Abstract
Microglial cells, resident macrophages in the central nervous system (CNS), are relatively quiescent but can respond to signals from the peripheral immune system and induce neuroinflammation. In aging, microglia tend to transition to the M1 pro-inflammatory state and become hypersensitive to messages emerging from immune-to-brain signaling pathways. Thus, whereas in younger individuals where microglia respond to signals from the peripheral immune system and induce a well-controlled neuroinflammatory response that is adaptive (e.g., when well controlled, fever and sickness behavior facilitate recovery from infection), in older individuals with an infection, microglia overreact and produce excessive levels of inflammatory cytokines causing behavioral pathology including cognitive dysfunction. Importantly, recent studies indicate a number of naturally occurring bioactive compounds present in certain foods have anti-inflammatory properties and are capable of mitigating brain microglial cells. These include, e.g., flavonoid and non-flavonoid compounds in fruits and vegetables, and n-3 polyunsaturated fatty acids (PUFA) in oily fish. Thus, dietary bioactives have potential to restore the population of microglial cells in the senescent brain to a more quiescent state. The pragmatic concept to constrain microglia through dietary intervention is significant because neuroinflammation and cognitive deficits are co-morbid factors in many chronic inflammatory diseases. Controlling microglial cell reactivity has important consequences for preserving adult neurogenesis, neuronal structure and function, and cognition.
Keywords: aging, behavior, flavonoids, n-3 polyunsaturated fatty acids, neuroinflammation, obesity
Channels of communication between the immune system and brain
Neurobehavioral changes due to influenza infection have been well documented (Hart, 1988). One explanation is that influenza virus enters the brain where it is detected by neurons that control behavior. This is possible since neurons express toll-like receptors (TLR) that are up regulated and rendered more sensitive to TLR ligands upon exposure to the anti-viral cytokine interferon-γ (Tang et al., 2007). However, most influenza strains, including those responsible for pandemics, are non-neurotropic (Kobasa et al., 2007; Schlesinger et al., 1998; Wang et al., 2010), suggesting that neurobehavioral symptoms associated with influenza infection are not due to virus entering the CNS. Instead, peripheral sentinel immune cells such as monocytes and macrophages play a critical role. These cells are also equipped with TLRs that recognize unique molecules associated with groups of pathogens (i.e., pathogen associated molecular patterns; see (Moresco et al., 2011)). Stimulation of TLRs that recognize viruses (TLR3 and TLR7) and bacteria (TLR4) on immune sentinel cells can have profound neurobehavioral effects, indicating the immune system conveys a message to the brain after detecting an infectious agent. This message is cytokine based, as macrophages and monocytes produce inflammatory cytokines [e.g., interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNFα)] that facilitate communication between the periphery and brain. Several cytokine-dependent pathways that enable the peripheral immune system to transcend the blood-brain barrier have been dissected (Banks, 2012; Quan, 2008).
First, there is evidence that inflammatory cytokines present in blood can be actively transported by the endothelial cells of the blood-brain barrier into the brain parenchyma (Banks and Kastin, 1991; Banks et al., 1995; Banks et al., 1994a; Banks et al., 1994b; Gutierrez et al., 1993). A fundamental point, however, is that inflammatory cytokines produced in the periphery need not enter the brain to elicit neurobehavioral changes. This is because inflammatory cytokines in the periphery can induce microglia—macrophage-like cells present in the brain—to produce a similar repertoire of inflammatory cytokines, thus recapitulating the message from the peripheral immune system (Ban et al., 1992; Laye et al., 1994). This often involves peripheral inflammatory cytokines acting on peripheral tissues to release chemical mediators of inflammation such as the prostaglandins (Saper et al., 2012). Hence, in a second pathway inflammatory cytokines in the periphery bind receptors on blood-brain barrier endothelial cells (Ching et al., 2007; Li et al., 2011) and either directly or indirectly with prostaglandin intermediates induce perivascular microglia or macrophages to express cytokines that are released into the brain parenchyma (van Dam et al., 1995; van Dam et al., 1992). Furthermore, in a third pathway inflammatory cytokines in the periphery convey a message to the brain via sensory nerves. After immune challenge, dendritic cells and macrophages that are closely associated with the abdominal vagus have been shown to express IL-1β protein (Goehler et al., 1999); IL-1 binding sites have been identified in several regions of the vagus as well (Goehler et al., 1997). When activated by immune stimuli, the vagus can activate specific neural pathways that are involved in perception of pain (Watkins et al., 1994), fever (Romanovsky et al., 1997), food intake (Bret-Dibat et al., 1995), and sickness behavior (Bluthe et al., 1996). However, activation of the vagus by peripheral immune stimuli also leads to the expression of inflammatory cytokines in the brain (Laye et al., 1995), presumably by microglia. Vagal afferents project to the nucleus of the solitary tract which in turn projects to other CNS locations including the locus coeruleus, the primary site of norepinephrine production (Berridge and Waterhouse, 2003). This is noteworthy because microglia express adrenergic receptors. Resting microglia primarily express β2 receptors but switch to α2A receptors under pro-inflammatory conditions (Gyoneva and Traynelis, 2013). Norepinephrine was shown to enhance motility of resting and activated microglia via the β2 and α2A receptors, respectively (Gyoneva and Traynelis, 2013). Furthermore, intracerebroventricular injection of isoproterenol, a β1 and β2 receptor agonist, enhanced the sensitivity of microglia to an inflammatory stimulus (Johnson et al., 2013). Finally, a fourth pathway provides a slower immune-to-brain signaling mechanism based on volume transmission (Dantzer et al., 2000; Konsman et al., 1999). In this method of immune-to-brain communication, production of IL-1β by the brain first occurs in the choroid plexus and circumventricular organs—brain areas devoid of an intact blood-brain barrier. The cytokines then slowly diffuse throughout the brain by volume transmission, along the way activating microglia, neurons and neural pathways that induce sickness behavior and inhibit cognition. Figure 1 summarizes the channels of communication, lists several neurobehavioral effects of infection, and highlights the neurobehavioral effects with a picture of Michael Ancher’s 1882 oil painting entitled “The Sick Girl.”
