Abstract
Bone metastasis represents the leading cause of breast cancer related-deaths. However, the effect of skeleton-associated biomechanical signals on the initiation, progression, and therapy response of breast cancer bone metastasis is largely unknown. This review seeks to highlight possible functional connections between skeletal mechanical signals and breast cancer bone metastasis and their contribution to clinical outcome. It provides an introduction to the physical and biological signals underlying bone functional adaptation and discusses the modulatory roles of mechanical loading and breast cancer metastasis in this process. Following a definition of biophysical design criteria, in vitro and in vivo approaches from the fields of bone biomechanics and tissue engineering will be reviewed that may be suitable to investigate breast cancer bone metastasis as a function of varied mechano-signaling. Finally, an outlook of future opportunities and challenges associated with this newly emerging field will be provided.
1. Introduction
Breast cancer, the most costly type of cancer in the US [1], primarily metastasizes to the skeleton and causes not only increased morbidity (e.g. pain and bone fracture), but ultimately represents the leading cause of breast cancer-related deaths among women worldwide [2]. Following dissemination to bone, cancer cells support their own growth by appropriating the bone remodeling process. More specifically, they stimulate osteolytic bone degradation, which activates the ‘vicious cycle’ of bone metastasis [3]. During this process, cancer cells increase the release of pro-tumorigenic growth factors from the bone matrix that further stimulate tumor growth (e.g. transforming growth factor-β, TGF-β) [4, 5]. Interestingly, bone metastasis typically initiates in the marrow spaces of cancellous bone, such as the spine and hip, a feature that is commonly attributed to the unique cellular and molecular composition of the cancellous compartment (e.g. vasculature, stem cell niches, sites of active remodeling) [6]. However, the irregular architecture of cancellous bone tissue, comprised of interconnecting plate- and rod-like struts interspersed with bone marrow, results in a complex, dynamic mechanical environment. Yet, how these physical cues affect the initiation, progression, and therapy response of bone metastasis is largely unexplored. In this review, we seek to establish that a relationship exists between skeletal mechanical signals and breast cancer bone metastasis, which likely plays an important role in secondary tumor growth, and also discuss appropriate experimental approaches to interrogate this relationship.
To provide structural support for the human body, the skeleton continually adjusts its mass and architecture in response to mechanical loads, and increasing evidence suggests that these physical forces may also play a role during the pathogenesis of bone metastasis. Daily habitual activities, such as walking and even muscle contractions when standing still, exert forces on the skeleton, giving rise to a variety of stresses and strains within the skeleton. Typically, these stresses and strains maintain bone homeostasis by balancing bone-forming and -degrading cellular activities, directly through deformations of the bone matrix and indirectly through fluid flow that imparts shear stresses and fluid pressure [7, 8] However, not only bone, but also tumor cells are capable of responding to these stimuli with immediate consequences on disease progression. For example, solid stress can inhibit tumor cell proliferation [9], increased interstitial fluid pressure stimulates tumor intravasation [10-12], and exposure of cells to shear stresses and pressures regulates their interactions with the vasculature at secondary sites [13]. In addition, results from our and other labs suggest that in vivo mechanical loading inhibits secondary tumor growth in bone [14, 15]. Consequently, biomechanical cues play an important modulatory role in bone metastasis, but more mechanistic studies are needed to better understand how mechanical loads alter bone-tumor interactions and develop therapies based on these principles.
Conventional approaches to studying bone metastasis typically rely on two-dimensional (2-D) cell culture and mouse models as well as simplified mechanical conditions. While these systems have generated critical knowledge regarding the biochemical underpinnings of bone metastasis, they lack dynamic mechanical stimuli, and frequently also other microenvironmental conditions inherent to human disease. Engineering-based approaches have the potential to overcome these shortcomings and provide humanized culture microenvironments and animal models mimicking functional loading conditions. When developing relevant loading models of bone metastasis, a number of critical biological and physical design parameters needs to be considered. Here, we will provide a short introduction to bone biology and mechanics as they pertain to bone metastasis, review current in vitro and in vivo approaches from the field of bone tissue engineering that may be suitable to examine breast cancer bone metastasis as a function of biomechanics, and finally, highlight outstanding challenges and opportunities associated with this newly emerging field.
2. Bone Functional Adaptation
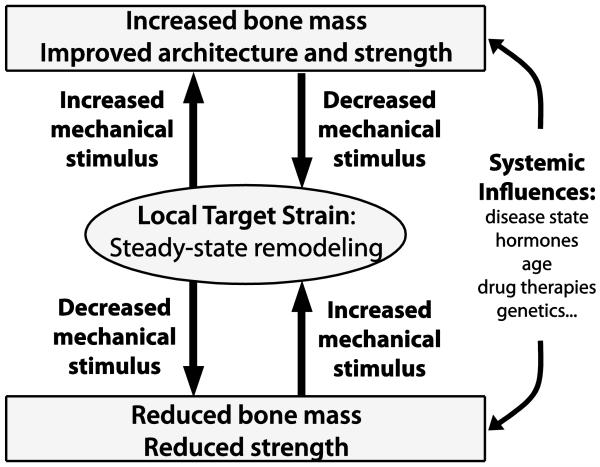
The skeleton is a dynamic, load-bearing tissue that continually undergoes remodeling, whereby ‘old’ bone is degraded (osteolysis) and replaced by new bone (osteogenesis), to meet the mechanical demands of daily activities. Functional adaptation, or more colloquially ‘Wolff’s Law’, is tightly regulated by the local strain environment that arises in bone tissue due to functional loading. The feedback loop governing adaptation, coined the ‘mechanostat’ [16], allows the skeleton to meet mechanical demands by optimizing bone mass and structure, with steady-state remodeling occurring within a target physiological (non-zero) strain range (Figure 1). Reductions in mechanical stimuli (e.g. due to bed rest, increased bone mass, and/or stress-resistant architecture) lead to osteolysis, while increases in mechanical stimuli (e.g. due to sports, reduced bone mass, and/or stress-increasing architecture) promote osteogenesis. Because both physiological and pathological remodeling is a surface-based process, rates are disproportionately high in cancellous regions (i.e. the porous bone found in vertebrae and the ends of long bones) relative to cortical sites. Therefore, it is not surprising that cancellous bone is particularly sensitive to shifts in the remodeling balance due to mechanical stimulation, but also in the presence of tumors as described in more detail below.
Figure 1. Schematic representation of the ‘mechanostat’ [16].
Steady-state remodeling occurs continuously within a target, non-zero strain range. Increased mechanical stimulus (e.g. exercise, reduced bone mass) increases the local strain environment and promotes osteogenesis, which then brings the local strain stimulus down to steady-state. Conversely, reduced mechanical stimulus (e.g. bed rest, microgravity) decreases the local strain environment and results in osteolysis, which then brings the local strain stimulus up to steady-state. Systemic effects, such as disease state, will alter the efficacy of the feedback loop to optimize mechanical integrity of the skeleton. Adapted from Lanyon BoneKey 2009.
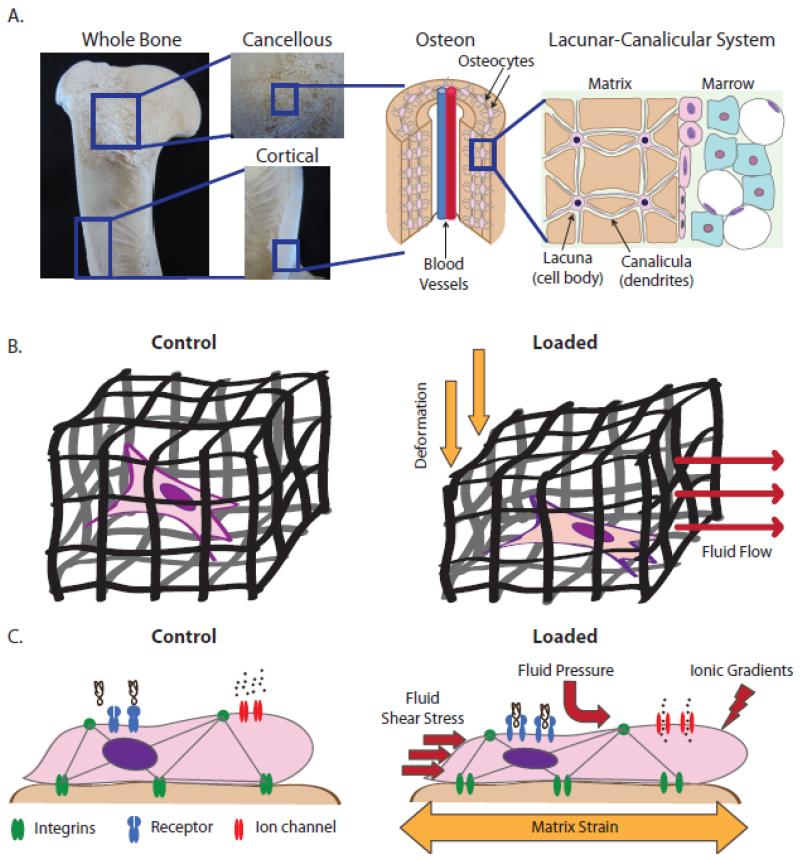
When investigating changes in cell behavior due to bone mechanical loading, two types of mechanical stimulus need to be considered: (1) substrate deformations and (2) interstitial fluid flow. Bone is fundamentally a fluid-filled porous matrix; a cortical shell surrounds cancellous bone and bone marrow in the medullary cavity, and the bone matrix itself is porous as well (Figure 2A). When external forces are applied to a whole bone, the fluid within each level of porosity is instantly pressurized and the resulting pressure gradients cause net fluid flow from high to low pressure (Figure 2B). Fluid flowing over cells, in turn, imposes hydrodynamic shear stresses and drag forces on the cells as well as electric signals resulting from ion gradients at the cell surface-interstitial fluid interface (stress-generated potential, SGP) [17] (Figure 2C). In addition, fluid flow mediates convective transport necessary for enhanced nutrient supply and metabolic waste removal [18]. Collectively, loading-induced movement of fluid through bone transmits chemical, mechanical, and electric signals [19], which, in addition to matrix deformation, functionally couple loading-induced mechanical forces and cell signaling.
Figure 2. Porosity within the skeleton and the cellular mechanical environment under mechanical loading.
A) The skeleton contains porosity at each length scale. A whole bone is comprised of two tissue types: porous cancellous bone and compact cortical bone. The matrix of each of these two bone tissues types is comprised of a vascular porosity called the osteon (~20-40 μm). The osteon is surrounded by concentric layers of matrix containing embedded osteocytes. The cell body of the osteocyte resides in a lacuna (~15-20 μm) while its dendrites reside in canaliculae (~250-300 nm). B) Due to these levels of porosity, bone is fundamentally a fluid-filled porous matrix containing embedded cells. When the porous matrix is deformed, the fluid within is instantly pressurized, causing pressure gradients and subsequent fluid flow from high to low pressure. C) At the cellular level, cells experience substrate strain, hydrodynamic shear stress, pressure gradients, and mass transport-associated ionic gradients. These forces cause deformation of transmembrane proteins as well as changes to cytoskeletal tension, both of which alter cell signaling.
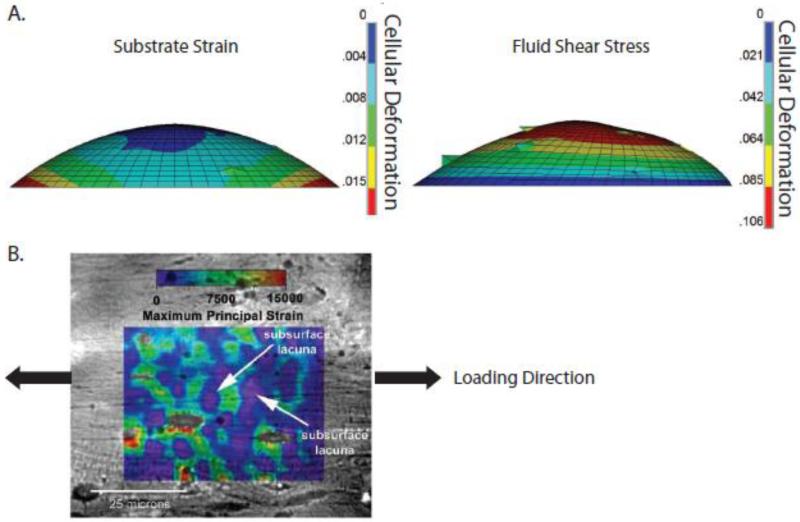
Based on results from 2-D in vitro studies, bone cells are generally considered to be more responsive to fluid flow than substrate strains [20-23]. In fact, a computational model suggested that hydrodynamic loading conditions significantly increase cellular deformation relative to those due to substrate strain [23] (Figure 3A). However, experimental approaches to study cancer and bone regeneration have increasingly turned to 3-D in vitro models that better recapitulate the in vivo environment. Yet in 3-D fluid-filled porous structures (either scaffolds or tissues), matrix strains and fluid shear forces cannot be readily de-coupled. Additionally, pore size strongly affects the morphology of adhered cells, which has downstream consequences on stress-induced cellular deformation [24]. Therefore, the importance of matrix strain versus interstitial flow to mechanotransduction may be underestimated. Indeed, digital imaging correlation revealed that local bone matrix strains around osteocyte lacunae were significantly greater (up to 30,000 με) than the corresponding average macroscopic strains (~2000 με), suggesting that local matrix strains may affect cell behavior more than previously thought [25] (Figure 3B). Nevertheless, compression-induced fluid flow can stimulate expression of mechanosensitive, osteogenic genes relative to substrate strain alone [26]. Model systems in which the relevant mechanical signals are applied in 3-D may help to better understand these disparities and elucidate how loading modulates tumor cell behavior in the skeleton. In order to engineer relevant loading models, an understanding of the resulting stresses and strains at multiple length scales is required.
Figure 3. Hydrodynamic loading may increase cellular deformation relative to substrate strain.
A) A computational model of individual cells undergoing substrate strain and hydrodynamic loading suggested that the effects of hydrodynamic loading dominate cellular deformation [23]. B) Macroscopic tensile strains of 1500 με applied to cortical bone specimens revealed that the local strain field is highly hetereogenous and can be several orders of magnitude greater than the applied strain, as shown by a microstructural strain field overlaid on digital micrographs. Shown here, the local perilacunar strain can reach peaks of over 15,000 με [25]. Reproduced with permission from The Journal of the Federation of American Societies for Experimental Biology and Elsevier.
2.1 Bone Strain
Physiological bone matrix strains can vary significantly depending on bone location (e.g. weight-bearing vs. non-weight bearing bones) as well as the magnitude, frequency, and history of loading. For example, physiological bone matrix strains due to normal daily activity are typically on the order of 0.05% strain (500 με) and occur at a frequency of 1-3 Hz [27, 28], whereas strain events due to muscle contractions occur continually and are characterized by high-frequency (10-50 Hz) and low strain-magnitude (<5 με) [29]. Interestingly, strenuous physical activities such as running and jumping can lead to bone strains as high as 0.2 – 0.35% strain (2000 – 3500 με) in a range of locations [27]. Although such high magnitude strain events occur relatively rarely, they are commonly believed to have the greatest impact on loading-induced bone remodeling and, therefore, may also play a role in bone metastasis [27, 30, 31]. Finally, the strain limit of bone before plastic deformation occurs is 0.7% [32] and the failure strain is 1-3% [33]. Paradoxically, results from in vitro experiments imply that cellular strains much higher than bone failure strain (i.e. up to 10%) are required to elicit intracellular responses. Hence, this suggests that tissue-level strain must be amplified at the cellular level, and the above-described significant differences in locally detected vs. macroscopically applied strains may play a role in this process [25] as well as local fluid flow over cell adhesion sites [34].
