Abstract
C57BL/6 inbred mice are frequently used as models in auditory research, mostly the C57BL/6J and C57BL/6N substrains. Genetic variation and phenotypic disparities between these two substrains have been extensively investigated, but conflicting information exists about differences in their auditory and vestibular phenotypes. Literature-based comparisons are rendered difficult or impossible because most auditory publications do not designate the substrain used. We therefore evaluated commercial C57BL/6N and C57BL/6J mice for their baseline auditory brainstem response (ABR) thresholds at 3 months of age as well as their susceptibility to noise exposure and aminoglycoside antibiotics. Both substrains have similar thresholds at 4 and 12 kHz, but C57BL/6N show significantly higher baseline thresholds at 24 and 32 kHz. Because of these elevated thresholds, the N substrain is unsuitable as a model for drug ototoxicity, which primarily affects high frequencies. Exposure to 2–20 kHz broadband noise for 2 h at 110 dB produced significantly higher threshold shifts in the J substrain. These results suggest caution in the selection of C57BL/6 substrains for auditory research and indicate the need to specify substrains, age and the breeding source in all publications.
Keywords: auditory thresholds, ototoxicity, noise exposure, genetic drift, NAD(P) transhydrogenase
1. Introduction
C57BL/6 inbred mice are routinely used in biological studies, in part because C57BL/6-derived genetic backgrounds are common in many spontaneous mutant and transgenic mouse strains. Several C57 substrains have evolved with distinct genetic phenotypes (Mekada et al., 2009; Zurita et al., 2011) and among these, C57BL/6J and C57BL/6N are frequently employed as models in auditory research. C57BL/6J mice were originally bred at Jackson Laboratories in 1948 and then became widely distributed. Such breeding colonies derived from the Jackson strain were designated as the C57BL/6N line. Among other spontaneous mutations, the original C57BL/6J mice acquired a null mutation between 1976 and 1984 in the Nnt gene resulting in the absence of an NAD(P) transhydrogenase (Freeman et al., 2006). This mutation is not found in C57BL/6N mice.
Disparities in vestibular and auditory phenotypes have been well established for different strains of mice (Jones et al., 2006; Zheng et al., 1999) but less is known about such differences between the C57BL/6J and C57BL/6N substrains. However, phenotypic variations between C57BL/6J and C57BL/6N can be considerable, including, for example, different susceptibilities to colon cancer (Diwan et al., 1980) or fetal alcohol syndrome (Green et al., 2007). Unfortunately, published studies on auditory and vestibular physiology and pathology often do not specify which C57 substrain is used, making it difficult to evaluate potential differences between the substrains and their relative suitability as research models. From a PubMed search spanning the time period of 2010–2013, we extracted 23 studies from different laboratories on cochlear physiology or experimental pathology with C57 mice. Of these, only ten defined the substrain and only eight provided complete documentation of both substrain and source.
In a direct comparison, Johnson and colleagues (Kane et al., 2012) found no significant difference in auditory thresholds between young C57BL/6N and C57BL/6J mice. However, vestibular and auditory differences between J and N were suggested by diverging performances in the rotarod test and in the startle response/prepulse inhibition test (Matsuo et al., 2010). Likewise, Simon et al. (2013) in a comprehensive genetic and phenotypic characterization of the two substrains reported differences in acoustic startle and pre-pulse inhibition.
We have undertaken a study of commercial C57BL/6N and C57BL/6J mice, comparing their baseline auditory brain stem responses at 3 months of age as well as their susceptibility to aminoglycoside antibiotics and noise exposure. Our results as well as comparisons with literature data suggest that there are significant differences between the two substrains that might confound auditory research.
2. Materials and methods
2.1. Animals
Thirty-six male C57BL6/J mice were received from Jackson Laboratories, Bar Harbor, Maine (http://www.jax.org/) and 36 male C57BL/6NHsd mice (referred to as C57BL/6N) from Harlan Laboratories, Indianapolis, Indiana (www.harlan.com) at an age of 12 to 12.5 weeks. They were housed at 22 ± 1 °C under a 12-h:12-h light-dark cycle with free access to water and a regular mouse diet (Purina 5025, St. Louis, MO). All animals were acclimated for one week before experiments began. Research protocols were approved by the University of Michigan Committee on Use and Care of Animals and animal care was under the supervision of the Unit for Laboratory Animal Medicine at the University of Michigan.
