Abstract
Background
The benzodiazepine lorazepam is widely utilized in the treatment of elderly individuals with anxiety disorders and related conditions. Negative effects of acute lorazepam administration on cognitive performance, especially memory, have been reported in both previously untreated elderly and in individuals who have received short term (up to three weeks) treatment with therapeutic doses. However, it remains unclear if these adverse cognitive effects also persist after long-term use, which is frequently found in clinical practice.
Methods
Cognitively intact elderly individuals (n=37) on long-term (at least three months) daily treatment with lorazepam were studied using a double-blind placebo-controlled cross-over study design. Subjects were administered their highest daily unit dose of lorazepam (0.25 – 3.00 mg) orplacebo on different days, approximately 1 week apart in a random order, and were assessed on memory, psychomotor speed, and subjective mood states
Results
Subjects had significantly poorer recall and slowed psychomotor performance following acute lorazepam administration. There were no significant effects on self-ratings of mood, sedation, or anxiety in the whole group, but secondary analyses suggested a differential response in subjects with Generalized Anxiety Disorder.
Conclusions
The reduced recall and psychomotor slowing that we observed, along with an absence of significant therapeutic benefits, following acute lorazepam administration in elderly long-term users reinforces the importance of cognitive toxicity as a clinical factor in benzodiazepine use, especially in this population.
Keywords: Aging, Lorazepam, Cognitive Toxicity, Memory Loss, Psychomotor Slowing, Benzodiazepines
Introduction
Benzodiazepines (BZPs) are among the most widely prescribed drugs in the rapidly increasing elderly population. A number of surveys indicate that 13%-25% of community-dwelling individuals (aged 65 or over) report current or recent BZP use (Jackson et al., 2014; Lister and File, 1984; Tamblyn et al., 2005; Woods et al., 1992). However, it is a concern that impairments in multiple cognitive domains (e.g., memory, psychomotor performance) have been demonstrated consistently following acute doses of BZPs, in both healthy and anxious participants (Curran, 1986; Mintzer and Griffiths, 2007; Satzger et al., 1990; Woods et al., 1992).These impairments have been observed with lorazepam, the BZP frequently recommended for the elderly, due in part to a lack of active metabolites, a relatively shorter elimination half-life, and a presumed better safety profile compared to other BZPs (Tamblyn et al., 2005).
In the elderly, administration of even a single dose of a BZP impairs performance (Bertz et al., 1997; Nikaido et al., 1990; Pomara et al., 2005, 1998, 1988; Satzger et al., 1990), and elderly individuals may show greater sensitivity than younger subjects to the adverse effects of BZPs on psychomotor performance (Bertz et al., 1997; Nikaido et al., 1990; Satzger et al., 1990) and memory (Pomara et al., 1989). Following chronic treatment with BZPs for 1-3 weeks, significant adverse effects can be observed following an acute dose – although partial tolerance may develop (Curran, 1986; Ghoneim, 1981; Pomara et al., 1998, 1989). However, clinical treatment often extends beyond 3 weeks (e.g., years), increasing the associated morbidity and mortality (Hampton et al., 2014; Tamblyn et al., 2005; Pariente et al., 2008). Conversely, discontinuation of BZPs in long-term users is generally associated with an improvement in cognitive function, with no significant adverse effects (Kitajima et al., 2012; Rickels, 1999).
In spite of the prevalence of long-term administration of BZPs, few studies have examined the impact of acute doses of BZPs on cognitive performance in populations of elderly long-term users, and none have included a placebo condition. (Curran, 1992; Gorenstein et al., 1994; Lucki et al., 1986, van Stevenick et al., 1997). Their findings suggest that acute administration of the patient's usual daily unit dose may still result in significant impairment, even after several years of continuous BZP treatment. One study only examined saccadic eye movements and body sway (van Stevenick et al., 1997) and another did not report psychiatric diagnoses (Curran et al., 1992). Older participants were either not included (Gorenstein et al., 1994) or were underrepresented (Curran et al., 1992; Lucki et al., 1986; van Stevenick, 1997), questioning the relevance of these results in the elderly population.
In the present study, we examined the effects of a single acute dose of lorazepam in elderly long-term users treated with this drug for anxiety and related conditions. Memory and psychomotor performance was assessed and self-report measures of mood states and anxiety levels were obtained. We also determined the degree to which various factors (e.g., strength of daily unit dose, total daily dose, dosing frequency, and duration of treatment) contributed to the acute adverse effects. Because prolonged use of benzodiazepines is reported to be more prevalent in older individuals, especially women (Kruse, 1990), we also examined if age and gender influenced the effects of an acute lorazepam challenge.
Methods
Subjects
Thirty-seven psychiatric outpatients on long-term (between 3 and 252 months of treatment; median = 60 months) treatment with lorazepam for anxiety and related conditions were recruited for participation from outpatient psychiatric clinics, newspaper advertisements, and outreach efforts to senior citizen groups in the New York City area and Rockland County, NY. The study was conducted at the NYU-Bellevue General Clinical Research Center in New York City and the Nathan S. Kline Institute in Orangeburg, NY. Subjects ranged in age from 60 – 91 years (mean = 70.7, standard deviation (SD) 8.1). Absence of current DSM-IV psychotic illness, dementia, and current alcohol or substance abuse/dependence were also inclusion criteria. DSM-IV diagnoses were determined by clinical psychiatric interview and the Structured Clinical Interview (First et al., 2002). Subjects with severe neurological or medical illnesses, as determined by medical history, physical evaluation and routine laboratory tests, were excluded. All subjects were free of cognitive impairment, as determined by a score ≥ 28 on the Mini Mental State Examination (Folstein et al., 1975), an age-corrected score of at least 7 in the vocabulary subtest of the Wechsler Adult Intelligence Scale-Revised (Wechsler, 1981), and a score ≥ 85 on the General Memory Index of the Wechsler Memory Scale-Revised (Wechsler, 1987). Other demographic characteristics and screening measures are presented in Table 1. Each participant was paid $200 for their participation.