2.2 Interstitial Fluid Flow
Theoretical modeling approaches predict that bone loading induces fluid flow-related shear stresses on the order of 8 – 30 dyn/cm2 (0.8 – 3 Pa) [7]. In fact, these stresses, rather than other flow-induced signals (e.g. electrokinetic forces or drag forces) [35, 36], seem to be primarily responsible for the ability of mesenchymal cells in the skeleton to respond to fluid flow (e.g. osteocytes [37-40], osteoblasts and their progenitors [41-43], bone marrow stromal cells [44-46]). Interstitial fluid flow and pressures also affect breast cancer cells. In breast tumors, the interstitial fluid pressure is elevated as high as 1.3 – 5.3 kPa (as compared to normal mammary tissue pressures ~0.1 kPa [47]) [48, 49], thereby generating pressure gradients at the tumor margin [50, 51] and resulting in greater fluid flow in the peritumoral tissue [52]. Increasing the rate of interstitial flow, in turn, correlates with the percentage of migratory breast cancer cells [53, 54]. Moreover, the bone marrow may also be considered a fluid-filled porous tissue that experiences fluid flow due to pressure differentials, and these stimuli may be important for bone metastasis since cancer cells typically localize in the marrow space. This notion is supported by the observation that reduction in interstitial pressure in primary breast tumors can decrease tumor cell proliferation [55-57]. Nevertheless, current lack of information about bone marrow physicochemical characteristics including rheology means this is purely speculative [58]. Intramedullary pressurization of bone marrow can induce fluid flow [59, 60], and pressurization resulting from externally applied loads can peak as high as 130 Pa [61] and can generate shear stresses up to 5 Pa at the interface between marrow and bone tissue [62]. Importantly, such changes in intramedullary pressure can modulate cellular functions in the bone marrow independent of matrix strains [59, 63, 64], suggesting that breast cancer cells may similarly change their behavior.
3. Biology of Bone Remodeling
3.1 Cells and Signals Involved in Bone Remodeling
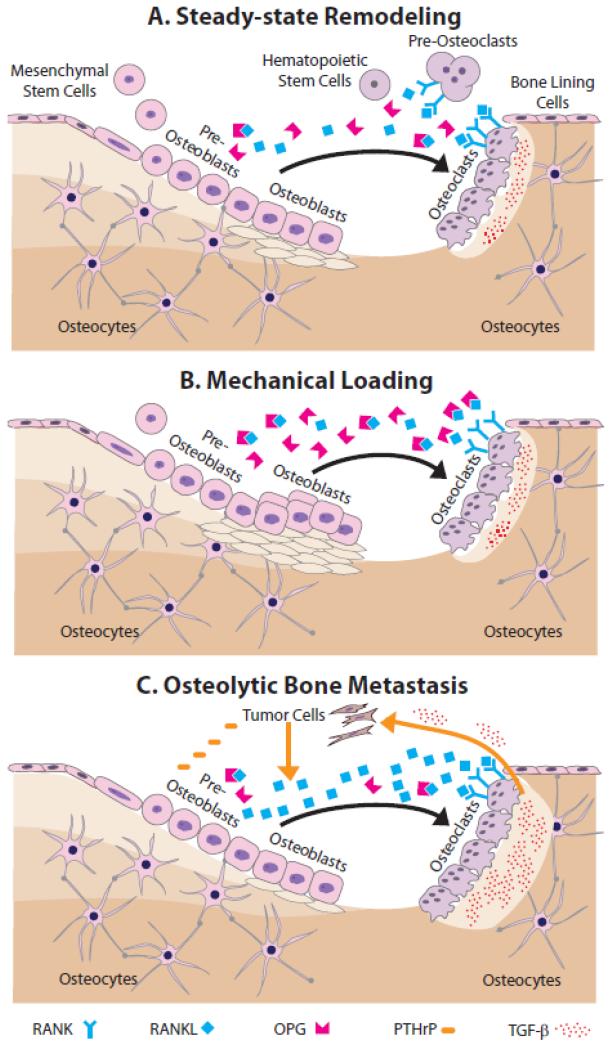
At the cellular level, remodeling depends on the coordinated actions of bone-forming osteoblasts, bone-degrading osteoclasts, and matrix-embedded osteocytes, collectively comprising the ‘basic multicellular unit’ (BMU) (Figure 4A). Under physiological conditions, mesenchymal stem cell-derived osteoblast precursors recruit hematopoietic cells of the macrophage/monocyte lineage from circulation and then induce their differentiation into large, multinucleated osteoclasts. These mature osteoclasts adhere to the bone surface, remove matrix via acidification and proteolytic digestion, and finally apoptose. Active osteoblasts proceed to the excavated area and secrete osteoid, a collagen type I-rich matrix necessary for subsequent mineralization. Following osteoid secretion, osteoblasts become quiescent bone lining cells, undergo apoptosis, or terminally differentiate into osteocytes [65]. The paracrine signals regulating the above-described bone cell interactions during remodeling are manifold.
Figure 4. Steady-state remodeling and the effects of mechanical loading and bone metastasis.
A) During steady-state remodeling, mesenchymal stem cell-derived pre-osteoblasts recruit hematopoietic stem cells and induce their differentiation via RANK-RANKL signaling into large, multinucleated osteoclasts. RANK-RANKL signaling is modulated by osteoblastic secretion of OPG, which is a decoy receptor for RANKL. Mature osteoclasts remove matrix, and then apoptose. Next, active osteoblasts secrete new bone matrix, which subsequently mineralizes. After this, osteoblasts become quiescent bone lining cells, undergo apoptosis, or terminally differentiate into osteocytes. B) During mechanical loading, more mesenchymal precursors commit to the osteoblastic lineage, their differentiation and matrix deposition is enhanced, and apoptosis is inhibited. C) During breast cancer bone metastasis, tumor cells secrete a variety of osteolytic factors including PTHrP, which increases osteoblastic secretion of RANKL thus leading to greater osteoclastogenesis. Elevated resorption of the bone matrix, in turn, releases more pro-tumorigenic growth factors, most notably TGF-β, that further stimulates tumor growth.
Of particular importance to osteoclastogenesis are macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB ligand (RANKL), proteins that are expressed by osteoblasts and their precursors [66] as well as osteocytes [67, 68]. M-CSF stimulates replication while RANKL controls differentiation of monocytes/macrophages into osteoclasts [69]. Importantly, RANKL signaling can be inhibited by osteoblasts and their precursors through expression of the RANKL decoy receptor osteoprotegerin (OPG) [70]. Hence, the local RANKL/OPG ratio plays a critical role in bone remodeling due to its direct effect on osteoclastogenesis. Interestingly, the detected effects of many growth factors and cytokines on osteoclastogenesis similarly occur through altering the OPG/RANKL ratio thus providing another microenvironmental mechanism that affects osteoblast activity during health and disease [71, 72].
Osteoblast differentiation also depends on the coordinated spatiotemporal interplay between growth factors and cytokines, with bone morphogenetic proteins (BMPs), members of the TGF-β superfamily, being the most widely investigated ones. In fact, due to their strong osteogenic effects, BMP-2 and BMP-7 specifically are approved for clinical use in bone fracture healing. BMPs mediate osteoblast differentiation by inducing runt-related transcription factor-2 (Runx2, also known as core-binding factor subunit alpha-1, Cbfa 1) and osterix (Osx) gene expression. Runx2 plays a role throughout the differentiation process and represents the earliest known osteoblast-specific marker during MSC differentiation [73]. Runx2 works in combination with Osx to push precursor cells down the osteoblast lineage [74]. Differentiation into bone-forming osteoblasts is governed by several intracellular pathways, such as mitogen-activated protein kinase (MAPK), nitric oxide (NO), and Wnt signaling (for excellent reviews, see [8, 75]). Sclerostin and dickkopf-1 (DKK1), which are constitutively expressed by osteocytes, decrease osteoblast differentiation and function by inhibiting canonical Wnt signaling as well as BMP signaling [76, 77].
As mature osteoblasts deposit osteoid and advance forward, a portion will remain behind to become embedded within the matrix as osteocytes. Early osteocytes start forming long dendrites, or processes, that reside in very small tunnels called canaliculae (~250-300 nm) while the cell body resides in lacunae (~15-20 μm). Together, these characteristic morphological features form the inter-connected lacunar-canalicular system (LCS), a syncytium-like network (Figure 2A) through which osteocytes communicate with each other and their surrounding cells via gap junctions [78]. Osteocytes regulate bone homeostasis via several mechanisms. In particular, their ability to regulate osteoblast differentiation and function due to constitutive secretion of inhibitory proteins sclerostin and DKK1, which is modulated by systemic (e.g. parathyroid hormone [79]) as well as autocrine and paracrine factors (e.g. prostaglandin E2 [80]). Moreover, osteocytes may exert greater control over osteoclastogenesis than osteoblasts by secreting RANKL in greater abundance [68]. Finally, osteocyte apoptosis is a critical signal that initiates local bone remodeling, presumably to repair local matrix damage [81].
3.2 Loading-induced Changes of Bone Cell Signaling
Loading affects both osteoblast and osteoclast functions, whereby the latter remains controversial as mechanical strain can both suppress and stimulate osteoclastogenesis [82-84]. Furthermore, osteoclasts and their progenitors do not reside in the bone marrow space, but are (i) recruited from circulation and (ii) primarily regulated by osteoblast-derived signals. Hence, their functional adaptation to loading may be limited and primarily mediated through secondary mechanisms [8, 85]. In fact, loading primarily interferes with osteoclastogenesis by increasing osteoblastic secretion of OPG, which ultimately reduces osteoclast differentiation due to a local decrease in the RANKL/OPG ratio [86-88], though the effects of loading on M-CSF secretion are less clear [86, 89]. Furthermore, loading stimulates new bone formation as mechanical stimulation can push mesenchymal precursors down the osteoblastic lineage [90], enhance osteoblast differentiation [91, 92], and inhibit osteoblast apoptosis [93] (Figure 4B). Wnt signaling is likely heavily involved in this process because (i) mice with nonfunctional Wnt receptors respond poorly to mechanical loading of the skeleton [94] and (ii) mechanical strain and fluid shear stress can enhance Wnt signaling [95, 96].
Despite the above-described connections between loading and osteoblast/osteoclast phenotypic changes, osteocytes are widely regarded the main mechanosensors of the skeleton [34, 97, 98]. The LCS is filled with interstitial fluid, which continually moves throughout the ‘syncitium’ with habitual loading and not only changes mass transport of key signaling proteins, but also imposes mechanical forces to the osteocytes. In this way, osteocytes integrate mechanical and chemical cues, and then appropriately direct osteoblast and osteoclast functions. Moreover, mechanical loading inhibits osteocyte apoptosis [99, 100] and promotes new bone formation by decreasing osteocyte secretion of sclerostin and DKK1 [101-103].
The mechanisms of mechanotransduction, which convert mechanical signals into biochemical ones, are relatively poorly understood although altered cell membrane topography as well as cytoskeletal tension likely play a key role [104, 105]. In both cases, transmembrane proteins such as integrins, ion channels, and gap junctions can serve as mechanotransducers (Figure 2C). Integrins are extracellular matrix (ECM) receptors mediating cellular attachment to bone surfaces via the formation of adhesion complexes [106]. In the particular case of osteocytes, these adhesion complexes are termed tethering elements and not only connect osteocyte dendrites with the canalicular wall, but also mediate osteocyte mechanosensing when altered interstitial fluid flow through the LCS puts tensional drag forces on tethering elements [107, 108]. Similarly, loading-induced changes in cell morphology can induce deformations to stretch- or voltage-activated channels and connexons altering intracellular signaling due to varied transport of ions or molecules [109, 110]. For example, voltage-gated channels in bone can depolarize in response to ionic and electric gradients arising from interstitial fluid flow (independent of membrane distortion) as well as in response to the opening of stretch-activated channels [111, 112].
3.3 Breast Cancer Cells and Their Role in Remodeling
Once metastatic breast cancer cells arrive in the skeletal microenvironment, they shift the bone remodeling process towards resorption by interfering with many of the same signaling mechanism that are affected by mechanical loading, just in an opposite manner (Figure 4C). In particular, a tumor-associated increase in parathyroid hormone-related protein (PTHrP) [113] stimulates secretion of RANKL from pre-osteoblasts and inhibits osteoblastic production of OPG [114]. Collectively, this increases the RANKL/OPG ratio, osteoclastogenesis, and ultimately osteolysis. The effect of tumor cells on osteoblasts is not as clearly defined although it has become evident that tumor cells exploit osteoblast-derived niche signals in a hematopoietic stem cell-like manner to home to and survive in bone [115]. Nevertheless, whether breast cancer cells inhibit or stimulate osteoblast differentiation remains unclear as both phenomena have been reported independently [116, 117]. Moreover, tumor cells decrease osteoblast survival [118], which may be responsible for the recent observation that tumor-associated osteolysis is due to reduced osteoblast activity rather than increased osteoclastic resorption [119]. Finally, breast cancer cells secrete factors that stimulate osteoblasts to release pro-inflammatory morphogens. These morphogens, in turn, exert spatial control over tumor cell homing to bone due to their chemoattractive function and sequestration within the cancellous bone matrix [120, 121].
Even less is known about the effect of cancer cells on osteocytes. Yet recent evidence from patients with multiple myeloma (MM), a blood cancer similarly associated with osteolytic lesions, indicates that osteocytes likely play a key role in cancer-related bone degradation. For example, osteocyte apoptosis, and subsequent osteoclastogenesis and osteolytic lesions, were all increased in MM patients [122]. Furthermore, circulating levels of sclerostin were elevated in MM patients and inversely correlated with bone mass, suggesting a direct connection between cancer-mediated differences in osteocyte signaling and osteolysis [123]. However, whether osteocytes were indeed the source of sclerostin and responsible for bone degradation in this specific setup was not verified. On the other hand, it is likely that osteocytes change their phenotype in the presence of a tumor because they can respond to changes in their soluble factor environment [78], form gap junctions with bone metastatic cancer cells [124], and function as progenitor cells for osteosarcoma [125]. Collectively, these results suggest that bone-metastatic cancers inversely regulate many of the same signaling pathways that are controlled by mechanical loading, and that these differences activate the vicious cycle of bone metastasis. As a consequence, leveraging the mechanosensitivity of the bone remodeling process may be a viable method of inhibiting cancer-associated bone disease by preserving normal bone cell fate decisions, viability and function.
4. Models of Applied Mechanical Loading
4.1 In Vivo Models
In vivo models of loading have revealed important principles governing osteogenesis in response to mechanical stimulation [126]. Most importantly, these models have shown that the applied forces must be dynamic to promote osteogenic bone remodeling [127, 128]. More specifically, cyclically-applied loading readily increases bone mass while static loading (e.g. applying a force and holding it constant) has no effect on bone formation. Increasing the magnitude [129, 130], the rate of application [131], and the frequency [132] of mechanical loading are additional parameters that also enhance the osteogenic response. Furthermore, bone cells desensitize to continual mechanical loading; therefore, inserting periods of rest can restore or enhance mechanosensitivity. For example, inserting ~10 seconds of either zero or low magnitude load in between single loading bouts amplified bone formation in two different in vivo loading models [133, 134]. Importantly, this technique lowered the strain magnitude required for activating bone formation as well as returned loading sensitivity to senescent mice [135, 136]. Whether or not these principles have any direct bearing on bone metastatic tumor progression or tumor cell function has not been explored. Nevertheless, due to their ability to modulate resident bone cell behavior and thus, the interplay of these cells with a tumor, it is likely that mechanical signals exert modulatory effects on bone metastasis that may be explored with in vivo models of loading.
