to study the chemical biology of HNO. Angeli’s salt spontaneously releases HNO in aqueous solution [20,21], with a pH-independent rate between pH 4–8 [20]. Despite the utility of Angeli’s salt as an HNO donor, the short half-life of ~2 min [22] under physiological conditions inhibits analysis of the effects of chronic exposure to HNO. Derivatization of Angeli’s salt to form a more stable HNO donor has been unsuccessful to date. Moreover, decomposition of Angeli’s salt produces nitrite, which also has biological activity [23]. Continuous progress in the pharmacology of HNO requires development of a versatile platform for systematically generating reliable, tunable, controlled fluxes of HNO in physiological media.
Scheme 1.
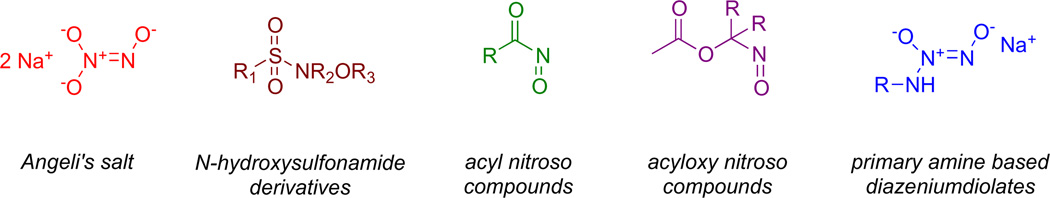
Common HNO donor structures.
A variety of organic donors have been produced (Scheme 1). For example Piloty’s acid (C6H5SO2NHOH) [24] and derivatives have long been known to function as base-sensitive HNO donors [25]. Although the rate of HNO release is tunable, these compounds often tend to generate NO under aerated conditions [26]. Acyl nitroso compounds generate HNO on reaction with nucleophiles [27], but are unstable intermediates [28]. The most general route of synthesizing such compounds is via oxidation of N-acyl hydroxylamine derivatives [29]. Acyloxy nitroso compounds, which are synthesized by oxidation of oximes [30], have more recently been found to function as HNO donors [31,32]. HNO release can be mediated either by enzymatic or spontaneous hydrolysis of the ester bond [33].
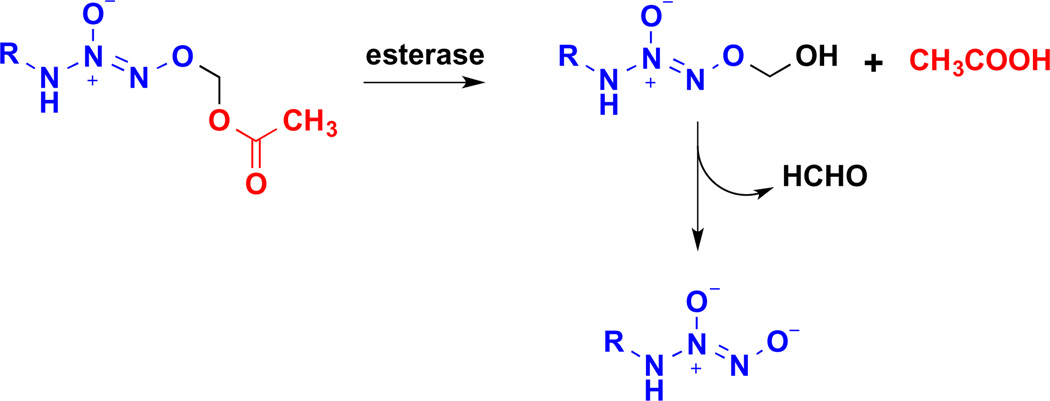
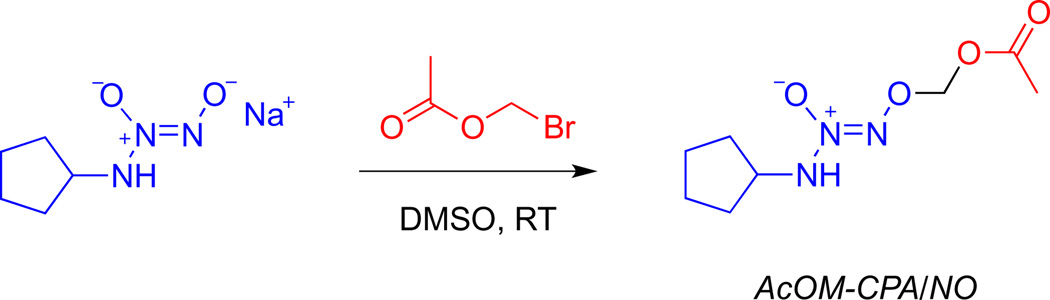
Diazeniumdiolates, also known as NONOates, are adducts of an NO dimer with nitrogen, carbon, oxygen or sulfur-based nucleophiles [34,35]. Angeli’s salt is formally in this class. Secondary amine-based diazeniumdiolates generate NO upon spontaneous decomposition, with reliable half-lives of decomposition ranging from 2 s to 20 h, depending on the amine backbone [36–38]. Importantly, diazeniumdiolates can be readily O2-derivatized [39–42], which facilitates purification and increases stability and decomposition half-life, among other effects. Moreover, derivatization allows conversion of diazeniumdiolates to prodrugs that can be bioactivated, thus providing a route to rational design for targeted delivery [43]. For instance, acetoxymethylation produces compounds that are sensitive to esterase-mediated hydrolysis [44] to release the parent diazeniumdiolate along with acetic acid and formaldehyde (Scheme 2).
Scheme 2.
Esterase-mediated decomposition of O2-acetoxymethyl protected diazeniumdiolates.
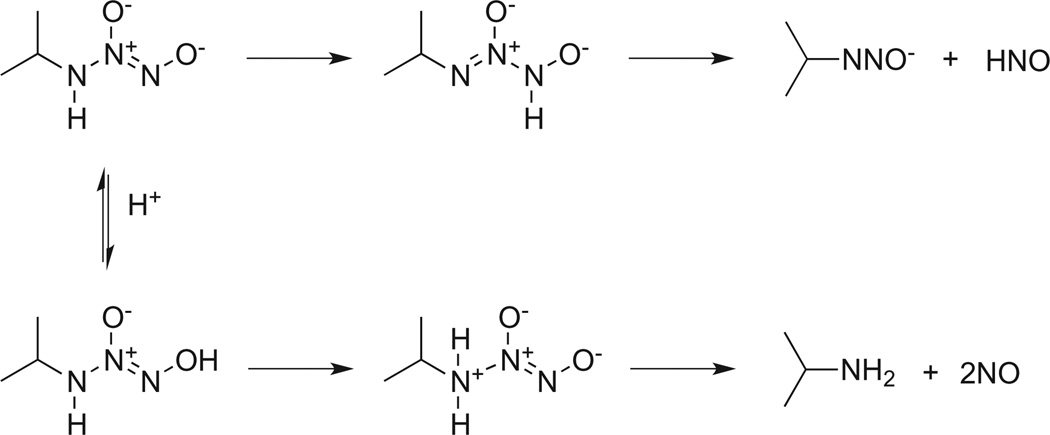
Unlike the large number of secondary amine based diazeniumdiolates, isopropylamine (IPA/NO) and cyclohexylamine (CHA/NO) diazeniumdiolates are currently the only examples of stable primary amine analogues in the literature [45]. Very recently, the unstable methylamine diazeniumdiolate was characterized and was trapped as the more stable O2-benzyl derivative [42]. Under physiological conditions, IPA/NO has a short half-life of 6.7 min [46] and generates HNO via a tautomerization pathway (Scheme 3) [46–48]. At lower pH, protonation of the nitroso oxygen, followed by tautomerization and N-N bond cleavage, leads to NO production. This mechanism of NO production is applicable to secondary amine diazeniumdiolates as well [49].
Scheme 3.
Mechanism of NO and HNO release from IPA/NO.
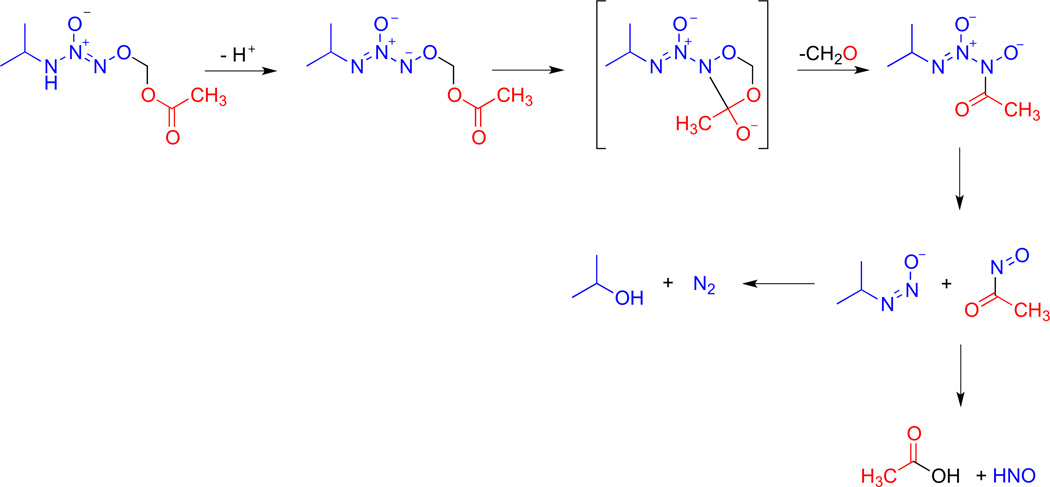
O2-derivatization such as acetoxymethylation of IPA/NO increases HNO production over the parent diazeniumdiolate due to access to a unique decomposition pathway (Scheme 4) [50,51]. After deprotonation of the amine proton, 1–4 acyl migration occurs via a cyclic intermediate and expulsion of formaldehyde. Subsequent fragmentation by N-N bond cleavage produces an acylnitroso derivative that on hydrolysis produces HNO. Esterase-mediated decomposition also occurs as shown in Scheme 2. A β-galactosidase-sensitive derivative has also recently been described [52].
Scheme 4.
Spontaneous decomposition mechanism of acetoxymethyl-protected IPA/NO.
Currently, whether other primary amine diazeniumdiolates release NO or HNO on decomposition is not known. To establish primary amine diazeniumdiolates as an alternative to currently existing HNO donors and to verify NO/HNO production, herein, we report the synthesis and characterization of a series of structurally related primary alicyclic amine-based diazeniumdiolates.