Figure 1.
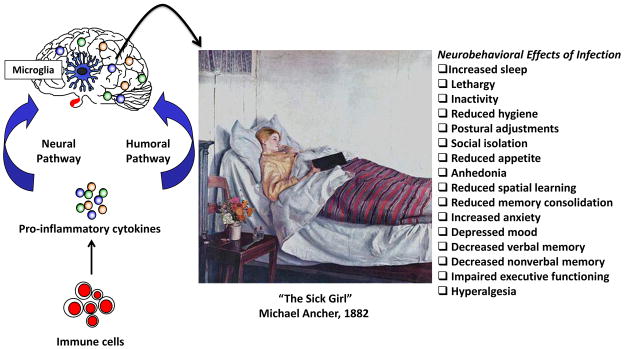
The peripheral immune system conveys information to the brain via humoral and neural pathways. Brain microglia respond to signals from the peripheral immune system and produce pro-inflammatory cytokines that induce the neurobehavioral changes associated with infection. Some of the neurobehavioral effects are evident in Michael Ancher’s 1882 oil painting entitled “The Sick Girl” (This work is in the United States public domain).
What are microglia, what do they do, and what goes wrong during aging?
An important point is that the aforementioned communication pathways share a common need to activate microglial cells and induce neuroinflammation. Celsus’ original definition of inflammation was based on four cardinal signs: dolor (pain), calor (heat), rubor (redness), and tumor (swelling); functio laesa (loss of function) was added later by Galen. While neuroinflammation can resemble its peripheral counterpart in circumstances such as viral and bacterial meningitis, head trauma, or autoimmune diseases of the CNS, the term neuroinflammation is increasingly used to identify a fundamentally different event that is exclusively driven by microglial cells and shows few if any of the cardinal signs originally described by Celsus (Aguzzi et al., 2013).
Microglia account for 12–15% of the cells in the brain. They originate from macrophages produced by primitive hematopoiesis in the yolk sac and migrate to the neural tube, where they give rise to microglia (Ginhoux et al., 2010). Bone marrow-derived monocytes do not contribute to the mature microglia pool in the healthy brain, suggesting microglia numbers are sustained by local progenitors. Microglia serve at least two vital functions during development. First, microglia were recently proven to be “gate keepers” regulating the number of neural precursor cells in the developing cerebral cortex. In an elegant sequence of experiments, Cunningham et al. (Cunningham et al., 2013) demonstrated that microglia selectively colonize the cortical proliferative zones in the developing neocortex, have an activated morphology and express markers indicative of activation, and phagocytize neural precursor cells in the late stages of cortical neurogenesis. Maternal immune activation during pregnancy further activated fetal microglia and reduced the number of neural precursor cells; conversely, eliminating or inactivating fetal microglia with clodronate-filled liposomes or minocycline, respectively, increased the number of neural precursor cells (Cunningham et al., 2013). Second, precursor cells that differentiate into neurons grow axons and dendrites and form synapses with other neurons. Initially, neurons make far more synapses than needed, and many must be eliminated in a process called synaptic pruning. Neurons express the chemokine fractalkine, CX3CL1, whose receptor in the CNS is expressed exclusively by microglia (Harrison et al., 1998). Hence, through this chemical attraction microglia closely associate with neurons and are in direct apposition with synapses (Tremblay et al., 2010). Microglia carry out synaptic pruning by actively engulfing pre- and postsynaptic material; and in mice whose microglia lack the fractalkine receptor, the number of synaptic puncta is abnormally high, as is the density of dendritic spines on CA1 pyramidal neurons (Paolicelli et al., 2011). Recent evidence suggests some developing synapses get “tagged” for elimination with complement protein C3, which is a ligand for the phagocytic complement receptor expressed on the surface of microglia (Schafer et al., 2012). The now recognized fundamental role of microglia in neurodevelopment adds credence to the neuroimmune hypotheses of psychiatric illness.
In adulthood “resting” or “quiescent” microglia are highly dynamic, randomly extending and contracting arms with filopodia-like protrusions to survey the microenvironment (Nimmerjahn et al., 2005). Using in vivo two-photon imaging of fluorescent-labeled neurons and microglia, Wake et al. (Wake et al., 2009) estimated that resting microglial processes make direct contact with neuronal synapses about once per hour. They further showed that the contact frequency is positively correlated with neuronal activity, and that when the duration of the contact is markedly prolonged due to cerebral ischemia the presynaptic bouton tends to be eliminated. Hence, a fundamental role of resting microglia in the adult brain is to contribute to synaptic plasticity by monitoring and responding to the functional status of the synapses (Tremblay et al., 2010). They further serve to phagocytize apoptotic debris. For example, in the adult hippocampus neurogenesis persists and is enhanced by environmental enrichment (van Praag et al., 1999). As is the case for prenatal and postnatal development, most of the newborn neurons undergo apoptosis; and those that survive require synaptic pruning. The apoptotic cells release “find me” and “eat me” signals (Tremblay et al., 2011) that stimulate local microglia to perform phagocytosis (Sierra et al., 2010). This process does not require prior microglial cell activation and occurs in the absence of inflammation; however, inflammation nearly doubles the phagocytic capacity of microglia (Sierra et al., 2010).
