Abstract
With an increasing number of clinical trials looking at combination therapies in cancer, potential drug-drug interactions require particular attention. One such instance is the treatment of CD30+ tumors after previous vorinostat (SAHA) failure with the anti-CD30 antibody-drug conjugate brentuximab vedotin. Using B-, T- and NK-cell lines in vitro, we demonstrate that SAHA downregulates the expression of CD30 and lowers the efficacy of subsequent brentuximab vedotin treatment if baseline CD30 levels are reduced by 50% or more. Interestingly, low dose SAHA treatment that maintained 50% or more of basal CD30 expression followed by subsequent treatment with brentuximab vedotin led to enhanced anti-tumor activity. The downregulation of CD30 was short-lived upon SAHA removal, suggesting that allowing SAHA washout may circumvent any interactions with subsequent drug therapies. Our findings confirm the requirement of CD30 for brentuximab vedotin efficacy and suggest that combination treatment with SAHA in CD30dim tumors may decrease efficacy. Combination treatment in highly CD30+ tumors, however, increases efficacy and warrants further consideration as a new treatment paradigm.
Keywords: Vorinostat (SAHA), Brentuximab Vedotin (SGN-35), CD30, Lymphoid, drug-drug interactions
INTRODUCTION
The past decade has seen the introduction of several new classes of anti-cancer therapeutics. Among these are the histone deacetylase inhibitors (HDACi) and antibody-drug conjugates (ADC). These fields were pioneered by the approval of vorinostat (SAHA) in 2006 and Gemtuzumab ozogamicin in 2001, respectively.(1, 2) Since their introduction in the early 2000s, there has been an explosion of drug development surrounding HDACi and ADCs. By late 2013, there were at least five HDACi and 27 ADCs in development by various pharmaceutical companies to complement those already approved for use by the FDA.(3, 4)
SAHA (suberoylanilide hydroxyamic acid) paved the way for further development of the HDACi class of drugs.(2) It was developed through study of the differentiating properties of dimethyl sulfoxide (DMSO) in murine erythroleukemia cells. Further analysis showed specifically that the sulfhydryl group of DMSO was interacting with metal ions. Based on that analysis, hydroxyamic acids were screened and found to be better binding partners, and SAHA in particular showed promise in cell culture models.(5) The structure of SAHA is very similar to that of Trichostatin A, a known HDACi, and X-ray crystallography of SAHA verified interactions with several HDAC enzymes, confirming its mechanism of action.(5) Though its approval in 2006 was for second line use in cutaneous T-cell lymphomas (CTCL)(2), SAHA has been utilized in numerous clinical trials for various cancers and continues to be tested in combination with both newly emerging and traditional therapies.
Brentuximab Vedotin (SGN-35) was the second FDA-approved ADC. This drug was first developed as a monoclonal antibody targeting the protein CD30, a TNFα receptor superfamily member, with the hope that targeted downregulation of CD30 would lead to cancer cell death.(6) When this approach showed little therapeutic benefit, the antibody was turned into an ADC by conjugating it to mono-methyl auristatin E, a potent member of the auristatin family that destabilizes microtubule polymerization during mitosis.(6, 7) Brentuximab vedotin was granted accelerated approval in 2011 for treatment of Hodgkin lymphoma (HL) after autologous stem cell transplant failure or failure of two other chemotherapeutic regimens and for systemic Anaplastic Large Cell Lymphoma (sALCL) after failure of one chemotherapy regimen.(6, 7) Brentuximab vedotin is currently involved in numerous clinical trials against CD30+ malignancies.
Ongoing clinical trials and off label use of SAHA and brentuximab vedotin raise an important issue of drug compatibility. SAHA is used in the setting of relapsed CTCL, approximately 25% of which are CD30+.(8) As mentioned previously, brentuximab vedotin is approved as second or third line treatment for cases of HL and sALCL. Because ALCL is a CD30+ CTCL (8, 9), ALCL relapsed or refractory to SAHA treatment could be treated with brentuximab vedotin. Indeed, there are several ongoing trials of brentuximab vedotin that include relapsed or refractory CD30+ CTCL as inclusion criteria.
Vahdat et al. described off-target effects of the proteasome inhibitor bortezomib on CD30 in HL and ALCL cell lines. Their work proved that bortezomib-mediated upregulation of TNFα converting enzyme (TACE), a protease that cleaves membrane bound proteins such as CD30, decreased surface CD30 levels as well as sensitivity to the anti-CD30 antibody MDX-060. This effect could be rescued by TACE-specific inhibitors.(10) These results illustrate the importance of considering the effects of combination therapies on CD30 expression and subsequently anti-CD30 treatments.
This study addresses the importance of CD30 expression to the efficacy of brentuximab vedotin and the potential implications of SAHA use in the setting of CD30+ ALCL. We found that pretreatment of cancer cell lines with SAHA lowers both CD30 mRNA and protein levels. Subsequent treatment with brentuximab vedotin was not as effective when compared to cells treated with brentuximab vedotin but not exposed to SAHA. This loss of efficacy was only seen if CD30 levels were decreased by 40–50% from baseline. If this threshold was not met, then SAHA treatment could potentiate the effects of brentuximab vedotin. Attention to these threshold effects could offer an effective treatment paradigm for highly CD30+ tumors.
MATERIALS AND METHODS
Antibodies and drugs
Antibodies used were anti-CD30 (BerH2) (Santa Cruz Biotechnology, sc-19658) and β-Actin (Cell Signaling, 3700). Vorinostat (SAHA) was purchased from Selleckchem and resuspended in DMSO. Brentuximab vedotin (SGN-35) was graciously provided by the Penn State Hershey Cancer Institute Pharmacy at 50 mg/mL in saline.