2.2. Auditory brain stem response
Auditory thresholds were recorded from 13- to 14-week old animals by Auditory Brainstem Response (ABR) in an electrically and acoustically shielded chamber (Acoustic Systems, Austin, TX). Animals were anesthetized with an intraperitoneal injection of ketamine (65 mg/kg body weight), xylazine (3.5 mg/kg), and acepromazine (2 mg/kg). Body temperature was maintained through the use of heating pads and heat lamps. Sub-dermal needle electrodes were placed at vertex (active) and the test ear (reference) and contralateral ear (ground) pinnae. Stimuli were presented and responses recorded with Tucker Davis Technologies (TDT) System III hardware and SigGen/BioSig software (TDT, Alachua, FL). Tones were delivered through an EC1 driver (TDT) with the speculum placed just inside the external auditory canal. Stimulus presentations consisted of 15-ms tone bursts, with 1-ms rise/fall times, at a rate of 10 per second. Up to 1024 responses were averaged for each stimulus level in 10 dB steps, with 5 dB steps near threshold. Thresholds were interpolated between the lowest stimulus level where a response was observed and 5 dB lower where no response was observed. Amplitudes (peak to following trough) and latencies of waves I and IV were analyzed offline from stored waveforms (Saul et al, 2008). All ABR were obtained by the same investigator; input/output functions were analyzed by a different observer, blinded to the conditions.
2.3. Aminoglycoside injections
Animals were allowed one week of recovery after the initial ABR recording. They were then (i.e. at an age of ~15 weeks) injected subcutaneously with 800 mg/kg kanamycin base twice daily for 14 days (Wu et al., 2001). Kanamycin sulfate was purchased from Sigma-Aldrich (St Louis, MO), dissolved in water, pH-adjusted to 7.5 with HCl, and the solution was filtered into a sterile vial from which it could be dispensed without contamination. ABR thresholds were tested before treatment and three weeks after the last injection.
2.4. Noise exposure
One week after the initial ABR recording, at an age of ~15 weeks, awake mice were placed in individual wire cages in a ventilated chamber, and broadband noise (2–20 kHz) was presented for 2 h at 110 dB SPL through a loudspeaker (Parasound HCA-750A amplifier, JBL 2450H Compression Driver with 2385A horn) mounted on the top of the chamber. For calibration, the system response (62.5 Hz–22 kHz) to a white noise source (General Radio 1381) was measured with a 1/2-inch microphone (B&K 4134) and a Fast Fourier Transform (FFT) spectrum analyzer (Stanford Research SR760). The spectrum data was used to create an equalizing FFT filter in an audio file editor (Adobe Audition), which was then used to generate an audio CD for the noise used in this study. The system response to the audio CD was verified with an FFT spectrum analyzer and the noise level was measured within the cage at the position of the animal’s head using a B&K Sound level meter (model 2231, with type 4155 1/2″ microphone, and type 1625 octave band filter). Auditory thresholds were tested before and 2 weeks after the noise exposure.
2.5. Statistical analysis
Data were analyzed by two-way ANOVA with Sidak’s post-hoc test for significance of multiple comparisons.