As microglia constantly survey the brain parenchyma, they are never truly at rest or in a quiescent state. However, events that threaten brain homeostasis such as infection, trauma, ischemia, and neurodegenerative disease can cause microglia to transition from surveillance mode to activation mode. Activated microglia can direct the movement of the protrusions toward the insult (Nimmerjahn et al., 2005), take on a de-ramified morphology that enables motility (Perry et al., 2010), and/or express major histocompatibility complex class II (MHC class II) and other markers indicative of inflammation (Varnum and Ikezu, 2012). As highlighted in an exceptionally thorough review by Kettenmann et al. (Kettenmann et al., 2011), microglial activation is not an all-or-nothing event and it does not follow a linear path with a fixed uniform outcome. Instead, depending on the nature, intensity, and duration of a provocation, resting microglia can transition to alerted and activated states. Furthermore, activated microglia that first exhibit a M1 pro-inflammatory phenotype can convert to a M2 neuroprotective anti-inflammatory phenotype. Of note, activated (and most likely alerted) microglia constitutively produce inflammatory cytokines and are hypersensitive to insults, including signals from the peripheral immune system (Frank et al., 2010; Godbout et al., 2005; Ye and Johnson, 1999). Hence, microglial cell activation is frequently viewed to be tantamount to neuroinflammation. As neuroinflammation can be deleterious to neurological function if left unregulated, mitigating microglial cell activity in certain situations is vital for optimal brain health.
Major histocompatibility complex class II is frequently used to identify activated microglia. During aging the percentage of brain microglia that express MHC class II increases and signs of neuroinflammation emerge. For example, <3% of microglia isolated from the brain of healthy young adult mice stained positive for MHC class II (Henry et al., 2009). This pales in comparison to the >25% of microglia from brains of old but otherwise healthy mice that were MHC class II-positive (Henry et al., 2009). Both pro-inflammatory M1 and anti-inflammatory M2 microglia can express MHC class II, meaning this molecule alone is not sufficient to distinguish pro-inflammatory and anti-inflammatory cells. However, in the study by Henry et al., most of the MHC class II-positive microglia from old mice were also IL-1β-positive (Henry et al., 2009). This is consistent with a prior study where the proportion of IL-6-positive microglia was markedly higher if the donor mouse was 22–24 months old compared to 6-months or 1 wk old (Ye and Johnson, 1999). These cells were also shown to be hypersensitive to LPS in vitro (Ye and Johnson, 2001). It is important to note that aging per se does not increase the number of microglial cells in the brain but rather it increases the proportion of resident microglia that are inflammatory and reactive to insults (Norden and Godbout, 2012).
A consequence of age-induced microglial sensitization (i.e., priming) is that an exaggerated inflammatory response takes place in the old but otherwise healthy aged brain following challenge with peripheral or central immune stimuli (Godbout et al., 2005; Huang et al., 2008), psychological stress (Buchanan et al., 2008), and tissue trauma (Barrientos et al., 2012; Rosczyk et al., 2008). A recent study suggests that microglia from aged mice retain a prominent M1 profile and are less sensitive to the anti-inflammatory and M2-promoting effects of IL-4 (Fenn et al., 2012). Ye et al. (Ye and Johnson, 2001) reported glia production of another anti-inflammatory cytokine, IL-10, was reduced by aging. Thus, if microglia “get stuck” in the M1 state, this may explain the age-associated increase in neuroinflammation. The prolonged increase in pro-inflammatory cytokines including IL-1β, IL-6 and TNF-α in the aged brain is accompanied by an extended sickness response, impaired learning, and depressive behaviors (Abraham and Johnson, 2009; Buchanan et al., 2008; Chen et al., 2008; Godbout et al., 2008). Whereas sickness behavior is normally adaptive, the excessive production of cytokines in the aged brain can lead to pathological or maladaptive behavioral changes such as delayed recovery from infection and cognitive impairment. The hippocampus has a high density of pro-inflammatory cytokine receptors, and appears to be particularly vulnerable to both aging- and inflammation-induced disruption in cognitive processing (Mattson and Magnus, 2006; Parnet et al., 2002; Wilson et al., 2002). Following activation of the peripheral innate immune system, aged rodents have significantly increased levels of pro-inflammatory cytokines in the hippocampus and demonstrate deficits specific to hippocampal-associated learning and memory tasks when compared to adults (Abraham et al., 2008; Barrientos et al., 2010; Chen et al., 2008; Rosczyk et al., 2008). As pro-inflammatory cytokines can act directly on neurons in the hippocampus to impair synaptic plasticity, this provides a mechanism underlying inflammation-induced cognitive impairment (Balschun et al., 2004; Lynch, 2002; Pickering and O’Connor, 2007). Reducing the proportion of microglia that are reactive or that “get stuck” in the M1 state after stimulation is a priority for reducing age-related neuroinflammation that may contribute to cognitive aging and be a predisposing factor for neurodegenerative disease.
Effects of diet on microglia: Feeding the Beast
It is clear that diet can influence microglia and neuroinflammation. Compelling evidence for this comes from studies focused on overweight and obesity. According to the Centers for Disease Control and Prevention, approximately 17% of U.S. children and adolescents aged 2–19 are obese. The high prevalence of obesity in children and adolescents is a significant concern because of its negative effects on morbidity and mortality in young adulthood, and the fact that 70% of obese adolescents grow up to become obese adults. All told, nearly 35% of U.S. adults are obese. Obesity-related conditions include heart disease, stroke, type 2 diabetes and certain types of cancers. There is also a well-established link between obesity and cognitive decline. Among psychoneuroimmunology researchers, interest in the relationship between diet and brain health is high as evidenced by Brain, Behavior, and Immunity’s recent (2014) Named Series on Diet, Inflammation and the Brain; and the Presidential Symposium at the 2014 meeting of the PsychoNeuroImmunology Research Society on Nutrition: Implications for Psychoneuroimmunology.