Cell Culture
Kem I and Kem III (syngeneic EBV-positive Burkitt lymphoma cell lines with a restricted (Latency I) or complete (Latency III) profile of latency-associated gene expression, respectively)(11), Karpas 299 (ALCL)(12) and NKL (aggressive Natural Killer-Large Granular Lymphocyte)(13) cells were cultured in RPMI 1640 medium supplemented with 10% FBS. Kem I and Kem III were both obtained from the laboratory of Alan Rickinson (University of Birmingham, UK) in 2000 and have not since been formally validated other than phenotypically with expression patterns of latency I vs. latency III, done routinely with PCR and Western blot. Karpas 299 cells were obtained from the laboratory of Mark Kirschbaum (Penn State, PA) in 2012 and have not been validated other than for CD30 expression by Western blot. NKL cells were a gift from Howard Young (NCI) and were validated in August 2014 by Genetica DNA laboratories with short tandem repeat profiling and comparison to the DSMZ, ATCC, Riken and JCRB cell repository databases. The following reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: Human rIL-2 from Dr. Maurice Gately, Hoffman-La Roche Inc.(14) IL-2 was added to NKL cultures at 100 IU/mL to maintain cell growth as described previously.(13) Cells were incubated at 37°C in a humidified 5% CO2 atmosphere.
Immunoblotting
All cells were lysed in RIPA buffer (Sigma, R0278) with 1:100 protease inhibitor (Sigma, P8340) and phosphatase inhibitor cocktail 2 (Sigma, P5726). Protein concentrations of lysates were determined using the BCA Protein Assay kit (Thermo, 23225), and 30 or 40 µg of protein each was loaded on 10% precast Novex® gels (Life Technologies) and run in the Xcell SureLock system (Life Technologies). Electrophoresed proteins were transferred onto PVDF (Millipore) and stained in Ponceau S solution (Sigma, P7170) to confirm protein transfer. Blots were blocked in either 5% BSA or non-fat dry milk for 1 hour prior to incubation overnight with the appropriate antibody. Specific signal was detected using anti-mouse HRP-conjugated secondary antibody (Cell Signaling, 7076) and Clarity ECL (Bio-Rad, 170-5061) on the Chemidoc MP system (Bio-Rad). Protein bands were analyzed and quantified using the Image Lab software suite (Bio-Rad). All protein bands were within the linear range as determined by Image Lab.
Absolute CD30 quantification
Purified CD30 protein (Novus Biologicals, NBP2-22660) was used as a standard to measure the absolute amounts of CD30 expression in CD30+ cell lines before and after treatment with SAHA. This protein preparation was chosen based on known reactivity with the anti-CD30 (BerH2) antibody.(15) Standards and samples were analyzed by immunoblotting with anti-CD30 (BerH2) (Santa Cruz Biotechnology, sc-19658). The standards and cell line samples were run on the same gel and developed together to decrease inter-exposure variation. CD30 protein levels were determined by comparison of band intensities. Values are expressed as ng of CD30 per µg of total cellular protein.
Quantitative Real Time PCR (qPCR)
Cells were lysed in Trizol (Invitrogen) and stored at −80°C until phenol/chloroform extraction as per the manufacturer’s instructions. RNA was quantified using a nanodrop spectrophotometer (Thermo) and reverse transcribed to cDNA with the Omniscript RT kit (Qiagen, 205110) as per the manufacturer’s instructions. Actin and CD30 cDNA transcripts were quantified using AB&I Taqman primer and probe sets (Life Technologies) on a Bio-Rad CFX96 real time system and analyzed using the CFX manager software.
MTS Assay
Cells were plated at 1–2×106/ml and cultured in the presence of SAHA or DMSO for 24–48 h. Cells from these groups were plated in 96 well plates at a concentration of 2.5×104 cells/well in 100µl in the presence or absence of 1nM brentuximab vedotin for 72 hrs. MTS reagent (Promega, G3582) was added to each well and incubation continued for a further 2–4 h per the manufacturer’s instructions. Absorbance was measured at 590 nm on a Synergy HT plate reader (Biotek).
siRNA knockdown
To knockdown CD30 expression, a CD30 siRNA (Santa Cruz Biotechnology) was introduced into cells by nucleofection. The siRNA was used at 300 nM/nucleofection as per the manufacturer’s instructions with the Nucleofector II system and nucleofector kit V (Lonza, VCA-1003). Program G-016 was used for all transfections with a cell count of 2–3×106 cells/100uL. Cells were allowed to recover for 48 h in fresh media prior to analysis.
Data Analysis
Unless otherwise mentioned, all data were analyzed in Microsoft Excel and plotted in GraphPad Prism 5. All statistics were calculated using student’s t-test, and all errors are standard error of the mean. All figures are representative of triplicate experiments unless otherwise stated.