Table 1.
Demographics of the study population. The values represent group means with standard deviations in parentheses (WAIS-R = Wechsler Adult Intelligence Scale-Revised; WMS-R = Wechsler Memory Scale-Revised; BSRT = Buschke Selective Reminding Test.)
| Characteristics | Total Sample (N = 37) | Completer Group (n = 31) | Non-Completer Group (n = 6) |
|---|---|---|---|
| Age, yrs | 70.7 (8.1) | 70.0 (7.8) | 73.8 (9.4) |
| Weight, lbs | 169.3 (53.3) | 174.1 (54.2) | 145.7 (45.8) |
| Education | 15.3 (2.6) | 15.2 (2.1) | 15.7 (4.8) |
| Sex, No. M/F | 18/19 | 16/15 | 2/4 |
| Highest prescribed unit dose of lorazepam, mg | 1.0 (0.6) | 0.9 (0.6) | 1.3 (0.5) |
| Duration of lorazepam use, mos | 82.1 (67.6) | 79 (70.9) | 98 (48.8) |
| Total daily dose lorazepam, mg | 1.4 (1.3) | 1.2 (1.1) | 2.5 (1.9) |
| Hamilton Rating Scale for Anxiety (HAM-A) | 9.8 (5.6) | 9.3 (5.6) | 12.5 (5.5) |
| Hamilton Rating Scale for Depression (HAM-D) | 9.5 (8.4) | 9.0 (8.7) | 11.7 (6.9) |
| Mini-Mental Status Examination Score | 29.2 (0.8) | 29.4 (0.8) | 28.3 (0.5) |
| WAIS-R Total Vocabulary Score | 13.1 (2.3) | 13.2 (2.0) | 12.5 (3.6) |
| WMS-R Total Verbal Score | 99.3 (15.4) | 99.5 (16.0) | 98.2 (12.7) |
| WMS-R Total Visual Score | 105.4(20.1) | 104.1 (20.4) | 111.8 (18.5) |
| WMS-R General Memory Index Score | 102.7 (14.0) | 102.4 (13.7) | 104.3 (16.9) |
| BSRT Screening Total Recall | 62.2 (13.8) | 62.7 (13.6) | 59.6 (16.0) |
Procedure
All participants provided written informed consent prior to participation. A double-blind, placebo-controlled, crossover study design on two different days was used. Following diagnostic and screening evaluation, individuals participated in two five-hour experimental sessions on separate days, one-week apart. Lorazepam and placebo doses were prepared by the institutional pharmacy and dispensed at the experimental sessions by research staff. Subjects were randomly assigned to receive the sequence “lorazepam-placebo”, or “placebo-lorazepam”. Following a morning baseline assessment, each subject was either administered his/her highest daily unit dose of lorazepam as the challenge dose, or placebo. The highest daily unit doses ranged from 0.5 mg–3.0 mg lorazepam. Experimental sessions began at approximately 9:00 a.m. under non-fasting conditions. The Buschke Selective Reminding Test (BSRT; Buschke, 1973, 1974) and the Purdue Pegboard Test (PPT; Lezak, 1995; Tiffin, 1968) tests, and both the Mood Rating Scale (MRS; Bond and Lader, 1974) and the State-Trait Anxiety Inventory scale (STAI-S; Spielberger, 1983) were administered at baseline, and again at 1, 2.5 and 5 hours following oral administration of the drug or placebo. Vital signs and blood samples were also obtained at each assessment point.
Plasma lorazepam determination
Blood samples for determination of plasma lorazepam levels were collected at baseline, and at 1, 2.5 and 5 hours post-drug administration. Quantitation of plasma drug levels was determined by electron-capture gas chromatography, as previously described (Greenblatt et al., 1978).
Neuropsychological Measures
The BSRT consists of a list of 16 nouns presented verbally to the subject at a rate of one word every two seconds. The subject is asked to recall as many words as possible and to indicate when no more can be recalled. After the initial presentation, the subject is presented only with those words that were not recalled on the immediately preceding trial, although they are asked to recall the entire list on each trial. Seven presentation and recall trials of the same list are given in immediate succession. Total Recall is defined as the total number of words correctly recalled across the seven learning trials.
The PPT requires participants to place as many pegs as possible into a row of holes in a 30 sec period. Participants complete three pegboard trials that require 1) the sole use of a dominant hand, 2) the sole use of a non-dominant hand, and 3) both hands. The number of correctly inserted pegs are counted and recorded (score range = 0 – 25).
The MRS is composed of 16 visual analogue scales, each with a 100 mm horizontal line anchored by opposing mood-descriptive adjectives, e.g., “Happy – Sad”. Participants are instructed to make a perpendicular mark along the horizontal axis. Marks closer to the midpoint (50 mm) are representative of neutral states, whereas marks closer to the anchor adjectives (0 mm and 100 mm) represent increasing degrees of the indicated subjective mood state. The 16 visual analogue scales of the MRS have been shown to be sensitive to the effects of acute psychopharmacological challenge (Bond and Lader, 1974).
The STAI-S is a 20-item self-rating scale assessing affective and cognitive domains associated with anxiety as experienced in the present moment. Participants provide ratings on a 4-point scale ranging from 1 (“not at all”) to 4 (“very much so”) on items pertaining to subjective feelings of anxiety at the present time. Scores on the STAI-S range from 20 – 80, and the measure has been shown to be reliable (Spielberger, 1983).
Scores on the Hamilton Depression (HAM-D) and Anxiety (HAM-A) scales were also obtained at baseline.