The evidence showing that obesity promotes chronic inflammation (Gregor and Hotamisligil, 2011) and that inflammatory cytokines in the periphery can stimulate microglia and cause neuroinflammation (Dantzer et al., 2008), made it logical to predict that an obesigenic diet affects brain microglial cells. There is now substantial evidence supporting this notion. For example, rats fed a high-fat diet for 20 weeks beginning at 2 months of age demonstrated impaired memory in a hippocampal-dependent task and had higher IL-1β protein levels in the hippocampus after a mild foot shock stressor (Sobesky et al., 2014). Importantly, centrally administered IL-1RA prevented the diet-induced memory disruption. The fact that basal levels (i.e., levels in unstressed rats) of IL-1β protein were not elevated by high fat diet, suggests the obesigenic diet have affected microglia phenotype, making them more sensitive to a secondary insult (i.e., mild foot shock). This is reminiscent of what occurs with microglial cells during aging. In another study, PET imaging was used to assess microglial cell activation in a pre-clinical genetic rat model of obesity and in a small clinical study of patients with multiple risk factors for stroke, including obesity-related peripheral inflammation (e.g., increased plasma IL-6 and C-reactive protein) (Drake et al., 2011). Both obese rats and patients with increased risk for stroke exhibited increased microglial cell activation. Finally, evidence of microgliosis in feeding-regulatory brain regions (e.g., paraventricular nucleus and arcuate nucleus) has been reported in rats overfed in the neonatal period (Ziko et al., 2014) and in adult mice given a high-fat diet (Gao et al., 2014). In adult rats that were overfed in the neonatal period, microglia were hypersensitive to peripheral immune stimulation with LPS (Ziko et al., 2014). The potential for overweight and obesity to be associated with altered development of microglia adds another layer of concern regarding maternal obesity during pregnancy and childhood obesity.
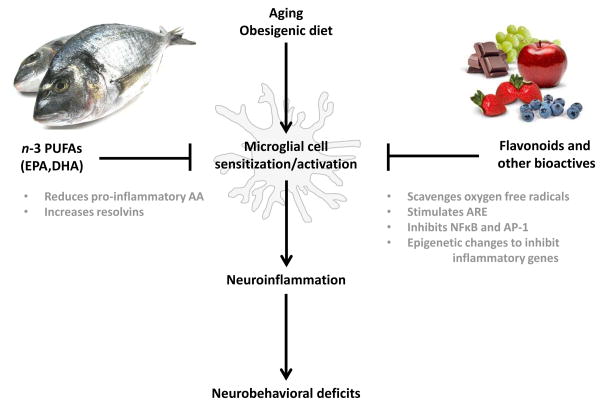
While it seems clear that a high fat diet can directly or indirectly result in microglial cell activation and neuroinflammation, a more provocative idea, in my opinion, is the potential for diet to constrain microglial cell activity (Figure 2). This is particularly intriguing in the context of aging, where microglia tend to adopt a sensitized phenotype and overreact to signals from the peripheral immune system and stress. Good evidence that this might be possible comes from studies of dietary flavonoids. Flavonoids are naturally occurring poly-phenolic compounds present in plants. The major sources of flavonoids in the human diet include fruits, vegetables, tea, wine and cocoa (Harnly et al., 2006). Significant evidence has emerged to indicate that consuming a diet rich in flavonoids may inhibit or reverse cognitive aging. For example, in a prospective study of individuals aged 65 years or older, dietary flavonoid intake (i.e., mg/d of 5 flavonoids: apigenin, kaempferol, luteolin, myricetin, and quercetin) was associated with improved cognitive function over a 10-year period (Letenneur et al., 2007). Furthermore, analyses of data from the Chicago Health and Aging Project—a cohort study of older residents residing on the south side of Chicago—suggested that adherence to a Mediterranean dietary pattern reduced the rate of cognitive decline (Tangney et al., 2011). Numerous other studies have yielded consistent results, with older rats or mice showing improved cognitive function when fed a flavonoid-rich diet (Jang et al., 2010; Joseph et al., 1999; Williams et al., 2008). Furthermore, pomegranate polyphenols and extract were recently found to reduce neuroinflammation, microgliosis, and Aβ plaque deposition in amyloid precursor protein/presenilin 1 transgenic mice (a transgenic mouse model of Alzheimer’s disease (Rojanathammanee et al., 2013). Importantly, another recent study indicates that flavonoids are the causal agents mediating the cognitive effects of flavonoid-rich foods (Rendeiro et al., 2013).
Figure 2.
Aging and an obesigenic diet can alter microglia phenotype, rendering them more sensitive to various insults. Consuming a diet rich in flavonoid and non-flavonoid bioactives and n-3 PUFAs can inhibit microglia and thereby reduce neuroinflammation. Abbreviations: ARE, anti-oxidant response element; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; PUFA, polyunsaturated fatty acid.