2. Materials and methods
Unless otherwise noted, chemicals were purchased from Sigma-Aldrich and used without further purification. NO was purchased from Matheson or Air Liquide. Concentrations of ionic diazeniumdiolate stock solutions (>10 mM; prepared in 10 mM NaOH and stored at −20 °C) [22] were determined directly prior to use from the measured molar extinction coefficient at 250 nm. Concentrations of O2-derivitized diazeniumdiolates were similarly ascertained, but in ethanol. Typically, the assay buffer consisted of the metal chelator diethylenetriaminepentaacetic acid (DTPA; 50 µM) in calcium- and magnesium-free Dulbecco’s phosphate-buffered saline (PBS; pH 7.4). By sequestering contaminating metals, addition of DTPA quenched the oxidation of HNO to NO [53]. Stock solutions of glutathione (GSH; > 10 mM) were made in assay buffer and stored at 4 °C. All reactions were performed at 37 °C except those involving measurement with an NO-specific electrode (room temperature). Thin layer chromatography was carried out using Analtech silica gel GF (250 µm) glass-backed plates. Flash chromatography was performed using the indicated solvent system on silica gel 60 (230–450 mesh size; Alfa Aesar).
2.1. Instrumentation
UV-visible spectroscopy was performed with an Agilent Hewlett-Packard 8453 diode-array spectrophotometer equipped with an Agilent 89090A thermostat. BioTek Synergy 2 microplate readers were also utilized for absorbance and fluorescence measurements. Electrochemical detection was accomplished with a World Precision Instruments Apollo 4000 system equipped with NO, O2 and H2O2 sensitive electrodes (Sarasota, FL). Solution pH was determined by use of a ThermoElectron Orion 420A+ pH meter. 1H and 13C NMR was carried out on a Bruker DRX-500 instrument. Low resolution mass spectra were recorded on a JEOL HX1 10A instrument. CHN analysis was performed at Midwest Microlab (Indianapolis, IN).
2.2. Synthesis of alicyclic amine diazeniumdiolates
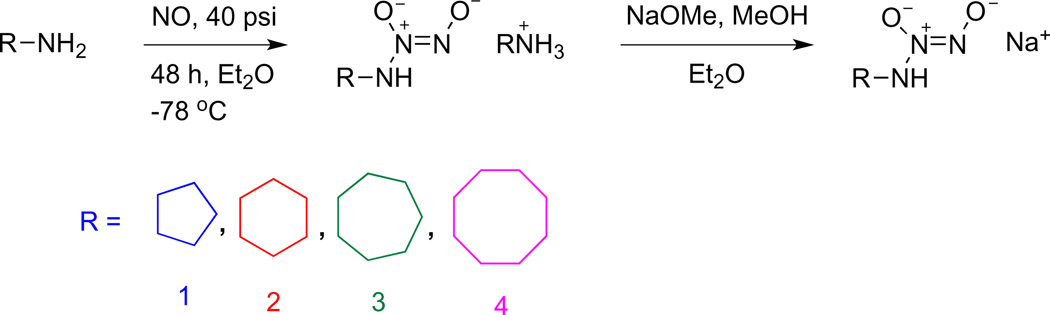
Sodium 1-(N-cyclohexylamino)diazen-1-ium-1,2-diolate (CHA/ NO; 2) was synthesized according to a previously published procedure [45]. For synthesis of other diazeniumdiolates, in general, a solution of the appropriate alicyclic amine in diethyl ether was placed in a 250 mL Parr bottle. The solution was deaerated with argon, cooled in dry ice, charged with 40 psi of NO, and allowed to stir for 24–48 h. The resulting solid precipitate was collected by filtration and washed with diethyl ether. The ammonium salt was converted to the sodium salt by dissolution in methanol and addition of one equivalent of 25% methanolic sodium methoxide. After several minutes of stirring, the sodium salt was precipitated by addition of diethylether and was collected as a white solid upon vacuum filtration. Note that as hygroscopic sodium salts, ionic diazeniumdiolates are not amenable to characterization by high resolution mass spectrometry or elemental analysis. Caution: Preparations of primary amine diazeniumdiolates can be unstable in the solid state, sometimes decomposing suddenly without warning. Minimize the storage amount (typically <250 mg) and store in containers with large head space. Additionally, using exactly 1 eq of base is a crucial step to avoid generation of highly reactive species. See reference 46 for additional details.
2.2.1. Sodium 1-(N-cyclopentylamino)diazen-1-ium-1,2-diolate (CPA/NO; 1)
A solution of cyclopentylamine (15 mL, 0.15 mol) in 25 mL of diethylether gave 3.7 g (14% yield) of the desired sodium salt. 1H NMR (DMSO): δ 1.48–1.56 [m, 4H], 1.59–1.66 [m, 4H], 3.86–3.88 [m, 1H], 6.00–6.01 [b, 1H]; 13C NMR (DMSO): δ 24.67, 31.27, 58.19; UV (10 mM NaOH): λmax 250 nm (ε = 8,200 M−1cm −1).
2.2.2. Sodium 1-(N-cycloheptylamino)diazen-1-ium-1,2-diolate (CHPA/NO; 3)
A solution of cycloheptylamine (30 mL, 0.22 mol) in 60 mL of diethylether gave 4.2 g (19% yield) of the desired sodium salt. 1H NMR (DMSO): δ 1.23–1.52 [m, 8H], 1.58–1.67 [m, 4H], 3.40–3.43 [m, 1H], 5.89–5.90 [b, 1H]; 13C NMR (DMSO): δ 23.79, 28.00,31.63, 56.00; UV (10 mM NaOH): λmax 250 nm (ε = 8,700 M−1 cm −1).
2.2.3. Sodium 1-(N-cyclooctylamino)diazen-1-ium-1,2-diolate (COA/NO;4)
A solution of cyclooctylamine (15 mL, 0.11 mol) in 25 mL of diethylether gave 2.9 g (17% yield) of the desired sodium salt. 1H NMR (DMSO): δ 1.46–1.65 [14H, m], 3.44–3.46 [1H, m], 5.86–5.87 [b, 1H]; 13C NMR (DMSO): δ 23.57, 25.32, 26.82, 29.43, 55.14; UV (10 mM NaOH): λmax 250 nm (ε = 8,300 M−1 cm−1).
2.2.4. Attempted synthesis of sodium 1-(N-cyclobutylamino)diazen-1-ium-1,2-diolate and sodium 1-(N-cyclopropylamino) diazen-1-ium-1,2-diolate
Similar methodology as above was used in order to synthesize the corresponding diazeniumdiolate salts. However, the final products could not be isolated due to instability.
2.3. General synthesis of acetoxymethylated diazeniumdiolates
A solution of bromomethyl acetate in THF was added to a slurry of the relevant sodium salt in dry DMSO at room temperature. The reaction mixture was stirred overnight, whereupon 15 mL of water was added, and stirring was continued for another 10 min. The residue was extracted with dichloromethane, washed with NaHCO3 (5%), dried over anhydrous sodium sulfate, filtered, and concentrated under vacuum. Column chromatography was performed using hexane: acetone (4:1) to give the desired product.
2.3.1. O2-(Acetoxymethyl) 1-(cyclopentylamino)diazen-1-ium-1,2-diolate (AcOM-CPA/NO;5)
A solution of bromomethyl acetate (183 mg, 1.19 mmol) in 3 mL of THF was added to a slurry of 1 (200 mg, 1.19 mmol in 10 mL of DMSO) according to the above general procedure to give the desired product as a colorless oil (187 mg, 72%). 1H NMR (CDCl3) δ 1.47–1.53 (m, 2H), 1.61–1.72 (m, 4H), 1.94–2.00 (m, 2H), 2.12 (s, 3H), 4.15–4.21 (m, 1H), 5.75 (s, 2H), 6.22 (d,J = 7.5 Hz, 1H); 13C NMR (CDCl3) δ 20.83, 23.91, 31.13, 58.71, 87.08, 169.31; UV (ethanol) λmax (ε) 239 nm (10,500 M−1 cm −1); MS (LCQ, ESI ionization method): 240.1 (MNa+peak). Anal. Calcd for C8H15N3O4: C, 44.23; H, 6.96; N, 19.34. Found: C, 44.44; H, 6.95; N, 19.40.
2.4. Decomposition profile
The rate constants of decomposition for ionic diazeniumdiolates at 37 °C were measured spectrophotometrically by monitoring the decrease in absorbance near 250 nm in assay buffer of the desired pH. The rate of decomposition of 5 in the presence or absence of the esterase in 2% guinea pig serum (by volume) was similarly determined at 238 nm. Kinetic analysis was performed by fitting the data to an exponential decay (A = ΔAe −kt+A∞).
2.5. Detection of nitrogen oxides
Trapping of HNO or NO by metmyoglobin (metMb) [17,54–56] or 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM-2DA) [51,57] assays, electrochemical detection [17,58], and quantitation of HNO [59] were performed as previously described. Details are presented in the Supporting Information. Figures are representative data sets, each from n ≥ 3 individual experiments.
2.6. Cell culture
Estrogen receptor α-negative (ER-; MDA-MB-231) or positive (ER+; MCF-7) human breast cancer cells (American Type Culture Collection, Manassas, VA) were grown as monolayers in RPMI 1640 media supplemented with 10% fetal bovine serum (FBS, Hyclone), penicillin (50 units/mL), and streptomycin (50 units/mL; Life Technologies, Inc., Grand Island, NY) at 37 °C in 5% CO2 and 80% relative humidity. Single cell suspensions were obtained by trypsinization (0.05% trypsin/EDTA, Life Technologies), and cells were counted using a Beckman cell counter or bright line hemocytometer (Sigma-Aldrich).
2.7. Clonogenic cell viability assay
Cells were plated at 400,000 cells per 60-mm dish and grown for 48 h. Cells were treated in growth media containing different concentrations (0.5–20 mM) of diazeniumdiolates for 24 h. After treatment, the cells were washed twice with PBS, trypsinized, counted and plated at a density of 100, 1,000 or 10,000 per 60-mm plate. For each condition, cells were plated in triplicate, and each experiment was repeated at least twice. After 10–12 d, the colonies were stained with crystal violet (0.5% w/v) and counted using a Stemi microscope.
2.8. MTT viability assay
Cytotoxicity was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Cells were plated at 8,000–10,000 cells per well in a 96-well plate and grown overnight. Cells were treated with different concentrations (10–100 µM) of 5 or control (ethanol, < 0.1% by volume) for 48 h. Thereafter, 10 µL of 5 mg/ mL MTT was added to each well, and the plate was incubated for 1 h at 37 °C. After removal of the media, 100 µL of DMSO was added to each well, and the absorbance was recorded at 550 nm. Growth inhibition is reported as the percentage of the corresponding control. Figures are representative data sets, each from n ≥ 3 individual experiments. For analysis of combined effects of 5 with tamoxifen, cells were treated with 10 µM tamoxifen, 75 µM of 5 or a combination for 48 h.
3. Results and discussion
The utility of NO-donating diazeniumdiolates is extensive due to ease of use as well as controllable release time. Here, we strove to expand the available number of HNO-releasing diazeniumdiolates. Since CHA/NO has been previously synthesized [45], we focused on a small series of alicyclic primary amine analogues. Alicyclic amine diazeniumdiolates were synthesized by exposing solutions of amine in diethyl ether to high pressures of NO (Scheme 5). Evidence of diazeniumdiolate formation was verified by the presence of a 250 nm peak, characteristic of the [N(O)NO]-moiety [22]. The molar extinction coefficients for these compounds (7,900–8,700 M−1 cm−1) were in good agreement with those of other diazeniumdiolates (6,000–10,000 M−1 cm−1) [22,34,37,40,46]. Cyclobutylamine- and cyclopropylamine-based diazeniumdiolates could not be isolated as the sodium salts due to low stability.