RESULTS
SAHA silences CD30 expression
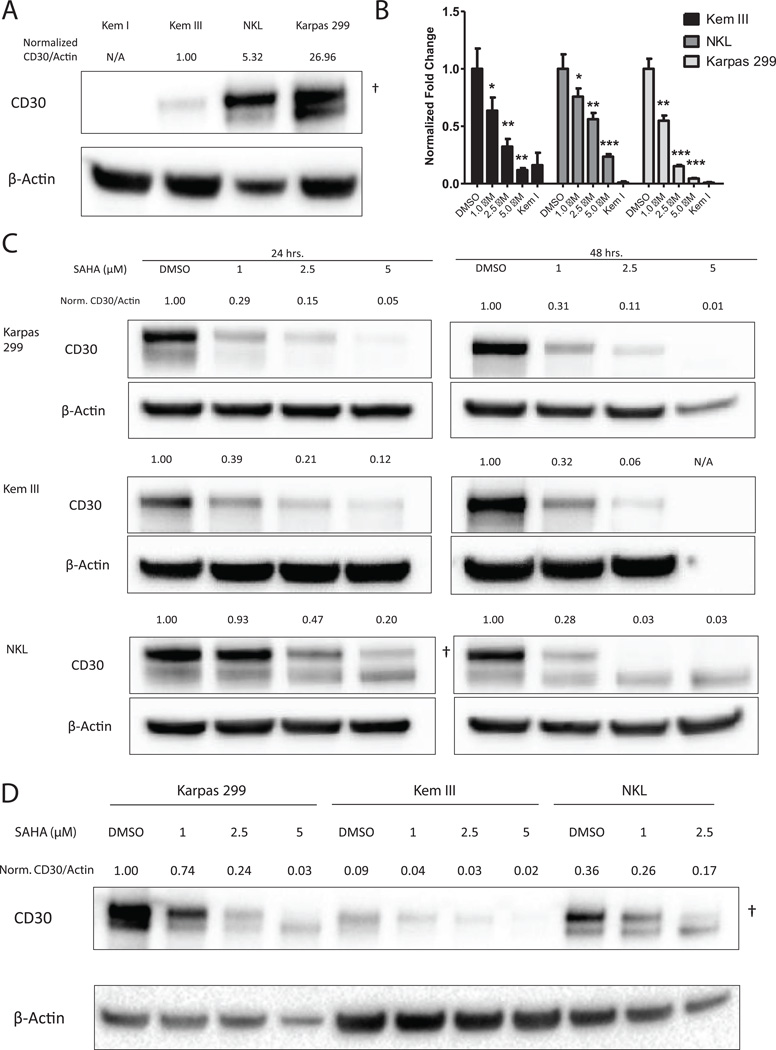
In order to test our hypothesis that downregulation of CD30 could lead to decreased efficacy of brentuximab vedotin, we first sought to confirm the ability of SAHA to decrease CD30 expression. Kem III, NKL and Karpas 299 were chosen as representative CD30+ B-, NK- and T-cell lines respectively, and Kem I was used as a CD30-negative control. Immunoblot analysis showed baseline CD30 protein levels were 5-fold or 27-fold higher in NKL and Karpas 299 cells, respectively, than in Kem III cells (Figure 1A). All cell lines were treated with increasing doses of SAHA and assayed for CD30 protein and mRNA expression at 24 and 48 hours post treatment, and each showed a statistically significant and dose dependent decrease in CD30 mRNA expression with SAHA treatment (Figure 1B). All SAHA treatment groups except Kem III at 5µM were significantly higher (p<0.05) than the Kem I negative control.
Figure 1. SAHA downregulates CD30 mRNA and protein.
(A) Kem III, NKL and Karpas 299 cells were assayed for basal CD30 expression by immunoblot. Kem I served as a negative control. Actin served as a loading control. Kem III, NKL and Karpas 299 cells were treated with SAHA (1 µM, 2.5 µM and 5 µM) or DMSO for 24 or 48 hours. (B) Cells were assayed for CD30 mRNA expression 24 hours post treatment by qPCR using the ΔΔCt method.. Each cell line is normalized to its DMSO control. *p<0.05, **p<0.005, ***p<0.0005 vs. vehicle control as determined by t-test. (C,D) Protein levels were also measured after treatment with SAHA, and CD30 to actin ratios normalized to (A) Kem I or (D) DMSO control are indicated above the figure. Quantification was limited to membrane bound CD30 (†) and does not include unprocessed cytoplasmic protein (lower band) (25).
The decrease in mRNA was consistent with decreases in protein levels. All three cell lines demonstrated a dose and time dependent decrease in CD30 protein expression (Figure 1C). Kem III cells exhibited up to 15-fold decrease in protein expression by 48 h of treatment with 2.5 µM SAHA, but cells experienced high levels of toxicity after 48 hours of 5 µM treatment, leaving little sample for protein analysis (Figure 1C). SAHA treatment of Karpas 299 cells had even more pronounced effects on CD30, showing 20 to 120-fold decrease in protein levels. Similar decreases were seen at 48 hours post-treatment in NKL cells (Figure 1 C), though downregulation of CD30 took longer in NKL cells than in other cell types, with significant reduction only evident after 48 hours (Figure 1C).
Because the absolute amount of CD30 protein varied greatly between cell lines, we compared the amounts of CD30 in each cell line following SAHA treatment by analyzing samples on the same immunoblot after 24 h and normalized to the Karpas 299 DMSO control (Fig. 1D). Although each cell line exhibited decreased CD30 protein in response to SAHA treatment, CD30 levels in SAHA-treated Karpas 299 cells, especially 1µM, remained much higher than those in untreated Kem III and NKL cells.
SAHA-mediated downregulation of CD30 is a transient phenomenon
Because SAHA can downregulate CD30, we next sought to determine whether the effects of SAHA on CD30 were long-lasting, perhaps due to chromatin remodeling events. To test this possibility, Kem III, Karpas 299 and NKL cells were treated with either DMSO or 1 µM SAHA for 48 hours, and CD30 expression was monitored at specified time points. A 1 µM dose of SAHA was chosen to limit toxicity to cells but still mediate CD30 knockdown. After 48 hours, cells were washed in PBS and put in fresh medium for an additional 4 days. CD30 expression was decreased by 48 h SAHA treatment in all cell lines, consistent with our previous observations. Following removal of SAHA, CD30 expression returned to pretreatment levels or higher (Figure 2).
Figure 2. SAHA-mediated downregulation of CD30 is reversible.
Cells were treated with 1 µM SAHA for 48, washed and cultured for 6 days. DMSO-treated cells were used as a control, indicated day 0. Upper band represents membrane bound CD30 (†). The lower band is unprocessed cytoplasmic protein (25), which was excluded from analysis.
