Abstract
Adolescence is a period of high vulnerability for alcohol use and abuse. Early alcohol use has been shown to increase the risk for alcohol-related problems later in life; therefore effective preventive treatments targeted toward adolescents would be very valuable. Many epidemiological and longitudinal studies in humans have revealed the beneficial effects of exercise for prevention and treatment of alcohol addiction. Pre-clinical studies have demonstrated that access to a running wheel leads to decreased voluntary alcohol consumption in adult mice, hamsters, and rats. However, age and sex may also influence the effects of exercise on alcohol use. Herein, we studied male and female C57BL/6 adolescent mice using a 24-h two bottle choice paradigm to evaluate 21 days of concurrent voluntary exercise on alcohol consumption and preference. Given previously known effects of exercise in increasing the expression of brain-derived neurotrophic factor (BDNF) in the hippocampus and its role in regulating the reward system, BDNF mRNA and protein levels were measured at the end of the behavioral experiment. Our results demonstrate sex differences in the efficacy of voluntary exercise and its effects on decreasing alcohol consumption and preference. We also report increased BDNF expression after 21 days of voluntary exercise in both male and female mice. Interestingly, the distance travelled played an important role in alcohol consumption and preference in female mice but not in male mice. Overall, this study demonstrates sex differences in the effects of voluntary exercise on alcohol consumption in adolescent mice and points out the importance of distance travelled as a limiting factor to the beneficial effects of wheel running in female mice.
Keywords: Ethanol consumption, Wheel running, Adolescent mice, Two-bottle choice, Sex differences, Brain-derived neurotrophic factor
1. Introduction
It is well known that drinking alcohol beverages is a common feature of social gatherings due to its effects on improving mood and increasing self-confidence and sociability. However, the harmful use of alcohol leads to the death of 2.5 million people worldwide year (http://www.who.int/en/). The risk for alcohol use and abuse among adolescents may be particularly high since this is a period during which social contexts exert powerful influences. Moreover, behavioral characteristics common among adolescents, such as enhanced novelty seeking, sensation seeking and reward seeking behavior along with poor judgment and lack of impulsive control, are likely to contribute to increased risk for alcohol use [reviewed in 1,2,3,4]. Importantly, earlier initiation of alcohol use (prior to age 15) has been associated with increased risk for alcohol-related problems later in life [5]. Therefore, it is necessary to find preventive treatments targeted toward adolescents.
Physical activity has been proposed to be a potential non-pharmacological treatment for alcohol addiction. Epidemiological data obtained from adolescents show that, when engaged in regular exercise, teens are less likely to consume alcohol [6,7,8,9,10,11,12,13]. Longitudinal studies also show that persistently active individuals report lower levels of alcohol use, during both adolescence and early adulthood [14], as well as fewer alcohol-related problems, particularly in women [15]. However, exercise does not always prevent the use of alcohol and, in some specific situations where high level of physical activity is required, is associated with increased use of alcohol. For example, female athletes involved in mixed-gender sports and male athletes involved in male-dominated sports show higher rates of alcohol use [16,17,18], suggesting that gender and both the type and intensity of exercise may also influence alcohol use.
Studies from animal models have shown variability in the effects of exercise on voluntary alcohol consumption, depending on the behavioral model and gender examined [reviewed in 19]. The majority of these studies have been performed using adult animals, and little is known about the effects of wheel running on alcohol consumption during adolescence. It is important to characterize these effects, because not only is adolescence a period during which experimentation with alcohol begins, but it is also a period of brain maturation, making it highly vulnerable to environmental changes. Brain-derived neurotrophic factor (BDNF) has been shown to play a significant role in neuronal development and synaptic plasticity whose levels peak during adolescence with the highest level of BDNF detected in the hippocampus [reviewed in 20]. Furthermore, BDNF has been suggested to be a key molecule in regulating hippocampal functions, such as its role in the reward system [21] or the HPA axis activity [22]. Importantly, differences in expression of genes involved in stress-related pathways have been demonstrated in hippocampi from brains of deceased alcoholics [23]. On the other hand, exercise is rewarding, has been shown to increase BDNF levels in the hippocampus [reviewed in 24] and to alter the HPA axis responsiveness [25]. Collectively, these studies justify careful examination of this neurotrophin in the hippocampus to determine its possible mechanistic role on the effects of exercise on alcohol consumption during adolescence.
Thus, the first goal of this study was to examine the effects of 21 days of concurrent voluntary wheel running on ethanol consumption and preference in adolescent C57BL/6 mice. Our results demonstrate sex differences in the effects of exercise on alcohol drinking. In a second goal, we measured mRNA and protein levels of BDNF expression in an attempt to understand some of the underlying neuronal mechanisms contributing to these behaviors. As seen in previous animal studies [26], we observed differences in BDNF expression after ethanol and exercise exposure, suggesting that BDNF may be involved in responses to exercise and alcohol, which merits further study.
2. Material and Methods
2.1. Animals
Male and female adolescent (21–25 days) C57BL/6Ibg mice were obtained from breeder pairs at the Institute for Behavioral Genetics at the University of Colorado, Boulder (Boulder, Colorado). Animals were housed in standard mouse cages with ad libitum water and standard rodent chow (Harlan Laboratories, Indianapolis, Indiana) until the start of the experiment. The lighting in the animal colony was maintained on a 12-hour light/dark cycle with lights on at 7:00 AM. Room temperature was maintained at 23 ± 1.5°C.
This study was conducted with approval from the Institutional Animal Care and Use Committee at the University of Colorado, Boulder (Boulder, Colorado) following guidelines established by the Office of Laboratory Animal Welfare (OLAW). All possible measures were taken to minimize discomfort.
2.2. Behavioral paradigm
Male (n = 15–18/condition) and female (n = 14–18/condition) C57BL/6Ibg mice were weaned at 21–25 days old and placed in individual cages (332 × 150 × 130 mm), with or without running wheels (Coulbourn Instruments, PA, USA) and two cylinder tubes (25 mL) of water. After 2 days of acclimation, mice were tested using previously established 24-hour two-bottle choice paradigm conditions that lead to differences in voluntary ethanol consumption [27]. The four conditions included cages with 1) water only (Sedentary-Water), 2) water and 10% ethanol two-bottle choice (Sedentary-Ethanol), 3) water only with a running wheel (Exercised-Water), and 4) water and 10% ethanol two-bottle choice with a running wheel (Exercised-Ethanol). Mice housed with a running wheel had 24-hour access to the wheel. The protocol lasted 21 days. The side of the tubes was alternated every two days in order to account for any side preference. Individual consumption of water and ethanol (if applicable) were recorded daily. Distance and time were counted and recorded daily using a Cat Eye Mity 8 cyclocomputer (Cateye America, CO, USA). On day 21, mice were sacrificed by cervical dislocation, and brains were removed for subsequent BDNF expression analysis. The hippocampi of a group of mouse brains were dissected and stored at −70°C until the time to perform the below BDNF ImmunoAssay.
2.3. BDNF Immunoassay
BDNF protein was measured in the hippocampus of mice at the end of the behavioral experiment using a Promega BDNF Emax ImmunoAssay System (Promega Co., WI, USA) according to manufacturer’s protocol.