In fact, two examples of physiological loading models specifically targeting cancellous bone, whole body high-frequency vibration (WBV) and tibial compression, suggest beneficial effects of this regiment on bone metastasis. In WBV, the loading frequency is greatly increased relative to physiological conditions, thereby permitting reductions in strain magnitude (e.g. in human studies, application rate is ~30Hz in comparison to the typical stride frequency of 1 Hz), which is beneficial to a patient population with skeletal fragility [75]. Only modest gains in bone density, if any, have been achieved using this approach in adults and post-menopausal women [137, 138]. Yet WBV may confer other important benefits, such as pushing bone marrow progenitor cells down the osteoblast rather than adipocyte lineage [139] or preserving osteogenic potential in precursor cells [140]. When applied to a mouse model of spontaneous ovarian cancer, WBV increased bone volume in the spine and proximal tibia, but did not affect the incidence or progression of the disease [15]. Since this particular cancer model does not typically involve metastasis to the skeleton, it remains to be elucidated whether the detected benefits were mediated by modulating bone marrow-derived MSC fate rather than directly affecting bone-tumor cell interactions.
Tibial compression applies compressive forces to cancellous, but also cortical bone. This model was adapted from the ulnar compression model, which was developed to apply forces in a physiological direction (i.e. along the primary axis of the bone) in a limb with a relatively large volume of cancellous bone. Tibial compression increased cortical and cancellous bone mass and improved structural indices in a variety of mouse models, such as hormone-deficient male mice [141] and mice with age-related bone loss [14]. When combined with intratibial injection of breast cancer cells, a common model of metastasis-related secondary tumor growth [142], tibial compression prevented osteolysis and secondary tumor formation in tumor-bearing tibiae [143], suggesting that anabolic loading can be used to inhibit osteolytic remodeling during breast cancer bone metastasis (Figure 5). Nevertheless, it remains to be defined whether these differences were due to interference with tumor cell homing and/or growth. Hence, future studies need to evaluate whether tibial loading may be capable of preventing the bone tropism of breast cancer and/or exhibit a therapeutic effect when applied to already-established, osteolytic bone metastases.
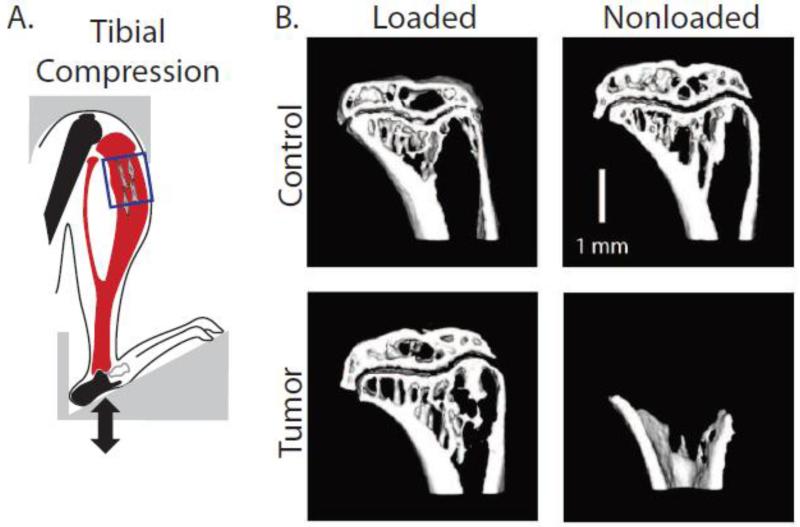
Figure 5. In vivo tibial compression prevents osteolysis and secondary tumor formation.
A) Schematic of compression of tumor-bearing tibiae. The box indicates region of interest for analysis in the proximal compartment. B) Representative microCT images of sham-injected (Control) and tumor-injected (Tumor) tibiae revealed that nonloaded tumor-bearing tibiae exhibited osteolytic degradation, whereas loading inhibited these adverse changes [143]. Reproduced with permission from John Wiley and Sons.
Although these animal models (i) have revealed that the mechanical microenvironment of breast cancer bone metastasis plays an important role in tumor progression and (ii) are well suited to study diseases in the context of the complexities of the whole organism (e.g. intact circulatory system), several limitations exist in their use to investigate cancer processes. For example, creating a human model of bone metastasis necessitates the use of immuno-compromised mice, though the immune system plays a key role in tumorigenesis and metastasis [144]. Additionally, transgenic rodent models are frequently used to study the effect of specific signaling mechanisms on cancer progression, invasion, and finally metastasis to the skeleton, but species-dependent differences in cell-signaling cast doubt on the relevance of such models for human disease. Finally, identifying isolated responses of individual cell types to controlled mechanical stimuli is precluded, and thus elucidating cellular and molecular mechanisms contributing to loading-induced changes of bone metastasis remains challenging. To overcome some of these limitations, engineering strategies have been utilized to create in vitro models of the human tumor and bone microenvironment. Adding mechanical stimulation to these platforms offers promise to reveal loading-induced cellular and molecular mechanisms that may interfere with bone metastasis and that may be explored therapeutically.
4.2 2-D In Vitro Models
2-D models represent the simplest case for investigating mechanical loading, whereby cells are cultured in monolayer on a 2-D surface to which stretching, bending, fluid flow or pressure may be applied. Typical 2-D substrates include tissue culture polystyrene [21], glass [145], silicone membrane [146], bone slices [147], and these substrates can also be surface-coated (e.g. with collagen, fibronectin) to study the integrated effects of cell adhesion and loading on mechanotransduction [148]. A significant advantage of 2-D models is that the strain environment can be readily determined using microscopy, such as digital image correlation [149], or computational approaches, such as finite element analysis (see [150]), and correlated with cell function. For example, when stretching a deformable membrane, the central region undergoes a uniform strain distribution while strains at the periphery are ~50% lower and heterogenous, conditions that may result in non-uniform cell responses [149, 151]. Nevertheless, adjusting geometry (e.g. rectangular versus circular) or loading profile (e.g. cyclic versus steady flow) can be utilized to prescribe a desired stress or strain profile and test the resulting cellular behavior.
Substrate stretch
2-D mechanical strain is typically achieved by seeding cells on a deformable membrane (e.g. silicone), which is then stretched uni-axially or bi-axially on the order of 1-10% strain. Several devices using this approach are commercially available (e.g. the Flexcell® line), and their application has revealed that stretching significantly changes cell behavior. For example, mechanical stretching induces osteogenic differentiation, as detected via increased expression of ALP [146, 152], Runx2 [146, 148, 152, 153], and collagen [146, 148, 153], as well as elevated calcium deposition [154, 155], even in the absence of osteogenic growth factors [156, 157]. As with physiological mechanical loading, this response is enhanced with increasing strain magnitude [153, 157]. Interestingly, the osteogenic response is also dependent on mode of stretching (uniaxial versus biaxial) [158] and cell differentiation stage. More specifically, stretching applied at early stages of osteoblast differentiation increases Runx2 and collagen expression and suppressed proliferation relative to committed osteoprogenitors [159, 160]. To date, only a few studies have investigated the response of cancer cells to dynamic cyclic stretching. For example, cyclic strain increased the proliferative ability of and decreased apoptosis in Lewis lung cancer cells [161]. Furthermore, cyclic strain increased expression of alpha-smooth muscle actin in myofibroblasts and their ability to accelerate cancer cell migration [162], which is of significant interest as these cells compose a large part of the tumor stroma also in secondary tumors in bone [163]. Furthermore, responses to cyclic strain are cell type-dependent and could depend on malignant capacity as leiomyoma cells (uterine cancer) exhibit attenuated mechanosensitivity to cyclic strain relative to their healthy counterparts [164].
Fluid flow
The effects of fluid flow across a monolayer of cells can be investigated using a variety of devices, such as rotating disc or radial flow devices [165, 166], cone and plate viscometers [167], as well as microfluidic strategies to deliver laminar fluid flow to cells attached to channel walls [168]. Nevertheless, the most commonly utilized set-up so far has been a parallel-plate flow chamber, with which both steady and dynamic (e.g. oscillatory and pulsatile) flow regimes can be applied. Using this approach, it could be demonstrated that fluid flow stimulates osteogenic differentiation due to its ability to increase ALP [41, 44], Runx2 [169-171], osteopontin [43, 172-174], osteocalcin [172, 174], and collagen [89, 104, 171, 174, 175]. Generally, 2-D osteogenic flow rates range from 0.01 dyn/cm2 to 20 dyn/cm2 [176], resulting in shear stresses from 0.1 – 2 Pa [177], and inserting rests may enhance osteogenesis similar to in vivo studies. For example, inserting rest periods of 10-15 seconds into an oscillatory fluid flow regime enhances the osteogenic response of MC3T3 cells, as indicated by Ca2+ signaling as well as upregulated osteopontin expression [178]. Finally, it is interesting to note that cyclic flows in 2-D are not definitively more osteogenic than steady flows despite the cyclic nature of stresses and strains intrinsic to habitual physiological activities [170, 179, 180].
Despite their obvious benefits and contribution to a better understanding of mechanically-mediated changes in cell behavior, 2-D culture models are limited due to their inability to recapitulate the dynamic interactions of cells with their 3-D surrounding. These differences in and of themselves can alter cell behavior due to varied cell polarity, cell-cell and cell-matrix interactions [181, 182], but, in addition, may affect responses to mechanical loading [150]. For example, osteoblast expression of osteopontin increases when cultured in 3-D relative to 2-D while the opposite is true in the presence of fluid flow [145]. Similarly, culture dimensionality significantly changes tumor cell behavior [183]; however, the additional effect of loading in these settings remains largely unclear. Engineered 3-D culture models that appropriately mimic the complexity of the tumor microenvironment under appropriate mechanical loading conditions will be critical and have been increasingly pursued in the recent past.
4.3 3-D In Vitro Models
Historically, tissue engineering strategies have been developed to generate 3-D constructs for regenerative medicine applications [184]. More recently, this approach is also increasingly utilized to develop model systems for studying the structure-function relationships in diseases and for testing therapeutic interventions [185]. In particular, various experimental strategies have been developed to mimic cancer and bone tissue as extensively reviewed elsewhere (e.g. [186, 187]). Most commonly, these approaches combine cells and scaffolds, whereby the latter provides 3-D structural support as well as biochemical motifs mimicking cell-ECM interactions. These scaffolds are typically porous and prepared from naturally-derived (e.g. Matrigel and collagen type I) or synthetic (e.g. polyethylene glycol [PEG], polylactide-co-glycolide [PLG]) biomaterials, or a combination thereof. In addition, cell-derived matrices may be used, but this approach usually requires extensive culture periods [188] or involves the use of detergents to decellularize these matrices prior to application of cells [189]. Furthermore, decellularized matrices are typically deposited on conventional cell culture plates and therefore considered 2.5-D, which may not fully eliminate the limitations of unnatural cell polarity. Nevertheless, dissolving decellularized ECMs and reconstituting them in a 3-D manner can overcome these challenges [189]. Clearly, these approaches have yielded important new insights into tumor-bone interactions. However, the additional application of mechanical forces to 3-D materials using various bioreactor technologies can be extremely challenging. In particular, the highly irregular geometries (e.g. pore size) intrinsic to conventional scaffolds and cell-derived ECMs make it difficult to determine the stress and strain fields, in turn, limiting the ability to correlate mechanical and cellular signals. Hence, advances in modeling (e.g. computational fluid dynamics) as well as sophisticated material fabrication methods may be needed [186].
Bioreactors
Bioreactors have initially been developed to overcome transport challenges associated with culturing cells in large 3-D constructs, but may also be used to impart hydrodynamic shear stresses. In particular, diffusion-limited transport of oxygen and nutrients has imposed restrictions to the size of constructs that could be generated: absent of convection, cells remain viable only within 200-800 μm of the scaffold surface, and therefore large (≥~ 1mm) cell-scaffold constructs develop a hypoxic and necrotic core [190-192]. To exert mechanical cues, bioreactors either apply fluid flow to cells or deform a cell-seeded, porous, fluid-filled scaffold.
The spinner flask or stirred flask, the simplest bioreactor for agitating culture media, was designed to improve cell seeding [193] and mass transport [194] over static cultures. In the spinner flask, cells within a 3-D scaffold are suspended via needle, thread, or wire in a large volume of media, and the media is mixed via magnetic stir bar at the bottom (Figure 6A). However, cells still remain localized to the scaffold surface [195], possibly due to the generation of turbulent eddies at the periphery of the scaffold and/or insufficient mass transport to the center of the scaffold [194, 196]. Despite these limitations, osteoblast differentiation is enhanced over static culture [92, 197-199]. Furthermore, this system was used to investigate the effect of fluid shear stress on interactions between prostate cancer and bone-derived cells. Interestingly, the viability of prostate cancer cells was decreased in the presence of media collected from spinner flask-cultured MSCs relative to media from statically cultured MSCs [200]. This suggests that appropriate mechanical stimuli enable MSCs to decrease tumor cell homing to bone during metastasis.
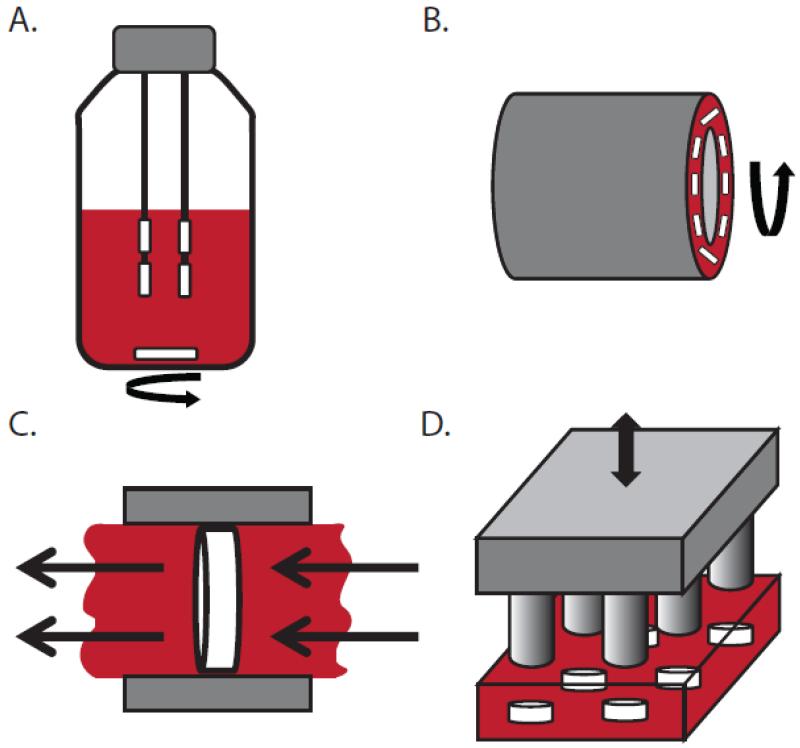
Figure 6. Schematics of commonly used bioreactors.