3. Results
3.1. Baseline auditory functions
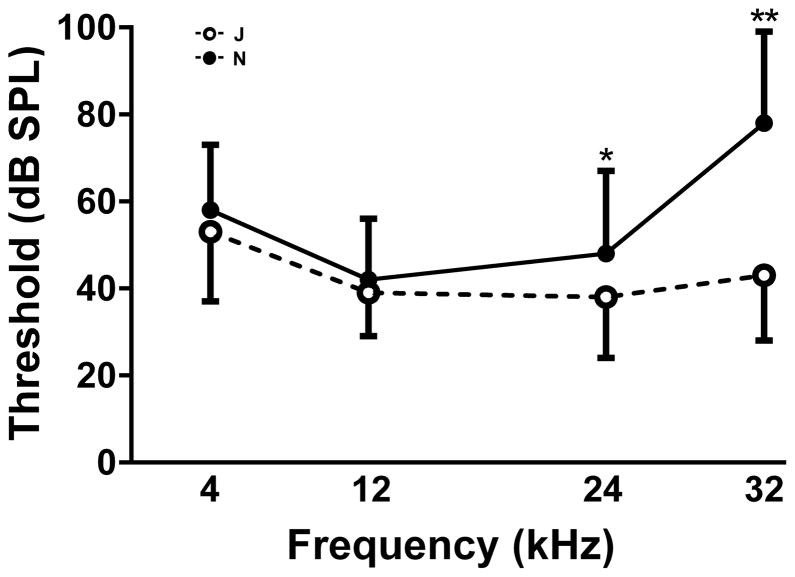
ABR data (Fig. 1a) revealed significant differences between the auditory thresholds of C57BL/6N (N) and C57BL/6J (J) mice. The two substrains performed nearly identically at the lower stimulus frequencies of 4 kHz and 12 kHz but the thresholds diverged significantly at 24 and 32 kHz. While the divergence was small at 24 kHz (~11 dB), the N substrain showed a profoundly higher threshold of approximately 80 dB SPL at 32 kHz, an elevation of 35 dB over the J substrain.
Fig. 1. Baseline ABR data of young adult C57BL/6J and C57BL/6N mice.
1a. Baseline ABRs were taken at the age of 13–14 weeks. Open circles: C57BL/6J; filled circles: C57BL/6N. Number of animals: n = 36 for C57BL/6N except at 24 kHz where n = 17; n = 44 for C57BL/6J except at 24 kHz where n = 23. Data are means + s.d. for N, means − s.d. for J. Significance of differences by two-way ANOVA with Sidak’s post-hoc test for multiple comparisons: F = 40.13; *0.001 < P < 0.01; **P < 0.001.
1b. Examples of amplitude & latency functions. Amplitude vs. intensity and latency vs. intensity functions were analyzed for peak I and peak IV at 12 kHz and 24 kHz. There were no significant differences in any of the parameters between J and N. Representative slopes are shown for peak I at 12 kHz. Open circles: C57BL/6J; filled circles: C57BL/6N. Number of animals: n = 12 of each strain. Data are means − s.d. for N, means + s.d. for J.
Input/output functions were analyzed for a subset of animals with thresholds of 35 ± 5 dB SPL at 12 kHz (n=12 for each J and N) and thresholds of 40 ± 5 dB SPL at 24 kHz (n=13 for each J and N). There were no differences in the slopes of the amplitude vs. intensity or the latency vs. intensity functions of peak I and peak IV at either frequency. Examples of the analyses are given in figure 1b.
3.2. Kanamycin treatment
In agreement with our previous studies (Wu et al., 2001) and consistent with the pattern of aminoglycoside-induced ototoxicity, animals receiving 800 mg kanamycin base/kg body weight twice daily showed high-frequency threshold shifts (Fig. 2). Significant elevations of up to 50 dB were recorded in each substrain: at 24 and 32 kHz in C57BL/6J but only at 24 kHz in C57BL/6N. The latter strain was unsuitable for any evaluation at 32 kHz because of the exceedingly high baseline threshold which masked any potential effect of drug treatment. Even at 24 kHz a comparison of the two substrains also appears misleading. The threshold shift is greater in the J strain (40 dB) than in the N strain (<30 dB), suggesting heightened sensitivity of the J mice. However, the disparity is due to the lower baseline threshold in J mice. There is no significant difference between J and N substrains in the thresholds attained after drug treatment.
Fig. 2. Effect of kanamycin on ABR thresholds.