Scheme 5.
Synthesis of alicyclic amine diazeniumdiolates.
3.1. Decomposition rate
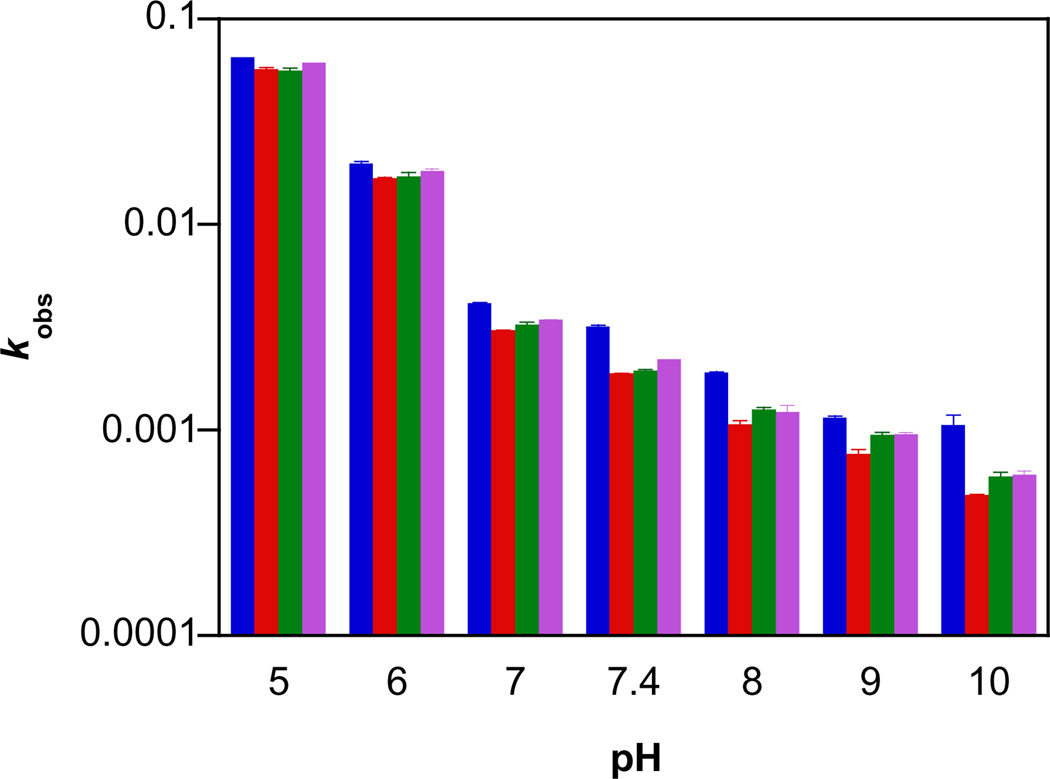
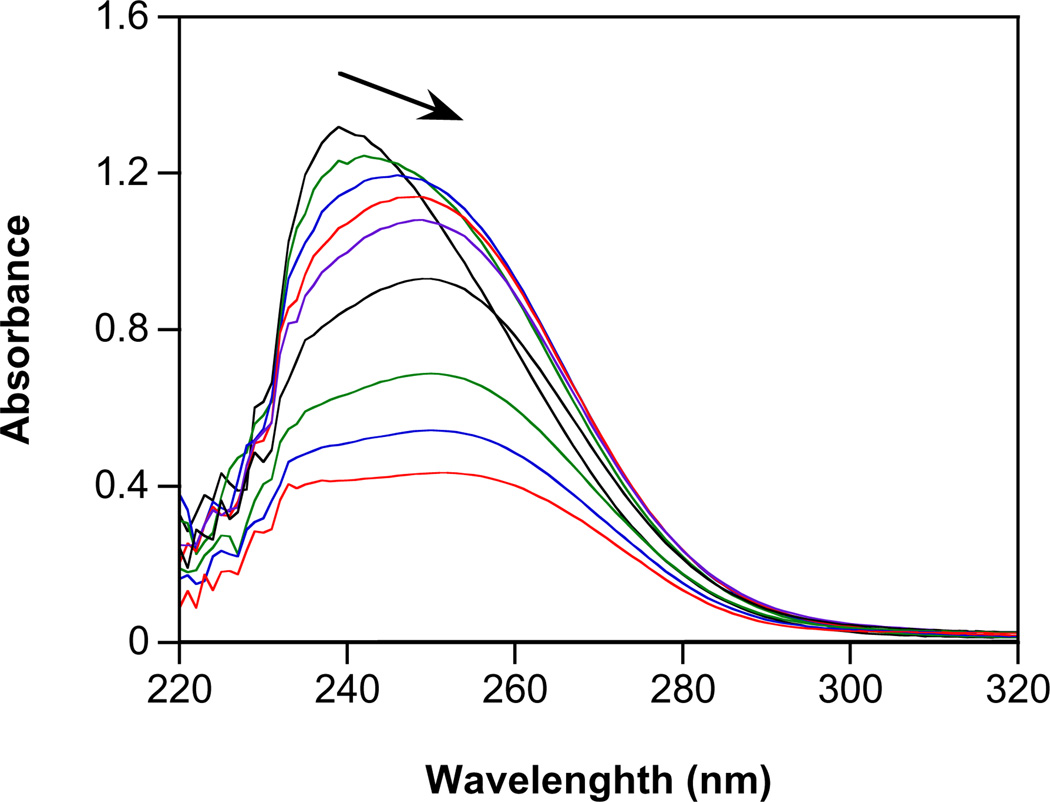
Diazeniumdiolates are typically stable as solids and in highly basic solution and can be stored at −20 °C for long periods [22]. Decomposition, which follows first order kinetics, is accelerated with decreasing pH. The newly prepared alicyclic amine diazeniumdiolates decayed as expected (Fig. 1, with 1 as the representative example). The rate constants of decomposition and half-lives at pH 7.4 and 37 °C are summarized in Table 1. Similar decay rates indicate a lack of a significant ring effect on the stability of these diazeniumdiolates. The deceleration of decomposition with increasing pH (Fig. 2; 100-fold variance in rate constant) is also consistent with other known ionic diazeniumdiolates [46,60].
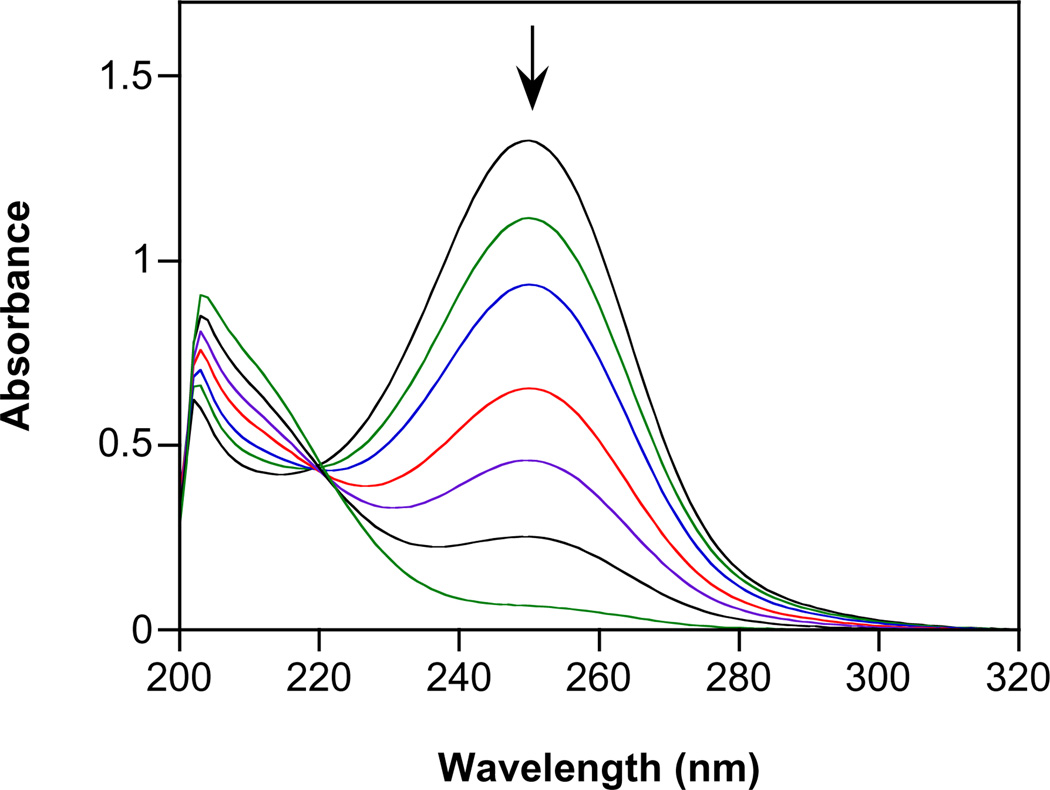
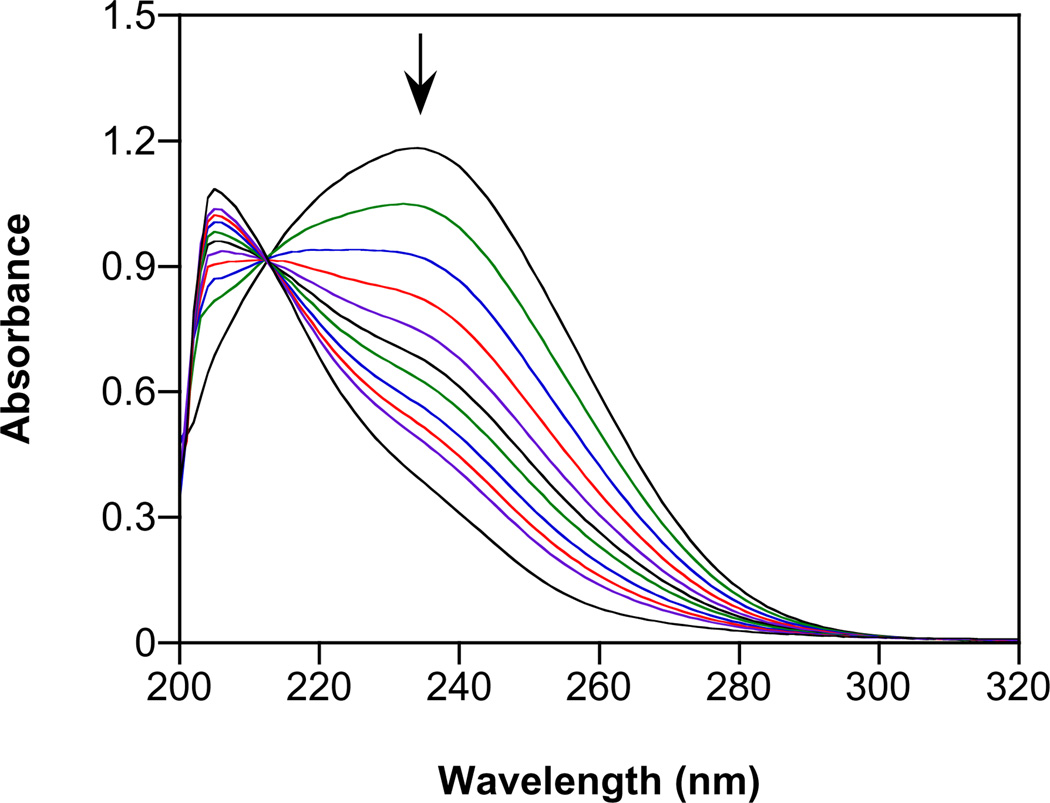
Fig. 1.