Flavonoids may improve cognition in the aged through a number of physiological mechanisms, including scavenging of reactive oxygen and nitrogen species (Rice-Evans and Miller, 1996) and interactions with intracellular signaling pathways (Spencer et al., 2012). Through these physiological mechanisms, flavonoids also impart an anti-inflammatory effect that may improve cognition. This seems likely for the flavone, luteolin, which is most prominent in parsley, celery, and green peppers. Whereas luteolin inhibits several transcription factors that mediate inflammatory genes (e.g., NFκB (Xagorari et al., 2001) and AP-1 (Jang et al., 2008)), it is a potent activator of nuclear factor erythroid 2-related factor 2 (Nrf2) which induces the expression of genes encoding anti-oxidant enzymes (Lim et al., 2007). A recent study of old but otherwise healthy mice found improved learning and memory and reduced expression of inflammatory genes in the hippocampus when luteolin was included in the diet (Jang et al., 2010). Thus, dietary luteolin may improve cognitive function in the aged by reducing brain microglial cell activity, although a microglia-dependent mechanism has not been established. Indirect support for a microglia-dependent mechanism, however, comes from an in vitro study where luteolin stimulated the formation of filopodia and caused ramification of BV-2 cells (a microglia cell line) even when they were activated with E. coli LPS (Dirscherl et al., 2010). Furthermore, supernatants from LPS-stimulated BV-2 cells caused discernible cell death in Neuro.2a cells even if Neuro.2a cells were incubated with luteolin; however, treating BV-2 cells with luteolin prior to LPS reduced neuronal cell death caused by conditioned supernatants (Jang et al., 2010). Similar beneficial effects of luteolin have been reported for other conditions associated with chronic inflammation. For example, in mice fed a high-fat diet, the addition of luteolin improved learning and memory and reduced TNFα, IL-1β, and several markers of oxidative stress in the hippocampus and cortex (Liu et al., 2014). Hence, the flavonoid luteolin is a naturally occurring immune-modulator that may be effective in reducing inflammatory microglia.
Luteolin is just one of many flavonoids with anti-inflammatory properties (others include, e.g., apigenin, quercetin, and epigallocatechin gallate); and it is important to recognize that whole foods can contain numerous flavonoids at various concentrations. Harnly et al. (Harnly et al., 2006) measured 20 flavonoids in more than 60 fresh fruits, vegetables, and nuts collected from four regions across the U.S. and found significant variation attributed to, in part, source of cultivar (i.e., geneotype) and growing and processing conditions. Thus, understanding the effects and mechanisms of individual flavonoids and various combinations and concentrations of flavonoids may be important for understanding the total impact of consuming flavonoid-rich foods. Furthermore, numerous other non-flavonoid compounds have been isolated from plants and shown to have potent anti-inflammatory properties. Several notable examples include resveratrol found in grapes, which acts through SIRT1 (a member of the sirtuins superfamily) to deacetylate several transcription factors including NFκB (Ayissi et al., 2014); and curcumin found in turmeric, which inhibits NFκB and AP-1 DNA binding activity (Bisht et al., 2010). Thus, what several plant-based flavonoid and non-flavonoid molecules have in common is an ability to protect against various stress-induced toxicities. They do so by modulating intracellular signaling pathways that inhibit synthesis of inflammatory mediators while stimulating the anti-oxidant response element that regulates expression of several detoxifying genes (Vauzour, 2012).
Another dietary component that may afford health benefits is oily fish rich in n-3 PUFA. Large population studies suggest that greater fish consumption may help control or protect against diseases associated with chronic inflammation including cardiovascular diseases, stroke, and rheumatoid arthritis. Accordingly, the Dietary Guidelines for Americans (United States. Department of Health and Human Services. et al., 2010) recommend consuming 8–12 ounces of seafood each week. Similar beneficial effects of diets high in n-3 PUFAs on psychiatric illnesses with an inflammatory component have also been suggested. For example, several epidemiological studies have demonstrated a negative correlation between annual fish consumption and depression (Golding et al., 2009; Hibbeln, 1998). A meta-analysis of randomized controlled trials published in 2010 concluded that n-3 PUFA supplementation is beneficial in individuals with diagnosed depressive illness but not in individuals without a diagnosis of depressive illness (Appleton et al., 2010). However, a more recent meta-analysis concluded that n-3 PUFA supplementation is effective in patients with diagnosis of major depressive disorder and patients with depressive symptomology but no diagnosis of major depressive disorder (Grosso et al., 2014). Consistent with this finding, n-3 PUFA supplementation had anxiolytic benefits in medical students without an anxiety disorder diagnosis (Kiecolt-Glaser et al., 2011).
As inflammatory mechanisms have been implicated in the pathophysiology of depression (Dantzer et al., 2008), n-3 PUFAs may impart their anti-depressant effects by dampening inflammation (Kiecolt-Glaser et al., 2012). On the one hand, arachidonic acid (AA) is an n-6 PUFA that gives rise to pro-inflammatory eicosanoids (e.g., prostaglandins). On the other hand, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are n-3 PUFAs that give rise to eicosanoids and docosanoids, respectively, that yield resolvins—bioactive products that counter pro-inflammatory signals (Serhan et al., 2002). In rodents maintained on normal laboratory chow, immune cells typically contain 15–20% of fatty acids as AA and contain little n-3 PUFA. The phospholipid of immune cells of humans consuming a typical Western diet also contains about 20% of fatty acids as AA, with about 1% EPA and 2.5% DHA (Calder, 2007). The phospholipid composition of immune cells, however, is diet-dependent so increasing intake of n-3 PUFAs from fish oil, e.g., can increase n-3 PUFAs in the cells at the expense of AA (Yaqoob et al., 2000). Thus, n-3 PUFAs reduce inflammation by (1) reducing the amount of pro-inflammatory AA in immune cells; and (2) giving rise to resolvins.