2.3.1. Extraction of protein from mouse brain hippocampi
Male (n = 5–6/condition) and female (n = 5–6/condition) hippocampi were removed from the freezer, and then weighed. Two milliliters of Promega lysis buffer (137 mM NaCl, 20 mM Tris-HCl (pH 8.0), 1% NP40, 10% Glycerol) were added to each 100 mg of tissue along with a protease inhibitor cocktail (Thermo Fisher Scientific, IL, USA). Samples were homogenized with a syringe and then sonicated with a Branson 1210 sonication water bath (EquipNet, MA, USA). Samples then were centrifuged at 16,000 × g for 30 min at 4°C. The resulting supernatant was pipetted into a new tube and stored at −70 °C until use.
2.3.2. BDNF protein quantification
Each well of a 96-well polystyrene plate, included in the Promega BDNF Emax ImmunoAssay kit, was coated overnight at 4°C, with anti-BDNF monoclonal antibody (mAb), by mixing 10 µL of mAb with 9.99 mL of carbonate coating buffer (25 mM sodium bicarbonate and 25 mM sodium carbonate, pH 9.7). Unabsorbed mAb was removed and plates were washed once with TBST wash buffer (20 mM Tris-HCl (pH 7.6), 150 mM NaCl and 0.05% (v/v) Tween 20). Plates were blocked using 200 µL Promega 1X Block & Sample buffer and incubated for 1-hour at room temperature. In the meantime, tissue extracts were removed from the freezer and thawed on ice. After 1-hour of incubation, plates were emptied and washed once in TBST. One hundred microliters of each sample or standard (1000, 750, 500, 300, 200, 100, 0 pg/mL) were added in triplicate to the plates and then incubated with shaking for 2-hours at room temperature. Plates were emptied and washed five times using TBST. One hundred microliters of 1:500 Anti-human BDNF polyclonal antibody (pAb), diluted in 1X Block & Sample buffer, was added to each well plate. Plates then were incubated again with shaking for 2-hours at room temperature. Plates were emptied and washed five times using TBST. One hundred microliters of 1:200 Anti-IgY horseradish peroxidase conjugate, diluted in 1X Block & Sample buffer, was added to each well plate. Plates then were incubated again with shaking for 1-hour at room temperature. Plates were emptied and washed again five times using TBST. Finally, plates were developed using 100 µL Promega TMB One Solution and the reaction was stopped using 100 µL 1 N HCl. Absorbance was measured at 450nm. BDNF levels are reported in pg/mg total protein ± SEM.
2.3.3. Total amount of protein quantification
Total amount of protein was measured for each sample using a BCA Protein Assay Kit (Thermo Fisher Scientific, IL, USA) according to manufacturer’s protocol. Briefly, 25 µl of each standard (albumin) or unknown sample and replicates (three per sample) were pipetted into an untreated 96 well microplate and mixed with 200 µl of working reagent (50:1, reagent A: reagent B). Samples were then incubated at 37°C for 30 min, and the absorbance was measured at 562 nm on an Epoch Microplate Spectrophotometer (BioTek Instruments, VT, USA). The albumin standards were used to construct a standard curve relating absorbance with protein concentration.
2.4. Bdnf in situ hybridization
In situ hybridization was performed in order to examine whether Bdnf expression in the hippocampus was subregion-specific. Frozen male (n = 3–4/condition) and female (n = 3–4/condition) mouse brains were sectioned coronally (14 µm) using a cryostat (Leica, Wetzlar, Germany), mounted on poly-L-lysine coated glass slides (ThermoFisher Scientific, MA, USA) and stored at −70°C. A previously established method for in situ hybridization of radiolabeled antisense riboprobes has been used [28]. Total Bdnf expression was measured using a probe from Bdnf exon IX. Briefly, C57BL/6Ibg mouse DNA was transcribed in vitro with 35S-UTP (PerkinElmer, MA, USA), as the sole source of UTP, using T7 RNA polymerase (Promega, WI, USA) and specific primers (Forward 5’-ggcgcccatgaaagaagtaa-3’; Reverse 5’-gaaattaatacgactcactatagggaagtgtacaagtccgcgtc-3’). Hybridizations were performed within 1 day of transcription. On the day of hybridization, after warming to room temperature, tissue was fixed first with a 4% paraformaldehyde solution (15 min), rinsed with 1X phosphate buffered saline (3 × 5 min), and then rinsed with 0.1 M TEA (2 min). Tissue was then acetylated with 0.25% acetic anhydride in 0.1 M TEA (15 min) and dehydrated in graded ethanol solutions of 50%, 70%, 95%, 100% and 100% (3 min each). Radiolabeled Bdnf riboprobe was diluted in a hybridization buffer containing 50% formamide, 10% dextran sulfate, 300 mM NaCl, 10 mM Tris, 1 mM EDTA, and 1X Denhardt’s solution, and ∼100 µL were pipetted onto a 24 mm × 60 mm coverslip, then placed upside down on a slide containing the tissue. Coverslips were sealed to slides using DPX mountant (Sigma-Aldrich, MO, USA). Tissue sections were hybridized with riboprobe solution for 16 hours at 60°C. After hybridization, tissue section slides were washed with 4X saline sodium citrate (SSC) before being treated with RNase A (20 µg/mL) for 1 hour at 37°C. Then, tissue sections were desalted by incubation in graded SSC solutions (all with 1 mM DTT) to a final stringency of 0.1 × SSC at 65°C. Finally, sections were dehydrated with graded ethanol solutions, dried, and exposed to PhosphoScreens (Packard BioScience, CT, USA) for at least 1 week. Slides were assayed at the same time to allow for direct comparisons between mice.
In order to relate the intensity of each screen image to a relative measure of tissue radioactivity, tissue standards containing known amounts of 35S were exposed along with tissue on each film. Ten tissue standards were prepared by mixing measured amounts of isotope with a homogenate prepared from whole brain. Actual concentrations of radioactivity were measured in weighted aliquots. The 35S standards contained from 0 to 25 nCi/mg. The tissue standards were used to construct standard curves relating optical density and a measure of radioactivity (counts per minute per mg). Exposed PhosphoScreens were imaged with a Cyclone PhosphoImage reader (Packard BioScience, CT, USA), and image .tif files (600 dpi) were imported into the OptiQuant analysis suite (Packard BioScience, CT, USA) for analysis. Intensity measures were performed, in the whole hippocampus (from bregma −0.94 mm to −2.80 mm), by someone blind to the testing conditions. Background intensity was subtracted from each sample, and values obtained were averaged for each animal.
2.5. Statistical analyses
All the statistical analyses were done using the R 3.0.1 Statistics Software. Three dependent variables with two groups in each variable (Sex (Male vs. Female), Treatment (Sedentary vs. Exercised), and Drink (Water vs. Ethanol)) were used to analyze the data. Two-way repeated measures ANOVAs (Sex x Treatment) were used to identify differences between groups in ethanol consumption and preference. Two-way repeated measures ANOVA (Sex x Drink) was also used to identify differences in daily distance travelled and time running. Three-way repeated measures ANOVA (Sex x Treatment x Drink) was used to determine differences in total fluid intake. Linear regression and Pearson’s correlation analyses were used to determine if significant correlations existed between ethanol preference or consumption and distance travelled. Protein and mRNA BDNF expression were analyzed using the three-way ANOVA (Sex x Treatment x Drink). Male and female data were analyzed separately when significant main effects of sex were found.