A) Spinner flask: scaffolds are suspended in media that is circulated via magnetic stir bar. B) Rotating-wall vessel: the outer wall rotates relative to the stationary inner wall to circulate media. C) Direct perfusion: media is driven directly through the porous scaffold, which is housed in a cartridge that is fitted to the scaffold shape such that media cannot flow around it. D) Direct compression: scaffolds are directly compressed via a loading platen.
The rotating-wall vessel (RWV) bioreactor was developed to eliminate turbulence intrinsic to the use of spinner flasks (Figure 6B). RWV bioreactors are typically comprised of concentric walls filled with culture media; the outer wall rotates relative to the stationary inner wall such that the scaffolds are essentially maintained in a state of free fall (i.e. centrifugal forces balanced by gravitational forces) [176, 196]. This design improves mass transport over the spinner flask, but yields inferior osteoblast culture outcomes possibly due to low shear stresses [198, 199, 201]. Nevertheless, when scaffolds are fixed to the bioreactor wall, osteoblast differentiation can be enhanced and is even better than in spinner flask cultures [202]. Alternatively, similar benefits can be achieved by employing semi-permeable vessel walls over which media is perfused [203]. This approach has also been used to generate 3-D prostate, melanoma, and breast cancer cell spheroids or organoids that mimicked in vivo cancerous tissue growth [204-209], further demonstrating that fluid shear is an important microenvironmental parameter for a variety of cancers.
Perfusion bioreactors directly address persistent limitations in mass transport to the interior of 3-D cultures in spinner flask and RWV designs [210] (Figure 6C). Direct perfusion bioreactors typically utilize pumps to drive media directly through a porous scaffold, which is housed in a cartridge that is fitted to the scaffold shape such that media cannot flow around it (in contrast to indirect perfusion in which fluid can flow around). Direct perfusion exerts shear stresses throughout the entire cell-scaffold construct in addition to providing superior mass transport, which results in even distribution of cells throughout the scaffold. However, a trade-off exists between enhancing mass transport with higher flow rates and allowing for cell attachment and more uniform ECM formation with lower flow rates [197, 211, 212] (Figure 7A). For cancer and bone tissue engineering applications, perfusion bioreactors most commonly employ steady or continuous flow [177, 213]. For example, steady flow applied via microfluidic device to MDA-MB231 breast cancer cells in 3-D hydrogels increased the percentage of breast cancer cells that migrate and their speed in response to flow [53] (Figure 7B), although this effect may be modulated by seeding density [54]. In the context of bone tissue engineering, steady perfusion enhances growth, differentiation, and mineralization of scaffolds seeded with bone cells relative to static controls [92, 201, 212, 214-216], even in the absence of osteogenic differentiation factors [91].
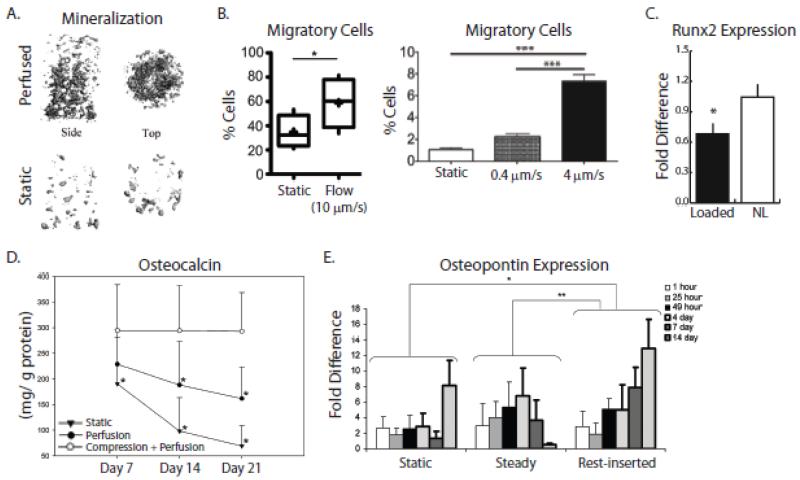
Figure 7. Exemplary 3-D studies demonstrating that interstitial flow affects cell behavior.
A) Perfusion of marrow stromal cells cultured in polycaprolactone (PCL) scaffolds resulted in more uniform ECM and mineralization, as indicated by microCT analysis [212]. B) (Left) Interstitial fluid flow through a collagen gel, applied via a microfluidic device, increased overall percentage of human breast cancer cells that migrate. (Right) Additionally, this percentage increased with increasing flow rate [53]. C) Cyclic compression applied to human breast cancer cells cultured in mineral-containing poly(lactide-co-glycolide) (PLG) scaffolds reduced their expression of Runx2, a gene associated with initiating osteolysis [143]. D) Production of osteocalcin was greatest when both compression and perfusion was applied to bone marrow-derived mesenchymal stem cells cultured in spongiosa disks [222]. E) Inserting periods of rest during flow enhanced expression of osteopontin in MC3T3 pre-osteoblasts cultured in collagen-glycosaminoglycan constructs [227]. Reproduced with permission from Elsevier, John Wiley and Sons, Royal Society of Chemistry, and Mary Ann Liebert, Inc.
Direct compression of cell-seeded scaffolds is another common approach for stimulating osteogenesis and typically applies cyclic loads in the range of 1-5 Hz (Figure 6D). For example, compression of mesenchymal stem cells seeded in cancellous bone-fibrin ‘sandwiches’ [217] increases expression of osteogenic signaling molecules [218], and a similar effect was noted for mature osteoblasts [219]. Interestingly, these responses may be further modified by imposing high frequency vibrations (~25 Hz) onto cyclic compression (3 Hz) [220]. Because compression using this approach simultaneously alters fluid flow and substrate deformation, it is difficult to attribute the detected changes to one vs. the other, though compression-induced fluid flow upregulates mechanosensitive, osteogenic genes relative to substrate strain alone [26]. To date, the effects of dynamic scaffold compression on breast cancer cells are not widely investigated. Yet human breast cancer cells subjected to 50% static compression, corresponding to ~0.1 kPa in agarose gels, increase expression of genes related to ECM proteolysis, adhesion, and migration [221]. In contrast, human breast cancer cells subjected to 10% cyclic compression of a 3-D model of the bone microenvironment decreased expression of genes interfering with bone homeostasis [143] (Figure 7C). Applying compression and perfusion simultaneously may best mimic the in vivo bone mechanical microenvironment, and indeed this approach increases osteoblast differentiation relative to perfusion alone [196]. Finally, adding cyclic compression to steady perfusion may foster osteogenic commitment and differentiation of mesenchymal stem cells [42, 222] (Figure 7D). Whether or not these changes alter downstream breast cancer cell behavior remains to be elucidated.
The modulatory effect of key loading characteristics (e.g. varied strain magnitude) on cellular functions relevant to bone metastasis is currently being investigated. These studies have, for example, revealed that increasing the medium flow rate can enhance osteogenesis due to the elevated hydrodynamic shear stresses [215, 223]. Similarly, breast cancer cells become more migratory in response to increased flow rate [53] (Figure 7B). Though cyclic flow best reflects conditions in the bone microenvironment in vivo, its superiority in tissue engineered bone constructs is not clear because few studies incorporate these conditions and due to the inability to compare results across disparate scaffold systems [224, 225] (see Computational Modeling). Yet, inserting periods of rest in steady flow regulates cell function in 3-D scaffolds cultured in perfusion bioreactors. More specifically, interposing high rate steady flow with periods of low flow increases osteoblast production of PGE2, a biomolecule involved in bone formation [224, 226]. Similarly, osteopontin is upregulated with 10 second rest periods between loading cycles as well as with 7-8 hours of rest in between loading sessions [224, 227] (Figure 7E), while other markers of bone differentiation were not affected by intermittently reduced loading [211]. The effects of cyclic fluid flow on cancer cell behavior has been largely unexplored, though such studies are important for understanding the interplay between bone metastasis and mechanical signals in the skeleton.
Delineating the relative effects of fluid flow on mass transport versus shear stresses is difficult in perfusion experiments because the two are intimately coupled in 3-D porous scaffolds. Yet, these two effects can be isolated by altering fluid viscosity without changing flow rate. More specifically, the addition of dextran to culture media increases its viscosity (and thus related shear stresses) while flow rate (and thus mass transport) remains constant [228]. Interestingly, this approach elucidated that shear stresses dominate cell behavior: Increasing shear stress enhances osteoblast differentiation and matrix mineralization [228, 229]. In contrast, increasing mass transport enhances osteogenic differentiation only up to a certain point (6-9 ml/min), after which tissue formation is inhibited, again demonstrating that there is an optimum range for proper nutrient supply and cell retention [228]. The relevance of these findings to bone metastasis remains to be determined. As stated previously, fluid flow increases breast cancer cell migration, but whether these effects are dominated by shear stress or mass transport effects is unclear. Furthermore, these findings were based on the relatively small fluid shear stresses in tumor-associated mammary tissue (in which mass transport effects may be more important than shear stresses [213]), but a different scenario may occur in bone. Finally, other microenvironmental variations may significantly affect how cells interpret shear stress vs. mass transport. For example, small changes in the ECM architecture, such as fiber alignment, can vastly alter local shear stresses [230, 231] and thus cancer cell function.
Computational Modeling
Characterizing the stress and strain fields in porous 3-D constructs with highly irregular geometries is incredibly difficult, even when ignoring the fact that tissue formation and remodeling over time will continually alter the stress and strain environment. The majority of scaffolds have highly irregular as well as non-uniform pore geometries; therefore, the stresses and strains within will also be irregular, further leading to heterogeneous cellular responses, even if similar mechanical signals are applied (e.g. the same averaged flow rate or bulk strains). Furthermore, comparing results between different labs is intractable. Computational modeling, such as computational fluid dynamics (CFD) and finite element modeling (FE), can help to address these challenges, and, in addition, define design parameters for bioreactor or scaffold design to achieve a specific mechanical environment.
Computational models revealed that pore size strongly affects the morphology of adhered cells, hydrodynamic shear strains, and mass transport. In large pores, cells tend to spread whereas in smaller pores, they exhibit rounder morphologies due to forming several attachment points [232], and this greatly affects cell deformation. More specifically, shear strains in flat cells are on the order of 1×10−7% strain (based on 1% scaffold deformation), while shear strains in bridged cell are on the order of 0.1% [233]. This may mean cells adhering in a 3-D manner are more likely to detach under medium flow, which may significantly impact tumor cell homing to bone during metastasis. Accordingly, cell detachment was found to be proportional to flow rate and inversely proportional to pore size [234] (Figure 8A). Furthermore, this implies that reduced shear stresses (~20 mPa) are necessary to induce osteogenesis in scaffolds with decreased pore size (< 100um) [224]. However, larger pore sizes improved mass transport and distribution of cells throughout the scaffold [235, 236]. Pore size strongly predicts shear stress magnitude, where shear stresses increase with decreasing pore sizes, though overall scaffold porosity strongly affects the statistical distribution of the shear stresses [237]. Finally, regular distribution of pores (e.g. PCL scaffold) results in asymmetric distribution of wall shear stresses (with a peak and plateau), whereas an irregular distribution of pores (e.g. scaffolds fabricated from particulate leaching) yields symmetric distribution of wall shear stresses and thus, ‘conditions’ the flow better [238] (Figure 8B). Collectively, these findings suggest that the integrated effects of local ECM architecture and mechanical conditions impact whether or not disseminated tumor cells form secondary tumors in bone, and that computational strategies will help to accurately predict these possibilities. For example, mathematical calculation of these features may shed light on why certain skeletal sites (e.g. spine and pelvis) are preferred for metastasis relative to others (e.g. wrist).
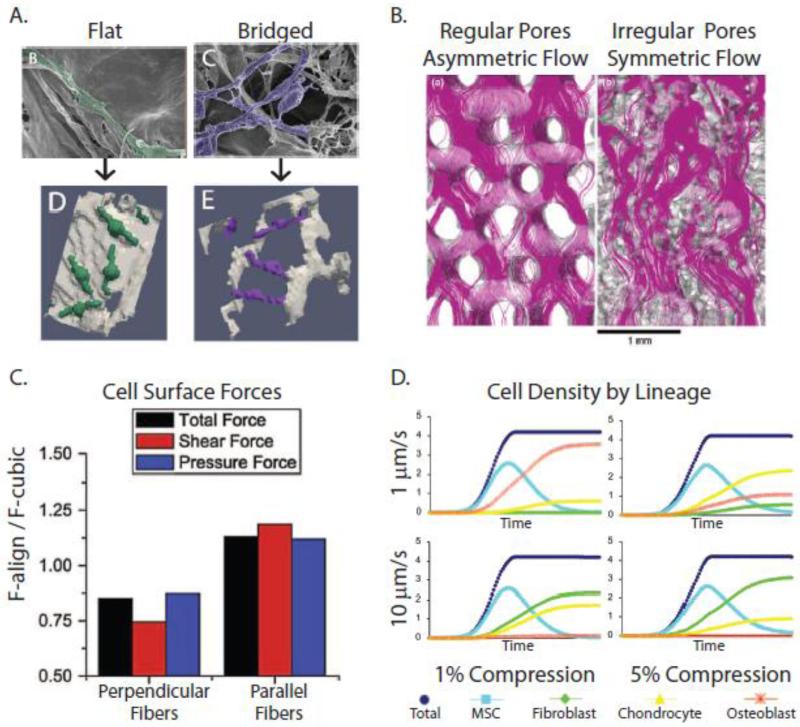
Figure 8. Computational simulations of loading-induced changes of cell behavior in 3-D porous scaffolds.
A) Computational models of MC3T3 pre-osteoblasts within collagen-glycosaminoglycan (CG) scaffolds (Bottom) rendered from high-resolution scanning electron microscopy (Top) revealed that cell detachment is proportional to flow rate and inversely proportional to pore size [234]. B) Flow patterns through porous scaffolds depend on pore orientation. In polycaprolactone (PCL) scaffolds, pores are more regularly-oriented, resulting in more asymmetric flow and shear stresses. In contrast, in silk fibroin scaffolds, the pores are more irregularly-oriented, ‘conditioning’ the flow, and shear stresses are more uniform [238]. C) Perpendicular alignment of ECM fibers results in lower cellular strains (stress-shielding), while parallel alignment of the fibers increases shear stresses on cells, as demonstrated using computational fluid dynamics simulations [231]. D) When FE and CDF approaches were combined to model the effect of specific combinations of compression and perfusion on MSCs cultured within CG scaffolds, differing patterns of MSC differentiation into fibroblasts, chrondrocytes, or osteoblasts were predicted [233]. Reproduced with permission from John Wiley and Sons, Springer, and Elsevier.
Computational models also predict that fiber orientation in ECM-like scaffolds may modulate the mechanical environment of cells. Perpendicular alignment of ECM fibers generates higher stresses on the ECM, and lower shear stress and pressure forces on cells, relative to cubic lattice arrangement, and this may be due to stress shielding. Conversely, parallel fiber alignment leads to higher stresses on cells [231]. Importantly, these results will not be predicted if simply using calculations based on the bulk average flow characteristics (Figure 8C). Additionally, small modifications to the local ECM can lead to large changes in the mechanical environment. For example, minor remodeling of fibers near the cell surface exhibits major effects on the cellular shear stress profile [230]. Again, how cells attach to the fibers, whether flat or bridged, will likely influence resulting hydrodynamic shear stresses. But in addition, this may mean that pathological ECM remodeling may generate a mechanical environment that regulates homing, dormancy, and growth of bone metastases.