Statistical Analysis
The comparisons between lorazepam and placebo over the 5 hours following their administration, with respect to all outcomes of interest, were based on mixed effects models analyses (Diggle et al., 2013). The outcomes included lorazepam plasma levels, total recall on memory testing, motor performance, mood, and anxiety ratings. Time was considered a factor with 4 levels (baseline, and 1, 2.5 and 5 hours post-treatment administration). To account for the correlation between the repeated observations on a subject over the two conditions, random subject effects were included in the models. The outcomes over time were modeled as a function of time, treatment (i.e., drug vs placebo), and their interaction. A significant treatment-by-time interaction would indicate that the difference in the mean outcome under highest daily unit dose (challenge dose) of lorazepam vs. placebo depends on the time elapsed since treatment administration; such significant effects are followed by pair-wise comparisons between the means for the two conditions at each time point. If the interaction between treatment and time was not significant, the model was refit with only main effects for time and treatment, and the effects of treatment and of time were judged based on this model. Cohen's d values are reported as indices of effect size for statistically significant F-tests and t-tests; these indices are computed as the ratio of the model based estimate (i.e., the maximum likelihood estimates from the mixed effects models) of the difference in the means, divided by the standard deviation of the respective measure at baseline.
To establish whether the challenge dose (ranging from 0.5 to 3.0 mg across study subjects) was associated with the magnitude of memory and psychomotor impairments following acute lorazepam administration, we modeled the outcome at 2.5 hours (i.e., time of peak effect) as a function of treatment, challenge dose and their interaction. We also explored whether the association between challenge dose and impairment depended on (i) duration of treatment, (ii) dosing frequency and (iii) total daily dose. This was done by modeling the outcome at time 2.5 hours as a function of treatment, challenge dose, each of the factor (i), (ii) and (iii) and their 2- and 3-way interactions. A backward step-wise elimination procedure was employed to arrive at a final model, preserving the hierarchical principle. A significant 3-way (treatment)-by-(covariate)-by-(challenge) dose interaction would indicate that the effect of lorazepam at 2.5 hours after administration depends on the covariate and this dependence is different for different challenge doses; similarly, a significant 2-way (treatment)-by-(covariate) interaction would indicate that the effect of lorazepam depends on the covariate, and this dependence is the same across the levels of the challenge dose. When there was an association between one of the factors characterizing subjects’ lorazepam use and the drug's effect on performance, we explored whether the association depended on age or gender by fitting models that included interactions with these demographic characteristics. An analogous strategy was used to evaluate whether (iv) baseline anxiety (measured by the Hamilton Anxiety scale) or (v) baseline depression (measured by Hamilton Depression scale) were related to how lorazepam affected memory and psychomotor performance.
Associations between study measures (all of them continuous) are estimated with Pearson's correlation coefficients. Statistical significance was set at α = 0.05, two-tailed. Following significant omnibus tests for overall differences, p-values for the post-hoc tests are reported without adjustment for multiple comparisons. For all exploratory analyses involving individual items on the self-report inventories that assessed mood states and anxiety levels and for the Generalized Anxiety Disorder (GAD) and Major Depressive Disorder (MDD) subgroup analyses, we report significant effects, and indicate which effects remain statistically significant after control of False Discovery Rate (FDR). All analyses were performed using SAS® software. The sample size of this cross-over study allows 80% power of a two-sided test with α=0.05 to detect lorazepam effects of magnitude that depend on the correlations between the measurements under lorazepam and placebo on the same subject. For example, if the correlation between the outcomes on a subject at a given time point under lorazepam and under placebo is ρ=0.5, the detectable effect (with 80% power of a 2-sided test with α=0.05) is of size Cohen's d=0.47; if that correlation is ρ=0.8 the detectable effect is Cohen's d=0.2. Thus the sample size allows the detection of medium to small effects.
Results
Of the 37 subjects meeting inclusion criteria, 31 completed all measures at all assessment points during both placebo and lorazepam sessions. Five of the six non-completing subjects were too physically and/or mentally fatigued to continue through to the fifth hour assessment, and the sixth withdrew after the 1st week. One subject was unable to complete tests of psychomotor performance due to neuropathy in the hands and wrists and was excluded from analyses of the PPT. Subject demographic and other characteristics assessed at screening are presented in Table 1 for the full sample, and for both the study completers (n = 31), and non-completers (n = 6). All analyses included data from all 37 subjects in the study, when applicable. The subjects fell into the following SCID DSM-IV diagnoses: GAD, n=11; MDD, n=8; GAD and MDD, n=4; panic disorder, n=7; bipolar disorder, n=2; insomnia, n=4; adjustment disorder with anxiety, n=1. Finally, the 37 subjects had the following prescribed maximum unit lorazepam doses (which was their challenge dose): 0.25 mg, n=1; 0.50 mg, n=10; 1.00 mg, n=21; 2.00 mg, n=4; 3.00 mg, n=1.