3. Results
3.1. Voluntary exercise decreases ethanol preference and consumption in adolescent C57Bl/6 mice
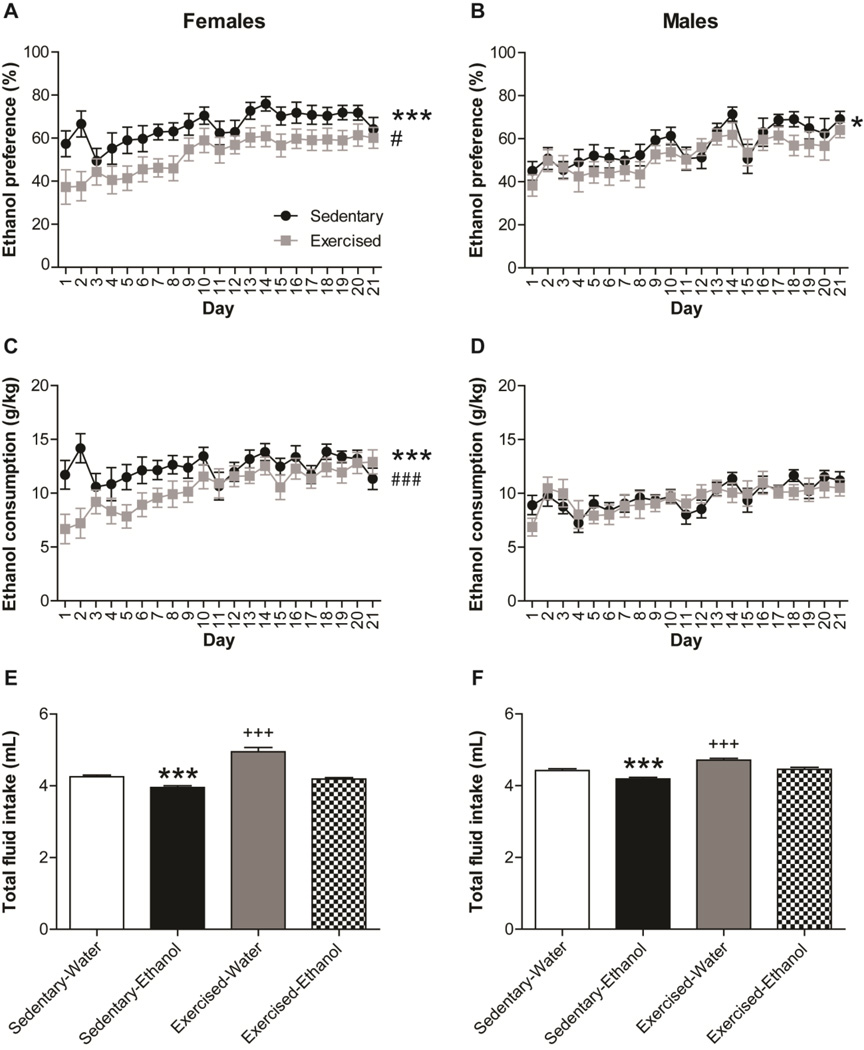
Two-way repeated measures ANOVA (Sex x Treatment) showed significant main effects of sex in ethanol preference (F(1,1334) = 8.573, p < 0.01; Fig. 1A, B) and consumption (F(1,1334) = 64.826, p < 0.001; Fig. 1C, D). Separate one-way repeated measures ANOVA showed a significant decrease in ethanol preference (F(1,666) = 26.842, p < 0.001; Fig. 1A) and consumption (F(1,666) = 15.027, p < 0.001; Fig. 1C) in female mice with access to exercise. However, the effect of voluntary exercise on ethanol preference and consumption decreased after several days of exposure as revealed by a statistically significant interaction (Treatment x Day), (Ethanol preference, F(1,666) = 5.685, p < 0.05; Ethanol consumption, F(1,666) = 11.771, p < 0.001; Fig. 1A, C). Male mice showed a less robust response to exercise. Indeed, a significant main effect of exercise was found in ethanol preference (F(1,666) = 5.704, p < 0.05; Fig. 1B) but not in ethanol consumption (F(1,666) = 0.070, ns; Fig. 1D).
Fig. 1.
Voluntary exercise decreases ethanol preference and consumption in adolescent C57Bl/6 mice. Graphs show (A, B) ethanol preference (%), and (C, D) ethanol consumption (g/kg) in (A, C) male and (B, D) female mice along 21 days. Black dots represent sedentary mice, and grey squares represent exercised mice. Total fluid intake (mL) is represented as the mean of the 21 days in (E) male and (F) female mice of the four different groups (Sedentary-Water, Sedentary-Ethanol, Exercised-Water, and Exercised-Ethanol). Data are shown as mean ± SEM; *p<0.05, and ***p<0.001 (Sedentary vs. Exercised); #p<0.05, and ###p<0.001 (Day x Treatment); +++p<0.001 (Water vs. Ethanol).
The three-way repeated measures ANOVA (Sex x Treatment x Drink) also showed significant sex differences in the total fluid intake (F(1,2670) = 8.780, p < 0.01; Fig. 1E, F). Separate two-way repeated measures ANOVA (Treatment x Drink) showed a significant increase in total fluid intake in exercised female (F(1,1313 = 11.949, p < 0.001; Fig. 1E) and male (F(1,1355 = 20.499, p < 0.001; Fig. 1F) mice compared to the “Sedentary” group, while access to ethanol showed a decrease in total fluid intake in both female (F(1,1313 = 64.989, p < 0.001; Fig. 1E) and male (F(1,1355 = 26.107, p < 0.001; Fig. 1F) mice compared to the “Water” group. Only female mice showed significant interaction (Treatment x Drink), (F(1,1313 = 14.493, p < 0.001; Fig. 1E).