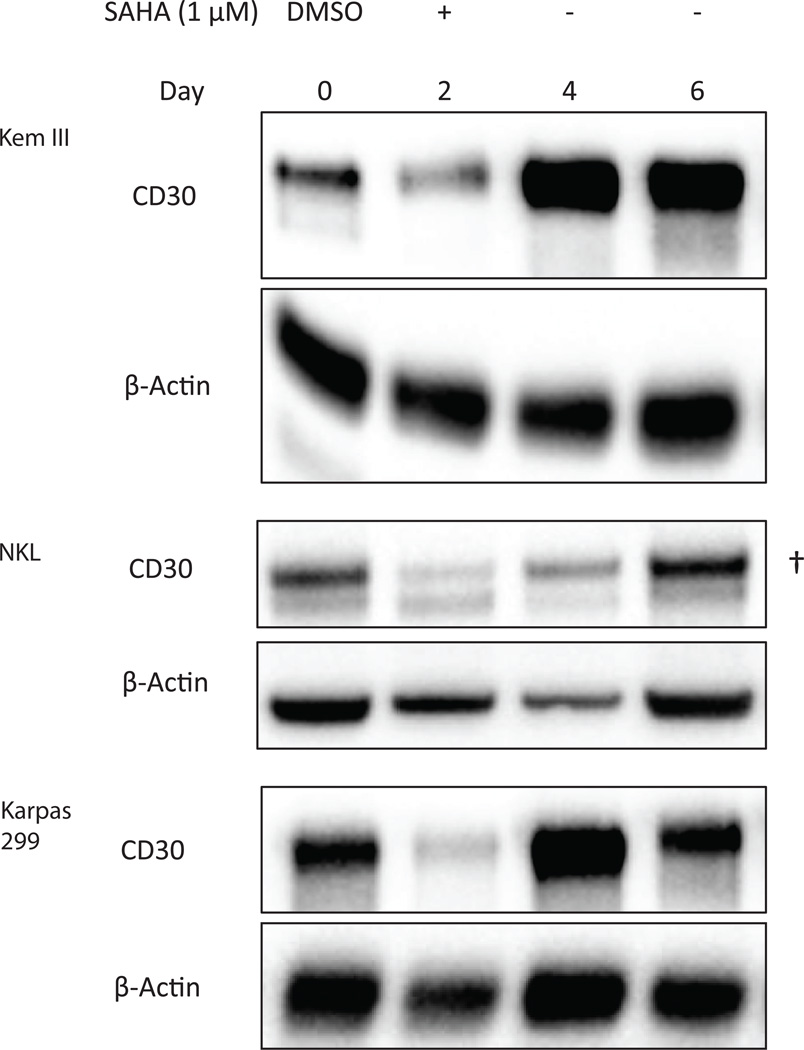
SAHA decreases brentuximab vedotin efficacy in a CD30-dependent manner
Because SAHA treatment decreases CD30 levels, we hypothesized that this could reduce the efficacy of the CD30-binding ADC brentuximab vedotin. To test this hypothesis, Kem III, Karpas 299 and NKL cells were treated with either DMSO or 1 µM SAHA for 48 hours, followed by treatment with 1 nM brentuximab vedotin for an additional 72 hours. This dose of brentuximab vedotin was determined empirically to result in specific differences in viability between CD30+ and CD30− cell lines, with 72 h as the optimal time to see those effects. All three CD30+ cell lines were susceptible to brentuximab vedotin, albeit to different levels. Kem I was used as negative control and was not sensitive (Figure 3A). Because CD30 is the target of brentuximab vedotin and is decreased in SAHA-treated Kem III cells, we anticipated that Kem III cells would show increased viability in the presence of brentuximab vedotin, and this was the case (Fig. 3A). By contrast, both Karpas 299 and NKL cells showed the opposite effect, becoming more susceptible to brentuximab vedotin after SAHA treatment (Figure 3A).
Figure 3. High dose SAHA treatment lowers the efficacy of brentuximab vedotin.
Cells were treated with (A) 1 µM SAHA for 48 hours or (B) 2.5 µM SAHA for 24 hours or DMSO control prior to treatment for 72 hours with 1 nM brentuximab vedotin. Viability was assessed by MTS assay. Each data point was normalized to its own untreated control to account for viability differences unrelated to brentuximab vedotin treatment. *p<0.05, **p<0.005, ***p<0.0005 as determined by t-test.
Because SAHA treatment decreased brentuximab vedotin efficacy in Kem III cells but not in Karpas 299 or NKL cells, we questioned whether decreasing CD30 expression through increasing the SAHA dose to 2.5 µM (Figure 3B) could rescue the latter two cell lines from the effects of brentuximab vedotin as in the case of Kem III. This hypothesis was based on the higher levels of CD30 in 1 µM SAHA-treated NKL and Karpas 299 versus Kem III (Figure 1D). In addition to increasing the dose, the treatment time for SAHA was reduced to 24 h to prevent excessive cytotoxicity. Brentuximab vedotin was maintained at 1 nM. At 72 h post-treatment with brentuximab vedotin, the viability of Karpas 299 and NKL cells was significantly increased by pre-treatment with SAHA (Figure 3B). These data suggested that CD30 knockdown below a certain threshold can lead to resistance to brentuximab vedotin treatment.