Plasma Lorazepam Levels
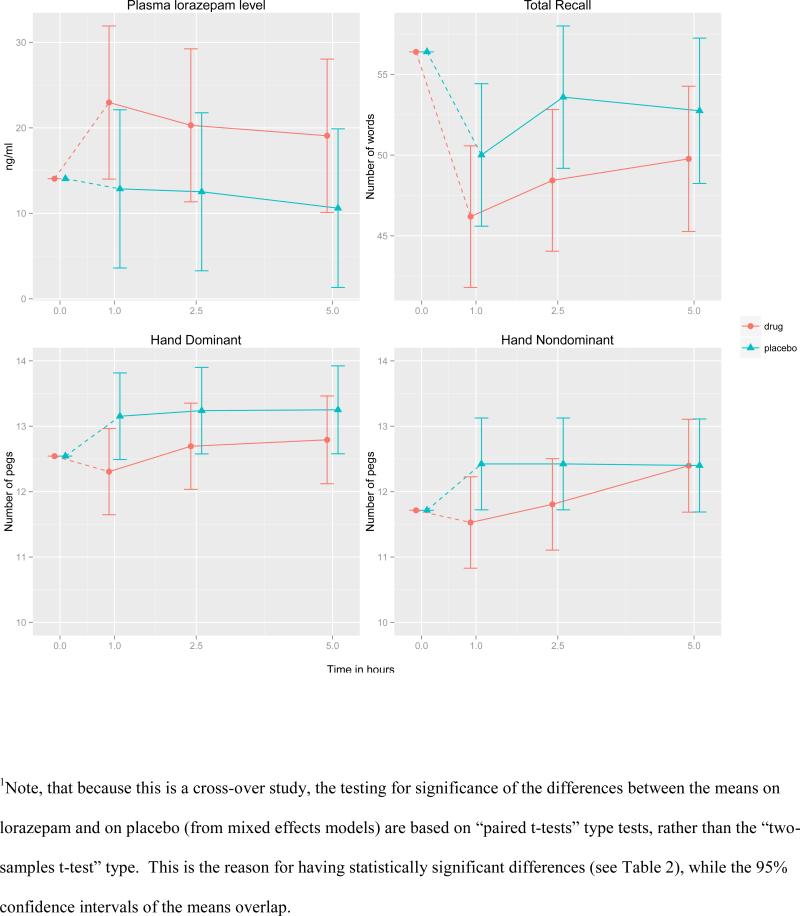
Lorazepam levels could not be determined in six participants due to insufficient plasma. Results of the mixed model with repeated measures on available plasma lorazepam levels revealed a significant interaction effect of time and drug [F (3, 80) = 10.25, p < 0.001]. While subjects had detectable plasma lorazepam levels throughout the placebo condition as a consequence of long-term administration, levels were higher in the acute lorazepam challenge condition (Figure 1). Follow-up tests revealed significant increases in plasma lorazepam levels from baseline to 1 hour [7.9 ng/mL increase, Wald's test z = 7.3, p <0.001] and then significant decreases from 1 hour to 2.5 hours [2.7 ng/mL decrease, z = −2.5, p = 0.015] under drug administration. There was no significant change in plasma lorazepam levels from baseline under placebo condition at any time.
Figure 1.
Model-based1 estimates of the means and 95% confidence intervals over time under placebo and lorazepam conditions for lorazepam plasma concentration levels (top left panel), total recall (top right), number of pegs inserted using dominant hand (bottom left) and number of pegs using non-dominant hand (bottom, right); cross-over design n=37.
Memory performance
Total recall scores are reported in Table 2. The effect of lorazepam on Total Recall depended on time post drug administration. Compared to placebo, significant decline in total recall was evident at 1 and 2.5 hours. At 1 hour, the mean difference for lorazepam minus placebo was -3.8 items [standard error (SE)=1.8, 95% confidence interval (CI) (−7.3, −0.3), Cohen's d=0.27], and at 2.5 hours, the mean difference was −5.2 items [SE=1.8, 95% CI (−8.7, −1.7), Cohen's d=0.36] (Figure 1).
Table 2.
Performance on memory and pegboard tests for lorazepam and placebo conditions at each study time point (PPT = Purdue Pegboard Test).
| Outcome measure | Hours1 post treatment | Raw data summaries: | Model-based inferences for Lorazepam – Placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lorazepam | Placebo | Difference (st. err) | 95% CI | p-value | ES2 | ||||
| n | Mean (sd) | n | Mean (sd) | ||||||
| BSRT Learning Total | Base | 37 | 56.4 (14.8) | 36 | 56.4 (14.8) | ||||
| 1 | 37 | 47.0 (13.2) | 36 | 50.1 (15.1) | −3.8 (1.8) | (−7.3, −0.3) | 0.032 | 0.27 | |
| 2.5 | 37 | 48.9 (13.1) | 36 | 53.9 (12.9) | −5.2 (1.8) | (−8.7, −1.7) | 0.004 | 0.36 | |
| 5 | 32 | 52.7 (11.8) | 32 | 54.4 (11.1) | −3.0 (1.9) | (−6.7, 0.7) | 0.115 | 0.21 | |
| PPT Dominant Hand | Base | 36 | 12.5 (2.0) | 34 | 12.5 (2.0) | ||||
| 1 | 36 | 12.4 (2.0) | 35 | 13.1 (1.9) | −0.9 (0.2) | (−1.3,−0.4) | 0.001 | 0.44 | |
| 2.5 | 36 | 12.7 (2.0) | 35 | 13.2 (2.1) | −0.5 (0.2) | (−1.0,−0.1) | 0.024 | 0.28 | |
| 5 | 31 | 13.0 (2.1) | 31 | 13.5 (2.0) | −0.5 (0.3) | (−1.0,0.0) | 0.071 | 0.24 | |
| PPT Non-Dominant Hand | Base | 36 | 11.7 (2.2) | 34 | 11.7 (2.2) | ||||
| 1 | 36 | 11.5 (2.1) | 35 | 12.4 (2.0) | −0.9 (0.2) | (−1.4,−0.4) | 0.001 | 0.41 | |
| 2.5 | 36 | 11.8 (2.2) | 35 | 12.3 (1.9) | −0.6 (0.2) | (−1.1,−0.1) | 0.012 | 0.29 | |
| 5 | 31 | 12.7 (2.1) | 31 | 12.6 (2.1) | −0.0 (0.3) | (−0.5,0.5) | 0.990 | 0.001 | |
| PPT Both Hands | Base | 36 | 9.6 (1.8) | 34 | 9.6 (1.8) | ||||
| 1 | 36 | 9.7 (1.8) | 35 | 10.1 (1.9) | −0.4 (0.2) | (−0.7,−0.1) | 0.013 | 0.20 | |
| 2.5 | 36 | 9.8 (1.8) | 35 | 10.1 (2.0) | −0.4 (0.2) | (−0.7,−0.1) | 0.013 | 0.20 | |
| 5 | 31 | 9.9 (2.0) | 31 | 10.4 (1.7) | −0.4 (0.2) | (−0.7,−0.1) | 0.013 | 0.20 | |
Base refers to pre-treatment baseline, 1, 2.5, and 5 refer to hours post-treatment.