When CFD is combined with FE analysis of the scaffold material, even richer results can be achieved [233, 239, 240]. For example, specific combinations of matrix strains and inlet fluid flows led to predictive differentiation patterns of MSCs in collagen-glycosaminoglycan scaffolds based on matrix and hydrodynamic strains [233] (Figure 8D). More specifically, osteogenic differentiation was greatest at low inlet velocity (1 μm/second) and low scaffold strains (1-2%), while chondrogenic differentiation dominated with increasing scaffold strain (5%) and fibroblastic differentiation dominated with increasing inlet velocity (100 μm/second). Using PLA + glass scaffolds, similar predictive power was achieved when modeling dynamic compression [239]. Homogeneous mature bone tissue formation occurred under strain levels of 0.5-1% and at rates of 0.0025-0.005 strain per second. Conversely, under higher levels of strain and strain rates, heterogeneous stresses and strains led to the formation of a heterogeneous tissue with a mixture of mature bone and fibrous tissue. These studies reveal that local tissue mechanics intimately regulate stem cell commitment and differentiation, which may significantly impact the establishment of metastatic tumors. For example, in bone marrow, MSCs and their progeny participate in creating and maintaining the hematopoietic stem cell niche, and disseminated cancer cells compete for occupancy of these niches [241, 242], though the role of mechanical loading in this regard is unknown.
5. Conclusions and Future Perspectives
In conclusion, mechanical forces modulate the reciprocal interplay between bone and tumor cells critical to bone metastasis, and engineering approaches offer potential to evaluate the underlying biophysical mechanisms. However, to develop effective therapeutic strategies based on these principles, much more sophisticated experimental and computational strategies will be needed. For example, ‘humanized mice’ can overcome challenges associated with studying human cells in a murine organism, and additionally may recapitulate the full range of (human) primary tumor development and metastatic cascade to the skeleton [243]. In addition, human bone fragments or tissue-engineered bone substitutes provide attractive alternatives for studies of human bone metastasis [244, 245]. However, these xenograft models are typically implanted at ectopic sites (e.g. subcutaneously), which currently makes the application of external mechanical forces difficult. Development of an in vivo loading model that is compatible with murine ‘humanized’ microenvironments, or vice versa, will greatly contribute to understanding the role of mechanical signals in bone metastasis. Additionally, in vitro model systems that incorporate multiple cell types, rather than monoculture or at best tri-culture, are likely to generate more advanced insights. In particular, characterization of the mechanical environment within the LCS, and how it facilitates or inhibits osteocyte-tumor cell signaling, is a relatively unexplored area. Sophisticated multi-physics modeling at several length scales will expand our knowledge in this regard, which can be leveraged to determine osteocyte-tumor cell interactions in the presence of mechanical loading.
Another future goal should be to more thoroughly characterize the ECM architecture and mechanical conditions in the bone marrow given its physical interactions with metastasized tumor cells. The bone marrow comprises numerous cell types (hematopoietic stem cells and their progeny [e.g. macrophages, platelets, and osteoclasts], MSCs and their progeny [e.g. adipocytes, fibroblasts, and osteoblasts], endothelial cells) that are both mechano-sensitive and known to regulate tumor cell signaling. For example, mechanically loading MSCs enhances their secretion of pro-angiogenic factors [246], especially when co-cultured with endothelial cells [247], and this may have both direct (via modulating tumor cell functions) and indirect consequences (via enhancing angiogenesis) on secondary tumor growth. Similarly, osteoblastic cells serve a supportive function in maintaining normal hematopoiesis [248], which is likely modulated by mechanical signals and disseminated tumor cells [249]. Better model systems of the bone marrow and its constituent cells will provide more insight in this regard [250, 251].
Another objective should be to evaluate the effect of mechanical loading on bone material properties and which modulatory roles tumor cells assume in this process. In fact, mechanical loading can alter bone material quality [252], and it remains to be investigated (i) which cellular mechanisms underlie these changes and (ii) what their functional consequences are on bone metastasis. For example, osteocyte network properties critically influence bone mineral quality, but neither the effects of loading nor tumor cells on these network properties are clear [253]. Nevertheless, tumor cell adhesion, growth and osteolytic capability are all modulated by varied bone mineral properties [254]. Therefore, adapting advanced materials characterization and synthesis techniques to study the integrated effects of loading and metastasis on bone materials properties will be critical.
Finally, metastasis is directed by premetastatic remodeling of target sites even prior to initial homing to these locations [255]. Hence, designing mechanically relevant culture models that not only recapitulate the complex interactions between bone and tumor cells, but also integrate other organ compartments of the body should be a priority. Organ-on-a-chip microfluidic systems may help to address these integrative effects and have the potential to provide mechanistical insights that may ultimately contribute to improved therapeutic strategies that may help to prevent and treat bone metastasis.
Acknowledgements
Funding was provided by an Individual Biomedical Research Award for M. Lynch from The Hartwell Foundation, a Humboldt Fellowship for Experienced Researchers for C. Fischbach, the NIH/MRRCC 5P30 AR046121-09 as well as the National Cancer Institute (R01CA173083 and the Cornell Center on the Microenvironment & Metastasis through Award Number U54CA143876). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1]. www.cancer.gov.
- [2].Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–180. doi: 10.1007/s10911-005-5399-8. [DOI] [PubMed] [Google Scholar]
- [3].Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, Chirgwin JM. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- [4].Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guise TA. The vicious cycle of bone metastases. J Musculoskelet Neuronal Interact. 2002;2:570–572. [PubMed] [Google Scholar]
- [6].Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- [7].Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- [8].Robling AG, Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009;19:319–338. doi: 10.1615/critreveukargeneexpr.v19.i4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK. Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol. 1997;15:778–783. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- [10].Boucher Y, Jain RK. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: implications for vascular collapse. Cancer Res. 1992;52:5110–5114. [PubMed] [Google Scholar]
- [11].Fleury ME, Boardman KC, Swartz MA. Autologous morphogen gradients by subtle interstitial flow and matrix interactions. Biophys J. 2006;91:113–121. doi: 10.1529/biophysj.105.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, Munn LL, Jain RK. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- [13].Richter U, Wicklein D, Geleff S, Schumacher U. The interaction between CD44 on tumour cells and hyaluronan under physiologic flow conditions: implications for metastasis formation. Histochem Cell Biol. 2012;137:687–695. doi: 10.1007/s00418-012-0916-5. [DOI] [PubMed] [Google Scholar]
- [14].Lynch ME, Main RP, Xu Q, Schmicker TL, Schaffler MB, Wright TM, van der Meulen MC. Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging. Bone. 2010;49:439–446. doi: 10.1016/j.bone.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pagnotti GM, Adler BJ, Green DE, Chan ME, Frechette DM, Shroyer KR, Beamer WG, Rubin J, Rubin CT. Low magnitude mechanical signals mitigate osteopenia without compromising longevity in an aged murine model of spontaneous granulosa cell ovarian cancer. Bone. 2012;51:570–577. doi: 10.1016/j.bone.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- [17].Qin YX, Lin W, Rubin C. The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Ann Biomed Eng. 2002;30:693–702. doi: 10.1114/1.1483863. [DOI] [PubMed] [Google Scholar]
- [18].Knothe Tate ML, Knothe U, Niederer P. Experimental elucidation of mechanical load-induced fluid flow and its potential role in bone metabolism and functional adaptation. Am J Med Sci. 1998;316:189–195. doi: 10.1097/00000441-199809000-00007. [DOI] [PubMed] [Google Scholar]
- [19].Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269:80–82. doi: 10.1038/269080a0. [DOI] [PubMed] [Google Scholar]
- [20].Owan I, Burr DB, Turner CH, Qiu J, Tu Y, Onyia JE, Duncan RL. Mechanotransduction in bone: osteoblasts are more responsive to fluid forces than mechanical strain. Am J Physiol. 1997;273:C810–815. doi: 10.1152/ajpcell.1997.273.3.C810. [DOI] [PubMed] [Google Scholar]
- [21].Smalt R, Mitchell FT, Howard RL, Chambers TJ. Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. Am J Physiol. 1997;273:E751–758. doi: 10.1152/ajpendo.1997.273.4.E751. [DOI] [PubMed] [Google Scholar]
- [22].You J, Yellowley CE, Donahue HJ, Zhang Y, Chen Q, Jacobs CR. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng. 2000;122:387–393. doi: 10.1115/1.1287161. [DOI] [PubMed] [Google Scholar]
- [23].McGarry JG, Klein-Nulend J, Mullender MG, Prendergast PJ. A comparison of strain and fluid shear stress in stimulating bone cell responses--a computational and experimental study. Faseb J. 2005;19:482–484. doi: 10.1096/fj.04-2210fje. [DOI] [PubMed] [Google Scholar]
- [24].Stops AJ, Harrison NM, Haugh MG, O’Brien FJ, McHugh PE. Local and regional mechanical characterisation of a collagen-glycosaminoglycan scaffold using high-resolution finite element analysis. J Mech Behav Biomed Mater. 2010;3:292–302. doi: 10.1016/j.jmbbm.2009.12.003. [DOI] [PubMed] [Google Scholar]
- [25].Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J. Osteocyte lacunae tissue strain in cortical bone. J Biomech. 2006;39:1735–1743. doi: 10.1016/j.jbiomech.2005.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tanaka SM, Sun HB, Roeder RK, Burr DB, Turner CH, Yokota H. Osteoblast responses one hour after load-induced fluid flow in a three-dimensional porous matrix. Calcif Tissue Int. 2005;76:261–271. doi: 10.1007/s00223-004-0238-2. [DOI] [PubMed] [Google Scholar]
- [27].Burr DB, Milgrom C, Fyhrie D, Forwood M, Nyska M, Finestone A, Hoshaw S, Saiag E, Simkin A. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18:405–410. doi: 10.1016/8756-3282(96)00028-2. [DOI] [PubMed] [Google Scholar]
- [28].Fritton SP, McLeod KJ, Rubin CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech. 2000;33:317–325. doi: 10.1016/s0021-9290(99)00210-9. [DOI] [PubMed] [Google Scholar]
- [29].Huang RP, Rubin CT, McLeod KJ. Changes in postural muscle dynamics as a function of age. J Gerontol A Biol Sci Med Sci. 1999;54:B352–357. doi: 10.1093/gerona/54.8.b352. [DOI] [PubMed] [Google Scholar]
- [30].Rubin CT, Lanyon LE. Limb mechanics as a function of speed and gait: a study of functional strains in the radius and tibia of horse and dog. J Exp Biol. 1982;101:187–211. doi: 10.1242/jeb.101.1.187. [DOI] [PubMed] [Google Scholar]
- [31].Rubin CT, Lanyon LE. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J Theor Biol. 1984;107:321–327. doi: 10.1016/s0022-5193(84)80031-4. [DOI] [PubMed] [Google Scholar]
- [32].Carter DR, Caler WE, Spengler DM, Frankel VH. Fatigue behavior of adult cortical bone: the influence of mean strain and strain range. Acta Orthopaedica Scandinavica. 1981;52:481–490. doi: 10.3109/17453678108992136. [DOI] [PubMed] [Google Scholar]
- [33].Reilly DT, Burstein AH. The elastic and ultimate properties of compact bone tissue. J Biomech. 1975;8:393–405. doi: 10.1016/0021-9290(75)90075-5. [DOI] [PubMed] [Google Scholar]
- [34].Fritton SP, Weinbaum S. Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction. Annu Rev Fluid Mech. 2009;41:347–374. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hung CT, Allen FD, Pollack SR, Brighton CT. What is the role of the convective current density in the real-time calcium response of cultured bone cells to fluid flow? J Biomech. 1996;29:1403–1409. doi: 10.1016/0021-9290(96)84535-0. [DOI] [PubMed] [Google Scholar]
- [36].Bakker AD, Soejima K, Klein-Nulend J, Burger EH. The production of nitric oxide and prostaglandin E(2) by primary bone cells is shear stress dependent. J Biomech. 2001;34:671–677. doi: 10.1016/s0021-9290(00)00231-1. [DOI] [PubMed] [Google Scholar]
- [37].Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts--correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217:640–648. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- [38].Lu XL, Huo B, Park M, Guo XE. Calcium response in osteocytic networks under steady and oscillatory fluid flow. Bone. 2012;51:466–473. doi: 10.1016/j.bone.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tan SD, de Vries TJ, Kuijpers-Jagtman AM, Semeins CM, Everts V, Klein-Nulend J. Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone. 2007;41:745–751. doi: 10.1016/j.bone.2007.07.019. [DOI] [PubMed] [Google Scholar]
- [40].You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42:172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kapur S, Baylink DJ, Lau KH. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone. 2003;32:241–251. doi: 10.1016/s8756-3282(02)00979-1. [DOI] [PubMed] [Google Scholar]
- [42].Liu C, Abedian R, Meister R, Haasper C, Hurschler C, Krettek C, von Lewinski G, Jagodzinski M. Influence of perfusion and compression on the proliferation and differentiation of bone mesenchymal stromal cells seeded on polyurethane scaffolds. Biomaterials. 2012;33:1052–1064. doi: 10.1016/j.biomaterials.2011.10.041. [DOI] [PubMed] [Google Scholar]
- [43].You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem. 2001;276:13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- [44].Grellier M, Bareille R, Bourget C, Amedee J. Responsiveness of human bone marrow stromal cells to shear stress. J Tissue Eng Regen Med. 2009;3:302–309. doi: 10.1002/term.166. [DOI] [PubMed] [Google Scholar]
- [45].Kim CH, You L, Yellowley CE, Jacobs CR. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone. 2006;39:1043–1047. doi: 10.1016/j.bone.2006.05.017. [DOI] [PubMed] [Google Scholar]