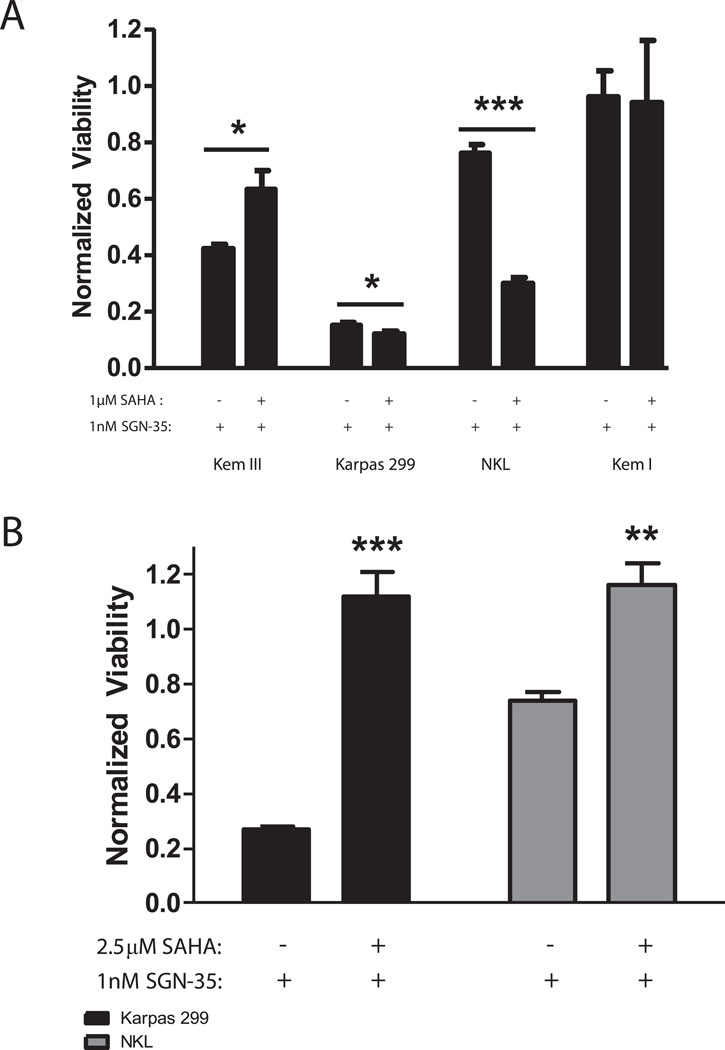
50–60% of baseline membrane CD30 protein expression defines the threshold for brentuximab vedotin efficacy
The inverse correlation between SAHA dose and efficacy of brentuximab vedotin (Figure 3) led us to determine the absolute amount of CD30 protein that is required in order for brentuximab vedotin to be efficacious. By comparison of the SAHA-treated Karpas 299, NKL and Kem III samples with purified CD30, we verified that the level of expression in Karpas 299 cells > NKL> Kem III, and determined that CD30 protein expression decreased in a dose dependent manner with SAHA treatment (Fig. 4A&B). Doses of SAHA used for Kem III were lower than for NKL and Karpas 299 due to the lower initial level of CD30 (Figure1A). Concurrently, NKL, Karpas 299 and Kem III cells were tested for viability after pre-treatment with these same doses of SAHA followed by 72 h of brentuximab vedotin. The viability was plotted against absolute CD30 levels to determine the correlation between CD30 and brentuximab vedotin efficacy. NKL and Karpas 299 cells both showed dose-dependent effects with nearly complete resistance to brentuximab vedotin as SAHA concentration increased (Figure 4C), consistent with Figure 3B. Kem III cells (Figure 3A) showed a similar resistance to brentuximab vedotin at SAHA doses above 1 µM. Though all cell lines had different absolute amounts of CD30 expression, the efficacy of brentuximab vedotin was fully abolished when CD30 levels dropped below 50–60% of the baseline (Figure 4C) as summarized in Table 1. All three cell lines also showed an increase in brentuximab vedotin efficacy at the lowest dose of SAHA, consistent with findings shown in Figure 3A.
Figure 4. SAHA decreases the efficacy of brentuximab vedotin when CD30 levels decrease to 50–60%.
(A) Purified CD30 peptide (serial dilutions between 2-0 µg/lane) was run and quantified by immunoblotting to generate a standard curve. (B) Karpas 299, Kem III and NKL cells were treated for 24 hours with differing doses of SAHA or DMSO, and CD30 protein levels were quantified using a standard curve. The amount of CD30 (ng/µg of total cellular protein) is shown above each lane. Upper band represents membrane bound CD30 (†). The lower band is unprocessed cytoplasmic protein(25) and was excluded from analysis. (C) Following exposure to SAHA, cells were treated with 1 nM brentuximab vedotin for 72 hours and viability was assessed by MTS assay (indicated by vertical bars). The absolute amount CD30 protein per µg of total cellular protein, an average of two separate quantifications, is plotted above each viability data point (indicated by line graph).
Table 1.
Summary of CD30 Threshold for Brentuximab Vedotin Efficacy
| Baseline CD30 | CD30 level at decreased brentuximab vedotin efficacy |
% knockdown | |
|---|---|---|---|
| NKL | 2.41 | 1.32 | 45% |
| Karpas 299 | 5.11 | 2.57 | 50% |
| Kem III | 1.67 | 1.14 | 32% |
This table summarizes the absolute amount of baseline CD30 present in each cell line, the amount of CD30 left when brentuximab vedotin efficacy begins to decrease and the percent knockdown that the decrease in CD30 represents. CD30 is measured in ng/µg total cellular protein.