Mice received 800 mg/kg of kanamycin base twice daily for 14 days. Solid bars: baseline ABR thresholds before drug administration; open bars: thresholds 3 weeks after the end of kanamycin treatment. N indicates the C57BL/6N substrain; J indicates C57BL/6J. Number of animals: n = 5 for C57BL/6N and n = 6 for C57BL/6J. Data are means + s.d. Significance of treatment effects within each strain (by two-way ANOVA with Sidak’s post-hoc test for multiple comparisons): N strain, F = 10.64, *0.001 < P < 0.01; J strain, F = 64.48, **P < 0.001. In contrast, there was no significant difference in the post-treatment thresholds between N and J substrains.
3.3. Noise exposure
We also explored potential differences between the J and N substrains with respect to their susceptibility to noise-induced hearing loss (Fig. 3). An exposure to broadband noise (2–20 kHz, 110 dB SPL) for 2 hours produced significant threshold shifts in each strain. Two weeks after the exposure, thresholds were elevated at all frequencies in the J mice but only at 12 and 24 kHz in the N mice. The lack of an effect at 32 kHz in the latter strain, again, needs to be considered with caution: the high baseline is a confounding factor. A comparison between strains showed that post-noise thresholds were significantly higher at 4 and 12 kHz in C57BL/6J than in C57BL/6N. A total of seven mice received sham treatment, remaining in the booth for 2 hours with no noise exposure. There were no significant changes at any frequency in these control animals.
Fig. 3. Effect of noise exposure on ABR thresholds.

One week after baseline ABRs (filled bars) mice were exposed to broadband noise (2–20 kHz) at 110 dB SPL for 2 h. A second ABR was taken 2 weeks after the noise exposure (open bars). N in the graphs indicates C57BL/6N; J indicates C57BL/6J. Number of animals: n = 6 for each strain. Data are means + s.d. Significance of treatment effects within each strain (by two-way ANOVA with Sidak’s post-hoc test for multiple comparisons): N strain, F = 10.64, *0.001 < P < 0.01; J strain, F = 64.48, **P < 0.001. In addition, post-exposure thresholds differ significantly between J and N strains at 4 kHz and 12 kHz: F = 13.77; *0.001 < P < 0.01; **P < 0.001.
A greater sensitivity of the J substrain to noise trauma was also borne out by the number of non-responses, i.e. when an ABR could not be elicited by the most intense tone stimulus of 115 dB, inferring a threshold above 115 dB (Table 1). Following the noise exposure, ABR traces were still obtained from all C57BL/6N animals, and only three individual measurements (one at 24 kHz and two at 32 kHz) yielded no response. In contrast, only three responses (of a possible total of 24) could be obtained from C57BL/6J mice.
Table 1.
Number of animals without ABR response after 110-dB noise exposure.
| Frequency | N substrain | J substrain |
|---|---|---|
| 4 kHz | 0/6 | 3/6 |
| 12 kHz | 0/6 | 6/6 |
| 24 kHz | 1/6 | 6/6 |
| 32 kHz | 2/6 | 6/6 |
For details, see legend to fig. 3.
4. Discussion
The salient point of this study is the demonstration that commercially available C57BL/6J and C57BL/6N mouse substrains show significant differences in auditory physiology and pathology that preclude interchangeable use or comparisons between them. This point is significant because—despite documented genetic and phenotypic differences between these two substrains—reports of auditory studies frequently lack such identifying information.
All C57BL/6 mice carry an ahl mutation that leads to accelerated hearing loss (Noben-Trauth et al., 2003). Young C57 mice still show audiograms with thresholds similar to those of CBA/J mice, but elevated thresholds are evident already during the first 6 months of life, starting at high frequencies (Li and Borg, 1991; Frisina et al., 2011; Kane et al., 2012; Zhang et al., 2013). The animals in this study were tested at 13 to 14 weeks of age; mature but still young, they are frequently used at this age for studies of drug- or noise-induced pathology. Already at this time, the C57BL/6N substrain exhibits a significant elevation of auditory thresholds compared to the J substrain.