- [46].Sikavitsas VI, Bancroft GN, Lemoine JJ, Liebschner MA, Dauner M, Mikos AG. Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Ann Biomed Eng. 2005;33:63–70. doi: 10.1007/s10439-005-8963-x. [DOI] [PubMed] [Google Scholar]
- [47].Wiig H. Evaluation of methodologies for measurement of interstitial fluid pressure (Pi): physiological implications of recent Pi data. Crit Rev Biomed Eng. 1990;18:27–54. [PubMed] [Google Scholar]
- [48].Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–949. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- [49].Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- [50].Flessner MF, Choi J, Credit K, Deverkadra R, Henderson K. Resistance of tumor interstitial pressure to the penetration of intraperitoneally delivered antibodies into metastatic ovarian tumors. Clin Cancer Res. 2005;11:3117–3125. doi: 10.1158/1078-0432.CCR-04-2332. [DOI] [PubMed] [Google Scholar]
- [51].Wiig H, Tveit E, Hultborn R, Reed RK, Weiss L. Interstitial fluid pressure in DMBA-induced rat mammary tumours. Scand J Clin Lab Invest. 1982;42:159–164. [PubMed] [Google Scholar]
- [52].Dafni H, Israely T, Bhujwalla ZM, Benjamin LE, Neeman M. Overexpression of vascular endothelial growth factor 165 drives peritumor interstitial convection and induces lymphatic drain: magnetic resonance imaging, confocal microscopy, and histological tracking of triple-labeled albumin. Cancer Res. 2002;62:6731–6739. [PubMed] [Google Scholar]
- [53].Haessler U, Teo JC, Foretay D, Renaud P, Swartz MA. Migration dynamics of breast cancer cells in a tunable 3D interstitial flow chamber. Integr Biol (Camb) 2012;4:401–409. doi: 10.1039/c1ib00128k. [DOI] [PubMed] [Google Scholar]
- [54].Polacheck WJ, Charest JL, Kamm RD. Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc Natl Acad Sci U S A. 2011;108:11115–11120. doi: 10.1073/pnas.1103581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hofmann M, Guschel M, Bernd A, Bereiter-Hahn J, Kaufmann R, Tandi C, Wiig H, Kippenberger S. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia. 2006;8:89–95. doi: 10.1593/neo.05469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hofmann M, Schultz M, Bernd A, Bereiter-Hahn J, Kaufmann R, Kippenberger S. Long-term lowering of tumour interstitial fluid pressure reduces Ki-67 expression. J Biomech. 2007;40:2324–2329. doi: 10.1016/j.jbiomech.2006.10.039. [DOI] [PubMed] [Google Scholar]
- [57].Nathan SS, DiResta GR, Casas-Ganem JE, Hoang BH, Sowers R, Yang R, Huvos AG, Gorlick R, Healey JH. Elevated physiologic tumor pressure promotes proliferation and chemosensitivity in human osteosarcoma. Clin Cancer Res. 2005;11:2389–2397. doi: 10.1158/1078-0432.CCR-04-2048. [DOI] [PubMed] [Google Scholar]
- [58].Gurkan UA, Akkus O. The mechanical environment of bone marrow: a review. Ann Biomed Eng. 2008;36:1978–1991. doi: 10.1007/s10439-008-9577-x. [DOI] [PubMed] [Google Scholar]
- [59].Kwon RY, Meays DR, Tang WJ, Frangos JA. Microfluidic enhancement of intramedullary pressure increases interstitial fluid flow and inhibits bone loss in hindlimb suspended mice. J Bone Miner Res. 2010;25:1798–1807. doi: 10.1002/jbmr.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Qin YX, Kaplan T, Saldanha A, Rubin C. Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J Biomech. 2003;36:1427–1437. doi: 10.1016/s0021-9290(03)00127-1. [DOI] [PubMed] [Google Scholar]
- [61].Zhang P, Su M, Liu Y, Hsu A, Yokota H. Knee loading dynamically alters intramedullary pressure in mouse femora. Bone. 2007;40:538–543. doi: 10.1016/j.bone.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Coughlin TR, Niebur GL. Fluid shear stress in trabecular bone marrow due to low-magnitude high-frequency vibration. J Biomech. 2012;45:2222–2229. doi: 10.1016/j.jbiomech.2012.06.020. [DOI] [PubMed] [Google Scholar]
- [63].Kwon RY, Meays DR, Meilan AS, Jones J, Miramontes R, Kardos N, Yeh JC, Frangos JA. Skeletal adaptation to intramedullary pressure-induced interstitial fluid flow is enhanced in mice subjected to targeted osteocyte ablation. PLoS One. 2012;7:e33336. doi: 10.1371/journal.pone.0033336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- [65].Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- [66].Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- [67].Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- [68].Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- [70].Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- [71].Martin T, Gooi JH, Sims NA. Molecular mechanisms in coupling of bone formation to resorption. Crit Rev Eukaryot Gene Expr. 2009;19:73–88. doi: 10.1615/critreveukargeneexpr.v19.i1.40. [DOI] [PubMed] [Google Scholar]
- [72].Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- [73].Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- [74].Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- [75].Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1–16. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- [77].Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. Embo J. 2003;22:6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Schaffler MB, Kennedy OD. Osteocyte signaling in bone. Curr Osteoporos Rep. 2012;10:118–125. doi: 10.1007/s11914-012-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37:148–158. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- [80].Genetos DC, Yellowley CE, Loots GG. Prostaglandin E2 signals through PTGER2 to regulate sclerostin expression. PLoS One. 2011;6:e17772. doi: 10.1371/journal.pone.0017772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jilka RL, Noble B, Weinstein RS. Osteocyte apoptosis. Bone. 2013;54:264–271. doi: 10.1016/j.bone.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kurata K, Uemura T, Nemoto A, Tateishi T, Murakami T, Higaki H, Miura H, Iwamoto Y. Mechanical strain effect on bone-resorbing activity and messenger RNA expressions of marker enzymes in isolated osteoclast culture. J Bone Miner Res. 2001;16:722–730. doi: 10.1359/jbmr.2001.16.4.722. [DOI] [PubMed] [Google Scholar]
- [83].Rubin J, Fan X, Biskobing DM, Taylor WR, Rubin CT. Osteoclastogenesis is repressed by mechanical strain in an in vitro model. J Orthop Res. 1999;17:639–645. doi: 10.1002/jor.1100170504. [DOI] [PubMed] [Google Scholar]
- [84].Rubin J, Biskobing D, Fan X, Rubin C, McLeod K, Taylor WR. Pressure regulates osteoclast formation and MCSF expression in marrow culture. J Cell Physiol. 1997;170:81–87. doi: 10.1002/(SICI)1097-4652(199701)170:1<81::AID-JCP9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- [85].Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012 doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Saunders MM, Taylor AF, Du C, Zhou Z, Pellegrini VD, Jr., Donahue HJ. Mechanical stimulation effects on functional end effectors in osteoblastic MG-63 cells. J Biomech. 2006;39:1419–1427. doi: 10.1016/j.jbiomech.2005.04.011. [DOI] [PubMed] [Google Scholar]
- [87].Kim CH, Kim KH, Jacobs CR. Effects of high frequency loading on RANKL and OPG mRNA expression in ST-2 murine stromal cells. BMC Musculoskelet Disord. 2009;10:109. doi: 10.1186/1471-2474-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kusumi A, Sakaki H, Kusumi T, Oda M, Narita K, Nakagawa H, Kubota K, Satoh H, Kimura H. Regulation of synthesis of osteoprotegerin and soluble receptor activator of nuclear factor-kappaB ligand in normal human osteoblasts via the p38 mitogen-activated protein kinase pathway by the application of cyclic tensile strain. J Bone Miner Metab. 2005;23:373–381. doi: 10.1007/s00774-005-0615-6. [DOI] [PubMed] [Google Scholar]
- [89].Kreja L, Liedert A, Hasni S, Claes L, Ignatius A. Mechanical regulation of osteoclastic genes in human osteoblasts. Biochem Biophys Res Commun. 2008;368:582–587. doi: 10.1016/j.bbrc.2008.01.106. [DOI] [PubMed] [Google Scholar]
- [90].David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–2562. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- [91].Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A. 2006;103:2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Stiehler M, Bunger C, Baatrup A, Lind M, Kassem M, Mygind T. Effect of dynamic 3-D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res A. 2009;89:96–107. doi: 10.1002/jbm.a.31967. [DOI] [PubMed] [Google Scholar]
- [93].Pavalko FM, Gerard RL, Ponik SM, Gallagher PJ, Jin Y, Norvell SM. Fluid shear stress inhibits TNF-alpha-induced apoptosis in osteoblasts: a role for fluid shear stress-induced activation of PI3-kinase and inhibition of caspase-3. J Cell Physiol. 2003;194:194–205. doi: 10.1002/jcp.10221. [DOI] [PubMed] [Google Scholar]
- [94].Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281:23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- [95].Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, Lanyon LE. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem. 2007;282:20715–20727. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- [96].Norvell SM, Ponik SM, Bowen DK, Gerard R, Pavalko FM. Fluid shear stress induction of COX-2 protein and prostaglandin release in cultured MC3T3-E1 osteoblasts does not require intact microfilaments or microtubules. J Appl Physiol (1985) 2004;96:957–966. doi: 10.1152/japplphysiol.00869.2003. [DOI] [PubMed] [Google Scholar]
- [97].Dallas SL, Prideaux M, Bonewald LF. The Osteocyte: An Endocrine Cell and More. Endocr Rev. 2013 doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: Master Orchestrators of Bone. Calcif Tissue Int. 2013 doi: 10.1007/s00223-013-9790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Noble BS, Peet N, Stevens HY, Brabbs A, Mosley JR, Reilly GC, Reeve J, Skerry TM, Lanyon LE. Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol. 2003;284:C934–943. doi: 10.1152/ajpcell.00234.2002. [DOI] [PubMed] [Google Scholar]
- [100].Tan SD, Kuijpers-Jagtman AM, Semeins CM, Bronckers AL, Maltha JC, Von den Hoff JW, Everts V, Klein-Nulend J. Fluid shear stress inhibits TNFalpha-induced osteocyte apoptosis. J Dent Res. 2006;85:905–909. doi: 10.1177/154405910608501006. [DOI] [PubMed] [Google Scholar]
- [101].Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- [102].Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- [103].Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–217. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Chen JC, Jacobs CR. Mechanically induced osteogenic lineage commitment of stem cells. Stem Cell Res Ther. 2013;4:107. doi: 10.1186/scrt318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Robling AG. The interaction of biological factors with mechanical signals in bone adaptation: recent developments. Curr Osteoporos Rep. 2012;10:126–131. doi: 10.1007/s11914-012-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- [107].You L, Cowin SC, Schaffler MB, Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34:1375–1386. doi: 10.1016/s0021-9290(01)00107-5. [DOI] [PubMed] [Google Scholar]
- [108].Cowin SC, Weinbaum S. Strain amplification in the bone mechanosensory system. Am J Med Sci. 1998;316:184–188. doi: 10.1097/00000441-199809000-00006. [DOI] [PubMed] [Google Scholar]
- [109].Duncan RL, Hruska KA. Chronic, intermittent loading alters mechanosensitive channel characteristics in osteoblast-like cells. Am J Physiol. 1994;267:F909–916. doi: 10.1152/ajprenal.1994.267.6.F909. [DOI] [PubMed] [Google Scholar]
- [110].Li J, Duncan RL, Burr DB, Turner CH. L-type calcium channels mediate mechanically induced bone formation in vivo. J Bone Miner Res. 2002;17:1795–1800. doi: 10.1359/jbmr.2002.17.10.1795. [DOI] [PubMed] [Google Scholar]
- [111].Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20:41–49. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Rawlinson SC, Mosley JR, Suswillo RF, Pitsillides AA, Lanyon LE. Calvarial and limb bone cells in organ and monolayer culture do not show the same early responses to dynamic mechanical strain. J Bone Miner Res. 1995;10:1225–1232. doi: 10.1002/jbmr.5650100813. [DOI] [PubMed] [Google Scholar]
- [113].Burtis WJ, Wu T, Bunch C, Wysolmerski JJ, Insogna KL, Weir EC, Broadus AE, Stewart AF. Identification of a novel 17,000-dalton parathyroid hormone-like adenylate cyclase-stimulating protein from a tumor associated with humoral hypercalcemia of malignancy. J Biol Chem. 1987;262:7151–7156. [PubMed] [Google Scholar]
- [114].Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, Gillespie MT. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140:4451–4458. doi: 10.1210/endo.140.10.7037. [DOI] [PubMed] [Google Scholar]
- [115].Iwasaki H, Suda T. Cancer stem cells and their niche. Cancer Sci. 2009;100:1166–1172. doi: 10.1111/j.1349-7006.2009.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Mercer RR, Miyasaka C, Mastro AM. Metastatic breast cancer cells suppress osteoblast adhesion and differentiation. Clin Exp Metastasis. 2004;21:427–435. doi: 10.1007/s10585-004-1867-6. [DOI] [PubMed] [Google Scholar]
- [117].Das S, Tucker JA, Khullar S, Samant RS, Shevde LA. Hedgehog signaling in tumor cells facilitates osteoblast-enhanced osteolytic metastases. PLoS One. 2012;7:e34374. doi: 10.1371/journal.pone.0034374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gregory LS, Choi W, Burke L, Clements JA. Breast Cancer Cells Induce Osteolytic Bone Lesions In vivo through a Reduction in Osteoblast Activity in Mice. PLoS One. 2013;8:e68103. doi: 10.1371/journal.pone.0068103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Mastro AM, Gay CV, Welch DR, Donahue HJ, Jewell J, Mercer R, DiGirolamo D, Chislock EM, Guttridge K. Breast cancer cells induce osteoblast apoptosis: a possible contributor to bone degradation. J Cell Biochem. 2004;91:265–276. doi: 10.1002/jcb.10746. [DOI] [PubMed] [Google Scholar]
- [120].Bussard KM, Okita N, Sharkey N, Neuberger T, Webb A, Mastro AM. Localization of osteoblast inflammatory cytokines MCP-1 and VEGF to the matrix of the trabecula of the femur, a target area for metastatic breast cancer cell colonization. Clin Exp Metastasis. 2010;27:331–340. doi: 10.1007/s10585-010-9330-3. [DOI] [PubMed] [Google Scholar]
- [121].Bussard KM, Venzon DJ, Mastro AM. Osteoblasts are a major source of inflammatory cytokines in the tumor microenvironment of bone metastatic breast cancer. J Cell Biochem. 2010;111:1138–1148. doi: 10.1002/jcb.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Giuliani N, Ferretti M, Bolzoni M, Storti P, Lazzaretti M, Dalla Palma B, Bonomini S, Martella E, Agnelli L, Neri A, Ceccarelli F, Palumbo C. Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia. 2012;26:1391–1401. doi: 10.1038/leu.2011.381. [DOI] [PubMed] [Google Scholar]
- [123].Terpos E, Christoulas D, Katodritou E, Bratengeier C, Gkotzamanidou M, Michalis E, Delimpasi S, Pouli A, Meletis J, Kastritis E, Zervas K, Dimopoulos MA. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int J Cancer. 2011;131:1466–1471. doi: 10.1002/ijc.27342. [DOI] [PubMed] [Google Scholar]