siRNA knockdown of CD30 decreases brentuximab vedotin efficacy
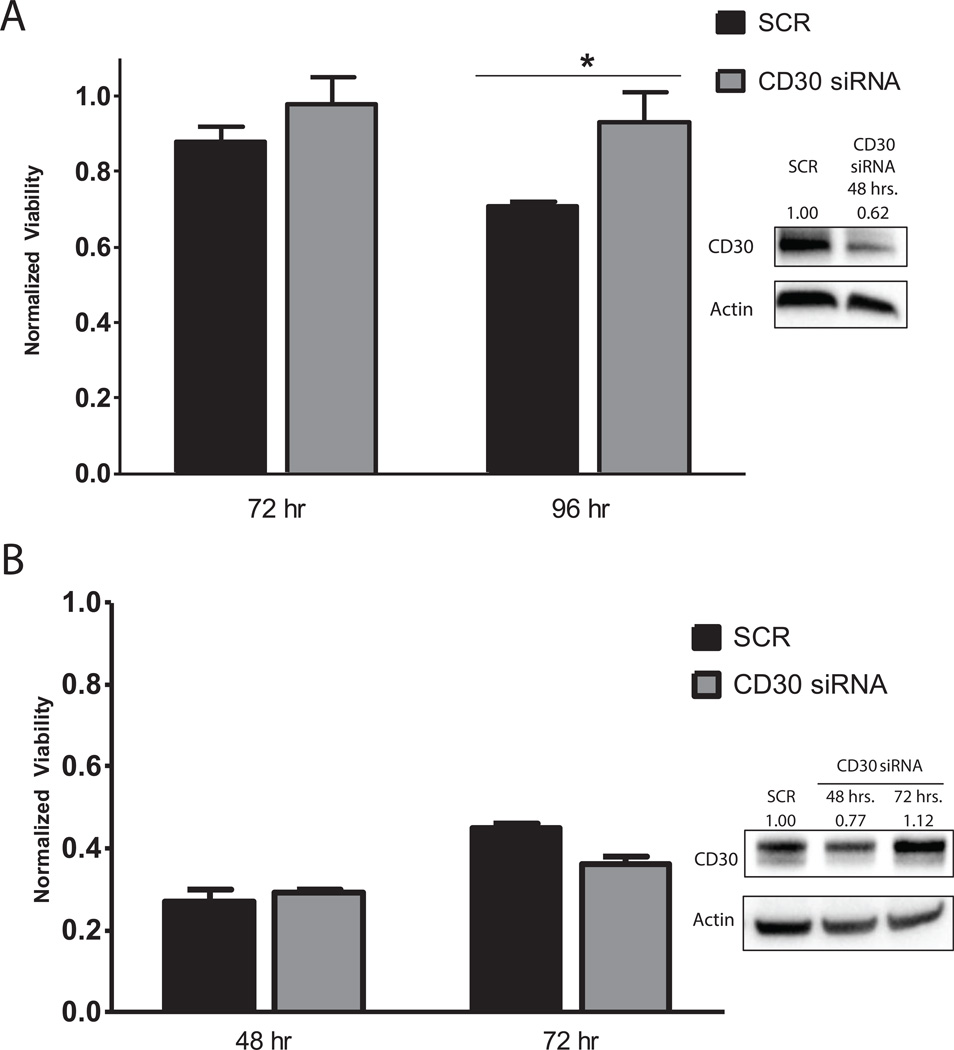
To determine whether the downregulation of CD30 was in fact the mechanism responsible for decreased brentuximab vedotin efficacy, Kem III and Karpas 299 cells were transfected with CD30 siRNA or scrambled siRNA (SCR). NKL cells are difficult to transfect and were not used in these experiments. After 24 hours, cells were analyzed for CD30 protein analysis and treated with 1 nM brentuximab vedotin for 48 and 72 (Karpas 299) or 72 and 96 (Kem III) hours. Kem III CD30 levels were decreased approximately 40% at 48 h (Fig. 5A), in line with the threshold for resistance to brentuximab vedotin we observed for Kem III. Indeed, CD30 siRNA transfected Kem III cells showed increased viability in the presence of brentuximab vedotin after 96 hours (Figure 5A).
Figure 5. CD30 knockdown reduces brentuximab vedotin efficacy in Kem III but not Karpas 299 cells.
Cells were nucleofected with scrambled (SCR) or CD30 siRNA and two days later, treated with 1 nM brentuximab vedotin for the indicated number of hours. Viability after brentuximab vedotin treatment was determined by MTS assay for (A) Kem III and (B) Karpas 299. Each data point was first normalized to its own untreated control to account for differences in viability unrelated to brentuximab vedotin treatment. *p<0.05 vs SCR control as determined by t-test. Knockdown efficiency was quantified by immunoblotting for CD30 (inset), where numbers represent CD30 to actin ratios normalized to the scrambled siRNA treated sample.
Despite knockdown of CD30 expression in Karpas 299 cells, no increased viability in the presence of brentuximab vedotin was observed compared to the siRNA control. Because the knockdown of CD30 in Karpas 299 was small (Figure 5B inset), we also checked viability at 48 and 72 h post brentuximab vedotin treatment but observed no increase (Figure 5B). The lack of response was likely due to the low level of CD30 knockdown, approximately 20%, which is well below the 50% threshold needed to see decreased brentuximab vedotin efficacy (Figure 4C).
DISCUSSION
This study demonstrates that SAHA can decrease the efficacy of brentuximab vedotin in an in vitro cell culture system through reduction of CD30. This effect occurs when CD30 levels are decreased to below 50–60% of baseline expression, whether by SAHA treatment or CD30 knockdown with siRNA. We also demonstrate that the SAHA-mediated downregulation of CD30 requires the continued presence of SAHA. Taken together, our findings suggest that treatment of tumors expressing low levels of CD30 with a combination of SAHA and brentuximab vedotin might impede the efficacy of anti-CD30 therapy. We also noted that low dose SAHA treatment that minimally affected CD30 levels could potentiate the effects of brentuximab vedotin, a phenomenon that warrants further study.
There are two rationales for studying the combination of SAHA and brentuximab vedotin. The first is to determine the plasticity of CD30 repression by SAHA and determine its effect on brentuximab vedotin efficacy, which is relevant given the growing number of clinical trials for brentuximab vedotin that are enrolling previously SAHA-treated patients. The second is to assess whether SAHA, when used at low doses that only slightly affect CD30 expression, has any potentiating properties when combined with brentuximab vedotin. This second rationale is based on our previous findings in a clinical trial of SAHA, the methyltransferase inhibitor cladribine, and the monoclonal antibody rituximab in Mantle Cell Lymphoma (MCL).(16) Data from this study shows much higher response rates with SAHA and cladribine in combination with rituximab than as reported with rituximab alone in MCL. Both these rationales were addressed in this study.