Cohen's d effects size: the difference of the means at each time point is divided by the standard deviation of the measure at baseline
Psychomotor performance
Table 2 shows the three psychomotor performance measures based on PPT. The effect of lorazepam on dominant and non-dominant hand trials was a function of the time since lorazepam administration (F-tests for the interaction terms had p-values 0.008 and 0.035 respectively). For the dominant hand the lorazepam-related slowing was significant at 1 and 2.5 hours post-drug administration, but the effect sizes differed with the largest effect being present at 1 hour (Cohen's d=0.44), and a smaller effect at 2.5 hours (d=0.28). Similarly, the results for the non-dominant hand showed significant slowing on lorazepam at 1 and 2.5 hours (d=0.41 and d=0.29, respectively), while at 5 hours the effect had disappeared (Figure 1). The effect of lorazepam on the PPT performance using both hands was significant (p= 0.013, d=0.20) with no relationship to time since drug administration. Total recall was not correlated with pegboard performance at any time point.
Subjective feelings and differential effects
For the whole group, there was no effect of treatment at any time point on any of the MRS items. There was no effect of treatment on any of the 20 STAI-S items, except item #11 “Self-confidence”, for which there was an interaction between treatment and time (p<0.001), indicating significant higher levels of self-confidence on lorazepam than on placebo at 2.5 hours [Wald's test z = -2.8, p = 0.007, d = 0.64], and at 5 hours [z = -2.9, p = 0.005, d = 0.68]. The self-confidence ratings on lorazepam at this time point were not correlated with total recall or with dominant or non-dominant hand performance on the pegboard.
Secondary analyses of subjects with GAD
A subgroup analysis was performed on the subjects with a diagnosis of GAD (n=11). With respect to memory and psychomotor performance, this subgroup's response to lorazepam was similar to that found in the main analysis. However, with respect to mood, the self-ratings on the “Calm-Excited” question of the Mood Rating Scale (item #2) differed between drug administration and placebo, indicating a higher calmness rating while on lorazepam [at all time points, difference (lorazepam - placebo) mean = -5.8, SE=2.5, t (10) =-2.3, p = 0.044, d = 1.46]. In addition, ratings on the “Troubled-Tranquil” question (item #8) were significantly higher on lorazepam than placebo, indicating a higher tranquility rating on lorazepam [at all time points, difference mean = 7.6, SE= 3.3, t (10) = 2.3, p = 0.044, d = 1.46]. The STAI-S ratings on the “I feel at ease” question (item #5) on lorazepam were marginally higher at 2.5 hours (p=0.066), and significantly lower at 5 hours [t (10) = 2.8, p = 0.009, d = 1.78] compared to placebo, indicating a subtle decline in this feeling in conjunction with lowering plasma drug levels. The ratings on the STAI-S question “I feel pleasant” (item #20) on lorazepam were lower at 1 hour, but higher at 2.5 and 5 hours compared to placebo; the change in this difference (lorazepam – placebo) from 1 to 2.5 hours was statistically significant, although the differences between lorazepam and placebo were not statistically significant at any time point. None of the significant findings for this subgroup analyses survived the control of FDR. As with the analysis of the entire group, neither total recall nor pegboard performance were correlated with subjective reports of mood or affective states by Pearson's correlation.
Secondary analysis of the effects of total daily dose, dosing frequency, strength of challenge dose, and duration of treatment
The effect of lorazepam on Total Recall at 2.5 hours was not related to the total daily dose, dosing frequency, strength of challenge dose, or duration of treatment. The effect of lorazepam on psychomotor performance measured by PPT dominant hand, non-dominant hand, and both hands did not depend on the dosing frequency. Lorazepam-related slowing on both dominant and non-dominant hands was associated with the challenge dose: higher challenge doses were associated with greater slowing on lorazepam (2-way interaction (treatment)-by-(challenge dose) p = 0.002 and 0.004, for dominant and non-dominant hand respectively). Total daily dose (which was highly correlated with the strength of the challenge dose, Pearson's correlation r=.7, p<0.001), did not have an effect on the lorazepam-related psychomotor slowing except on the dominant hand; the dependence was such that higher total daily doses were associated with less impairment due to lorazepam, controlling for the challenge dose (p=0.023). Controlling FDR, the only statistically significant results are those relating higher challenge doses to higher psychomotor impairment on dominant and non-dominant hands. Age and gender did not modulate the effects of these factors.
Secondary analyses of the effect of baseline anxiety and depression
The lorazepam effect on memory and motor performance did not depend on the level of anxiety at entry into the study, as measured by Ham-A. Depression at the entry into the study (measured by Ham-D) was only related to the effect of lorazepam on the non-dominant hand performance, with more depressed subjects having more slowing on lorazepam (HamD)-by-(treatment) interaction: F(1, 33)=7.99, p=0.008). However, this finding did not survive control for FDR.
General Discussion
Despite long-term use of lorazepam, subjects continue to experience significant negative cognitive effects in the hours following oral administration of their prescribed unit dose. In several instances, these adverse effects lasted up to five hours post administration and were independent of age or gender.