- [124].Sottnik J, Zhang H, Keller E. Osteocytes promote prostate cancer bone growth. Amer Soc Bone Miner ResBaltimore, MD. 2013 [Google Scholar]
- [125].Sottnik JL, Campbell B, Mehra R, Behbahani-Nejad O, Hall CL, Keller ET. Osteocytes Serve as a Progenitor Cell of Osteosarcoma. J Cell Biochem. 2014 doi: 10.1002/jcb.24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Turner CH. Three rules for bone adaptation to mechanical stimuli. Bone. 1998;23:399–407. doi: 10.1016/s8756-3282(98)00118-5. [DOI] [PubMed] [Google Scholar]
- [127].Hert J, Liskova M, Landa J. Reaction of bone to mechanical stimuli. 1. Continuous and intermittent loading of tibia in rabbit. Folia Morphol (Praha) 1971;19:290–300. [PubMed] [Google Scholar]
- [128].Rubin CT, Lanyon LE. Kappa Delta Award paper. Osteoregulatory nature of mechanical stimuli: function as a determinant for adaptive remodeling in bone. J Orthop Res. 1987;5:300–310. doi: 10.1002/jor.1100050217. [DOI] [PubMed] [Google Scholar]
- [129].Churches AE, Howlett CR. Functional adaptation of bone in response to sinusoidally varying controlled compressive loading of the ovine metacarpus. Clin Orthop Relat Res. 1982:265–280. [PubMed] [Google Scholar]
- [130].Goodship AE, Lanyon LE, McFie H. Functional adaptation of bone to increased stress. An experimental study. J Bone Joint Surg Am. 1979;61:539–546. [PubMed] [Google Scholar]
- [131].O’Connor JA, Lanyon LE, MacFie H. The influence of strain rate on adaptive bone remodelling. J Biomech. 1982;15:767–781. doi: 10.1016/0021-9290(82)90092-6. [DOI] [PubMed] [Google Scholar]
- [132].Hsieh YF, Turner CH. Effects of loading frequency on mechanically induced bone formation. J Bone Miner Res. 2001;16:918–924. doi: 10.1359/jbmr.2001.16.5.918. [DOI] [PubMed] [Google Scholar]
- [133].Srinivasan S, Ausk BJ, Poliachik SL, Warner SE, Richardson TS, Gross T. Rest-Inserted Loading Rapidly Amplifies the Response of Bone to Small Increases in Strain and Load Cycles. J Appl Physiol. 2007 doi: 10.1152/japplphysiol.00507.2006. [DOI] [PubMed] [Google Scholar]
- [134].Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res. 2002;17:1613–1620. doi: 10.1359/jbmr.2002.17.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Srinivasan S, Agans SC, King KA, Moy NY, Poliachik SL, Gross TS. Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. Bone. 2003;33:946–955. doi: 10.1016/j.bone.2003.07.009. [DOI] [PubMed] [Google Scholar]
- [136].Srinivasan S, Ausk BJ, Prasad J, Threet D, Bain SD, Richardson TS, Gross TS. Rescuing loading induced bone formation at senescence. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- [138].Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- [139].Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Ozcivici E, Luu YK, Rubin CT, Judex S. Low-level vibrations retain bone marrow’s osteogenic potential and augment recovery of trabecular bone during reambulation. PLoS One. 2010;5:e11178. doi: 10.1371/journal.pone.0011178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Fritton JC, Myers ER, Wright TM, van der Meulen MC. Bone mass is preserved and cancellous architecture altered due to cyclic loading of the mouse tibia after orchidectomy. J Bone Miner Res. 2008;23:663–671. doi: 10.1359/JBMR.080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Berlin O, Samid D, Donthineni-Rao R, Akeson W, Amiel D, Woods VL., Jr. Development of a novel spontaneous metastasis model of human osteosarcoma transplanted orthotopically into bone of athymic mice. Cancer Res. 1993;53:4890–4895. [PubMed] [Google Scholar]
- [143].Lynch ME, Brooks D, Mohanan S, Lee MJ, Polamraju P, Dent K, Bonassar L, van der Meulen MC, Fischbach C. In Vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J Bone Miner Res. 2013 doi: 10.1002/jbmr.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- [145].Barron MJ, Tsai CJ, Donahue SW. Mechanical stimulation mediates gene expression in MC3T3 osteoblastic cells differently in 2D and 3D environments. J Biomech Eng. 2010;132:041005. doi: 10.1115/1.4001162. [DOI] [PubMed] [Google Scholar]
- [146].Zhang P, Wu Y, Jiang Z, Jiang L, Fang B. Osteogenic response of mesenchymal stem cells to continuous mechanical strain is dependent on ERK1/2-Runx2 signaling. Int J Mol Med. 2012;29:1083–1089. doi: 10.3892/ijmm.2012.934. [DOI] [PubMed] [Google Scholar]
- [147].Morko J, Kiviranta R, Mulari MT, Ivaska KK, Vaananen HK, Vuorio E, Laitala-Leinonen T. Overexpression of cathepsin K accelerates the resorption cycle and osteoblast differentiation in vitro. Bone. 2009;44:717–728. doi: 10.1016/j.bone.2008.11.019. [DOI] [PubMed] [Google Scholar]
- [148].Kearney EM, Farrell E, Prendergast PJ, Campbell VA. Tensile strain as a regulator of mesenchymal stem cell osteogenesis. Ann Biomed Eng. 2010;38:1767–1779. doi: 10.1007/s10439-010-9979-4. [DOI] [PubMed] [Google Scholar]
- [149].Bieler FH, Ott CE, Thompson MS, Seidel R, Ahrens S, Epari DR, Wilkening U, Schaser KD, Mundlos S, Duda GN. Biaxial cell stimulation: A mechanical validation. J Biomech. 2009;42:1692–1696. doi: 10.1016/j.jbiomech.2009.04.013. [DOI] [PubMed] [Google Scholar]
- [150].Riehl BD, Park JH, Kwon IK, Lim JY. Mechanical stretching for tissue engineering: two-dimensional and three-dimensional constructs. Tissue Eng Part B Rev. 2012;18:288–300. doi: 10.1089/ten.teb.2011.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Wall ME, Weinhold PS, Siu T, Brown TD, Banes AJ. Comparison of cellular strain with applied substrate strain in vitro. J Biomech. 2007;40:173–181. doi: 10.1016/j.jbiomech.2005.10.032. [DOI] [PubMed] [Google Scholar]
- [152].Qi MC, Hu J, Zou SJ, Chen HQ, Zhou HX, Han LC. Mechanical strain induces osteogenic differentiation: Cbfa1 and Ets-1 expression in stretched rat mesenchymal stem cells. Int J Oral Maxillofac Surg. 2008;37:453–458. doi: 10.1016/j.ijom.2007.12.008. [DOI] [PubMed] [Google Scholar]
- [153].Haasper C, Jagodzinski M, Drescher M, Meller R, Wehmeier M, Krettek C, Hesse E. Cyclic strain induces FosB and initiates osteogenic differentiation of mesenchymal cells. Exp Toxicol Pathol. 2008;59:355–363. doi: 10.1016/j.etp.2007.11.008. [DOI] [PubMed] [Google Scholar]
- [154].Hanson AD, Marvel SW, Bernacki SH, Banes AJ, van Aalst J, Loboa EG. Osteogenic effects of rest inserted and continuous cyclic tensile strain on hASC lines with disparate osteodifferentiation capabilities. Ann Biomed Eng. 2009;37:955–965. doi: 10.1007/s10439-009-9648-7. [DOI] [PubMed] [Google Scholar]
- [155].Simmons CA, Matlis S, Thornton AJ, Chen S, Wang CY, Mooney DJ. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J Biomech. 2003;36:1087–1096. doi: 10.1016/s0021-9290(03)00110-6. [DOI] [PubMed] [Google Scholar]
- [156].Huang CH, Chen MH, Young TH, Jeng JH, Chen YJ. Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells. J Cell Biochem. 2009;108:1263–1273. doi: 10.1002/jcb.22356. [DOI] [PubMed] [Google Scholar]
- [157].Jagodzinski M, Drescher M, Zeichen J, Hankemeier S, Krettek C, Bosch U, van Griensven M. Effects of cyclic longitudinal mechanical strain and dexamethasone on osteogenic differentiation of human bone marrow stromal cells. Eur Cell Mater. 2004;7:35–41. doi: 10.22203/ecm.v007a04. [DOI] [PubMed] [Google Scholar]
- [158].Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88:359–368. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- [159].Jansen JH, Weyts FA, Westbroek I, Jahr H, Chiba H, Pols HA, Verhaar JA, van Leeuwen JP, Weinans H. Stretch-induced phosphorylation of ERK1/2 depends on differentiation stage of osteoblasts. J Cell Biochem. 2004;93:542–551. doi: 10.1002/jcb.20162. [DOI] [PubMed] [Google Scholar]
- [160].Weyts FA, Bosmans B, Niesing R, van Leeuwen JP, Weinans H. Mechanical control of human osteoblast apoptosis and proliferation in relation to differentiation. Calcif Tissue Int. 2003;72:505–512. doi: 10.1007/s00223-002-2027-0. [DOI] [PubMed] [Google Scholar]
- [161].Ma D, Lu H, Xu L, Xu X, Xiao W. Mechanical loading promotes Lewis lung cancer cell growth through periostin. In Vitro Cell Dev Biol Anim. 2009;45:467–472. doi: 10.1007/s11626-009-9214-5. [DOI] [PubMed] [Google Scholar]
- [162].Huang JW, Pan HJ, Yao WY, Tsao YW, Liao WY, Wu CW, Tung YC, Lee CH. Interaction between lung cancer cell and myofibroblast influenced by cyclic tensile strain. Lab Chip. 2013;13:1114–1120. doi: 10.1039/c2lc41050h. [DOI] [PubMed] [Google Scholar]
- [163].Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Norian JM, Owen CM, Taboas J, Korecki C, Tuan R, Malik M, Catherino WH, Segars JH. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31:57–65. doi: 10.1016/j.matbio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Goldstein AS, Dimilla PA. Application of fluid mechanic and kinetic models to characterize mammalian cell detachment in a radial-flow chamber. Biotechnol Bioeng. 1997;55:616–629. doi: 10.1002/(SICI)1097-0290(19970820)55:4<616::AID-BIT4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- [166].Deligianni DD, Katsala ND, Koutsoukos PG, Missirlis YF. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials. 2001;22:87–96. doi: 10.1016/s0142-9612(00)00174-5. [DOI] [PubMed] [Google Scholar]
- [167].Furukawa KS, Ushida T, Noguchi T, Tamaki T, Tateishi T. Development of cone and plate-type rheometer for quantitative analysis of endothelial cell detachment by shear stress. Int J Artif Organs. 2003;26:436–441. doi: 10.1177/039139880302600510. [DOI] [PubMed] [Google Scholar]
- [168].Roy B, Das T, Mishra D, Maiti TK, Chakraborty S. Oscillatory shear stress induced calcium flickers in osteoblast cells. Integr Biol (Camb) 2014;6:289–299. doi: 10.1039/c3ib40174j. [DOI] [PubMed] [Google Scholar]
- [169].Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation--the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122:546–553. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Case N, Sen B, Thomas JA, Styner M, Xie Z, Jacobs CR, Rubin J. Steady and oscillatory fluid flows produce a similar osteogenic phenotype. Calcif Tissue Int. 2011;88:189–197. doi: 10.1007/s00223-010-9448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Scaglione S, Wendt D, Miggino S, Papadimitropoulos A, Fato M, Quarto R, Martin I. Effects of fluid flow and calcium phosphate coating on human bone marrow stromal cells cultured in a defined 2D model system. J Biomed Mater Res A. 2008;86:411–419. doi: 10.1002/jbm.a.31607. [DOI] [PubMed] [Google Scholar]
- [172].Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005;36:1047–1055. doi: 10.1016/j.bone.2005.03.008. [DOI] [PubMed] [Google Scholar]
- [173].Kreke MR, Sharp LA, Lee YW, Goldstein AS. Effect of intermittent shear stress on mechanotransductive signaling and osteoblastic differentiation of bone marrow stromal cells. Tissue Eng Part A. 2008;14:529–537. doi: 10.1089/tea.2007.0068. [DOI] [PubMed] [Google Scholar]
- [174].Sharp LA, Lee YW, Goldstein AS. Effect of low-frequency pulsatile flow on expression of osteoblastic genes by bone marrow stromal cells. Ann Biomed Eng. 2009;37:445–453. doi: 10.1007/s10439-008-9632-7. [DOI] [PubMed] [Google Scholar]
- [175].McGarry JG, Klein-Nulend J, Prendergast PJ. The effect of cytoskeletal disruption on pulsatile fluid flow-induced nitric oxide and prostaglandin E2 release in osteocytes and osteoblasts. Biochem Biophys Res Commun. 2005;330:341–348. doi: 10.1016/j.bbrc.2005.02.175. [DOI] [PubMed] [Google Scholar]
- [176].Yeatts AB, Choquette DT, Fisher JP. Bioreactors to influence stem cell fate: augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim Biophys Acta. 2012;1830:2470–2480. doi: 10.1016/j.bbagen.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].McCoy RJ, O’Brien FJ. Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: a review. Tissue Eng Part B Rev. 2010;16:587–601. doi: 10.1089/ten.TEB.2010.0370. [DOI] [PubMed] [Google Scholar]
- [178].Batra NN, Li YJ, Yellowley CE, You L, Malone AM, Kim CH, Jacobs CR. Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. J Biomech. 2005;38:1909–1917. doi: 10.1016/j.jbiomech.2004.08.009. [DOI] [PubMed] [Google Scholar]
- [179].Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31:969–976. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Malone AM, Batra NN, Shivaram G, Kwon RY, You L, Kim CH, Rodriguez J, Jair K, Jacobs CR. The role of actin cytoskeleton in oscillatory fluid flow-induced signaling in MC3T3-E1 osteoblasts. Am J Physiol Cell Physiol. 2007;292:C1830–1836. doi: 10.1152/ajpcell.00352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- [182].Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- [183].Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- [185].Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- [186].Lee EJ, Kasper FK, Mikos AG. Biomaterials for tissue engineering. Ann Biomed Eng. 2014;42:323–337. doi: 10.1007/s10439-013-0859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Infanger DW, Lynch ME, Fischbach C. Engineered Culture Models for Studies of Tumor-Microenvironment Interactions. Annu Rev Biomed Eng. 2013 doi: 10.1146/annurev-bioeng-071811-150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Krishnan V, Dhurjati R, Vogler EA, Mastro AM. Osteogenesis in vitro: from pre-osteoblasts to osteocytes: a contribution from the Osteobiology Research Group, The Pennsylvania State University. In Vitro Cell Dev Biol Anim. 2010;46:28–35. doi: 10.1007/s11626-009-9238-x. [DOI] [PubMed] [Google Scholar]
- [189].DeQuach JA, Yuan SH, Goldstein LS, Christman KL. Decellularized porcine brain matrix for cell culture and tissue engineering scaffolds. Tissue Eng Part A. 2011;17:2583–2592. doi: 10.1089/ten.tea.2010.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G. Effects of oxygen on engineered cardiac muscle. Biotechnol Bioeng. 2002;78:617–625. doi: 10.1002/bit.10245. [DOI] [PubMed] [Google Scholar]
- [191].Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36:17–28. doi: 10.1002/(sici)1097-4636(199707)36:1<17::aid-jbm3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- [192].Volkmer E, Drosse I, Otto S, Stangelmayer A, Stengele M, Kallukalam BC, Mutschler W, Schieker M. Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue Eng Part A. 2008;14:1331–1340. doi: 10.1089/ten.tea.2007.0231. [DOI] [PubMed] [Google Scholar]
- [193].Vunjak-Novakovic G, Freed LE, Biron RJ, Langer R. Effects of mixing on the composition and morphology of tissue-engineered cartilage. AIChE J. 1996;42:850–860. [Google Scholar]
- [194].Martin I, Obradovic B, Freed LE, Vunjak-Novakovic G. Method for quantitative analysis of glycosaminoglycan distribution in cultured natural and engineered cartilage. Ann Biomed Eng. 1999;27:656–662. doi: 10.1114/1.205. [DOI] [PubMed] [Google Scholar]
- [195].Vunjak-Novakovic G, Obradovic B, Martin I, Bursac PM, Langer R, Freed LE. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998;14:193–202. doi: 10.1021/bp970120j. [DOI] [PubMed] [Google Scholar]