Spontaneous decomposition of 1 in assay buffer at pH 7.4 and at 37 °C. Spectra are shown at 0, 1, 2, 4, 6, 10 and 18 min.
Table 1.
Decomposition data for alicyclic amine diazeniumdiolates at pH 7.4 and 37 °C.
| Compound | k× 10−3 (s−1) | half-life (min) |
|---|---|---|
| 1 | 3.2 ± 0.07 | 3.6 ± 0.08 |
| 2 | 1. 9 ± 0.06 | 6.1 ± 0.02 |
| 3 | 1. 9 ± 0.01 | 5.9 ± 0.04 |
| 4 | 2.1 ± 0.02 | 5.4 ± 0.07 |
Fig. 2.
The pH-dependence of the first-order decomposition rate constants of 100 µM 1 (blue), 2 (red), 3 (green) or 4 (purple) at 37 °C in assay buffer measured at 250 nm (mean ± SD, n≥ 3).
3.2. NO/HNO release profile
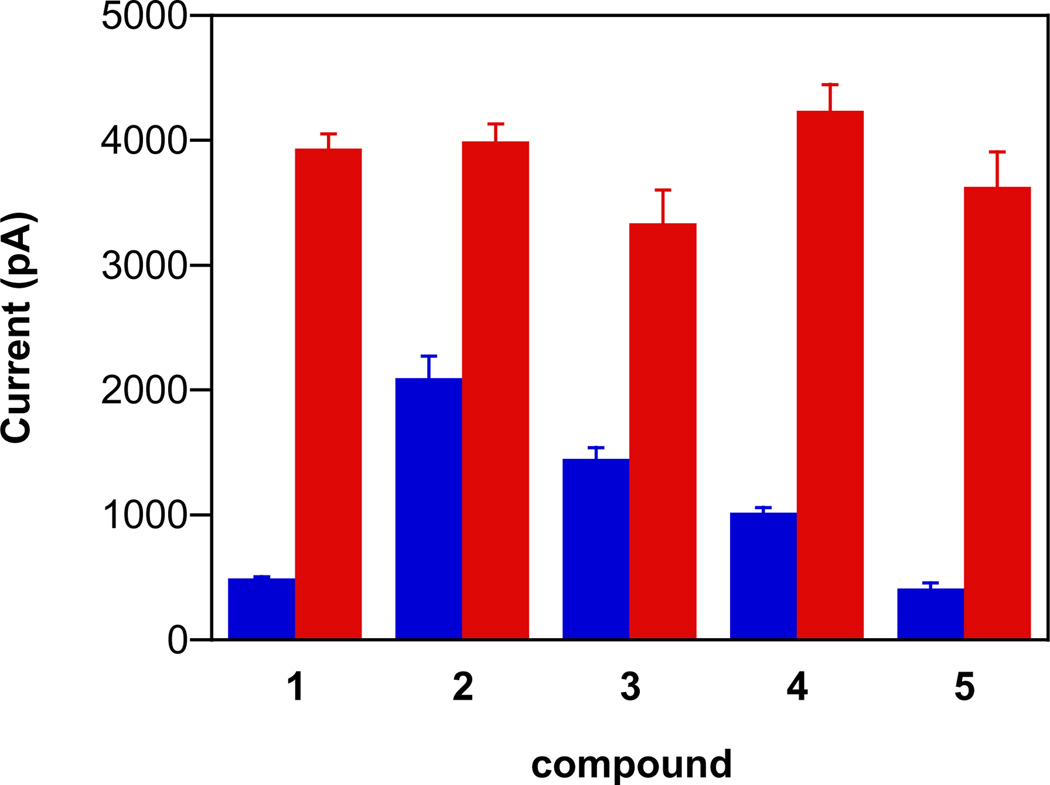
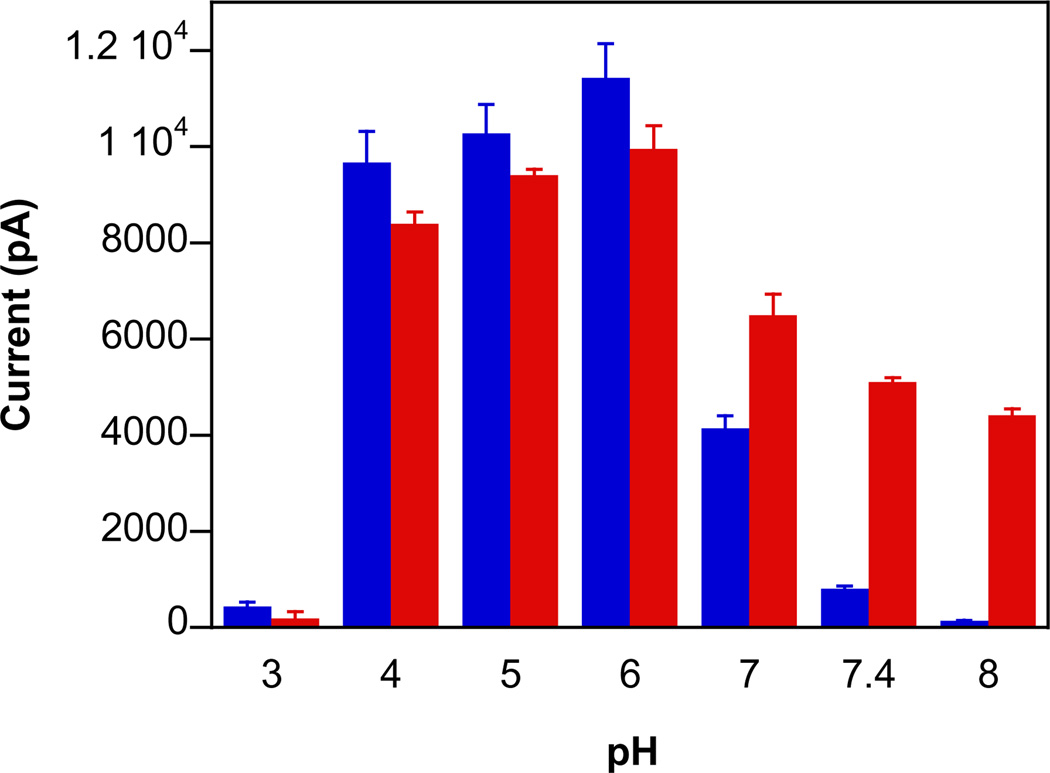
Numerous methods exist for detecting NO. Conversely, direct detection of HNO is complicated by irreversible dimerization [14,15], and assessment of HNO release is often indirect and qualitative. Our group has developed a protocol for HNO detection from donor compounds [47]. HNO production can be rapidly assessed with an NO-specific electrode in the presence of ferricyanide, which oxidizes HNO to NO [58]. The differences in the signal intensities for 1–4 in assay buffer alone (Fig. 3, blue bars) parallel the trend in decay rates (Table 1). The current intensity was elevated (2–8 fold) during decomposition of the ionic diazeniumdiolates in the presence of ferricyanide (Fig. 3, red bars). This elevation indicates significant production of HNO from these new primary amine-based diazeniumdiolates at physiological pH. The similar signal maxima also suggest similar product distribution for 1–4.
Fig. 3.
The maximum current intensity from an NO-specific electrode during decomposition of 50 µM 1–5 at pH 7.4 (blue bars) and with 1 mM ferricyanide (red bars) at room temperature (mean ± SD, n ≥ 3). For 5, the assay buffer contained 2% guinea pig serum.
As previously demonstrated with IPA/NO (5 µM) [47], the alicyclic amine diazeniumdiolates also exhibit a pH-dependent nitrogen oxide release profile (Fig. 4, with 1 as the representative example). At physiological pH and above, these alicyclic amine diazeniumdiolates primarily release HNO, as indicated by the increase of the signal intensity in the presence versus absence of ferricyanide. At lower pH, the independence of the signal intensity to ferricyanide denotes that NO is the major decomposition product. Thus, the release of HNO near physiological pH is established to be a general property of primary amine-based diazeniumdiolates. As these results are purely qualitative, the extent of HNO/NO production requires analysis by other techniques.
Fig. 4.
The pH-dependence of the maximum current intensity from an NO-specific electrode during decomposition of 5 µM 1 in assay buffer of pH 3–8 (blue bars) and with 1 mM ferricyanide (red bars) at room temperature (mean ± SD, n≥ 3).
3.3. Quantification of HNO release
We have recently developed a high throughput assay for quantitatively detecting HNO by trapping with GSH and labeling with a specific fluorogenic reagent [59]. At pH 7.4, the HNO yield was approximately 50% (Table 2). The small deviations between 1–4 are consistent with the trend in signal maximum as shown in Fig. 3 (red bars), and again suggesting little influence of ring size on the HNO donating capabilities. A decrease in pH to 7.0 reduced the yield of HNO by half. These HNO yields for 1–4 can be compared to 59 ± 2 and 36 ± 1% at pH 7.4 and 7.0, respectively, for IPA/NO [59]. Given the high sensitivity of HNO yield near neutral pH, use of primary amine diazeniumdiolates as HNO donors requires careful control of pH.
Table 2.
Percent HNO released from 1–5 at pH 7.0 or 7.4 using 50 µM GSH as the trapping agent.
| Compound | % HNO at pH 7.0 | % HNO at pH 7.4 |
|---|---|---|
| 1 | 28 ± 1 | 54 ± 4 |
| 2 | 25 ± 1 | 50 ± 4 |
| 3 | 24 ± 1 | 46 ± 4 |
| 4 | 31 ± 1 | 59 ± 2 |
| 5 | ND | 96 ± 3 |
A higher diazeniumdiolate concentration was used at pH 7.0 (60 µM) versus 7.4 (40 µM) to ensure that a detectable signal was generated. ND, not determined. For 5, the measurement was performed in the absence of serum.
