Abstract
Vocalizations serve as a conspecific social communication system among mammals. Modulation of acoustic features embedded within vocalizations is used by several mammalian species to signal whether it is safe or dangerous to approach conspecific and heterospecific mammals. As described by the Polyvagal Theory, the phylogenetic shift in the evolution of mammals involved an adaptive neuroanatomical link between the neural circuits regulating heart rate and the muscles involved in modulating the acoustic features of vocalizations. However, few studies have investigated the covariation between heart rate and the acoustic features of vocalizations. In the current study, we document that specific features of vocalizations covary with heart rate in a highly social and vocal mammal, the prairie vole (Microtus ochrogaster). Findings with the prairie vole illustrate that higher pitch (i.e., fundamental frequency) and less variability in acoustic features of vocalizations (i.e., less vocal prosody) are associated with elevated heart rate. The study provides the first documentation that the acoustic features of prairie vole vocalizations may function as a surrogate index of heart rate.
Keywords: heart rate, heart rate variability, Polyvagal Theory, prairie vole, prosody, respiratory sinus arrhythmia (RSA), ultrasonic vocalization (USV), vagus
1. Introduction
The Polyvagal Theory applies evolution as an organizing principle to interpret the adaptive significance of autonomic responses in promoting either defensive or social behaviors in response to environmental challenges. As described in the Polyvagal Theory, the phylogenetic shift in neural regulation of the autonomic nervous system in mammals is uniquely associated with a myelinated vagus with inhibitory pathways to the sino-atrial node of the heart. This unique feature enables mammals to rapidly and efficiently regulate heart rate by either removing the inhibitory action of the vagus on the heart to promote movement including fight/flight behaviors or to actively slow heart rate to foster pro-social behaviors. Consistent with the phylogenetic shift in neural regulation of the heart, the brainstem areas regulating the myelinated vagus are neuroanatomically linked to the special visceral efferent pathways of several cranial nerves (i.e., V, VII, IX, X, XI) forming an interconnected social engagement system regulating and coordinating heart rate with behaviors such as facial expression, listening, and vocalization.
1.1. Role of mammalian vocalizations
Vocalizations underlie a conspecific social communication system among mammals that evolved to communicate and signal environmental risk. Based on the Polyvagal Theory [1], the modulation of acoustic features of vocalizations may signal visceral state to other conspecific or heterospecific mammals. However, the prosodic features of vocalizations (i.e., acoustic features associated with emotion, affect, and intention) in nonhuman mammalian species are understudied and poorly defined. In general, previous research on animal vocalizations has focused either on descriptive strategies of quantifying the number of vocalizations or describing unique “language-like” frequency patterns that are associated with specific intentions [2-4].
The Polyvagal Theory leads to an alternative plausible model [1] that emphasizes the adaptive function of modulations in the vocalization as an effective and efficient form of communicating visceral state. The theory emphasizes that vocalizations convey the visceral state of the signaler to conspecifics and to other mammals that share an ability to process vocalizations within the same frequency band. Historically, in the animal behavior literature, vocalizations have been studied either as behavioral processes specific to an identifiable stimulus (e.g., fear of a predator) or as a global index of neurobehavioral state (e.g., pain, hunger, isolation, etc). The Polyvagal Theory expands beyond these traditional interpretations of vocalizations to focus on the communication of visceral state embedded in the acoustic structure of vocalizations.
1.2. A common neurophysiology underlying vocal prosody and autonomic regulation
The phylogenetic convergence of the neural circuits regulating the heart and the striated muscles of the face and head form an integrated functional social engagement system with mechanisms for feeling, expressing, and receiving social cues [5-8]. This integrated system receives inputs and outputs from a ventral vagal circuit that includes two brainstem nuclei: the nucleus ambiguus (NA) and the nucleus tractus solitarius (NTS). The vagal efferent pathways originate in NA and regulate heart rate and modulate vocalizations via the laryngeal and pharyngeal muscles [9, 10]. Both the heart and the muscles involved in vocalizations (i.e., laryngeal and pharyngeal) send afferent information back to the brainstem via NTS. This common circuit provides the underlying neuroanatomical and neurophysiological basis to hypothesize that vagal output to the laryngeal and pharyngeal muscles reflected in the prosodic features of vocalization would be mirrored in the vagal influence to the heart controlling heart rate. For example, metabolic challenges to homeostatic processes, often associated with movement (e.g., fight and flight behaviors), typically result in an increase in heart rate (i.e., due to a suppression of vagal influences and complementary increases in sympathetic tone), would hypothetically result in vocalizations characterized by a higher pitch [1].
The mechanism relating vagal tone to pitch is due to the dependence of pitch and the height (i.e., the contraction or relaxation) of the laryngeal muscles [11, 12]. Physiologically, laryngeal regulation (via the vagus) of the glottis (i.e., the combination of the vocal cords and the space in between the folds), which opens to enhance air flow during inspiration, and partially closes to reduce air flow and increase subglottal pressure during expiration. During expiration the posterior cricoarytenoid (PCA) muscle (regulated by the recurrent laryngeal branch of the vagus) is phasically active, while activity of the cricothyroid (CT) muscle (regulated by superior laryngeal nerve) tends to increase. The thyroarytenoid (TA) muscle (regulated by superior laryngeal nerve) is also more active during expiration. The action of the TA is dictated by two separate divisions: the external division (TA-X) to adduct the vocal fold, and the vocalis division (TA-V) to modulate sound quality [13]. Consistent with this model, the vagal efferent influence on the heart’s pacemaker (i.e., sinoatrial node) is greatest during exhalation.