- [196].Hutmacher DW, Singh H. Computational fluid dynamics for improved bioreactor design and 3D culture. Trends Biotechnol. 2008;26:166–172. doi: 10.1016/j.tibtech.2007.11.012. [DOI] [PubMed] [Google Scholar]
- [197].Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R, Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- [198].Sikavitsas VI, Bancroft GN, Mikos AG. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res. 2002;62:136–148. doi: 10.1002/jbm.10150. [DOI] [PubMed] [Google Scholar]
- [199].Wang TW, Wu HC, Wang HY, Lin FH, Sun JS. Regulation of adult human mesenchymal stem cells into osteogenic and chondrogenic lineages by different bioreactor systems. J Biomed Mater Res A. 2009;88:935–946. doi: 10.1002/jbm.a.31914. [DOI] [PubMed] [Google Scholar]
- [200].Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735–749. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- [201].Goldstein AS, Juarez TM, Helmke CD, Gustin MC, Mikos AG. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials. 2001;22:1279–1288. doi: 10.1016/s0142-9612(00)00280-5. [DOI] [PubMed] [Google Scholar]
- [202].Song K, Liu T, Cui Z, Li X, Ma X. Three-dimensional fabrication of engineered bone with human bio-derived bone scaffolds in a rotating wall vessel bioreactor. J Biomed Mater Res A. 2008;86:323–332. doi: 10.1002/jbm.a.31624. [DOI] [PubMed] [Google Scholar]
- [203].Terai H, Hannouche D, Ochoa E, Yamano Y, Vacanti JP. In vitro engineering of bone using a rotational oxygen-permeable bioreactor system. Mater Sci Eng. 2002;20:3–8. [Google Scholar]
- [204].Becker JL, Blanchard DK. Characterization of primary breast carcinomas grown in three-dimensional cultures. J Surg Res. 2007;142:256–262. doi: 10.1016/j.jss.2007.03.016. [DOI] [PubMed] [Google Scholar]
- [205].Clejan S, O’Connor K, Rosensweig N. Tri-dimensional prostate cell cultures in simulated microgravity and induced changes in lipid second messengers and signal transduction. J Cell Mol Med. 2001;5:60–73. doi: 10.1111/j.1582-4934.2001.tb00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Licato LL, Prieto VG, Grimm EA. A novel preclinical model of human malignant melanoma utilizing bioreactor rotating-wall vessels. In Vitro Cell Dev Biol Anim. 2001;37:121–126. doi: 10.1290/1071-2690(2001)037<0121:ANPMOH>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [207].Rhee HW, Zhau HE, Pathak S, Multani AS, Pennanen S, Visakorpi T, Chung LW. Permanent phenotypic and genotypic changes of prostate cancer cells cultured in a three-dimensional rotating-wall vessel. In Vitro Cell Dev Biol Anim. 2001;37:127–140. doi: 10.1290/1071-2690(2001)037<0127:PPAGCO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [208].Song H, David O, Clejan S, Giordano CL, Pappas-Lebeau H, Xu L, O’Connor KC. Spatial composition of prostate cancer spheroids in mixed and static cultures. Tissue Eng. 2004;10:1266–1276. doi: 10.1089/ten.2004.10.1266. [DOI] [PubMed] [Google Scholar]
- [209].Vamvakidou AP, Mondrinos MJ, Petushi SP, Garcia FU, Lelkes PI, Tozeren A. Heterogeneous breast tumoroids: An in vitro assay for investigating cellular heterogeneity and drug delivery. J Biomol Screen. 2007;12:13–20. doi: 10.1177/1087057106296482. [DOI] [PubMed] [Google Scholar]
- [210].Bancroft GN, Sikavitsas VI, Mikos AG. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003;9:549–554. doi: 10.1089/107632703322066723. [DOI] [PubMed] [Google Scholar]
- [211].Partap S, Plunkett NA, Kelly DJ, O’Brien FJ. Stimulation of osteoblasts using rest periods during bioreactor culture on collagen-glycosaminoglycan scaffolds. J Mater Sci Mater Med. 2010;21:2325–2330. doi: 10.1007/s10856-009-3966-z. [DOI] [PubMed] [Google Scholar]
- [212].Porter BD, Lin AS, Peister A, Hutmacher D, Guldberg RE. Noninvasive image analysis of 3D construct mineralization in a perfusion bioreactor. Biomaterials. 2007;28:2525–2533. doi: 10.1016/j.biomaterials.2007.01.013. [DOI] [PubMed] [Google Scholar]
- [213].Shieh AC, Swartz MA. Regulation of tumor invasion by interstitial fluid flow. Phys Biol. 2011;8:015012. doi: 10.1088/1478-3975/8/1/015012. [DOI] [PubMed] [Google Scholar]
- [214].Bjerre L, Bunger CE, Kassem M, Mygind T. Flow perfusion culture of human mesenchymal stem cells on silicate-substituted tricalcium phosphate scaffolds. Biomaterials. 2008;29:2616–2627. doi: 10.1016/j.biomaterials.2008.03.003. [DOI] [PubMed] [Google Scholar]
- [215].Grayson WL, Marolt D, Bhumiratana S, Frohlich M, Guo XE, Vunjak-Novakovic G. Optimizing the medium perfusion rate in bone tissue engineering bioreactors. Biotechnol Bioeng. 2011;108:1159–1170. doi: 10.1002/bit.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Hofmann A, Konrad L, Gotzen L, Printz H, Ramaswamy A, Hofmann C. Bioengineered human bone tissue using autogenous osteoblasts cultured on different biomatrices. J Biomed Mater Res A. 2003;67:191–199. doi: 10.1002/jbm.a.10594. [DOI] [PubMed] [Google Scholar]
- [217].Matziolis G, Tuischer J, Kasper G, Thompson M, Bartmeyer B, Krocker D, Perka C, Duda G. Simulation of cell differentiation in fracture healing: mechanically loaded composite scaffolds in a novel bioreactor system. Tissue Eng. 2006;12:201–208. doi: 10.1089/ten.2006.12.201. [DOI] [PubMed] [Google Scholar]
- [218].Matziolis D, Tuischer J, Matziolis G, Kasper G, Duda G, Perka C. Osteogenic predifferentiation of human bone marrow-derived stem cells by short-term mechanical stimulation. Open Orthop J. 2011;5:1–6. doi: 10.2174/1874325001105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Sittichockechaiwut A, Scutt AM, Ryan AJ, Bonewald LF, Reilly GC. Use of rapidly mineralising osteoblasts and short periods of mechanical loading to accelerate matrix maturation in 3D scaffolds. Bone. 2009;44:822–829. doi: 10.1016/j.bone.2008.12.027. [DOI] [PubMed] [Google Scholar]
- [220].Dumas V, Perrier A, Malaval L, Laroche N, Guignandon A, Vico L, Rattner A. The effect of dual frequency cyclic compression on matrix deposition by osteoblast-like cells grown in 3D scaffolds and on modulation of VEGF variant expression. Biomaterials. 2009;30:3279–3288. doi: 10.1016/j.biomaterials.2009.02.048. [DOI] [PubMed] [Google Scholar]
- [221].Demou ZN. Gene expression profiles in 3D tumor analogs indicate compressive strain differentially enhances metastatic potential. Ann Biomed Eng. 2010;38:3509–3520. doi: 10.1007/s10439-010-0097-0. [DOI] [PubMed] [Google Scholar]
- [222].Jagodzinski M, Breitbart A, Wehmeier M, Hesse E, Haasper C, Krettek C, Zeichen J, Hankemeier S. Influence of perfusion and cyclic compression on proliferation and differentiation of bone marrow stromal cells in 3-dimensional culture. J Biomech. 2008;41:1885–1891. doi: 10.1016/j.jbiomech.2008.04.001. [DOI] [PubMed] [Google Scholar]
- [223].Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A. 2002;99:12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Jaasma MJ, O’Brien FJ. Mechanical stimulation of osteoblasts using steady and dynamic fluid flow. Tissue Eng Part A. 2008;14:1213–1223. doi: 10.1089/ten.tea.2007.0321. [DOI] [PubMed] [Google Scholar]
- [225].Kavlock KD, Goldstein AS. Effect of pulsatile flow on the osteogenic differentiation of bone marrow stromal cells in porous scaffolds. Biomed Sci Instrum. 2008;44:471–476. [PubMed] [Google Scholar]
- [226].Vance J, Galley S, Liu DF, Donahue SW. Mechanical stimulation of MC3T3 osteoblastic cells in a bone tissue-engineering bioreactor enhances prostaglandin E2 release. Tissue Eng. 2005;11:1832–1839. doi: 10.1089/ten.2005.11.1832. [DOI] [PubMed] [Google Scholar]
- [227].Plunkett NA, Partap S, O’Brien FJ. Osteoblast response to rest periods during bioreactor culture of collagen-glycosaminoglycan scaffolds. Tissue Eng Part A. 2010;16:943–951. doi: 10.1089/ten.TEA.2009.0345. [DOI] [PubMed] [Google Scholar]
- [228].Li D, Tang T, Lu J, Dai K. Effects of flow shear stress and mass transport on the construction of a large-scale tissue-engineered bone in a perfusion bioreactor. Tissue Eng Part A. 2009;15:2773–2783. doi: 10.1089/ten.TEA.2008.0540. [DOI] [PubMed] [Google Scholar]
- [229].Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci U S A. 2003;100:14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Pedersen JA, Boschetti F, Swartz MA. Effects of extracellular fiber architecture on cell membrane shear stress in a 3D fibrous matrix. J Biomech. 2007;40:1484–1492. doi: 10.1016/j.jbiomech.2006.06.023. [DOI] [PubMed] [Google Scholar]
- [231].Pedersen JA, Lichter S, Swartz MA. Cells in 3D matrices under interstitial flow: effects of extracellular matrix alignment on cell shear stress and drag forces. J Biomech. 2010;43:900–905. doi: 10.1016/j.jbiomech.2009.11.007. [DOI] [PubMed] [Google Scholar]
- [232].Jungreuthmayer C, Jaasma MJ, Al-Munajjed AA, Zanghellini J, Kelly DJ, O’Brien FJ. Deformation simulation of cells seeded on a collagen-GAG scaffold in a flow perfusion bioreactor using a sequential 3D CFD-elastostatics model. Med Eng Phys. 2009;31:420–427. doi: 10.1016/j.medengphy.2008.11.003. [DOI] [PubMed] [Google Scholar]
- [233].Stops AJ, Heraty KB, Browne M, O’Brien FJ, McHugh PE. A prediction of cell differentiation and proliferation within a collagen-glycosaminoglycan scaffold subjected to mechanical strain and perfusive fluid flow. J Biomech. 2010;43:618–626. doi: 10.1016/j.jbiomech.2009.10.037. [DOI] [PubMed] [Google Scholar]
- [234].McCoy RJ, Jungreuthmayer C, O’Brien FJ. Influence of flow rate and scaffold pore size on cell behavior during mechanical stimulation in a flow perfusion bioreactor. Biotechnol Bioeng. 2012;109:1583–1594. doi: 10.1002/bit.24424. [DOI] [PubMed] [Google Scholar]
- [235].Byrne DP, Lacroix D, Planell JA, Kelly DJ, Prendergast PJ. Simulation of tissue differentiation in a scaffold as a function of porosity, Young’s modulus and dissolution rate: application of mechanobiological models in tissue engineering. Biomaterials. 2007;28:5544–5554. doi: 10.1016/j.biomaterials.2007.09.003. [DOI] [PubMed] [Google Scholar]
- [236].Tierney CM, Haugh MG, Liedl J, Mulcahy F, Hayes B, O’Brien FJ. The effects of collagen concentration and crosslink density on the biological, structural and mechanical properties of collagen-GAG scaffolds for bone tissue engineering. J Mech Behav Biomed Mater. 2009;2:202–209. doi: 10.1016/j.jmbbm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- [237].Boschetti F, Raimondi MT, Migliavacca F, Dubini G. Prediction of the micro-fluid dynamic environment imposed to three-dimensional engineered cell systems in bioreactors. J Biomech. 2006;39:418–425. doi: 10.1016/j.jbiomech.2004.12.022. [DOI] [PubMed] [Google Scholar]
- [238].Zermatten E, Vetsch JR, Ruffoni D, Hofmann S, Muller R, Steinfeld A. Micro-computed tomography based computational fluid dynamics for the determination of shear stresses in scaffolds within a perfusion bioreactor. Ann Biomed Eng. 2014;42:1085–1094. doi: 10.1007/s10439-014-0981-0. [DOI] [PubMed] [Google Scholar]
- [239].Milan JL, Planell JA, Lacroix D. Simulation of bone tissue formation within a porous scaffold under dynamic compression. Biomech Model Mechanobiol. 2010;9:583–596. doi: 10.1007/s10237-010-0199-5. [DOI] [PubMed] [Google Scholar]
- [240].Sandino C, Planell JA, Lacroix D. A finite element study of mechanical stimuli in scaffolds for bone tissue engineering. J Biomech. 2008;41:1005–1014. doi: 10.1016/j.jbiomech.2007.12.011. [DOI] [PubMed] [Google Scholar]
- [241].Mishra A, Shiozawa Y, Pienta KJ, Taichman RS. Homing of cancer cells to the bone. Cancer Microenviron. 2011;4:221–235. doi: 10.1007/s12307-011-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Bragado P, Sosa MS, Keely P, Condeelis J, Aguirre-Ghiso JA. Microenvironments dictating tumor cell dormancy. Recent Results Cancer Res. 2012;195:25–39. doi: 10.1007/978-3-642-28160-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Thibaudeau L, Quent VM, Holzapfel BM, Taubenberger AV, Straub M, Hutmacher DW. Mimicking breast cancer-induced bone metastasis in vivo: current transplantation models and advanced humanized strategies. Cancer Metastasis Rev. 2014 doi: 10.1007/s10555-014-9499-z. [DOI] [PubMed] [Google Scholar]
- [244].Kuperwasser C, Dessain S, Bierbaum BE, Garnet D, Sperandio K, Gauvin GP, Naber SP, Weinberg RA, Rosenblatt M. A mouse model of human breast cancer metastasis to human bone. Cancer Res. 2005;65:6130–6138. doi: 10.1158/0008-5472.CAN-04-1408. [DOI] [PubMed] [Google Scholar]
- [245].Thibaudeau L, Taubenberger AV, Holzapfel BM, Quent VM, Fuehrmann T, Hesami P, Brown TD, Dalton PD, Power CA, Hollier BG, Hutmacher DW. A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone. Dis Model Mech. 2014;7:299–309. doi: 10.1242/dmm.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Kasper G, Dankert N, Tuischer J, Hoeft M, Gaber T, Glaeser JD, Zander D, Tschirschmann M, Thompson M, Matziolis G, Duda GN. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells. 2007;25:903–910. doi: 10.1634/stemcells.2006-0432. [DOI] [PubMed] [Google Scholar]
- [247].Nishi M, Matsumoto R, Dong J, Uemura T. Engineered bone tissue associated with vascularization utilizing a rotating wall vessel bioreactor. J Biomed Mater Res A. 2013;101:421–427. doi: 10.1002/jbm.a.34340. [DOI] [PubMed] [Google Scholar]
- [248].Panaroni C, Wu JY. Interactions between B lymphocytes and the osteoblast lineage in bone marrow. Calcif Tissue Int. 2013;93:261–268. doi: 10.1007/s00223-013-9753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Berl) 2013;91:411–429. doi: 10.1007/s00109-013-1021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Torisawa Y.-s., Spina CS, Mammoto T, Mammoto A, Weaver JC, Tat T, Collins JJ, Ingber DE. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat Meth, advance online publication. 2014 doi: 10.1038/nmeth.2938. [DOI] [PubMed] [Google Scholar]
- [251].Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, Moretti M, Kamm RD. Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integr Biol (Camb) 2014;6:555–563. doi: 10.1039/c3ib40267c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Turunen MJ, Prantner V, Jurvelin JS, Kroger H, Isaksson H. Composition and microarchitecture of human trabecular bone change with age and differ between anatomical locations. Bone. 2013;54:118–125. doi: 10.1016/j.bone.2013.01.045. [DOI] [PubMed] [Google Scholar]
- [253].Kerschnitzki M, Kollmannsberger P, Burghammer M, Duda GN, Weinkamer R, Wagermaier W, Fratzl P. Architecture of the osteocyte network correlates with bone material quality. J Bone Miner Res. 2013;28:1837–1845. doi: 10.1002/jbmr.1927. [DOI] [PubMed] [Google Scholar]
- [254].Pathi SP, Lin DD, Dorvee JR, Estroff LA, Fischbach C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials. 2011;32:5112–5122. doi: 10.1016/j.biomaterials.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]