3.2. Distance travelled and time running increase along days
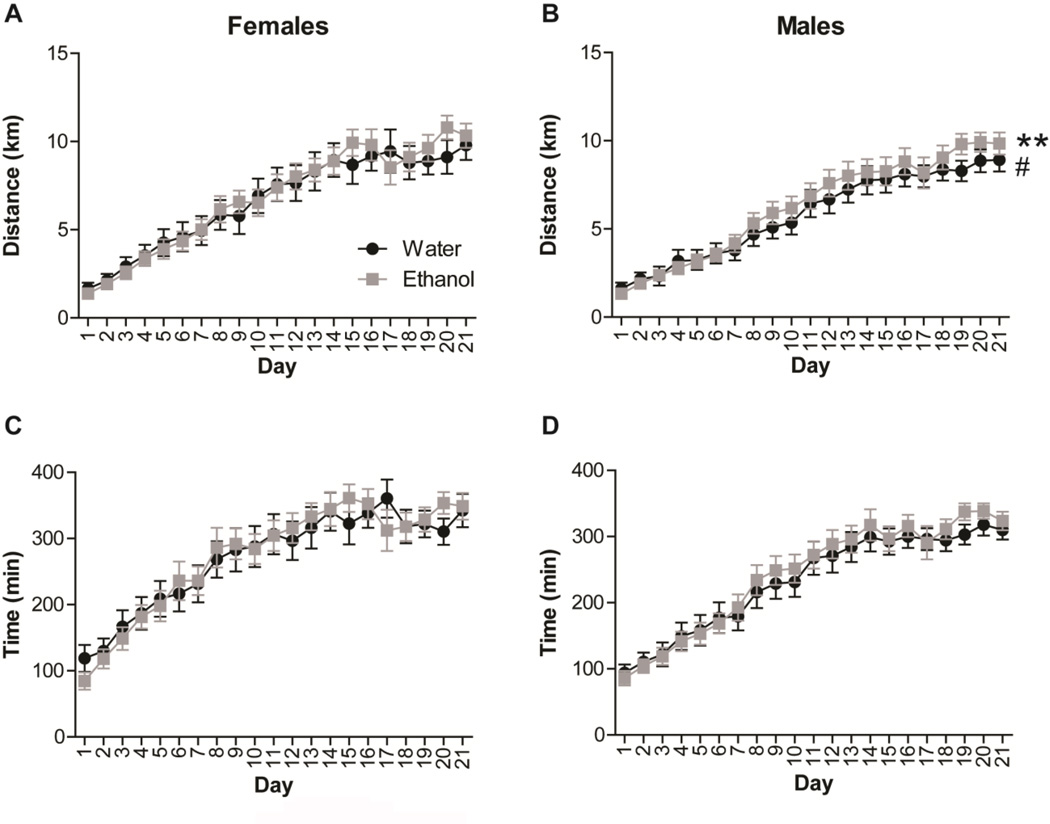
Two-way repeated measures ANOVA (Sex x Drink) revealed significant main effects of sex on distance travelled (F(1,1334) = 24.242, p < 0.001; Fig. 2A, B), and time running (F(1,1326) = 55.579, p < 0.001; Fig. 2C, D). Separate one-way repeated measures ANOVA showed a significant increase, along days, in distance travelled and time running in both female (Distance, F(1,666) = 21.490, p < 0.001; Time, F(1,663) = 11.583, p < 0.001; Fig. 2A, C) and male mice (Distance, F(1,666) = 77.094, p < 0.001; Time, F(1,661) = 48.886, p < 0.001; Fig. 2B, D). No significant differences between “Water” and “Ethanol” female mice groups were found in distance travelled (F(1,666) = 0.456, ns; Fig. 2A) or time running (F(1,663) = 0.170, ns; Fig. 2C). However, male mice with access to ethanol showed increased distance travelled (F(1,666) = 6.606, p = 0.01; Fig. 2B) and interaction (Day x Drink) (F(1,666) = 4.300, p > 0.05; Fig. 2B), although no significant differences were found in time running (F(1,661) = 3.329, ns; Fig. 2D).
Fig. 2.
Distance travelled and time running increase along days. Graphs show (A, B) distance travelled and (C, D) time running in (A, C) female and (B, D) male mice along 21 days of free access to a wheel running. Black dots represent mice with only access to water, and grey squares represent mice with access to ethanol. Data are shown as mean ± SEM; **p<0.01 (Sedentary vs. Exercised), #p<0.05 (Day x Drink).
3.3. The distance travelled in female mice is associated with its effects on ethanol preference and consumption
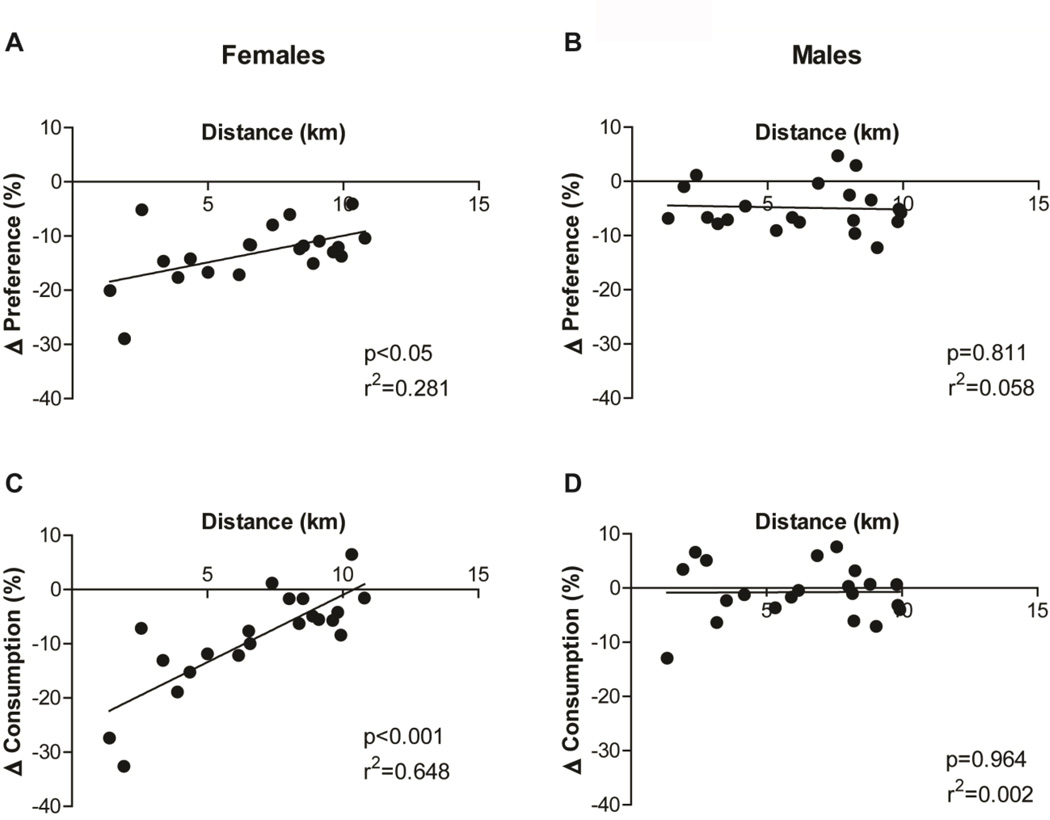
The effects of exercise on ethanol preference and consumption weakened, in female mice, as the distance travelled increased (Fig. 3). Pearson’s correlation analyses along with a linear regression test revealed significant correlations between the distance travelled and ethanol preference (r = 0.530, p < 0.05; Fig. 3A) or consumption (r = 0.805, p < 0.001; Fig. 3C) in female mice. In contrast, this correlation was not observed in male mice (Ethanol preference, r = −0.055, p = 0.811; Ethanol consumption, r = 0.01, p = 0.964; Fig. 3B, D)
Fig. 3.
The distance travelled in female mice is determinant to its effects on ethanol preference and consumption. Graphs show the correlation between the amount of distance travelled and the difference (Δ= “Exercised-Ethanol”-“Sedentary-Ethanol”) in (A, B) ethanol preference and (C, D) consumption of (A, C) female and (B, D) male mice. Linear regression p-value and r-square are shown on the right bottom of each graph.