This divergence is in apparent contrast with findings of Kane et al. (2012) showing no significant difference in auditory thresholds at 8, 16, or 32 kHz between 3-month-old C57BL/6J and C57BL/6N mice. Interestingly, thresholds at 32 kHz for the J substrain are essentially identical in Kane’s study (45 ± 6 dB SPL) and ours (43 ± 15). In their phenotypic characterization of the J and N substrains, Simon et al. (2013) also mentioned “no differences” in auditory thresholds but the authors do not include their ABR data or give test frequencies. However, in contrast to our study, Kane et al. (2012) had obtained both substrains from the Jackson Laboratory while the sources of Simon et al. (2013) were the Jackson Laboratory for J and Taconic for N, i.e. suppliers different from ours for the N substrain. Since the N strain has been maintained in several separate locations since the 1970s, sufficient generations have elapsed to allow for genetic drift not only between J and N but also between different N-strain populations.
The availability of substrains from a variety of sources raises the question not only of genetic but also of potential environmental variations (e.g., noise levels in breeding facilities and during transport) on the physiology of animals. We received our animals in three separate shipments over a period of 18 months, with equal numbers of C57BL/6N and C57BL/6J delivered each time. Regardless of purchasing date, baseline ABR data for the two strains were similar and the discrepancy in the 32-kHz thresholds was significant in all three batches of animals. This finding attests to the consistency of the local influences in our study. On the other hand, auditory performance can vary with breeding or housing conditions. Recently reported thresholds of C57BL/6J mice, originally from Jackson Laboratories but then bred “in-house” in two research facilities (Frisina et al., 2011; Zhang et al., 2013), differ from those that we have measured and also from each other. Frisina et al. record threshold means of <10 dB SPL at 12 kHz, <20 dB at 24 kHz and ~25 dB at 32 kHz for young adults of 3–4 months as compared to ~25 dB, >30 dB and ~40 dB, respectively, measured by Zhang et al. for 3-months-old animals. Interestingly, the 4-kHz thresholds are comparably high at 50–60 dB SPL in all three studies.
While the threshold discrepancies between J and N substrains documented here are important in their own right, they also render comparisons of otopathology studies between different C57BL/6 substrains essentially impossible. Aminoglycosides, for example, primarily affect the highest frequencies, which are no longer amenable to measurement in the N substrain at three months of age. Likewise, a striking difference between C57BL/6J and C57BL/6N mice is seen in their susceptibility to noise trauma. Even aside from problems evaluating the highest frequencies, the J strain is significantly more sensitive. Hence, if C57 mice have to be used in studies of otopathology, their substrains have to be carefully chosen. Furthermore, the fact that thresholds within substrains from different sources may vary clearly posits that data from published studies can only be compared if both the substrain and the breeder are defined. An additional complexity of working with C57 mice is introduced by the rapid progression of ABR threshold shifts in young animals. In a longitudinal month-by-month assessment of a C57BL/6J substrain, Li and Borg (1991) observed changes between the ages of 2 to 4 months approximating 10 db/year at high frequencies as well as large individual variability.
Consequently, our study reinforces the absolute need for using age-matched littermates as controls to ensure valid comparisons when evaluating phenotypes—including auditory assessments—of mutant strains of mice. This is especially relevant with respect to the international effort in generating targeted knockout mutations in a large proportion of known mouse genes, which makes use of embryonic stem cells derived from the C57BL/6N substrain (Skarnes et al., 2011).