The phospholipid composition of CNS tissue is also subject to dietary manipulation, suggesting a diet enriched with n-3 PUFAs may be able to inhibit neuroinflammation (Orr et al., 2013). Providing aged mice a diet enriched with DHA and EPA for 2 months increased n-3 PUFAs in brain, improved spatial learning, and reduced the age-related increase in CD11b, IL-1β, TNFα, and IL-6 in hippocampus (Labrousse et al., 2012). Similarly, an EPA-enriched diet prevented the age-related increase in cortical and hippocampal IL-1β in rats (Martin et al., 2002) and protected aged rats from further increases in hippocampal IL-1β induced by Aβ (Lynch et al., 2007). Additionally, mice fed a n-3 PUFA-enriched diet for 2 months exhibited attenuated short and long-term behavioral deficits and a reduced neuroinflammatory response after controlled cortical impact (i.e., traumatic brain injury) (Pu et al., 2013). A recent study that investigated the effects of an n-3 PUFA-deficient diet during development provides further proof that n-3 PUFAs mediate neuroinflammation. In this study, mouse pups born of dams provided a n-3 PUFA-deficient diet during gestation and lactation had decreased n-3 PUFA levels in brain at postnatal day (PD) 0 and 21; and at PD21, in the hippocampus expression of several pro-inflammatory genes was up regulated and the motility of microglia was decreased, suggesting an impairment in their ability to maintain homeostasis (Madore et al., 2014). Reinforcing these findings are several in vitro studies showing n-3 PUFAs to be potent inhibitors of LPS-stimulated cytokine production by microglia (De Smedt-Peyrusse et al., 2008; Moon et al., 2007).
Conclusion
In light of the recent evidence suggesting microglial cells become dysregulated due to aging and cause neuroinflammation which can disrupt neural structure and function, it is an interesting prospect to think dietary flavonoids, n-3 PUFAs, and other food-derived bioactives like resveratrol and curcumin can be used to constrain microglia. The Dietary Guidelines for Americans recommends consuming more of certain foods such as fruits, vegetables, whole grains, fat-free and low-fat dairy products and seafood; and suggests the Mediterranean diet as an eating plan that can reduce the incidence of cardiovascular disease. The Mediterranean diet, however, reduces the risk of Parkinson’s and Alzheimer’s diseases, indicating the benefits extend to the brain and probably microglial cells. As we continue to elucidate the anti-inflammatory mechanisms of individual dietary components, it becomes evident that the benefits are not attributable to a single component but rather a combination of components that provide a diverse group of bioactives that impart anti-inflammatory properties through different mechanisms. A better understanding of the additive, synergistic, and antagonistic effects of various dietary bioactives in microglia will be useful. Nonetheless, it is worth noting that it is not clear that the dietary bioactives shown to have anti-inflammatory activity access the brain to interact directly with microglia or other CNS cells. It is complicated to dissect because a healthy diet rich in flavonoids, e.g., can reduce inflammation in the periphery, and microglia seem to act like an “immunostat”, detecting and responding to signals emerging from immune-to-brain signaling pathways. Thus, whether anti-inflammatory dietary bioactives enter the brain and impart an anti-inflammatory effect on microglia is an interesting question, but it is one that is more theoretical than practical because what is most important is how the immunostat is adjusted, i.e., whether it occurs via a direct or an indirect route.
Research Highlights.
Microglia are important for brain development, homeostasis, and plasticity but must be regulated
Dysregulated microglia can cause chronic neuroinflammation and cognitive deficits
Microglia can become dysregulated during aging and be hypersensitive to stimuli
Dietary bioactives with anti-inflammatory represent a pragmatic way to constrain microglia
Acknowledgments
I thank current and former students, post-doctorates and research associates who studied and worked in my research laboratory. Their collective efforts are represented in this review. This work was partially supported by NIH R01 AG16710 and HD069899.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91:757–770. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- Ayissi VB, Ebrahimi A, Schluesenner H. Epigenetic effects of natural polyphenols: a focus on SIRT1-mediated mechanisms. Molecular nutrition & food research. 2014;58:22–32. doi: 10.1002/mnfr.201300195. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. FASEB J. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Ban E, Haour F, Lenstra R. Brain interleukin 1 gene expression induced by peripheral lipopolysaccharide administration. Cytokine. 1992;4:48–54. doi: 10.1016/1043-4666(92)90036-q. [DOI] [PubMed] [Google Scholar]
- Banks WA. Brain meets body: the blood-brain barrier as an endocrine interface. Endocrinology. 2012;153:4111–4119. doi: 10.1210/en.2012-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Blood to brain transport of interleukin links the immune and central nervous systems. Life Sci. 1991;48:PL117–121. doi: 10.1016/0024-3205(91)90385-o. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Ehrensing CA. Blood-borne interleukin-1 alpha is transported across the endothelial blood-spinal cord barrier of mice. J Physiol. 1994a;479 (Pt 2):257–264. doi: 10.1113/jphysiol.1994.sp020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett. 1994b;179:53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR, Maier SF. Memory impairments in healthy aging: Role of aging-induced microglial sensitization. Aging and disease. 2010;1:212–231. [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci. 2012;32:14641–14648. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain research. Brain research reviews. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bisht K, Wagner KH, Bulmer AC. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology. 2010;278:88–100. doi: 10.1016/j.tox.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Michaud B, Kelley KW, Dantzer R. Vagotomy attenuates behavioural effects of interleukin-1 injected peripherally but not centrally. Neuroreport. 1996;7:1485–1488. doi: 10.1097/00001756-199606170-00008. [DOI] [PubMed] [Google Scholar]
- Bret-Dibat JL, Bluthe RM, Kent S, Kelley KW, Dantzer R. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav Immun. 1995;9:242–246. doi: 10.1006/brbi.1995.1023. [DOI] [PubMed] [Google Scholar]
- Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755–765. doi: 10.1016/j.psyneuen.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins, leukotrienes, and essential fatty acids. 2007;77:327–335. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S, Zhang H, Belevych N, He L, Lai W, Pu XA, Jaeger LB, Chen Q, Quan N. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J Neurosci. 2007;27:10476–10486. doi: 10.1523/JNEUROSCI.3357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Konsman JP, Bluthe RM, Kelley KW. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Autonomic neuroscience : basic & clinical. 2000;85:60–65. doi: 10.1016/S1566-0702(00)00220-4. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt-Peyrusse V, Sargueil F, Moranis A, Harizi H, Mongrand S, Laye S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J Neurochem. 2008;105:296–307. doi: 10.1111/j.1471-4159.2007.05129.x. [DOI] [PubMed] [Google Scholar]
- Dirscherl K, Karlstetter M, Ebert S, Kraus D, Hlawatsch J, Walczak Y, Moehle C, Fuchshofer R, Langmann T. Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. Journal of neuroinflammation. 2010;7:3. doi: 10.1186/1742-2094-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C, Boutin H, Jones MS, Denes A, McColl BW, Selvarajah JR, Hulme S, Georgiou RF, Hinz R, Gerhard A, Vail A, Prenant C, Julyan P, Maroy R, Brown G, Smigova A, Herholz K, Kassiou M, Crossman D, Francis S, Proctor SD, Russell JC, Hopkins SJ, Tyrrell PJ, Rothwell NJ, Allan SM. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav Immun. 2011;25:1113–1122. doi: 10.1016/j.bbi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav Immun. 2012;26:766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol. 2010;226:181–184. doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Ottaway N, Schriever SC, Legutko B, Garcia-Caceres C, de la Fuente E, Mergen C, Bour S, Thaler JP, Seeley RJ, Filosa J, Stern JE, Perez-Tilve D, Schwartz MW, Tschop MH, Yi CX. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia. 2014;62:17–25. doi: 10.1002/glia.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, JOC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, Watkins LR. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, Watkins LR. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain research bulletin. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Golding J, Steer C, Emmett P, Davis JM, Hibbeln JR. High levels of depressive symptoms in pregnancy with low omega-3 fatty acid intake from fish. Epidemiology. 2009;20:598–603. doi: 10.1097/EDE.0b013e31819d6a57. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, Drago F, Caraci F. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PloS one. 2014;9:e96905. doi: 10.1371/journal.pone.0096905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993;47:169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- Gyoneva S, Traynelis SF. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J Biol Chem. 2013 doi: 10.1074/jbc.M113.458901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S. Flavonoid content of U.S. fruits, vegetables, and nuts. Journal of agricultural and food chemistry. 2006;54:9966–9977. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Dilger RN, Johnson RW. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr. 2010;140:1892–1898. doi: 10.3945/jn.110.123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci U S A. 2008;105:7534–7539. doi: 10.1073/pnas.0802865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Zimomra ZR, Stewart LT. Beta-adrenergic receptor activation primes microglia cytokine production. J Neuroimmunol. 2013;254:161–164. doi: 10.1016/j.jneuroim.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiological reviews. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25:1725–1734. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Hwang BS, Glaser R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun. 2012;26:988–995. doi: 10.1016/j.bbi.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Feldmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Kelley K, Dantzer R. Temporal and spatial relationships between lipopolysaccharide-induced expression of Fos, interleukin-1beta and inducible nitric oxide synthase in rat brain. Neuroscience. 1999;89:535–548. doi: 10.1016/s0306-4522(98)00368-6. [DOI] [PubMed] [Google Scholar]
- Labrousse VF, Nadjar A, Joffre C, Costes L, Aubert A, Gregoire S, Bretillon L, Laye S. Short-term long chain omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PloS one. 2012;7:e36861. doi: 10.1371/journal.pone.0036861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laye S, Bluthe RM, Kent S, Combe C, Medina C, Parnet P, Kelley K, Dantzer R. Subdiaphragmatic vagotomy blocks induction of IL-1 beta mRNA in mice brain in response to peripheral LPS. Am J Physiol. 1995;268:R1327–1331. doi: 10.1152/ajpregu.1995.268.5.R1327. [DOI] [PubMed] [Google Scholar]
- Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27:157–162. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. American journal of epidemiology. 2007;165:1364–1371. doi: 10.1093/aje/kwm036. [DOI] [PubMed] [Google Scholar]
- Li Q, Powell N, Zhang H, Belevych N, Ching S, Chen Q, Sheridan J, Whitacre C, Quan N. Endothelial IL-1R1 is a critical mediator of EAE pathogenesis. Brain Behav Immun. 2011;25:160–167. doi: 10.1016/j.bbi.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Park HS, Choi JK, Lee IS, Choi HJ. Isoorientin induces Nrf2 pathway-driven antioxidant response through phosphatidylinositol 3-kinase signaling. Archives of pharmacal research. 2007;30:1590–1598. doi: 10.1007/BF02977329. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fu X, Lan N, Li S, Zhang J, Wang S, Li C, Shang Y, Huang T, Zhang L. Luteolin protects against high fat diet-induced cognitive deficits in obesity mice. Behav Brain Res. 2014;267:178–188. doi: 10.1016/j.bbr.2014.02.040. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Loane DJ, Minogue AM, Clarke RM, Kilroy D, Nally RE, Roche OJ, O’Connell F, Lynch MA. Eicosapentaenoic acid confers neuroprotection in the amyloid-beta challenged aged hippocampus. Neurobiol Aging. 2007;28:845–855. doi: 10.1016/j.neurobiolaging.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Interleukin-1 beta exerts a myriad of effects in the brain and in particular in the hippocampus: analysis of some of these actions. Vitamins and hormones. 2002;64:185–219. doi: 10.1016/s0083-6729(02)64006-3. [DOI] [PubMed] [Google Scholar]