3.4. Voluntary exercise increases protein BDNF expression in the hippocampus
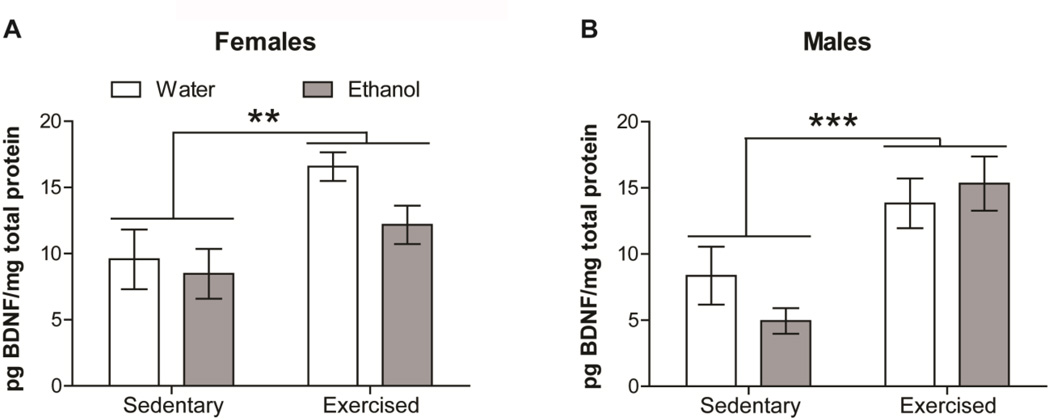
The three-way ANOVA (Sex x Treatment x Drink) revealed that voluntary exercise increases protein BDNF expression in the hippocampus (F(1, 35) = 28.272, p < 0.001; Fig. 4). No significant main differences between drinking groups (Water vs. Ethanol) were found (F(1, 35) = 2.166, ns). Although no main sex differences in protein BDNF expression were found (F(1, 35) = 0.879, ns), separate analyses and graphs were performed to follow the same structure as in the behavioral data. A significant main effect of exercise was found in both female (F(1, 16) = 9.631, p < 0.01; Fig. 4A) and male (F(1, 19) = 18.599, p < 0.001; Fig. 4B) mice. However, ethanol exposure did not affect BDNF protein expression (Female, F(1, 16) = 2.540, ns; Male, F(1, 19) = 0.352, ns). No significant interaction (Treatment x Drink) was found in any of the sexes (Female, F(1, 16) = 0.922, ns; Male, F(1, 19) = 1.813, ns).
Fig. 4.
Voluntary exercise increases protein BDNF expression in the hippocampus. Graphs show the amount of BDNF protein relative to the total amount of protein found in the hippocampus of (A) female and (B) male mice under the four experimental conditions (Sedentary-Water, Sedentary-Ethanol, Exercised-Water, and Exercised-Ethanol). Data are shown as mean ± SEM; **p<0.01, and ***p<0.001 (Sedentary vs. Exercised).
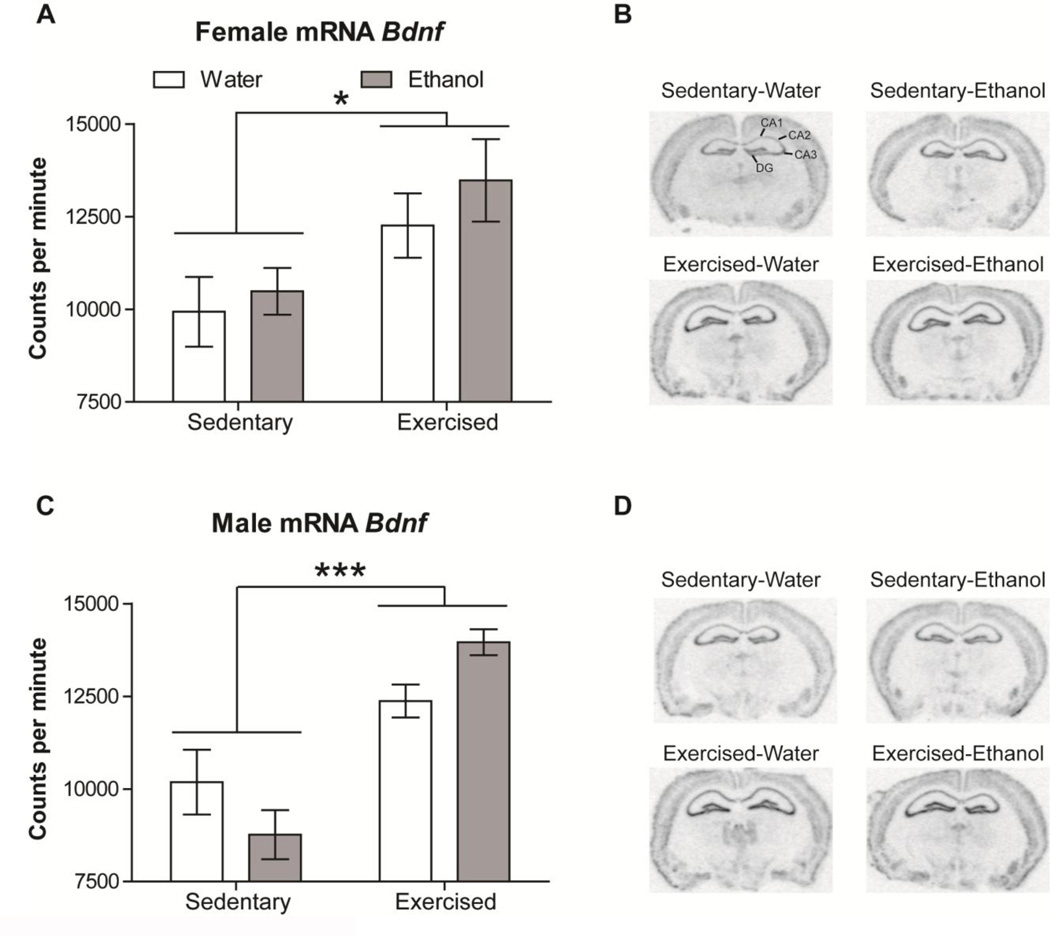
3.5. Voluntary exercise increases mRNA Bdnf expression in the hippocampus
Bdnf in situ hybridization showed similar results to those found at protein level. A three-way ANOVA (Sex x Treatment x Drink) showed a significant increase of Bdnf expression in mice with access to exercise (F(1, 20) = 31.513, p < 0.001; Fig. 5). However, no main effect of ethanol exposure (F(1, 20) = 0.693, ns; Fig. 5) was observed. Although no sex differences were found (F(1, 20) = 0.054, ns; Fig. 5), separate female and male analyses were performed to be consistent with other analyses. The increased Bdnf expression was found in both female (F(1, 10) = 8.408, p < 0.05; Fig. 5A, B) and male (F(1, 10) = 33.217, p < 0.001; Fig. 5C, D) exercised mice, when compared to the sedentary group. A significant interaction (Drink x Treatment) was found in male mice (F(1, 10) = 5.347, p < 0.05; Fig. 5C, D).
Fig. 5.
Voluntary exercise increases mRNA Bdnf expression in the hippocampus. Graphs show Bdnf mRNA expression (counts per minute) in the whole hippocampus of (A) female and (C) male mice under the four experimental conditions (Sedentary-Water, Sedentary-Ethanol, Exercised-Water, and Exercised-Ethanol). (B) Female and (D) male brain photographs obtained from 35S-UTP radiolabeled slices show intense mRNA Bdnf expression in the hippocampus. Data are shown as mean ± SEM; *p<0.05, and ***p<0.001 (Sedentary vs. Exercised).