1.3. The prairie vole: A model for investigating vocalizations as a noncontact surrogate of autonomic state
Prairie voles (Microtus ochrogaster) have comparatively low heart rates for their body size and high levels of heart rate variability. These observations reflect the strong influence of myelinated vagal pathways on the heart of prairie voles. The robust vagal output inherent to the prairie vole produces large rhythmic beat-to-beat changes in heart rate in frequencies similar to spontaneous respiration (i.e., respiratory sinus arrhythmia, RSA) [14, 15]. The amplitude of RSA for prairie voles is in the range of humans and significantly higher than other laboratory rodents, such as mice and rats, which during basal state express high sympathetic tone and low vagal tone to the heart [14]. In addition, prairie voles are highly vocal, possessing a disproportionately large auditory cortex [16-18]. Thus, prairie voles, by expressing atypically strong vagal influences to the heart [14, 15, 19] and by being atypically vocal, offer significant translational potential for investigating a potential covariation between the regulation of vocalizations and heart rate.
Due to the transfer function of the middle ear structures, each mammalian species has a frequency band of perceptual advantage in which social communication occurs (see [1]). Species specific transfer functions serve as a portal to signal safety to down regulate physiological state to foster proximity and the necessary physical contact to promote nurturance and the establishment and maintenance of social bonds. Alternatively, when the acoustic features of the vocalizations are outside the frequency band of perceptual advantage, vocalizations signal danger. Thus, depending on the transfer function of the specific mammalian species, modulation of the acoustic features of vocalizations across a narrow bandwidth of +/− 5-10 kHz could be meaningful [1]. Prairie voles may parallel humans, in which variations in acoustic features of vocalizations across a very narrow frequency band (i.e., between 500 and 3500 Hz) convey important information regarding emotional state and health in which meaningful information is conveyed. The physics of the prairie vole’s middle ear structures suggests that a frequency band between 20 and 40 kHz [1] would be equivalent to the frequencies conveying prosody in the human.
Prosody-based analyses of vocalizations, investigating both the pitch and the variations in pitch with the frequency band used for social communication (i.e., 20 to 40 kHz in the vole), may provide a surrogate to invasive and costly surgical implantation of radiotelemetry devices [14, 19]. The ability for vocalizations to offer an accurate, non-invasive means for acquiring information relating to autonomic state would be highly beneficial. Thus, consistent with the shared neural regulation between the structures producing vocalizations and heart rate, this study is the first conducted to demonstrate the feasibility of using prairie vole vocalizations as a surrogate for direct invasive measures of heart rate. Vocal prosody in the vole was quantified using a novel, high-throughput tool described below.
2. Methods and materials
2.1. Animals
Subjects were reproductively-naïve, laboratory-bred, female prairie voles (3 months of age), F2-3 descendants of a wild stock originally caught near Champaign, Illinois. This stock was systematically outbred with field-caught animals. Prior studies in the Brain-Body Center (University of Illinois at Chicago), including both sexes, found that the behavioral, neuroendocrine, and autonomic effects of certain social challenges were most reliably elicited in females. Consistent with these prior observations the study was limited to females. Animals were maintained on a 14/10 h light/dark cycle in a temperature- and humidity-controlled environment, and allowed food (Purina rabbit chow) and water ad libitum. Breeding pairs were maintained in large polycarbonate cages (25 × 45 × 60 cm), with cotton nesting material. At 21 days of age offspring were removed and housed in female sibling pairs in smaller cages (12 × 18 × 28 cm) where they remained until the start of the study.
In this study the voles were naïve to the procedures and were not used in any previous protocols. Six voles were initially implanted with radiotelemetry devices to monitor vagal regulation of the heart via the quantification of RSA from the beat-to-beat heart rate pattern. To optimize the opportunity to observe a covariation between heart rate and vocalization features, only voles that exhibited a strong vagal regulation of basal heart rate were tested. The relative degree of vagal regulation of heart rate can be quantified by evaluating the covariation between heart rate and RSA during sequential short time period (e.g., 5 s). Of the six voles, three exhibited a high degree of vagal regulation of basal heart rate (i.e., heart period positively correlating with RSA) when assessed 1 month post-surgery. Since the hypothesis being tested was dependent on heart rate being predominantly regulated via vagal pathways, the preliminary test of the hypothesis linking vocalizations to heart rate was conducted on the 3 voles with strong covariations between heart rate and RSA.