The study was not designed as an exhaustive characterization of C57BL/6J versus C57BL/6N mice nor to delineate the underlying causes for the observed differences. However, it is tempting to speculate that the genetic discrepancy in the nnt gene might affect auditory pathophysiology. Nicotinamide nucleotide transhydrogenase, for which this gene codes, is an oxidoreductase in the inner mitochondrial membrane. The enzyme has a ubiquitous tissue distribution (Humphrey, 1957) and participates in maintaining mitochondrial redox homeostasis by providing a link between mitochondrial respiration and H2O2 detoxification via the thioredoxin/peroxiredoxin system (Lopert et al., 2014). Mutations in this gene result in mitochondrial redox abnormalities in C57BL/6J mice (Ronchi et al., 2013) and have been implicated in disorders such as familial glucocorticoid deficiency (Meimaridou et al., 2012) and diabetes-like impaired glucose tolerance (Toye et al., 2005). There is compelling evidence for mitochondrial dysfunction as a major contributor to acquired hearing loss by age, noise, and drugs (Böttger and Schacht, 2013) and for an involvement of the thioredoxin/peroxiredoxin system in noise trauma, drug-induced and age-related hearing loss (Chen et al., 2013; Tadros et al., 2014). Complex interactions between external stressors and a genetically compromised mitochondrial homeostasis might further confound studies of auditory pathology in C57BL/6J and comparisons with C57BL/6N mice.
Highlights.
C57BL/6J and C57BL/6N mouse substrains differ in auditory physiology and pathology
C57BL/6N have significantly elevated ABR thresholds at high frequencies
The N substrain is unsuitable as a model for drug ototoxicity
The J substrain is significantly more sensitive to noise trauma
Acknowledgments
This work was supported by research grant R01 DC003685 and core grant P30 DC005188 from the National Institutes on Deafness and Other Communication Disorders, National Institutes of Health. The authors thank Dr. David Dolan, Jennifer Eberle and Karin Halsey for help with and discussions of ABR data; and Andra Talaska for expert editing.
Footnotes
Author contributions: AK and JS designed the study and wrote the manuscript; AK conducted the experiments.
Conflict of Interest: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ann Kendall, Email: akendall@umich.edu.
Jochen Schacht, Email: schacht@umich.edu.
References
- Böttger EC, Schacht J. The mitochondrion: a perpetrator of acquired hearing loss. Hear Res. 2013;303:12–19. doi: 10.1016/j.heares.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FQ, Zheng HW, Schacht J, Sha S-H. Mitochondrial peroxiredoxin 3 regulates sensory cell survival in the cochlea. PLoS One. 2013;8:e61999. doi: 10.1371/journal.pone.0061999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan BA, Blackman KE. Differential susceptibility of 3 sublines of C57BL/6 mice to the induction of colorectal tumors by 1,2-dimethylhydrazine. Cancer Lett. 1980;9:111–115. doi: 10.1016/0304-3835(80)90114-7. [DOI] [PubMed] [Google Scholar]
- Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes. 2006;55:2153–2156. doi: 10.2337/db06-0358. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Singh A, Bak M, Bozorg S, Seth R, Zhu X. F1 (CBA×C57) mice show superior hearing in old age relative to their parental strains: hybrid vigor or a new animal model for “golden ears”? Neurobiol Aging. 2011;32:1716–1724. doi: 10.1016/j.neurobiolaging.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green ML, Singh AV, Zhang Y, Nemeth KA, Sulik KK, Knudsen TB. Reprogramming of genetic networks during initiation of the Fetal Alcohol Syndrome. Dev Dyn. 2007;236:613–631. doi: 10.1002/dvdy.21048. [DOI] [PubMed] [Google Scholar]