Analyses of the different regions in the hippocampus were performed (Tables 1, 2). The dentate gyrus, CA2 and CA1 showed increased Bdnf expression in female (DG, F(1, 10) = 7.306, p < 0.05; CA2, F(1, 10) = 5.196, p < 0.05; CA1, F(1, 10) = 5.657, p < 0.05, Table 1) and male (DG, F(1, 10) = 82.855, p < 0.001; CA2, F(1, 10) = 20.079, p < 0.01; CA1, F(1, 10) = 5.602, p < 0.05, Table 2) mice with access to exercise. Although no effects of ethanol exposure were found, a significant interaction (Drink x Treatment) was observed in the dentate gyrus of male mice (F(1, 10) = 7.623, p < 0.05; Table 2).
Table 1.
Female hippocampal subregion-specific mRNA Bdnf expression. The table shows Bdnf expression of different hippocampal subregions (CA1, CA2, CA3 and Dentate gyrus (DG)) under the four experimental conditions (Sedentary-Water, Sedentary-Ethanol, Exercised-Water, and Exercised-Ethanol).
| Female Hippocampus Region |
Bdnf expression (counts per minute) | |||
|---|---|---|---|---|
|
Sedentary Water |
Sedentary Ethanol |
Exercised Water |
Exercised Ethanol |
|
| CA1 | 8952 ± 618 | 9571 ± 195 | 11429 ± 929 | 12815 ± 1953* |
| CA2 | 6262 ± 523 | 6614 ± 525 | 7925 ± 931 | 8694 ± 1136* |
| CA3 | 25082 ± 2105 | 28922 ± 2613 | 25493 ± 5567 | 28068 ± 1589 |
| DG | 14579 ± 1732 | 15272 ± 1372 | 17418 ± 1000 | 18925 ± 423* |
Data are shown as mean ± SEM;
p<0.05 (Sedentary vs. Exercised).
Table 2.
Male hippocampal subregion-specific mRNA Bdnf expression. The table shows Bdnf expression of different hippocampal subregions (CA1, CA2, CA3 and Dentate gyrus (DG)) under the four experimental conditions (Sedentary-Water, Sedentary-Ethanol, Exercised-Water, and Exercised-Ethanol).
| Male Hippocampus Region |
Bdnf expression (Counts per minute) | |||
|---|---|---|---|---|
|
Sedentary Water |
Sedentary Ethanol |
Exercised Water |
Exercised Ethanol |
|
| CA1 | 9202 ±1035 | 7627 ±789 | 9558 ± 675 | 11256 ±689* |
| CA2 | 6552 ± 796 | 5391 ±594 | 8311 ±415 | 8887 ± 273 ** |
| CA3 | 26449 ±1158 | 24619 ±2300 | 25113 ±375 | 28656 ±2136 |
| DG | 14796 ±894 | 13283 ±663 | 19248 ±596 | 21731 ±545***# |
Data are shown as mean ± SEM;
p<0.05,
p<0.01 and
p<0.001 (Sedentary vs. Exercised),
p<0.05 (Drink x Treatment).
4. Discussion
Numerous studies in human and animal models support the idea that aerobic exercise may be an effective treatment for reducing alcohol consumption in all phases of the alcohol addiction process [19]. However, few animal studies have been performed using adolescents, a period of high vulnerability to drug use. In this work, we have focused on the effects of concurrent exercise, in the form of voluntary wheel running, on ethanol consumption in adolescent C57BL/6 female and male mice. Our results reproduce the effects of exercise decreasing ethanol consumption and preference previously seen in adult mice [27,29,30], and hamsters [31,32]. As previously reported in other studies [reviewed in 24], exercise increased hippocampal BDNF expression in both female and male mice, suggesting a potential role of this neurotrophin in the adaptive response to wheel running.
Individually, ethanol consumption [reviewed in 33] and wheel running [34,35,36,37] are reinforcing and rewarding in rodents. Indeed, our results show an escalated ethanol consumption and preference in all groups of mice tested, and an increase in distance travelled and time on wheel running along experimental days, a characteristic of reinforcing and rewarding stimuli when chronically presented [reviewed in 38]. Therefore, the idea of a hedonic substitution between ethanol consumption and wheel running suggested by some authors in rats [39], mice [40], and hamsters [31] could potentially explain the decrease in ethanol consumption and preference observed in our exercised adolescent C57BL/6 mice. Interestingly, this effect was less robust in male mice, supporting previously reported sex differences in the effects of voluntary exercise decreasing alcohol consumption [27]. Part of this sex difference may be explained by the fact that female C57Bl/6 mice show higher alcohol preference compared to males so any decrease will be easier to observe [41,42]. Genetic predisposition may also contribute to individual differences based in part on baseline levels of alcohol preference. For example, DBA/2J mice, a strain that avoids alcohol, do not show any effects of exercise on voluntary consumption [29]. Therefore, overall effects of exercise on alcohol consumption in humans are likely to be influenced by many factors, including sex and genetic predisposition. It will be important to test other strains of mice to determine whether differences observed in C57BL/6 may be generalized in the genetic context, since such differences could have implications for treatment in a genetically heterogeneous human population.
Disruption of the HPA axis, a system implicated in stress-induced escalations of drug seeking behaviors [50] hyper-responsive during adolescence [51,52], has been associated with alcoholism in clinical and animal studies [23, 53]. Wheel running, although rewarding, is also considered a non-aversive stimulus that activates the hypothalamic-pituitary-adrenal (HPA) axis [44,45,46,47,48,49], suggesting the HPA axis may be a neurobiological pathway common to the adaptive effects of chronic alcohol exposure and exercise. However, the observed behavioral effects are different between males and females. It is possible that these differences may be mediated by sex-specific HPA axis responsiveness to exercise and alcohol exposure through differences in sex hormones. This is based on the fact that reproductive hormones predominately regulate the HPA axis activity in females but not in males [25]. Furthermore, one study showed that 17β-estradiol, the predominant sex steroid hormone in females, is required for the sexually dimorphic effects of repeated alcohol exposure on the HPA axis during adolescence [54].
The hippocampus is a brain region that has been shown to play an important role in the negative feedback regulation of the HPA axis [55,56], thus alterations or adaptive changes in this region due to chronic exposure to exercise may have a negative impact on its effects decreasing alcohol consumption. Specifically, the neurotrophin BDNF, involved in the regulation of the HPA axis [58,59], is a strong candidate for mediating the effects of ethanol [60] and exercise [24] in the hippocampus. Indeed, we found a negative correlation between mRNA BDNF expression in the hippocampus and alcohol preference in male mice, which supports our hypothesis that this neurotrophin may be involved in decreasing alcohol consumption (data not shown). In contrast, female mice did not show any correlation between mRNA BDNF expression and alcohol preference, maybe because the effects of distance travelled on alcohol consumption are masking the effects of BDNF. Importantly, several studies have demonstrated that alcohol exposure is associated with lower hippocampal volume in humans and a reduction of hippocampal neurogenesis in animals, and that these differences are more consistently found in adolescents than in adults [reviewed in 57] suggesting that voluntary exercise is likely maintaining or restoring the correct function of the hippocampus through increasing the expression of BDNF.