Our results of a significant impairment in total verbal recall in our sample of older individuals after acute challenge of their prescribed unit dose are consistent with previous studies in younger long-term benzodiazepine users, in which acute BZP challenge resulted in adverse memory effects. In addition, as similar protocols were used, it is possible to compare our findings with one of our previous studies on the effects of lorazepam on memory in previously untreated elderly subjects (Pomara et al., 2005). In the untreated group, declines in Total Recall under 1.0 mg of lorazepam compared to placebo at 1, 2.5, and 5 hours were 9.7%, 16.0%, and 9.1%, respectively, all of which were significant. In contrast, in this study, lorazepam administration induced smaller declines in Total Recall, which were 6.3%, 9.2%, and 3.2% for the same time periods, with a significant effect only at the 2.5 hour time point despite much higher plasma drug levels – these results are consistent with the development of tolerance. Though these sets of data were not from the same study, the comparison is made only to indicate the potential differences between lorazepam responses in long-term vs. drug naïve elderly subjects — while the negative effects of lorazepam on Total Recall are stronger for untreated participants, we still found negative effects on memory in the long-term treatment group. Because aging may be associated with some decline in memory, a further drug- induced impairment, as we have demonstrated, is more likely to result in clinically significant effects in this population.
Furthermore, while tolerance has been most convincingly demonstrated for sedation and the adverse effects of BZPs on psychomotor performance, these observations have generally been derived from studies in younger populations on long-term treatment with BZPs (Lucki et al., 1986). Our results of significant impairment in psychomotor performance in an older population following an acute lorazepam challenge suggests that a complete tolerance to this adverse effect may not develop in this population, possibly contributing to increased risk of falls and associated morbidity and mortality reported in older long-term BZP community users (Pariente et al., 2008; Puustinen et al., 2007; Rapoport et al., 2009).
Self-ratings of anxiety, as measured by the total score on STAI-S, were not significantly reduced by acute lorazepam doses, and it should be noted that all long-term users still exhibited significant residual symptoms of anxiety at baseline. This is in contrast to previous studies on younger populations, which have reported little to no tolerance developed towards anxiolytic activity in long-term BZP user populations (Rickels, 1978; Satzger et al., 1990), suggesting that anxiolytic efficacy may decline after long-term lorazepam treatment in older populations. Total recall and pegboard performance were neither correlated with each other nor with self-ratings of mood states and anxiety levels at any time point following lorazepam administration, implying that the declines in memory and psychomotor performance associated with acute lorazepam challenge in long-term users are not accompanied by changes in self-ratings of mood states, including increased sedation. Thus, while the therapeutic benefits of lorazepam may fade with long-term use, the adverse effects on memory and psychomotor performance appear to persist. However, in further exploratory analyses, we found that the subgroup of individuals with a GAD diagnosis did experience a significant therapeutic anxiolytic effect. Although these observations were the result of secondary analyses with relatively small sample sizes, they do raise the possibility of a mechanistic interpretation. The pharmacodynamic action of BZPs, including lorazepam, is in part related to benzodiazepine receptor densities, the concentration of presynaptically-released GABA, and an enhancement of post-synaptic ionotropic GABA-A receptor-mediated inhibitory currents (e.g., Jacob et al., 2012; Reynolds et al., 2012). Tolerance development after chronic benzodiazepine administration has been associated with reductions in benzodiazepine receptor densities, region- and GABA-A receptor subtype-specific reductions in both GABA-A receptor density and function (Fujita et al., 1999; Miller et al., 1988; Vinkers and Olivier, 2012), as well as changes in ionotropic glutamate receptors, other neurotransmitters and the neurosteroid system (Vinkers and Olivier, 2012). The aforementioned results of impairment in memory and psychomotor performance, along with the continued therapeutic effects seen in the GAD population after acute lorazepam challenges are not consistent with a significant down regulation of BZP receptor density and GABA-A receptor function in the specific brain circuits mediating these effects in this population of long-term lorazepam users (Sanacora et al., 2004). Therefore, future studies, including in vivo magnetic resonance spectroscopy neuro-imaging and other techniques, should be considered to elucidate the status of central benzodiazepine receptor density and GABA-A receptor function in long-term elderly users, and the degree to which possible receptor abnormalities associated with disorders such as GAD, MDD, panic disorder, and insomnia might influence anxiolytic BZP response and tolerance development. In addition, it would also be important to elucidate if psychosocial and situational factors implicated in long-term BZP use and addiction in younger populations (Konopka et al., 2013) contribute to their long-term use in older adults.
Finally, our results suggest that the decline in memory performance induced by an acute lorazepam challenge can reflect changes in central nervous system function that, while triggered by the drug, continue to worsen while plasma drug levels are already subsiding. The impact on psychomotor slowing, in which the magnitude of the effect was related to the time course of the plasma drug levels, is different than that on memory, which may reflect a form of short-term pharmacodynamic plasticity that has the potential to last for hours and be influenced by genetic factors (Pomara et al., 2005). Both the memory and psychomotor declines following lorazepam challenge contrast with the psychological effects in GAD, where self-ratings of calmness and tranquility were elevated across time points. The temporal characteristics of cognitive toxicity, which may be unlike other effects of BZPs, warrant further study, and the temporal differences in lorazepam's impact on memory, psychomotor function, and mood in long-term users not only has clinical relevance, but may lead to a better understanding of the differential effects of BZPs on different neurobiological systems.
Highlights.
In long-term older adult Lorazepam users, acute oral administration of their unit doses, produced poorer recall and slower psychomotor performance compared to placebo.
In contrast there were no significant effects on self-ratings of mood, sedation or anxiety
The impairments in memory and psychomotor performance in the absence of therapeutic effects, raise serious concerns about the long-term use of this medication in the elderly.
Acknowledgements
The authors wish to thank Drs. C. De la Pena and R. Hernando for their assistance in participant recruitment and medical evaluations, Dr. Kenneth Belzer for assistance with data analyses and an earlier version of this manuscript, and Ms. Chelsea Reichert, Ms. Leeann Tan, and Dr. Juan Young for assistance in creating the final version of this manuscript.