Although CD30 levels decreased with SAHA treatment, we found that SAHA-mediated CD30 silencing requires the continued presence of SAHA, suggesting that direct inhibition of HDACs and not chromatin remodeling may be responsible for these effects. Regulation of the CD30 promoter has been shown to involve an intricate interplay between activators and repressors that can be affected by HDACs 1 and 2.(17–19) It is possible, therefore, that the effects of SAHA on CD30 expression may be mediated through these HDACs. Further study of the effect of SAHA on acetylation of CD30 repressors such as YY1 is required to elucidate this mechanism.(17, 18) Regardless of the mechanism through which SAHA decreases CD30 expression, the finding that CD30 repression requires continued presence of SAHA has important implications, because an increasing number of clinical trials are exploring use of brentuximab vedotin as salvage therapy, including patients with prior SAHA treatment, alone or in combination with other drugs. Our findings suggest that if patients are allowed a washout period of more than a few days, the efficacy of brentuximab vedotin should not be adversely affected.
Recently, there has been some debate as to whether CD30 protein expression is important for the efficacy of brentuximab vedotin. Because of the ~1000 times higher affinity of brentuximab vedotin for CD30+ versus CD30− negative cells, it is important to understand the true importance of CD30 expression for its efficacy. (6) However, a recent interim analysis of a Phase II Study of brentuximab vedotin in patients with relapsed or refractory CD30+ Non-Hodgkin Lymphoma showed that there was no correlation between CD30 positivity and treatment response.(20) It is, therefore, important to clarify the true importance of CD30 expression for the efficacy of brentuximab vedotin. Our studies clearly demonstrate that levels of CD30 expression can affect the efficacy of brentuximab vedotin. Reducing CD30 levels on the surface of CD30+ cells by SAHA treatment resulted in decreased toxicity and increased viability of brentuximab vedotin treated cells. Most importantly, the loss of responsiveness to brentuximab vedotin after knockdown of CD30 with siRNA demonstrates that CD30 plays an essential role in mediating the response to brentuximab vedotin.
Notably, differences in basal CD30 expression affected the efficacy of brentuximab vedotin. Even low dose SAHA treatment of Kem III cells (low CD30 levels) significantly reduced the efficacy of brentuximab vedotin, whereas low dose SAHA treatment of Karpas 299 or NKL, which have much higher CD30 levels, did not. This latter result was likely due to the higher basal expression of CD30. By quantifying CD30 protein levels, we found that the actual amount of CD30 required for response to brentuximab vedotin was cell line dependent but that cells maintaining CD30 levels higher than 50–60% of basal levels remained susceptible to brentuximab vedotin. For example, CD30 siRNA treatment of Kem III cells achieved almost 40% knockdown and led to decreased brentuximab vedotin efficacy, whereas Karpas 299 only achieved a 20% knockdown and did not show decreased brentuximab vedotin efficacy. These observations correlate with the observed thresholds for brentuximab vedotin efficacy in Kem III and Karpas 299 cells, respectively. Similarly, siRNA knockdown of CD30 in Kem III reached the threshold for susceptibility, and Karpas 299 did not. In the Phase II study described above, the tumors of patients were all CD30+ by immunohistochemistry, although some tumors expressed low levels. Thus, it is likely that the threshold CD30 level required for efficacy was met in this study. Some of these differences in the CD30 threshold between cell lines could be attributed to the way that these cells respond to auristatin E, the cytotoxic component of brentuximab vedotin. A greater resistance to auristatin E could explain the need for higher amounts of CD30 for brentuximab vedotin efficacy. If there are more CD30 sites, then more brentuximab vedotin could bind and more auristatin could be delivered to overcome resistance.
An interesting finding of our study was that low dose SAHA treatment of cell lines that did not decrease CD30 levels below the outlined thresholds potentiated the effects of brentuximab vedotin. It is well known that SAHA can induce apoptosis and has the potential to sensitize previously resistant cells to apoptotic mechanisms.(21) A previous study from our lab also showed that SAHA and cladribine could potentiate the effects of rituximab, another monoclonal antibody, in MCL.(16) Therefore, it is conceivable that low dose treatment could potentiate the effects of auristatin E, a microtubule poison that induces apoptosis.(2, 22, 23) This finding suggests that low dose SAHA could be effective in combination with brentuximab vedotin in tumors with high levels of CD30, whereas SAHA in low CD30 expressing tumors may inhibit brentuximab vedotin if given concurrently. Maximum serum concentrations of SAHA in humans at the maximum oral 400 mg dose are in the range of 0.5–1 µM in the blood, potentially enough to silence CD30 expression in tumors with low CD30 expression.(2, 24) This reciprocal phenomenon of potentiation versus inhibition of brentuximab vedotin efficacy requires further study.
Our analyses have demonstrated that SAHA downregulates CD30 and negatively impacts brentuximab vedotin efficacy if CD30 levels fall below a threshold of 50–60% of baseline CD30 expression. If CD30 levels remain above the threshold, SAHA can potentiate the effects of brentuximab vedotin. These effects were observed in NK-, B- and T-cell cell lines, indicating that these phenomena apply broadly across different types of lymphoid cells. If extended to clinical applications, our findings imply that patients previously treated with SAHA and allowed washout of the drug should not have decreased efficacy of subsequent brentuximab vedotin treatment due to SAHA-mediated CD30 downregulation. Clinically, the combination of low dose SAHA with brentuximab vedotin may also lead to greater efficacy of the latter in highly CD30+ malignancy and provide a new tool for the fight against cancer. Our work provides a rationale for further study of HDACi and brentuximab vedotin combination treatments with attention to threshold expression of CD30 in these CD30+ hematologic malignancies.
Acknowledgments
Financial Support:
T. P. Loughran Jr.: NCI R01 CA94872
Elliot M. Epner received research support from Merck and is on the speakers’ board of Celgene and Seattle Genetics.
Footnotes
Conflict of Interest:
The other authors have no conflicts of interest to declare.