Based on the behavioral sex differences observed in the present study, we expected to find sex differences in BDNF expression. However, we did not find main effect sex differences on the protein expression of BDNF in the whole hippocampus. It is known that voluntary exercise increases BDNF expression in the hippocampus, but this increase is different among sub-regions. While BDNF is highly increased in the CA1 sub-region after voluntary exercise, it is sustained in CA3 and dentate gyrus [61]. In order to study whether the effects of voluntary exercise were sub-region specific, we studied the mRNA expression of BDNF in CA1, CA2, CA3 and dentate gyrus hippocampal sub-regions. We did not find main effect sex differences of voluntary exercise on BDNF expression levels in any of the sub-regions. One alternative explanation is that BDNF could be expressed in different neuronal locations in male and female mice, thus differently affecting the neuronal function and its consequences on behavior. Previous studies have shown that wheel running differentially modifies exon-specific BDNF expression in the hippocampus of male and female mice [62]. BDNF expression is regulated by different promoters [63], which generate different BDNF transcripts. These different transcripts are targeted to specific locations in the cell (e.g. dendrites, cell body) [64,65] and differ in their overall mRNA stability and translational efficiency [66]. Thus, the study of exon-specific BDNF expression would be needed to better understand its role on the sex-specific effects of ethanol consumption and voluntary exercise. Another possible explanation could be the involvement of other brain regions, such as the prefrontal cortex, ventral tegmental area, striatum, or amygdala, where BDNF expression has been also associated with alcohol addiction [26, 67, 68]. However, so far the hippocampus is the only region where a strong increase in BDNF has been observed after exposure to chronic voluntary exercise [61, 69].
5. Conclusions
In conclusion, concurrent voluntary exercise, in the form of wheel running, decreases ethanol consumption and preference in adolescent mice, likely maintaining the hedonic homeostasis system. The effect of voluntary exercise on this ethanol-behavior is more efficient in female than male mice, probably because of sex differences in the rewarding effects of exercise and the role of sex hormones on mediating the HPA axis activity. However, the distance travelled seems to contribute to ethanol consumption and preference in adolescent female mice. Although the mechanism underlying this effect is unknown, the stress responsiveness and adaptation to ethanol consumption and wheel running through its regulation by the hippocampus could play an important role. The increased expression of BDNF in the hippocampus after exercise could be a mechanism to protect the integrity and functionality of this region. However, the study of exon-specific BDNF expression may elucidate the sex differences observed in the effects of exercise on alcohol consumption. Additional studies are needed to better understand the effects of non-aversive (i.e. exercise) and aversive HPA axis-activating stimuli on ethanol consumption and the common mechanisms directing the behavioral response.
Highlights.
We studied the effects of wheel running on ethanol consumption in adolescent mice
We identified sex-differences in the effects of exercise on alcohol drinking
The amount of distance travelled affects ethanol consumption in female mice
Hippocampal BDNF expression increased after voluntary exercise
Acknowledgements
We thank Dr. Benjamin Greenwood for comments and advice.
Support - This works was supported by a Grant from National Institutes of Health (NIH R01AA017889 (MAE effort)) and the University of Colorado funds
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Blakemore SJ. The social brain in adolescence. Nature reviews Neuroscience. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 2.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology, biochemistry, and behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 4.Donovan JE. Adolescent alcohol initiation: a review of psychosocial risk factors. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2004;35:529. doi: 10.1016/j.jadohealth.2004.02.003. e527–518. [DOI] [PubMed] [Google Scholar]
- 5.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of substance abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 6.Field T, Diego M, Sanders CE. Exercise is positively related to adolescents’ relationships and academics. Adolescence. 2001;36:105–110. [PubMed] [Google Scholar]
- 7.Kirkcaldy BD, Shephard RJ, Siefen RG. The relationship between physical activity and self-image and problem behaviour among adolescents. Social psychiatry and psychiatric epidemiology. 2002;37:544–550. doi: 10.1007/s00127-002-0554-7. [DOI] [PubMed] [Google Scholar]
- 8.Kulig K, Brener ND, McManus T. Sexual activity and substance use among adolescents by category of physical activity plus team sports participation. Archives of pediatrics & adolescent medicine. 2003;157:905–912. doi: 10.1001/archpedi.157.9.905. [DOI] [PubMed] [Google Scholar]
- 9.Martinsen M, Sundgot-Borgen J. Adolescent elite athletes’ cigarette smoking, use of snus, and alcohol. Scandinavian journal of medicine & science in sports. 2012 doi: 10.1111/j.1600-0838.2012.01505.x. [DOI] [PubMed] [Google Scholar]
- 10.Pate RR, Heath GW, Dowda M, Trost SG. Associations between physical activity and other health behaviors in a representative sample of US adolescents. American journal of public health. 1996;86:1577–1581. doi: 10.2105/ajph.86.11.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pate RR, Trost SG, Levin S, Dowda M. Sports participation and health-related behaviors among US youth. Archives of pediatrics & adolescent medicine. 2000;154:904–911. doi: 10.1001/archpedi.154.9.904. [DOI] [PubMed] [Google Scholar]
- 12.Strohle A, Hofler M, Pfister H, Muller AG, Hoyer J, et al. Physical activity and prevalence and incidence of mental disorders in adolescents and young adults. Psychological medicine. 2007;37:1657–1666. doi: 10.1017/S003329170700089X. [DOI] [PubMed] [Google Scholar]
- 13.Terry-McElrath YM, O’Malley PM. Substance use and exercise participation among young adults: parallel trajectories in a national cohort-sequential study. Addiction. 2011;106:1855–1865. doi: 10.1111/j.1360-0443.2011.03489.x. discussion 1866–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson MC, Gordon-Larsen P. Physical activity and sedentary behavior patterns are associated with selected adolescent health risk behaviors. Pediatrics. 2006;117:1281–1290. doi: 10.1542/peds.2005-1692. [DOI] [PubMed] [Google Scholar]
- 15.Korhonen T, Kujala UM, Rose RJ, Kaprio J. Physical activity in adolescence as a predictor of alcohol and illicit drug use in early adulthood: a longitudinal population-based twin study. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2009;12:261–268. doi: 10.1375/twin.12.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore MJ, Werch CE. Sport and physical activity participation and substance use among adolescents. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2005;36:486–493. doi: 10.1016/j.jadohealth.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Rainey CJ, McKeown RE, Sargent RG, Valois RF. Patterns of tobacco and alcohol use among sedentary, exercising, nonathletic, and athletic youth. The Journal of school health. 1996;66:27–32. doi: 10.1111/j.1746-1561.1996.tb06254.x. [DOI] [PubMed] [Google Scholar]
- 18.Aaron DJ, Dearwater SR, Anderson R, Olsen T, Kriska AM, et al. Physical activity and the initiation of high-risk health behaviors in adolescents. Medicine and science in sports and exercise. 1995;27:1639–1645. [PubMed] [Google Scholar]
- 19.Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neuroscience and biobehavioral reviews. 2013;37:1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey BJ, Glatt CE, Tottenham N, Soliman F, Bath K, et al. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience. 2009;164:108–120. doi: 10.1016/j.neuroscience.2009.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasic GP, Smoller JW, Perlis RH, Sun M, Lee S, et al. BDNF, relative preference, and reward circuitry responses to emotional communication. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2009;150B:762–781. doi: 10.1002/ajmg.b.30944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taliaz D, Loya A, Gersner R, Haramati S, Chen A, et al. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:4475–4483. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClintick JN, Xuei X, Tischfield JA, Goate A, Foroud T, et al. Stress-response pathways are altered in the hippocampus of chronic alcoholics. Alcohol. 2013;47:505–515. doi: 10.1016/j.alcohol.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2010;61:533–541. [PubMed] [Google Scholar]