3.4. Cell viability
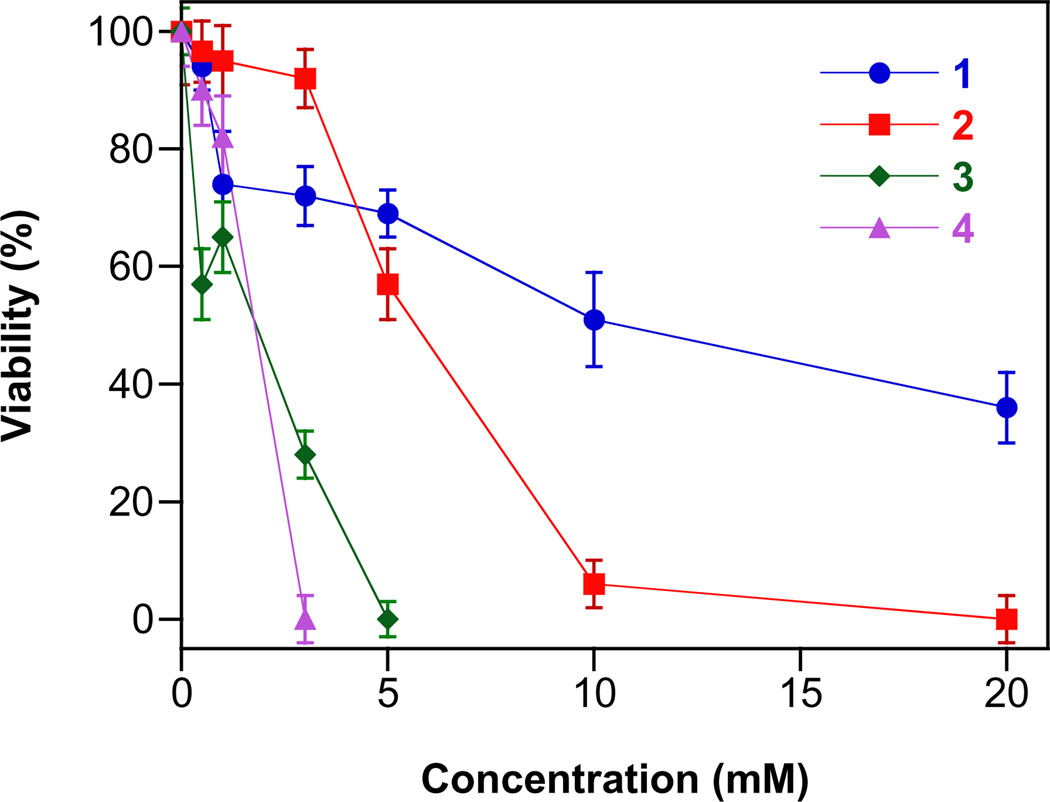
HNO donors have the potential to be applied in treatment of heart failure,7 as preconditioning agents against ischemia reperfusion injury [9] and as alcohol deterrents [11]. To be able to use primary amine-based diazeniumdiolates as pharmacological precursors of HNO, low cytotoxicity is a requirement. Typically, simple ionic diazeniumdiolates become cytotoxic at millimolar concentrations [13,58]. Similar cytotoxicity values were observed for 1–4 as evaluated by clonogenic assay in MCF-7 breast cancer cells (Fig. 5). The decomposed diazeniumdiolate products required higher initial concentrations of 1–4 (≥20 mM; data not shown), supporting a role of the released nitrogen oxides on cell viability.
Fig. 5.
The effects of 1–4 (0.5–20 mM) on the viability of MCF-7 cells (mean ± SD, n≥ 3).
HNO has also been shown to inhibit breast cancer [13] and neuroblastoma [61] proliferation in mouse xenografts as well as in culture, through increased apoptosis. Due in part to short half-life and spontaneous decomposition outside cells, ionic diazeniumdiolates may have limited potential from a chemotherapeutic point of view. The sensitivity of cells to released HNO will depend on the extent of HNO diffusion and consumption, for example by dimerization. Conversion to neutral species that can be activated (e.g., enzymatic, hydrolytic or photolytic) has been used as a strategy to overcome the limitations of the ionic precursors, including improvement of cellular uptake and delivery of HNO [43,50].
3.5. Synthesis of acetoxymethyl-protected diazeniumdiolates
The acetoxymethyl ester derivative of 1 was prepared (Scheme 6) by reacting with bromomethylacetate under anhydrous conditions. Acetoxymethyl derivatives of the other alicyclic diazeniumdiolates were also prepared similarly (data not shown), but AcOM-CPA/NO (5) in general was chosen for further detailed characterization due to the fact that the corresponding anion (1) had the lowest cytotoxicity among the four potential precursors (Fig. 5).
Scheme 6.
Synthesis of AcOM-CPA/NO (5).
3.6. Analysis of the decomposition of 5
In the presence of serum esterases, the half-life of decomposition of 5 at pH 7.4 and 37 °C (5.8 min) was similar to that of the parent diazeniumdiolate (1; 3.6 min). The initial peak at 238 nm shifted to 250 nm (Fig. 6), indicating cleavage of the ester bond and production of the free diazeniumdiolate (Scheme 2).
Fig. 6.
Hydrolysis of 5 in assay buffer containing 2% guinea pig serum at pH 7.4 and 37 °C. Spectra are shown at 0, 1, 2, 3, 4, 6, 10, 14 and 20 min. The noise at low wavelength is due the presence of serum proteins.
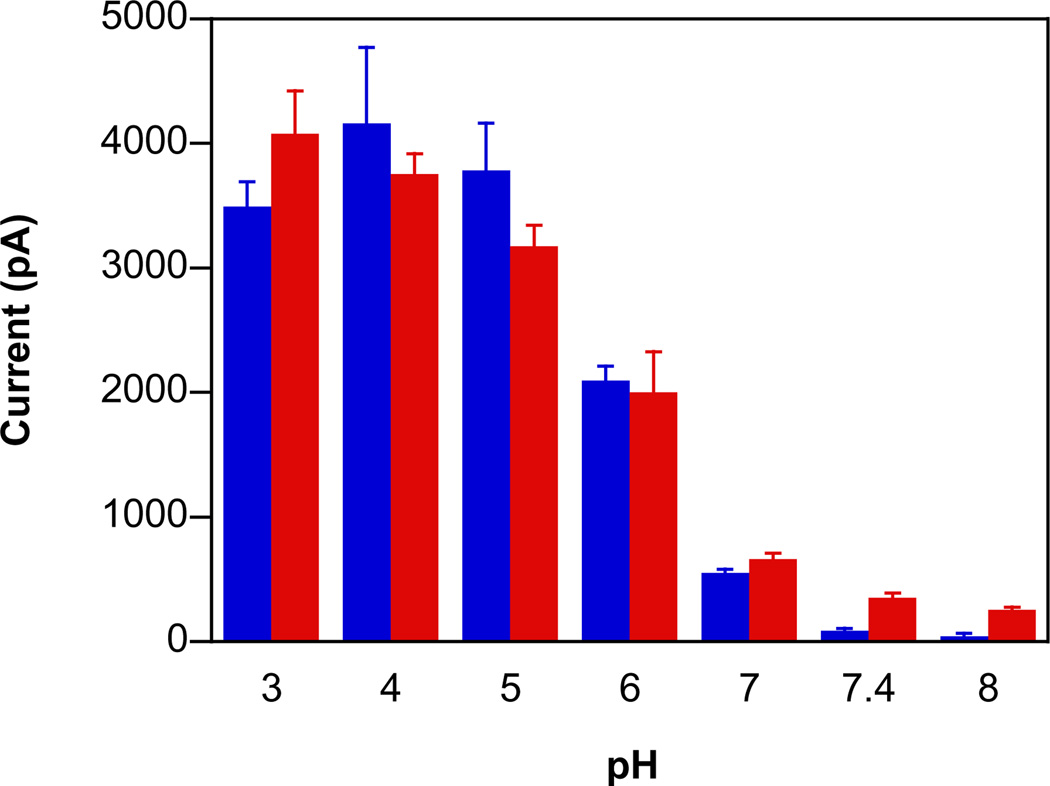
To further verify the esterase-mediated decomposition profile of 5, electrochemical assessment was performed in the presence of 2% guinea pig serum. The decomposition profile for 5 in the presence and absence of 1 mM ferricyanide (Fig. 7) at varied pH was also similar to that of the parent diazeniumdiolates (Figs 3 and Fig 4). This further reinforces the production of free 1 via Scheme 2.
Fig. 7.
The pH-dependent maximum current intensity from an NO-specific electrode during decomposition of 5 (100 µM) in assay buffer containing 2% guinea pig serum of pH 3–10 (blue bars) and with 1 mM ferricyanide (red bar) at room temperature (mean ± SD, n ≥ 3). The diminished signal at low pH is likely due to esterase deactivation. In order to observe signal at pH 3, all data were collected with a higher concentration than was used in Fig. 4.
In the absence of serum, the half-life of 5 increases to 21 min at pH 7.4 and 37 °C, and decomposition accelerates with increasing pH (0.3,1.3 and 5.0 × 10−3 s−1 at pH 7, 8 and 9, respectively). The lack of an apparent peak at 250 nm (Fig. 8) indicates that free diazeniumdiolate is not produced during decomposition. All of these data are consistent with the previously determined mechanism for acetoxymethyl protected IPA/NO (Scheme 4) [50].
Fig. 8.
Hydrolysis of 5 in assay buffer at pH 7.4 and 37 °C (n≥ 3). Spectra are shown at 0, 7, 13, 20, 27, 33, 40, 50, 60, 70 and 100 min.
Acetoxymethyl protected IPA/NO has been previously suggested to have enhanced HNO production over the parent compound due to a unique decomposition mechanism (Scheme 4) [50]. Here, the yield of HNO in the absence of serum was assessed by trapping with GSH [59] or metMb [17,55]. Under physiological conditions, HNO production from 5 was significantly enhanced compared to 1 (Table 2; 96 vs. 54%).
MetMb (50 µM) undergoes reductive nitrosylation (Eq. 1) with all the prepared alicyclic diazeniumdiolates (Fig. S1A) [10].
| (1) |
As with AcOM-IPA/NO [50], a higher yield of MbNO (Fig. S1B) was observed for 5 (100 µM), suggesting increased production of HNO. Trapping of HNO was quenched in the presence of 1 mM GSH (a known scavenger of HNO) [10,62], further confirming HNO production (Fig. S1A, B). Together, these data indicate that Scheme 4 is generally applicable for acetoxymethyl-protected primary amine diazeniumdiolates. Such species therefore provide a new class of donor for studies that require longer exposure to HNO.