- Madore C, Nadjar A, Delpech JC, Sere A, Aubert A, Portal C, Joffre C, Laye S. Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Martin DS, Lonergan PE, Boland B, Fogarty MP, Brady M, Horrobin DF, Campbell VA, Lynch MA. Apoptotic changes in the aged brain are triggered by interleukin-1beta-induced activation of p38 and reversed by treatment with eicosapentaenoic acid. J Biol Chem. 2002;277:34239–34246. doi: 10.1074/jbc.M205289200. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon DO, Kim KC, Jin CY, Han MH, Park C, Lee KJ, Park YM, Choi YH, Kim GY. Inhibitory effects of eicosapentaenoic acid on lipopolysaccharide-induced activation in BV2 microglia. Int Immunopharmacol. 2007;7:222–229. doi: 10.1016/j.intimp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol. 2011;21:R488–493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Microglia of the Aged Brain: Primed to be Activated and Resistant to Regulation. Neuropathol Appl Neurobiol. 2012 doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SK, Trepanier MO, Bazinet RP. n-3 Polyunsaturated fatty acids in animal models with neuroinflammation. Prostaglandins, leukotrienes, and essential fatty acids. 2013;88:97–103. doi: 10.1016/j.plefa.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Parnet P, Kelley KW, Bluthe RM, Dantzer R. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. J Neuroimmunol. 2002;125:5–14. doi: 10.1016/s0165-5728(02)00022-x. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nature reviews Neurology. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- Pickering M, O’Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Progress in brain research. 2007;163:339–354. doi: 10.1016/S0079-6123(07)63020-9. [DOI] [PubMed] [Google Scholar]
- Pu H, Guo Y, Zhang W, Huang L, Wang G, Liou AK, Zhang J, Zhang P, Leak RK, Wang Y, Chen J, Gao Y. Omega-3 polyunsaturated fatty acid supplementation improves neurologic recovery and attenuates white matter injury after experimental traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1474–1484. doi: 10.1038/jcbfm.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N. Immune-to-brain signaling: how important are the blood-brain barrier-independent pathways? Molecular neurobiology. 2008;37:142–152. doi: 10.1007/s12035-008-8026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendeiro C, Vauzour D, Rattray M, Waffo-Teguo P, Merillon JM, Butler LT, Williams CM, Spencer JP. Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor. PloS one. 2013;8:e63535. doi: 10.1371/journal.pone.0063535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ. Antioxidant activities of flavonoids as bioactive components of food. Biochemical Society transactions. 1996;24:790–795. doi: 10.1042/bst0240790. [DOI] [PubMed] [Google Scholar]
- Rojanathammanee L, Puig KL, Combs CK. Pomegranate polyphenols and extract inhibit nuclear factor of activated T-cell activity and microglial activation in vitro and in a transgenic mouse model of Alzheimer disease. J Nutr. 2013;143:597–605. doi: 10.3945/jn.112.169516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA, Simons CT, Szekely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am J Physiol. 1997;273:R407–413. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Experimental gerontology. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Romanovsky AA, Scammell TE. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci. 2012;15:1088–1095. doi: 10.1038/nn.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger RW, Husak PJ, Bradshaw GL, Panayotov PP. Mechanisms involved in natural and experimental neuropathogenicity of influenza viruses: evidence and speculation. Advances in virus research. 1998;50:289–379. doi: 10.1016/s0065-3527(08)60811-8. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell stem cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobesky JL, Barrientos RM, De May HS, Thompson BM, Weber MD, Watkins LR, Maier SF. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1beta, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP, Vafeiadou K, Williams RJ, Vauzour D. Neuroinflammation: modulation by flavonoids and mechanisms of action. Molecular aspects of medicine. 2012;33:83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr. 2011;93:601–607. doi: 10.3945/ajcn.110.007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS biology. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States. Department of Health and Human Services., United States. Department of Agriculture., United States. Dietary Guidelines Advisory Committee. Dietary guidelines for Americans, 2010. G.P.O; Washington, D.C: 2010. [Google Scholar]
- van Dam AM, Bauer J, Tilders FJ, Berkenbosch F. Endotoxin-induced appearance of immunoreactive interleukin-1 beta in ramified microglia in rat brain: a light and electron microscopic study. Neuroscience. 1995;65:815–826. doi: 10.1016/0306-4522(94)00549-k. [DOI] [PubMed] [Google Scholar]
- van Dam AM, Brouns M, Louisse S, Berkenbosch F. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain Res. 1992;588:291–296. doi: 10.1016/0006-8993(92)91588-6. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Varnum MM, Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Archivum immunologiae et therapiae experimentalis. 2012;60:251–266. doi: 10.1007/s00005-012-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauzour D. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative medicine and cellular longevity. 2012;2012:914273. doi: 10.1155/2012/914273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GF, Li W, Li K. Acute encephalopathy and encephalitis caused by influenza virus infection. Current opinion in neurology. 2010;23:305–311. doi: 10.1097/wco.0b013e328338f6c9. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer JP. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. The Journal of pharmacology and experimental therapeutics. 2001;296:181–187. [PubMed] [Google Scholar]
- Yaqoob P, Pala HS, Cortina-Borja M, Newsholme EA, Calder PC. Encapsulated fish oil enriched in alpha-tocopherol alters plasma phospholipid and mononuclear cell fatty acid compositions but not mononuclear cell functions. European journal of clinical investigation. 2000;30:260–274. doi: 10.1046/j.1365-2362.2000.00623.x. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- Ziko I, De Luca S, Dinan T, Barwood JM, Sominsky L, Cai G, Kenny R, Stokes L, Jenkins TA, Spencer SJ. Neonatal overfeeding alters hypothalamic microglial profiles and central responses to immune challenge long-term. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.06.014. [DOI] [PubMed] [Google Scholar]