Funding
This work was supported by a grant awarded to NP by the National Institute of Mental Health (#R01MH059142).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors have no financial, personal or other actual or potential conflicts of interest to disclose.
References
- Bertz RJ, Kroboth PD, Kroboth FJ, Reynolds IJ, Salek F, Wright CE, Smith RB. Alprazolam in young and elderly men: sensitivity and tolerance to psychomotor, sedative and memory effects. J Pharmacol and Exp Therap. 1997;281:1317–1329. [PubMed] [Google Scholar]
- Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–18. [Google Scholar]
- Buschke H. Selective reminding for analysis of memory and learning. J Verb Learn Verb Behav. 1973;12:543–50. [Google Scholar]
- Buschke H. Retrieval in human learning. Trans N.Y. Acad. Sci. 1974;36:721–729. [Google Scholar]
- Curran HV. Tranquillising memories: a review of the effects of benzodiazepines on human memory. Biol Psychol. 1986 Oct;23(2):179–213. doi: 10.1016/0301-0511(86)90081-5. [DOI] [PubMed] [Google Scholar]
- Curran HV. Memory functions, alertness and mood of long-term benzodiazepine users: a preliminary investigation of the effects of a normal daily dose. J Psychopharmacol. 1992;6(1):69–75. doi: 10.1177/026988119200600113. [DOI] [PubMed] [Google Scholar]
- Diggle P, Heagerty P, Liang KY, Zeger S. Analysis of Longitudinal Data. Oxford University Press; 2013. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fujita M, Woods SW, Verhoeff NP, Abi-Dargham A, Baldwin RM, Zoghbi SS, Soares JC, Jatlow PA, Krystal JH, Rajeevan N, Charney DS, Seibyl JP, Innis RB. Changes of benzodiazepine receptors during chronic benzodiazepine administration in humans. Eur J Pharmacol. 1999 Mar 5;368(2-3):161–72. doi: 10.1016/s0014-2999(99)00013-8. [DOI] [PubMed] [Google Scholar]
- Ghoneim MM, Mewaldt SP, Berie JL, Hinrichs JV. Memory and performance effects of single and 3-week administration of diazepam. Psychopharmacology. 1981;73:147–153. doi: 10.1007/BF00429206. [DOI] [PubMed] [Google Scholar]
- Gorenstein C, Bernik MA, Pompeia S. Differential acute psychomotor and cognitive effects of diazepam long-term benzodiazepine users. Int Cl Psychopharmacology. 1994;9:145–153. doi: 10.1097/00004850-199409000-00002. [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ, Franke K, Shader RI. Analysis of lorazepam and its glucuronide metabolite by electron-capture gas--liquid chromatography. Use in pharmacokinetic studies of lorazepam. J Chromatogr. 1978 Sep 1;146(2):311–20. [PubMed] [Google Scholar]
- Hampton LM, Daubresse M, Chang HY, Alexander GC, Budnitz DS. Emergency Department Visits by Adults for Psychiatric Medication Adverse Events. JAMA Psychiatry. 2014 Jul 9; doi: 10.1001/jamapsychiatry.2014.436. doi: 10.1001/jamapsychiatry.2014.436. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson G, Gerard C, Minko N, Parsotam N. Variation in benzodiazepine and antipsychotic use in people aged 65 years and over in New Zealand. N Z Med J. 2014 Jun 20;127(1396):67–78. [PubMed] [Google Scholar]
- Jacob TC, Michels G, Silayeva L, Haydon J, Succol F, Moss SJ. Benzodiazepine treatment induces subtype-specific changes in GABA(A) receptor trafficking and decreases synaptic inhibition. Proceedings of the National Academy of Sciences. 2012;109:18595–00. doi: 10.1073/pnas.1204994109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima R, Miyamoto S, Tenjin T, Ojima K, Ogino S, Miyake N, Fujiwara K, Funamoto Y, Arai J, Tsukahara S, Ito Y, Tadokoro M, Anai K, Kaneda Y, Yamaguchi N. Effects of tapering of long-term benzodiazepines on cognitive function inpatients with schizophrenia receiving a second-generation antipsychotic. Prog Neuropsychopharmacol Biol Psychiatry. 2012 Mar 30;36(2):300–6. doi: 10.1016/j.pnpbp.2011.11.008. doi:10.1016/j.pnpbp.2011.11.008. Epub 2011 Nov 21. [DOI] [PubMed] [Google Scholar]
- Konopka A, Pełka-Wysiecka J, Grzywacz A, Samochowiec J. Psychosocial characteristics of benzodiazepine addicts compared to not addicted benzodiazepineusers. Prog Neuropsychopharmacol Biol Psychiatry. 2013 Jan 10;40:229–35. doi: 10.1016/j.pnpbp.2012.09.001. doi:10.1016/j.pnpbp.2012.09.001. Epub 2012 Sep 15. [DOI] [PubMed] [Google Scholar]
- Kruse WH. Problems and pitfalls in the use of benzodiazepines in the elderly. Drug Saf. 1990 Sep-Oct;5(5):328–44. doi: 10.2165/00002018-199005050-00003. Review. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. Third Edition. Oxford University Press; New York, NY: 1995. [Google Scholar]
- Lister RG, File SE. The nature of lorazepam-induced amnesia. Psychopharmacology. 1984;83:183–187. doi: 10.1007/BF00429732. [DOI] [PubMed] [Google Scholar]
- Lucki I, Rickels K, Geller AM. Chronic use of benzodiazapines and psychomotor and cognitive test performance. Psychopharmacology. 1986;88:426–433. doi: 10.1007/BF00178503. [DOI] [PubMed] [Google Scholar]