REFERENCES
- 1.Ravandi F, Estey EH, Appelbaum FR, Lo-Coco F, Schiffer CA, Larson RA, et al. Gemtuzumab ozogamicin: time to resurrect? J Clin Oncol. 2012;30:3921–3923. doi: 10.1200/JCO.2012.43.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. The Oncologist. 2007;12:1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 3.DeWoskin VA, Million RP. The epigenetics pipeline. Nat Rev Drug Discov. 2013;12:661–662. doi: 10.1038/nrd4091. [DOI] [PubMed] [Google Scholar]
- 4.Sassoon I, Blanc V. Antibody-drug conjugate (ADC) clinical pipeline: a review. Methods Mol Biol. 2013;1045:1–27. doi: 10.1007/978-1-62703-541-5_1. [DOI] [PubMed] [Google Scholar]
- 5.Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- 6.Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012;30:631–637. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- 7.Newland AM, Li JX, Wasco LE, Aziz MT, Lowe DK. Brentuximab vedotin: a CD30-directed antibody-cytotoxic drug conjugate. Pharmacotherapy. 2013;33:93–104. doi: 10.1002/phar.1170. [DOI] [PubMed] [Google Scholar]
- 8.Li JY, Horwitz S, Moskowitz A, Myskowski PL, Pulitzer M, Querfeld C. Management of cutaneous T cell lymphoma: new and emerging targets and treatment options. Cancer Manag Res. 2012;4:75–89. doi: 10.2147/CMAR.S9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wozniak MB, Piris MA. Cutaneous T-cell lymphoma: two faces of the same coin. J Invest Dermatol. 2010;130:348–351. doi: 10.1038/jid.2009.373. [DOI] [PubMed] [Google Scholar]
- 10.Vahdat AM, Reiners KS, Simhadri VL, Eichenauer DA, Boll B, Chalaris A, et al. TNF-alpha-converting enzyme (TACE/ADAM17)-dependent loss of CD30 induced by proteasome inhibition through reactive oxygen species. Leukemia. 2010;24:51–57. doi: 10.1038/leu.2009.230. [DOI] [PubMed] [Google Scholar]
- 11.Gregory CD, Rowe M, Rickinson AB. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J Gen Virol. 1990;71(Pt 7):1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- 12.Fischer P, Nacheva E, Mason DY, Sherrington PD, Hoyle C, Hayhoe FG, et al. A Ki-1 (CD30)-positive human cell line (Karpas 299) established from a high-grade non-Hodgkin's lymphoma, showing a 2;5 translocation and rearrangement of the T-cell receptor beta-chain gene. Blood. 1988;72:234–240. [PubMed] [Google Scholar]
- 13.Robertson MJ, Cochran KJ, Cameron C, Le JM, Tantravahi R, Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp Hematol. 1996;24:406–415. [PubMed] [Google Scholar]
- 14.Lahm HW, Stein S. Characterization of recombinant human interleukin-2 with micromethods. J Chromatog. 1985;326:357–361. doi: 10.1016/s0021-9673(01)87461-6. [DOI] [PubMed] [Google Scholar]
- 15.Nagata S, Ise T, Onda M, Nakamura K, Ho M, Raubitschek A, et al. Cell membrane-specific epitopes on CD30: Potentially superior targets for immunotherapy. Proc Natl Acad Sci U S A. 2005;102:7946–7951. doi: 10.1073/pnas.0502975102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasanali Z, Sharma K, Spurgeon S, Okada C, Stuart A, Shimko S, Leshchenko V, Parekh S, Chen Y, Kirschbaum M, Epner EM. Combined epigenetic and immunotherapy produces dramatic responses in 100% of newly diagnosed mantle cell lymphoma patients. Cancer Res. 2013;73:8s. (suppl; abstr LB-140). [Google Scholar]
- 17.Yao YL, Yang WM, Seto E. Regulation of transcription factor YY1 by acetylation and deacetylation. Mol Cell Biol. 2001;21:5979–5991. doi: 10.1128/MCB.21.17.5979-5991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franchina M, Woo AJ, Dods J, Karimi M, Ho D, Watanabe T, et al. The CD30 gene promoter microsatellite binds transcription factor Yin Yang 1 (YY1) and shows genetic instability in anaplastic large cell lymphoma. J Pathol. 2008;214:65–74. doi: 10.1002/path.2258. [DOI] [PubMed] [Google Scholar]
- 19.Croager EJ, Gout AM, Abraham LJ. Involvement of Sp1 and microsatellite repressor sequences in the transcriptional control of the human CD30 gene. Am J Pathol. 2000;156:1723–1731. doi: 10.1016/S0002-9440(10)65043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharman JP, Oki Y, Advani RH, Bello CM, Winter JN, Yang Y, et al. A phase 2 study of brentuximab vedotin in patients with relapsed or refractory CD30-positive Non-Hodgkin lymphomas: Interim results in patients with DLBCL and other B-Cell lymphomas. Blood. 2013;122:848. [PubMed] [Google Scholar]
- 21.Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol. 2012;90:85–94. doi: 10.1038/icb.2011.100. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Richon V, Ni X, Talpur R, Duvic M. Selective induction of apoptosis by histone deacetylase inhibitor SAHA in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. J Invest Dermatol. 2005;125:1045–1052. doi: 10.1111/j.0022-202X.2005.23925.x. [DOI] [PubMed] [Google Scholar]
- 23.Butler LM, Zhou X, Xu WS, Scher HI, Rifkind RA, Marks PA, et al. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci U S A. 2002;99:11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly WK, O'Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Froese P, Lemke H, Gerdes J, Havsteen B, Schwarting R, Hansen H, et al. Biochemical characterization and biosynthesis of the Ki-1 antigen in Hodgkin-derived and virus-transformed human B and T lymphoid cell lines. J Immunol. 1987;139:2081–2087. [PubMed] [Google Scholar]