- Humphrey GF. The distribution and properties of transhydrogenase from animal tissues. Biochem J. 1957;65:546–550. doi: 10.1042/bj0650546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Jones TA, Johnson KR, Yu H, Erway LC, Zheng QY. A comparison of vestibular and auditory phenotypes in inbred mouse strains. Brain Res. 2006;1091:40–46. doi: 10.1016/j.brainres.2006.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane KL, Longo-Guess CM, Gagnon LH, Ding D, Salvi RJ, Johnson KR. Genetic background effects on age-related hearing loss associated with Cdh23 variants in mice. Hear Res. 2012;283:80–88. doi: 10.1016/j.heares.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Borg E. Age-related loss of auditory sensitivity in two mouse genotypes. Acta Oto-laryngol. 1991;111:827–834. doi: 10.3109/00016489109138418. [DOI] [PubMed] [Google Scholar]
- Lopert P, Patel M. Nicotinamide Nucleotide Transhydrogenase (Nnt) Links the Substrate Requirement in Brain Mitochondria for Hydrogen Peroxide Removal to the Thioredoxin/Peroxiredoxin (Trx/Prx) System. J Biol Chem. 2014;289:15611–15620. doi: 10.1074/jbc.M113.533653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. Behavioral profiles of three C57BL/6 substrains. Front Behav Neurosci. 2010;4:29. doi: 10.3389/fnbeh.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meimaridou E, Kowalczyk J, Guasti L, Hughes CR, Wagner F, Frommolt P, Nurnberg P, Mann NP, Banerjee R, Saka HN, Chapple JP, King PJ, Clark AJ, Metherell LA. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat Genet. 2012;44:740–742. doi: 10.1038/ng.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekada K, Abe K, Murakami A, Nakamura S, Nakata H, Moriwaki K, Obata Y, Yoshiki A. Genetic differences among C57BL/6 substrains. Exp Anim. 2009;58:141–149. doi: 10.1538/expanim.58.141. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchi JA, Figueira TR, Ravagnani FG, Oliveira HC, Vercesi AE, Castilho RF. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radical Biol Med. 2013;63:446–456. doi: 10.1016/j.freeradbiomed.2013.05.049. [DOI] [PubMed] [Google Scholar]
- Saul SM, Brzezinski JA, Altschuler RA, Shore SE, Rudolph DD, Kabara LL, Halsey KE, Hufnagel RB, Zhou J, Dolan DF, Glaser T. Math5 expression and function in the central auditory system. Mol Cell Neurosci. 2008;237:153–169. doi: 10.1016/j.mcn.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, Sorg T, Wong K, Bedu E, Cartwright EJ, Dacquin R, Djebali S, Estabel J, Graw J, Ingham NJ, Jackson IJ, Lengeling A, Mandillo S, Marvel J, Meziane H, Preitner F, Puk O, Roux M, Adams DJ, Atkins S, Ayadi A, Becker L, Blake A, Brooker D, Cater H, Champy MF, Combe R, Danecek P, di Fenza A, Gates H, Gerdin AK, Golini E, Hancock JM, Hans W, Holter SM, Hough T, Jurdic P, Keane TM, Morgan H, Muller W, Neff F, Nicholson G, Pasche B, Roberson LA, Rozman J, Sanderson M, Santos L, Selloum M, Shannon C, Southwell A, Tocchini-Valentini GP, Vancollie VE, Westerberg H, Wurst W, Zi M, Yalcin B, Ramirez-Solis R, Steel KP, Mallon AM, de Angelis MH, Herault Y, Brown SD. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013;14:R82. doi: 10.1186/gb-2013-14-7-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, Jackson D, Severin J, Biggs P, Fu J, Nefedov M, de Jong PJ, Stewart AF, Bradley A. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros SF, D’Souza M, Zhu X, Frisina RD. Gene expression changes for antioxidants pathways in the mouse cochlea: relations to age-related hearing deficits. PLoS One. 2014;9:e90279. doi: 10.1371/journal.pone.0090279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toye AA, Lippiat JD, Proks P, Shimomura K, Bentley L, Hugill A, Mijat V, Goldsworthy M, Moir L, Haynes A, Quarterman J, Freeman HC, Ashcroft FM, Cox RD. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia. 2005;48:675–686. doi: 10.1007/s00125-005-1680-z. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear Res. 2001;158:165–178. doi: 10.1016/s0378-5955(01)00303-3. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Liu H, McGee J, Walsh EJ, Soukup GA, He DZ. Identifying microRNAs involved in degeneration of the organ of corti during age-related hearing loss. PLoS One. 2013;8:e62786. doi: 10.1371/journal.pone.0062786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita E, Chagoyen M, Cantero M, Alonso R, Gonzalez-Neira A, Lopez-Jimenez A, Lopez-Moreno JA, Landel CP, Benitez J, Pazos F, Montoliu L. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011;20:481–489. doi: 10.1007/s11248-010-9403-8. [DOI] [PubMed] [Google Scholar]