3.7. Intracellular NO and HNO release
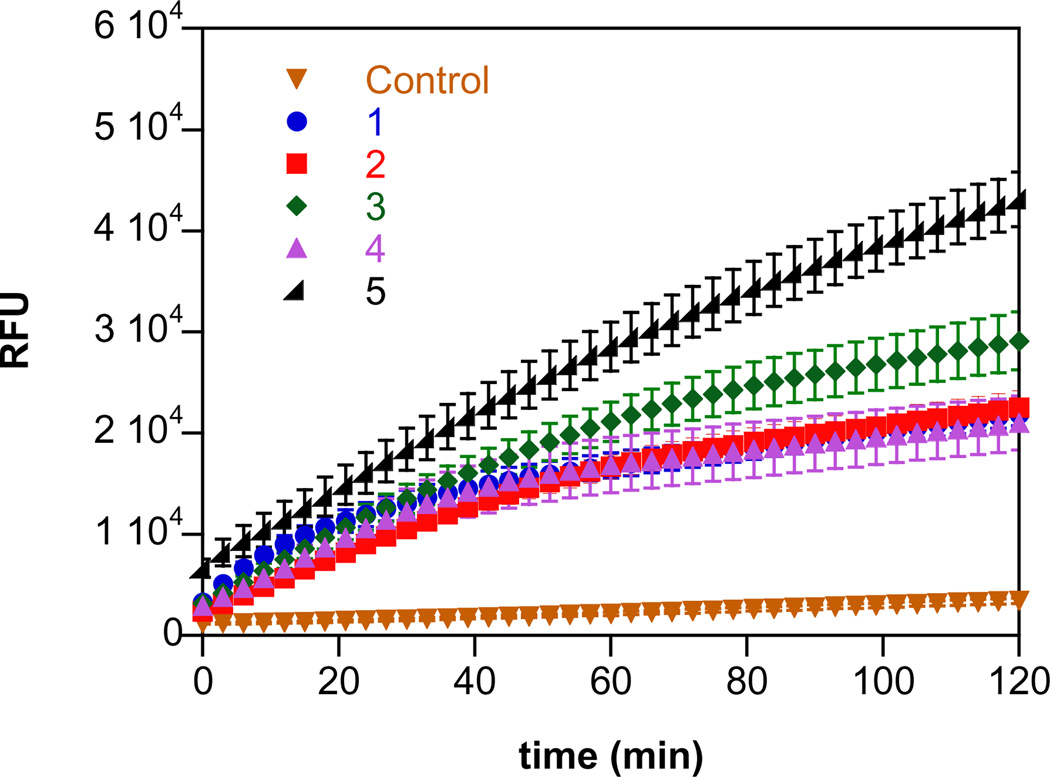
To determine whether hydrolysis of 5 will occur intracellularly, DAF-FM-2DA was used as a reporter molecule in MDA-MB-231 breast cancer cells. DAF reacts with the autoxidation products of both NO [63] and HNO [57], with higher fluorescence signal in the latter case. The diacetate DAF-FM-2DA is readily taken up by cells, where hydrolysis of the ester bonds by intracellular esterase produces the non-permeable DAF-FM. On treatment of DAF-FM-2DA loaded cells with 1–5, an increase in fluorescence intensity was observed in a time-dependent manner compared to control (Fig. 9). A similar signal was generated for the four ionic compounds while that for 5 was ~3 fold higher, signifying enhanced HNO release within the cells.
Fig. 9.
HNO/NO release measured in MB-231 cells. The cells were exposed to 100 µL of 10 µM DAF-FM-2DA (stock diluted in PBS) for 75 min at 37 °C and washed three times with PBS to remove excess dye. Upon addition of 10 µM 5 in ethanol (<0.1%; control) or 10 µM 1–4 in 10 mM NaOH, the increase in fluorescence intensity (RFU) at 535 nm was measured as a function of time at 37 °C following excitation at 485 nm. The data are expressed as mean ± SD (n = 2, six replicates per plate).
3.8. Cell viability
Under aerobic conditions HNO can be cytotoxic [58]. This is in part due to consumption of GSH but also due to formation of reactive nitrogen oxide species that mediate DNA double strand breaks [64,65]. HNO is also known to irreversibly inhibit enzymes containing critical thiols [66–68]. Inhibition of one such enzyme, glyceraldehyde phosphate dehydrogenase (GAPDH) [67], a key player in glycolysis, has chemotherapeutic potential, given that cancer cells have a higher dependence on glycolysis than normal cells (i.e., the Warburg effect).
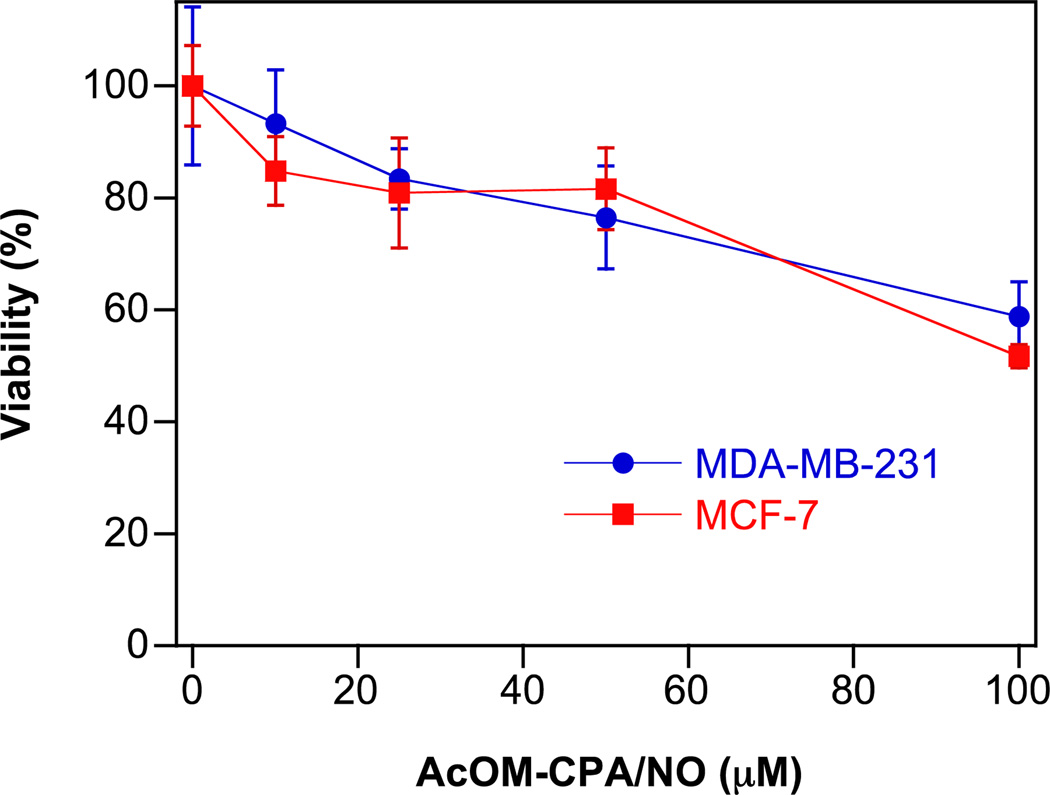
The cytotoxicity of 5 (Fig. 10) was substantially increased (IC50 of ca. 100 µM) compared to Angeli’s salt, which has been previously shown to inhibit the proliferation of human breast cancer cells (highly aggressive MDA-MB-231 and less aggressive MCF-7 cells) in the millimolar range [13]. The enhanced effect of 5 can be explained in part by increased uptake thereby leading to higher intracellular concentration of HNO.
Fig. 10.
The effect of 5 (10–100 µM) on the viability of MCF-7 or MDA-MB-231 cells (mean ± SD, n≥ 3).
3.9. Effect of 5 on the cytotoxicity of tamoxifen
NO releasing donor compounds have been previously reported to increase the cytotoxicity of cancer drugs such as cisplatin [69], melphalan [70] and doxorubicin [71] toward cancer cells. Such analysis has not been reported for HNO donors. Here, we evaluated the potential of using 5 in combination with tamoxifen for targeting ER-breast cancer.
Tamoxifen is used clinically worldwide in treatment of early and advanced stages of ER+ breast cancer [72]. Tamoxifen is also prescribed as a chemopreventive agent for women who are at high risk of developing breast cancer. Tamoxifen exerts its anticancer properties by competing with estrogen for binding to the estrogen receptor, which is crucial for breast cancer cell proliferation [73]. Tamoxifen has also been shown to induce apoptosis in ER-cancer cells in a receptor-independent manner, albeit at quite high concentrations [74]. Successful application of tamoxifen to a broader population of breast cancer patients requires development of effective therapeutic approaches in targeting more aggressive hormone-insensitive breast cancer patients.
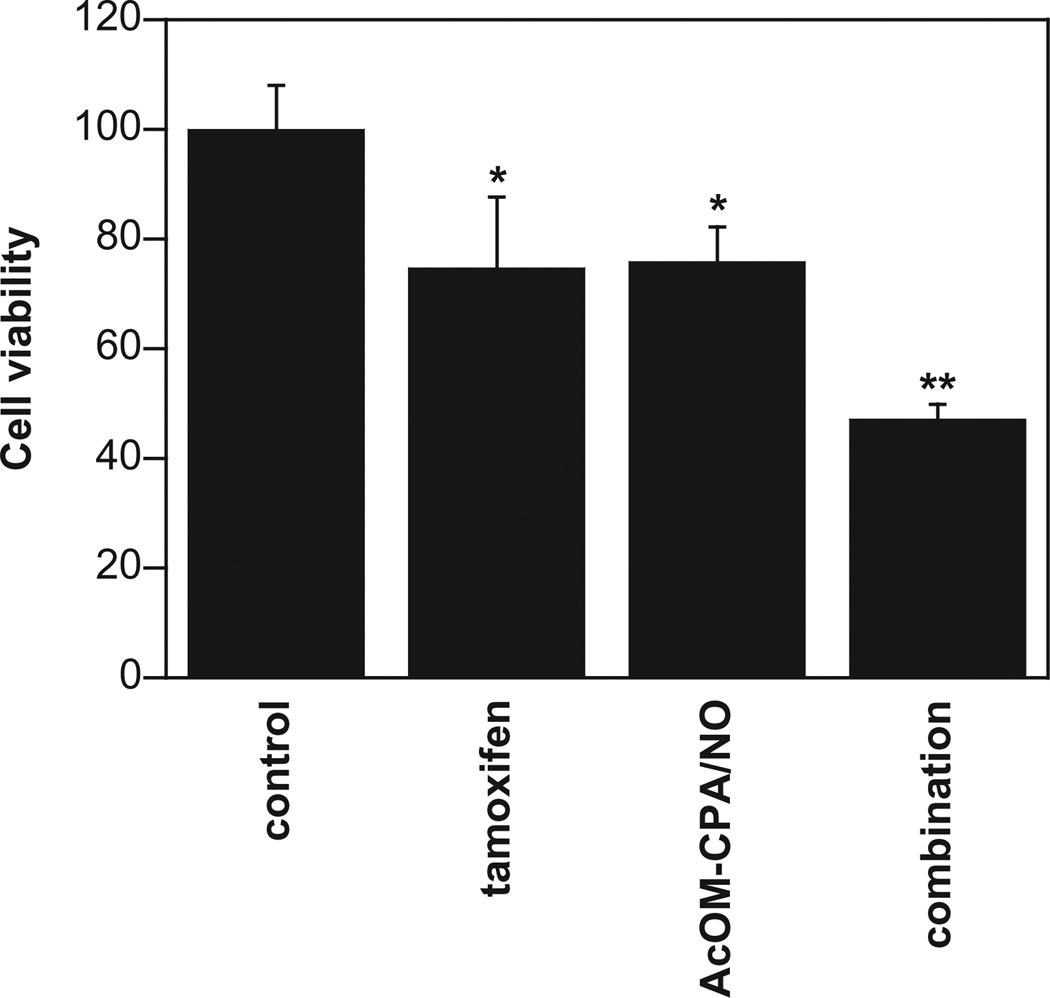
MB-231 cells were used for evaluating the combinatory potential of 5 with tamoxifen. Figure 11 shows the surviving fractions of MB-231 cells treated with tamoxifen (10 µM) or 5 (75 µM) with 76 ± 13 and 75 ± 6% viability, respectively. Co-administration of tamoxifen and 5 at these concentrations reduced cell viability to 47 ± 3%. It is expected that tamoxifen and HNO will have different molecular targets, thus providing multiple pathways to impact cell survival in combination. Moreover, long term, combined usage may lower occurrence of resistance compared to individual treatment. Further investigation into such antitumor effects may lead to a better understanding of the action of HNO in combination with other drugs and the intriguing possibility of using HNO in cancer treatment.