- 25.Stranahan AM, Lee K, Mattson MP. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromolecular medicine. 2008;10:118–127. doi: 10.1007/s12017-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, et al. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehringer MA, Hoft NR, Zunhammer M. Reduced alcohol consumption in mice with access to a running wheel. Alcohol. 2009;43:443–452. doi: 10.1016/j.alcohol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pichard C, Gorwood PA, Hamon M, Cohen-Salmon C. Differential effects of free versus imposed motor activity on alcohol consumption in C57BL/6J versus DBA/2J mice. Alcohol. 2009;43:593–601. doi: 10.1016/j.alcohol.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Darlington TM, McCarthy RD, Cox RJ, Ehringer MA. Mesolimbic transcriptional response to hedonic substitution of voluntary exercise and voluntary ethanol consumption. Behavioural brain research. 2014;259:313–320. doi: 10.1016/j.bbr.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammer SB, Ruby CL, Brager AJ, Prosser RA, Glass JD. Environmental modulation of alcohol intake in hamsters: effects of wheel running and constant light exposure. Alcoholism, clinical and experimental research. 2010;34:1651–1658. doi: 10.1111/j.1530-0277.2010.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brager AJ, Hammer SB. Impact of wheel running on chronic ethanol intake in aged Syrian hamsters. Physiology & behavior. 2012;107:418–423. doi: 10.1016/j.physbeh.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol- drinking behavior in rodents. Critical reviews in neurobiology. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- 34.Belke TW. Running and responding reinforced by the opportunity to run: effect of reinforcer duration. Journal of the experimental analysis of behavior. 1997;67:337–351. doi: 10.1901/jeab.1997.67-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behavioural processes. 2005;68:165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Iversen IH. Techniques for establishing schedules with wheel running as reinforcement in rats. Journal of the experimental analysis of behavior. 1993;60:219–238. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lett BT, Grant VL, Byrne MJ, Koh MT. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34:87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- 38.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nature reviews Neuroscience. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 39.Werme M, Lindholm S, Thoren P, Franck J, Brene S. Running increases ethanol preference. Behavioural brain research. 2002;133:301–308. doi: 10.1016/s0166-4328(02)00027-x. [DOI] [PubMed] [Google Scholar]
- 40.Ozburn AR, Harris RA, Blednov YA. Wheel running, voluntary ethanol consumption, and hedonic substitution. Alcohol. 2008;42:417–424. doi: 10.1016/j.alcohol.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grahame NJ, Li TK, Lumeng L. Limited access alcohol drinking in high- and low- alcohol preferring selected lines of mice. Alcoholism, clinical and experimental research. 1999;23:1015–1022. [PubMed] [Google Scholar]
- 42.Middaugh LD, Kelley BM, Bandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- 43.Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- 44.Makatsori A, Duncko R, Schwendt M, Moncek F, Johansson BB, et al. Voluntary wheel running modulates glutamate receptor subunit gene expression and stress hormone release in Lewis rats. Psychoneuroendocrinology. 2003;28:702–714. doi: 10.1016/s0306-4530(02)00062-8. [DOI] [PubMed] [Google Scholar]
- 45.Droste SK, Gesing A, Ulbricht S, Muller MB, Linthorst AC, et al. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144:3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- 46.Droste SK, Schweizer MC, Ulbricht S, Reul JM. Long-term voluntary exercise and the mouse hypothalamic-pituitary-adrenocortical axis: impact of concurrent treatment with the antidepressant drug tianeptine. Journal of neuroendocrinology. 2006;18:915–925. doi: 10.1111/j.1365-2826.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 47.Droste SK, Chandramohan Y, Hill LE, Linthorst AC, Reul JM. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 2007;86:26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- 48.Girard I, Garland T., Jr Plasma corticosterone response to acute and chronic voluntary exercise in female house mice. Journal of applied physiology. 2002;92:1553–1561. doi: 10.1152/japplphysiol.00465.2001. [DOI] [PubMed] [Google Scholar]
- 49.Fediuc S, Campbell JE, Riddell MC. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. Journal of applied physiology. 2006;100:1867–1875. doi: 10.1152/japplphysiol.01416.2005. [DOI] [PubMed] [Google Scholar]
- 50.Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCormick CM. An animal model of social instability stress in adolescence and risk for drugs of abuse. Physiology & behavior. 2010;99:194–203. doi: 10.1016/j.physbeh.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Klein ZA, Romeo RD. Changes in hypothalamic-pituitary-adrenal stress responsiveness before and after puberty in rats. Hormones and behavior. 2013;64:357–363. doi: 10.1016/j.yhbeh.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 53.Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. The European journal of neuroscience. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Przybycien-Szymanska MM, Gillespie RA, Pak TR. 17beta-Estradiol is required for the sexually dimorphic effects of repeated binge-pattern alcohol exposure on the HPA axis during adolescence. PloS one. 2012;7:e32263. doi: 10.1371/journal.pone.0032263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. 14. [Google Scholar]
- 56.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in neuro-psychopharmacology & biological psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 57.Welch KA, Carson A, Lawrie SM. Brain structure in adolescents and young adults with alcohol problems: systematic review of imaging studies. Alcohol and alcoholism. 2013;48:433–444. doi: 10.1093/alcalc/agt037. [DOI] [PubMed] [Google Scholar]
- 58.Givalois L, Naert G, Rage F, Ixart G, Arancibia S, et al. A single brain-derived neurotrophic factor injection modifies hypothalamo-pituitary-adrenocortical axis activity in adult male rats. Molecular and cellular neurosciences. 2004;27:280–295. doi: 10.1016/j.mcn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Naert G, Ixart G, Tapia-Arancibia L, Givalois L. Continuous i.c.v. infusion of brain- derived neurotrophic factor modifies hypothalamic-pituitary-adrenal axis activity, locomotor activity and body temperature rhythms in adult male rats. Neuroscience. 2006;139:779–789. doi: 10.1016/j.neuroscience.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 60.Davis MI. Ethanol-BDNF interactions: still more questions than answers. Pharmacology & therapeutics. 2008;118:36–57. doi: 10.1016/j.pharmthera.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 62.Zajac MS, Pang TY, Wong N, Weinrich B, Leang LS, et al. Wheel running and environmental enrichment differentially modify exon-specific BDNF expression in the hippocampus of wild-type and pre-motor symptomatic male and female Huntington’s disease mice. Hippocampus. 2010;20:621–636. doi: 10.1002/hipo.20658. [DOI] [PubMed] [Google Scholar]
- 63.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. Journal of neuroscience research. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiaruttini C, Sonego M, Baj G, Simonato M, Tongiorgi E. BDNF mRNA splice variants display activity-dependent targeting to distinct hippocampal laminae. Molecular and cellular neurosciences. 2008;37:11–19. doi: 10.1016/j.mcn.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 65.Baj G, Leone E, Chao MV, Tongiorgi E. Spatial segregation of BDNF transcripts enables BDNF to differentially shape distinct dendritic compartments. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16813–16818. doi: 10.1073/pnas.1014168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kerns RT, Ravindranathan A, Hassan S, Cage MP, York T, et al. Ethanol-responsive brain region expression networks: implications for behavioral responses to acute ethanol in DBA/2J versus C57BL/6J mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:2255–2266. doi: 10.1523/JNEUROSCI.4372-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain research. 1996;726:49–56. [PubMed] [Google Scholar]