- Miller LG, Greenblatt DJ, Barnhill JG, Shader RI. Chronic benzodiazepine administration. I. Tolerance is associated with benzodiazepine receptor downregulation and decreased gamma-aminobutyric acidA receptor function. J Pharmacol Exp Ther. 1988 Jul;246(1):170–6. [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. A triazolam/amphetamine dose-effect interaction study: dissociation of effects on memory versus arousal. Psychopharmacology. 2007;192(3):425–40. doi: 10.1007/s00213-007-0726-y. [DOI] [PubMed] [Google Scholar]
- Nikaido AM, Ellinwood EH, Heatherly DG, Gupta SK. Age-related increase in CNS sensitivity to benzodiazepines as assessed by task difficulty. Psychopharmacology. 1990;100:90–97. doi: 10.1007/BF02245796. [DOI] [PubMed] [Google Scholar]
- Pariente A, Dartigues JF, Benichou J, Letenneur L, Moore N, Fourrier-Réglat A. Benzodiazepines and injurious falls in community dwelling elders. Drugs & Aging. 2008;25(1):61–70. doi: 10.2165/00002512-200825010-00007. [DOI] [PubMed] [Google Scholar]
- Pomara N, Deptula D, Rubinstein S, Stanley B, Stanley M. The effects of diazepam and aging on intrusions. Psychopharmacol Bull. 1988;24(2):228–31. [PubMed] [Google Scholar]
- Pomara N, Deptula D, Medel M, Block RI, Greenblatt DJ. Effects of diazepam on recall memory: relationship to aging, dose, and duration of treatment. Psychopharmacol Bull. 1989;25(1):144–8. [PubMed] [Google Scholar]
- Pomara N, Tun H, DaSilva D, Hernando R, Deptula D, Greenblatt DJ. The acute and chronic performance effects of Alprazolam and Lorazepam in the elderly: relationship to duration of treatment and self-rated sedation. Psychopharmacol Bull. 1998;134:139–153. [PubMed] [Google Scholar]
- Pomara N, Willoughby LM, Wesnes K, Greenblatt DJ, Sidtis JJ. Apolipoprotein E epsilon 4 allele and lorazepam effects on memory in high functioning older adults. Arch Gen Psychiatry. 2005;62:209–216. doi: 10.1001/archpsyc.62.2.209. [DOI] [PubMed] [Google Scholar]
- Puustinen J, Nurminen J, Kukola M, Vahlberg T, Laine K, Kivelä SL. Associations between use of benzodiazepines or related drugs and health, physical abilities and cognitive function: a non-randomised clinical study in the elderly. Drugs Aging. 2007;24(12):1045–59. doi: 10.2165/00002512-200724120-00007. [DOI] [PubMed] [Google Scholar]
- Rapoport MJ, Lanctôt KL, Streiner DL, Bédard M, Vingilis E, Murray B, Schaffer A, Shulman KI, Herrmann N. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009 Apr 21;70(5):663–73. doi: 10.4088/JCP.08m04325. doi: 10.4088/JCP.08m04325. [DOI] [PubMed] [Google Scholar]
- Reynolds LM, Engin E, Tantillo G, Lau HM, Muschamp JW, Carlezon WA, Rudolph U. Differential Roles of GABA(A) Receptor Subtypes in Benzodiazepine-Induced Enhancement of Brain-Stimulation Reward. Neuropsychopharmacology. 2012:1–10. doi: 10.1038/npp.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickels K. Use of anti anxiety agents in anxious outpatients. Psychopharmacology. 1978;58:1–17. doi: 10.1007/BF00426784. [DOI] [PubMed] [Google Scholar]
- Rickels K, Lucki I, Schweizer E, García-España F, Case WG. Psychomotor performance of long-term benzodiazepine users before, during, and after benzodiazepine discontinuation. J Clin Psychopharmacol. 1999 Apr;19(2):107–13. doi: 10.1097/00004714-199904000-00003. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of {gamma}-aminobutyric acid and glutamate in patients with major depression. Archives of General Psychiatry. 2004;61(7):705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Satzger W, Engel RR, Ferguson E, Kapfhammer H, Eich FX, Hippius H. Effects of single doses of alpidem, lorazepam, and placebo, on memory and attention in healthy young and elderly volunteers. Pharmacopsychiatry. 1990;23:114–119. doi: 10.1055/s-2007-1014546. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State Trait Anxiety Inventory, Form Y. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Tamblyn R, Abrahamowicz M, du Berger R, McLeod P, Bartlett G. A 5-year prospective assessment of the risk associated with individual benzodiazepines and doses in new elderly users. J Am Geriatr Soc. 2005;53(2):233–41. doi: 10.1111/j.1532-5415.2005.53108.x. [DOI] [PubMed] [Google Scholar]
- Tiffin J. Purdue Pegboard: Examiner Manual. Science Research Associates; Chicago, Il: 1968. [Google Scholar]
- van Stevenick AL, Wallnöfer AE, Schoemaker RC, Pieters MSM, Danhof M, van Gerven JMA, Cohen AF. A study of the effects of long-term use on individual sensitivity to temazepam and lorazepam in clinical population. Br J Clin Pharmacol. 1997;44:267–275. doi: 10.1046/j.1365-2125.1997.t01-1-00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. The Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale--Revised. The Psychological Corporation; San Antonio, TX: p. 1987. [Google Scholar]
- Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44(2):151–347. [PubMed] [Google Scholar]
- Vinkers CH, Olivier B. Mechanisms Underlying Tolerance after Long-Term Benzodiazepine Use: A Future for Subtype-Selective GABA(A) Receptor Modulators?. Adv Pharmacol Sci. 2012;2012:416864. doi: 10.1155/2012/416864. [DOI] [PMC free article] [PubMed] [Google Scholar]