Fig. 11.
The effects of tamoxifen (10 µM), 5 (75 µM) or a combination on the viability of MDA-MB-231 cells (mean ± SD, n ≥ 3; *, p < 0.05 vs. control; **, p < 0.01 vs. control).
4. Conclusions
In conclusion, we have demonstrated that diazeniumdiolates provide a general class of HNO donor. Secondary amine-based diazeniumdiolates are commercially available, and our work suggests facile synthesis and potential commercialization of primary amine-based diazeniumdiolates. These diazeniumdiolates were shown to release NO/HNO in a manner similar to that reported for IPA/NO, thus establishing the decomposition mechanism to be common to primary amine-based diazeniumdiolates. Derivatization can dramatically change the production and release time of HNO, thus facilitating exploration of chronic HNO exposure. These features also hold promise in potential application for selectively targeting cancer cells. In the future, additional compounds will be synthesized to generate a library of HNO donors with a wide range of decomposition half-lives.
Supplementary Material
Acknowledgment
This work was supported by the National Institutes of Health (R01-GM076247 to KMM) and in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abbreviations
- AcOM-CPA/NO
O2-(acetoxymethyl)-1-(cyclopentylamino) diazen-1-ium-1,2-diolate
- Angeli’s salt
sodium trioxodinitrate
- CHA/NO
sodium 1-(N-cyclohexylamino)diazen-1-ium-1,2-diolate
- CHPA/NO
sodium 1-(N-cyclohep-tylamino)diazen-1-ium-1,2-diolate
- COA/NO
sodium 1-(N-cyclooctylamino) diazen-1-ium-1,2-diolate
- CPA/NO
sodium 1-(N-cyclopentylamino)diazen-1-ium-1,2-diolate
- DAF-FM-2DA
4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate
- DTPA
diethylenetriaminepentaacetic acid
- ER(−)
estrogen receptor α-negative
- ER(+)
estrogen receptor α-positive
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GSH
glutathione
- HNO
nitroxyl
- IPA/NO
sodium 1-(N-isopropylamino)diazen-1-ium-1,2-diolate
- metMb
ferric myoglobin
- MbNO
nitrosyl myoglobin
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NO
nitric oxide
- PBS
phosphate-buffered saline
- Piloty’s acid
N-hydroxylbenzenesulfonamide
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.niox.2014.08.013.
References
- 1.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl Acad. Sci. U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 3.MacMicking J, Xie Q, Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 4.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 5.Rees DD. In: Cardiovascular actions of nitric oxide, in. Fang C, editor. New York: Nitric Oxide and Infection Academic/Plenum; 1999. pp. 151–174. [Google Scholar]
- 6.Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, et al. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc. Natl Acad. Sci. U.S.A. 2001;98:10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paolocci N, Katori T, Champion HC, St John ME, Miranda KM, Fukuto JM, et al. Positive inotropic and lusitropic effects of HNO/NO-in failing hearts: independence from b-adrenergic signaling. Proc. Natl Acad. Sci. US.A. 2003;100:5537–5542. doi: 10.1073/pnas.0937302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis A, Li CG, Rand MJ. Differential actions of L-cysteine on responses to nitric oxide nitroxyl anions and EDRF in the rat aorta. Br. J. Pharmacol. 2000;129:315–322. doi: 10.1038/sj.bjp.0703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagliaro P, Mancardi D, Rastaldo R, Penna C, Gattullo D, Miranda KM, et al. Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radic. Biol. Med. 2003;34:33–43. doi: 10.1016/s0891-5849(02)01179-6. [DOI] [PubMed] [Google Scholar]
- 10.Miranda KM, Paolocci N, Katori T, Thomas DD, Ford E, Bartberger MD, et al. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc. Natl Acad. Sci. USA. 2003;100:9196–9201. doi: 10.1073/pnas.1430507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagasawa HT, DeMaster EG, Redfern B, Shirota FN, Goon DJ. Evidence for nitroxyl in the catalase-mediated bioactivation of the alcohol deterrent agent cyanamide. J. Med. Chem. 1990;33:3120–3122. doi: 10.1021/jm00174a001. [DOI] [PubMed] [Google Scholar]
- 12.DeMaster EG, Redfern B, Nagasawa HT. Mechanisms of inhibition of aldehyde dehydrogenase by nitroxyl the active metabolite of the alcohol deterrent agent cyanamide. Biochem. Pharmacol. 1998;55:2007–2015. doi: 10.1016/s0006-2952(98)00080-x. [DOI] [PubMed] [Google Scholar]
- 13.Norris AJ, Sartippour MR, Lu M, Park T, Rao JY, Jackson MI, et al. Nitroxyl inhibits breast tumor growth and angiogenesis. Int. J. Cancer. 2008;122:1905–1910. doi: 10.1002/ijc.23305. [DOI] [PubMed] [Google Scholar]
- 14.Smith PAS, Hein GE. The alleged role of nitroxyl in certain reactions of aldehydes and alkyl halides. J. Am. Chem. Soc. 1960;82:5731–5740. [Google Scholar]
- 15.Kohout FC, Lampe FW. On the role of the nitroxyl molecule in the reaction of hydrogen atoms with nitric oxide. J. Am. Chem. Soc. 1965;87:5795–5796. [Google Scholar]
- 16.King SB, Nagasawa HT. Chemical approaches toward generation of nitroxyl. Methods Enzymol. 1999;301:211–220. doi: 10.1016/s0076-6879(99)01084-8. [DOI] [PubMed] [Google Scholar]
- 17.Miranda KM, Nagasawa HT, Toscano JP. Donors of HNO. Curr. Top. Med. Chem. 2005;5:649–664. doi: 10.2174/1568026054679290. [DOI] [PubMed] [Google Scholar]
- 18.DuMond JF, King SB. The chemistry of nitroxyl-releasing compounds. Antioxid. Redox Signal. 2011;14:1637–1648. doi: 10.1089/ars.2010.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angeli A. Sopra la nitroidrossilammina. Gazz. Chim. Ital. 1896;26:17–25. [Google Scholar]
- 20.Hughes MN, Wimbledon PE. The chemisry of trioxodinitrates. Part I. Decompostion of sodium trioxodinitrate (Angeli’s salt) in aqueous solution. J. Chem. Soc. Dalton Trans. 1976:703–707. [Google Scholar]
- 21.Dutton AS, Fukuto JM, Houk KN. Mechanisms of HNO and NO production from Angeli’s salt: density functional and CBS-QB3 theory predictions. J. Am. Chem. Soc. 2004;126:3795–3800. doi: 10.1021/ja0391614. [DOI] [PubMed] [Google Scholar]
- 22.Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, et al. Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide - vasorelaxant effects. J. Med. Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 23.Gladwin MT. Nitrite as an intrinsic signaling molecule. Nat. Chem. Biol. 2005;1:245–246. doi: 10.1038/nchembio1005-245. [DOI] [PubMed] [Google Scholar]
- 24.Piloty O. Ueber eine oxydation des hydroxylamins durch benzolsulfochlorid. Ber. Dtsch. Chem. Ges. 1896;29:1559–1567. [Google Scholar]
- 25.Bonner FT, Ko YH. Kinetic, isotopic and N15 NMR-study of N-hydroxybenzenesulfonamide decomposition - an HNO source reaction. Inorg. Chem. 1992;31:2514–2519. [Google Scholar]
- 26.Zamora R, Grzesiok A, Weber H, Feelisch M. Oxidative release of nitric oxide accounts forguanylyl cyclase stimulating, vasodilator and anti-platelet activity of Piloty’sacid: a comparison with Angeli’s salt. Biochem. J. 1995;312:333–339. doi: 10.1042/bj3120333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkinson RN, Storey BM, King SB. Reactions of acyl nitroso compounds with amines: production of nitroxyl (HNO) with the preparation of amides. Tetrahedron Lett. 1996;37:9287–9290. [Google Scholar]
- 28.Cohen AD, Zeng BB, King SB, Toscano JP. Direct observation of an acyl nitroso species in solution by time-resolved IR spectroscopy. J. Am. Chem. Soc. 2003;125:1444–1445. doi: 10.1021/ja028978e. [DOI] [PubMed] [Google Scholar]
- 29.Howard JA, Ilyashenko G, Sparkes HA, Whiting A. Development of new transition metal catalysts for the oxidation of a hydroxamic acid with in situ Diels-Alder trapping of the acyl nitroso derivative. Dalton Trans. 2007:2108–2111. doi: 10.1039/b704728b. [DOI] [PubMed] [Google Scholar]
- 30.Lown JW. The reaction of lead tetraacetate with oximes. Part I. Alicyclic and aliphatic oximes. J. Chem. Soc. B. 1966:441–446. [Google Scholar]
- 31.Rehse K, Herpel M. New NO-donors with antithrombotic vasodilating activities Part 20. Azodioxides activated by electron acceptors in geminal or vicinal position. Arch. Pharm. 1998;331:104–110. doi: 10.1002/(sici)1521-4184(199803)331:3<104::aid-ardp104>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 32.Rehse K, Herpel M. New NO-donors with antithrombotic vasodilating activities Part 21. Pseudonitrosites and other azodioxides with vicinal electron acceptors. Arch. Pharm. 1998;331:111–117. doi: 10.1002/(sici)1521-4184(199803)331:3<111::aid-ardp111>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 33.Sha X, Isbell TS, Patel RP, Day CS, King SB. Hydrolysis of acyloxy nitroso compounds yields nitroxyl (HNO) J. Am. Chem. Soc. 2006;128:9687–9692. doi: 10.1021/ja062365a. [DOI] [PubMed] [Google Scholar]
- 34.Keefer LK, Flippen-Anderson JL, George C, Shanklin AP, Dunams TM, Christodoulou D, et al. Chemistry of the diazeniumdiolates. 1. Structural and spectral characteristics of the [N(O)NO](−) functional group. Nitric Oxide 5. 2001:377–394. doi: 10.1006/niox.2001.0359. [DOI] [PubMed] [Google Scholar]
- 35.Keefer LK. Fifty years of diazeniumdiolate research. From laboratory curiosity to broad-spectrum biomedical advances. ACS Chem. Biol. 2011;6:1147–1155. doi: 10.1021/cb200274r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hrabie JA, Klose JR, Wink DA, Keefer LK. New nitric oxide-releasing zwitterions derived from polyamines. J. Org. Chem. 1993;58:1472–1476. [Google Scholar]
- 37.Keefer LK, Nims RW, Davies KM, Wink DA. NONOates (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 38.Keefer LK. Progress toward clinical application of the nitric oxide-releasing diazeniumdiolates. Annu. Rev. Pharmacol. Toxicol. 2003;43:585–607. doi: 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]
- 39.Saavedra JE, Billiar TR, Williams DL, Kim YM, Watkins SC, Keefer LK. Targeting nitric oxide (NO) delivery in vivo. Design of a liver-selective NO donor prodrug that blocks tumor necrosis factor-alpha-induced apoptosis and toxicity in the liver. J. Med. Chem. 1997;40:1947–1954. doi: 10.1021/jm9701031. [DOI] [PubMed] [Google Scholar]
- 40.Hrabie JA, Keefer LK. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chem. Rev. 2002;102:1135–1154. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- 41.Saavedra JE, Bohle DS, Smith KN, George C, Deschamps JR, Parrish D, et al. Chemistry of the diazeniumdiolates. O-versus N-alkylation of the RNH[N(O)NO] - ion. J. Am. Chem. Soc. 2004;126:12880–12887. doi: 10.1021/ja031538i. [DOI] [PubMed] [Google Scholar]
- 42.Biswas D, Holland RJ, Deschamps JR, Cao Z, Keefer LK, Saavedra JE. O2-functionalized methylamine diazeniumdiolates: evidence for E ⇄ Z equilibration in an acyclic system. J. Org. Chem. 2012;77:10804–10810. doi: 10.1021/jo3020837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keefer LK. Nitric oxide (NO)- and nitroxyl (HNO)-generating diazeniumdiolates (NONOates): emerging commercial opportunities. Curr. Top. Med. Chem. 2005;5:625–636. doi: 10.2174/1568026054679380. [DOI] [PubMed] [Google Scholar]
- 44.Saavedra JE, Shami PJ, Wang LY, Davies KM, Booth MN, L Citro M, et al. Esterase-sensitive nitric oxide donors of the diazeniumdiolate family: in vitro antileukemic activity. J. Med. Chem. 2000;43:261–269. doi: 10.1021/jm9903850. [DOI] [PubMed] [Google Scholar]
- 45.Drago RS, Karstetter BR. The reaction of nitrogen(II) oxide with various primary and secondary amines. J. Am. Chem. Soc. 1961;83:1819–1822. [Google Scholar]
- 46.Salmon DJ, Torres de Holding CL, Thomas L, Peterson KV, Goodman GP, Saavedra JE, et al. HNO and NO release from a primary amine-based diazeniumdiolate as a function of pH. Inorg. Chem. 2011;50:3262–3270. doi: 10.1021/ic101736e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miranda KM, Katori T, Torres de Holding CL, Thomas L, Ridnour LA, McLendon WJ, et al. Comparison of the NO and HNO donating properties of diazeniumdiolates: primary amine adducts release HNO in vivo. J. Med. Chem. 2005;48:8220–8228. doi: 10.1021/jm050151i. [DOI] [PubMed] [Google Scholar]
- 48.Dutton AS, Miranda KM, Wink DA, Fukuto JM, Houk KN. Theoretical investigation of the mechanism of decomposition of monoalkylamine diazeniumdiolates: density functional theory and CBS-QB3 predictions. Inorg. Chem. 2006;45:2448–2456. doi: 10.1021/ic051505z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dutton AS, Fukuto JM, Houk KN. The mechanism of NO formation from the decomposition of dialkylamino diazeniumdiolates: density functional theory and CBS-QB3 predictions. Inorg. Chem. 2004;43:1039–1045. doi: 10.1021/ic0349609. [DOI] [PubMed] [Google Scholar]
- 50.Andrei D, Salmon DJ, Donzelli S, Wahab A, Klose JR, L Citro M, et al. Dual mechanisms of HNO generation by a nitroxyl prodrug of the diazeniumdiolate (NONOate) class. J. Am. Chem. Soc. 2010;132:16526–16532. doi: 10.1021/ja106552p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basudhar D, Bharadwaj G, Cheng RY, Jain S, Shi S, Ridnour LA, et al. Synthesis and comparison of nitroxyl and nitric oxide releasing diazeniumdiolate-based aspirin derivatives. J. Med. Chem. 2013;56:7804–7820. doi: 10.1021/jm400196q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holland RJ, Paulisch R, Cao Z, Keefer LK, Saavedra JE, Donzelli S. Enzymatic generation of the NO/HNO-releasing IPA/NO anion at controlled rates in physiological media using β-galactosidase. Nitric Oxide. 2013;35:131–1316. doi: 10.1016/j.niox.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez BE, Shinyashiki M, Han TH, Fukuto JM. Antioxidant actions of nitroxyl (HNO) Free Radic. Biol. Med. 2007;42:482–491. doi: 10.1016/j.freeradbiomed.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 54.Doyle MP, Mahapatro SN. Nitric oxide dissociation from trioxodinitrate(II) in aqueous solution. J. Am. Chem. Soc. 1984;106:3678–3679. [Google Scholar]
- 55.Doyle MP, Mahapatro SN, Broene RD, Guy JK. Oxidation and reduction of hemoproteins by trioxodinitrate(II): the role of nitrosyl hydride and nitrite. J.Am. Chem. Soc. 1988;110:593–599. [Google Scholar]
- 56.Miranda KM, Nims RW, Thomas DD, Espey MG, Citrin D, Bartberger MD, et al. Comparison of the reactivity of nitric oxide and nitroxyl with heme proteins. A chemical discussion of the differential biological effects of these redox related products of NOS. J. Inorg. Biochem. 2003;93:52–60. doi: 10.1016/s0162-0134(02)00498-1. [DOI] [PubMed] [Google Scholar]
- 57.Espey MG, Miranda KM, Thomas DD, Wink DA. Ingress and reactive chemistry of nitroxyl-derived species within human cells. Free Radic. Biol. Med. 2002;33:827–834. doi: 10.1016/s0891-5849(02)00978-4. [DOI] [PubMed] [Google Scholar]
- 58.Wink DA, Feelisch M, Fukuto J, Christodoulou D, Jourd’heuil D, Grisham MB, et al. The cytotoxicity of nitroxyl: possible implications for the pathophysiological role of NO. Arch. Biochem. Biophys. 1998;351:66–74. doi: 10.1006/abbi.1997.0565. [DOI] [PubMed] [Google Scholar]
- 59.Johnson GM, Chozinski TJ, Salmon DJ, Moghaddam AD, Chen HC, Miranda KM. Quantitative detection of nitroxyl upon trapping with glutathione and labeling with a specific fluorogenic reagent. Free Radic. Biol. Med. 2013;63:476–844. doi: 10.1016/j.freeradbiomed.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 60.Davies KM, Wink DA, Saavedra JE, Keefer LK. Chemistry of the diazeniumdiolates. 2. Kinetics and mechanism of dissociation to nitric oxide in aqueous solution. J. Am. Chem. Soc. 2001;123:5473–5481. doi: 10.1021/ja002899q. [DOI] [PubMed] [Google Scholar]
- 61.Stoyanovsky DA, Schor NF, Nylander KD, Salama G. Effects of pH on the cytotoxicity of sodium trioxodinitrate (Angeli’s salt) J. Med. Chem. 2004;47:210–217. doi: 10.1021/jm030192j. [DOI] [PubMed] [Google Scholar]
- 62.Wong PSY, Hyun J, Fukuto JM, Shirota FN, DeMaster EG, Shoeman DW, et al. Reaction between S-nitrosothiols and thiols: generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry. 1998;37:5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- 63.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, et al. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal. Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- 64.Miranda KM, Yamada K, Espey MG, Thomas DD, DeGraff W, Mitchell JB, et al. Further evidence for distinct reactive intermediates from nitroxyl and peroxynitrite: effects of buffer composition on the chemistry of Angeli’s salt and synthetic peroxynitrite. Arch. Biochem. Biophys. 2002;401:134–144. doi: 10.1016/S0003-9861(02)00031-0. [DOI] [PubMed] [Google Scholar]
- 65.Chazotte-Aubert L, Oikawa S, Gilibert I, Bianchini F, Kawanishi S, Ohshima H. Cytotoxicity and site-specific DNA damage induced by nitroxyl anion (NO) in the presence of hydrogen peroxide - implications for various pathophysiological conditions. J. Biol. Chem. 1999;274:20909–20915. doi: 10.1074/jbc.274.30.20909. [DOI] [PubMed] [Google Scholar]
- 66.Shen B, English AM. Mass spectrometric analysis of nitroxyl-mediated protein modification: comparison of products formed with free and protein-based cysteines. Biochemistry. 2005;44:14030–14044. doi: 10.1021/bi0507478. [DOI] [PubMed] [Google Scholar]
- 67.Lopez BE, Wink DA, Fukuto JM. The inhibition of glyceraldehyde-3-phosphate dehydrogenase by nitroxyl (HNO) Arch. Biochem. Biophys. 2007;465:430–436. doi: 10.1016/j.abb.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 68.Väänänen AJ, Salmenperä P, Hukkanen M, Rauhala P, Kankuri E. Cathepsin B is a differentiation-resistant target for nitroxyl (HNO) in THP-1 monocyte/ macrophages. Free Radic. Biol. Med. 2006;41:120–131. doi: 10.1016/j.freeradbiomed.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Wink DA, Cook JA, Christodoulou D, Krishna MC, Pacelli R, Kim S, et al. Nitric oxide and some nitric oxide donor compounds enhance the cytotoxicity of cisplatin. Nitric Oxide. 1997;1:88–94. doi: 10.1006/niox.1996.0108. [DOI] [PubMed] [Google Scholar]
- 70.Cook JA, Krishna MC, Pacelli R, DeGraff W, Liebmann J, Mitchell JB, et al. Nitric oxide enhancement of melphalan-induced cytotoxicity. Br. J. Cancer. 1997;76:325–334. doi: 10.1038/bjc.1997.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riganti C, Miraglia E, Viarisio D, Costamagna C, Pescarmona G, Ghigo D, et al. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005;65:516–525. [PubMed] [Google Scholar]
- 72.Osborne CK. Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 73.Jordan VC, Morrow M. Tamoxifen, raloxifene and the prevention of breast cancer. Endocr. Rev. 1999;20:253–278. doi: 10.1210/edrv.20.3.0368. [DOI] [PubMed] [Google Scholar]
- 74.Gelmann EP. Tamoxifen induction of apoptosis in estrogen receptor-negative cancers: new tricks for an old dog? J. Natl Cancer Inst. 1996;88:224–226. doi: 10.1093/jnci/88.5.224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.