2.2. Experimental apparatus
Two microphones (Ultramic250K, Dodotronic, Interdisciplinary Center for Bioacoustics, University of Pavia, Italy) sensitive to sound within the ultrasonic range were used in this study to record vole USVs. The microphones incorporated an integrated analogue-to-digital converter and anti-aliasing algorithm, and were set to sample at the maximum 250 kHz with a medium-gain amplifier. The microphone was placed 2 cm over the center of the testing cage. Audio recording was controlled with Adobe Audition (Adobe Systems, San Jose, CA).
While developing the protocol, it was noted that prairie voles produced very few USVs during a 30 min trial defined by being separated from their cage mate. In order to elicit a higher frequency of USVs, it was therefore necessary to have a familiar vole in proximity. Pilot experiments documented that the highest number of USVs was evoked when the cage mate was present in the same cage and the frequency of USVs was reduced when the animals were placed in separate side-by-side cages. USV frequency was reduced as the cages were moved further away from each other (e.g., approximately 5, 10, 15, 30, 60, 90, and 120 cm increments).
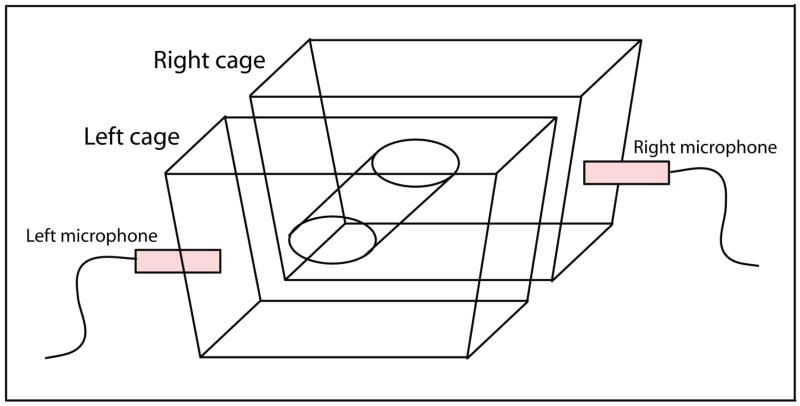
The ability to obtain a significant number of USVs for analysis had to be balanced with the need to localize the vocalizations from each vole (i.e., determine which vocalization corresponded to which animal, enabling for the specific comparison of the implanted vole’s autonomic and vocalization data). Based on pilot research, we confirmed that 15 cm of separation between testing cages evoked a considerable number of USVs and allowed a distinction to be made between the two microphones (as visualized in their corresponding spectrograms). Operating under the assumption that USVs evoked through proximity with a familiar conspecific may be, in part, due to olfactory cues, the two testing cages were integrated into a novel experimental apparatus which served to further localize the USVs while still permitting the transfer of olfactory cues (Fig. 1). Visual inspection of resulting spectrograms verified that the energy overlap between the left and right microphones (recording from each vole in the dyad) was significantly reduced using this configuration of cages.
Figure 1.
Experimental apparatus. While providing sufficient separation and sound buffering to effectively localize individual vole USVs, the apparatus also allowed the passage of olfactory cues between cages, presumably facilitating communicatory vocalizations between the two conspecifics.
2.3. Cage relocation paradigm
In addition to the novelty challenge of being placed in the testing cages, cage relocation was used as a mild stimulus to elicit USVs by inducing emotional stress. The cage relocation protocol was based on observations by Yee et al. [20] and our pilot research, whereby disrupted behavior in the vole occurs when the home cage was moved from a room housing other voles to a separate behavioral testing room in which no other voles were present. The act of relocation lasted for 15-20 s. The number of USVs evoked in response to cage relocation was similar to the number elicited through ethological challenges piloted in the laboratory to elicit vocalizations such as cage flooding. Immediately following relocation to the behavioral testing room, the animals were gently moved to the vocalization testing apparatus (i.e., side-by-side cages; Fig. 1). Synchronized heart rate and acoustic features were recorded for 30 min in each radiotelemetry implanted vole. The experimenter was absent from the room during recording.
2.4. Implantation of radiotelemetry transmitters
Telemetric transmitters were implanted intraperitoneally 2 weeks prior to the start of the study under aseptic conditions for long-term electrocardiogram (ECG) recordings using methods similar to those described elsewhere [14, 19, 21, 22]. Prairie voles were anesthetized with ketamine (67 mg/kg, sc) and xylazine (13.33 mg/kg, sc), placed under a warming lamp, and the surgical area was shaved and scrubbed with Betadine. A small rostral-to-caudal skin incision was made on the ventral surface of the animal, slightly lateral to midline. The skin was separated from underlying fascia, and another rostral-to-caudal incision was made through the underlying muscle. A wireless radiofrequency transmitter (Data Sciences International (DSI), St. Paul, MN; model TA10ETA-F20; 2.1 cm length, 3.9 g weight, 1.9 cm3 volume) was inserted into the abdominal cavity. The transmitter body was sutured to muscle and wound closed with nonabsorbable 5-0 silk (NLS Animal Health, Owings Mills, MD). The leads were directed rostrally using a trochar with a sleeve. The cathodal lead was brought through the sleeve, and anchored in place on the right side of the heart with a permanent suture. The anodal lead was brought through the sleeve to rest near the heart apex on the left side of the heart, and anchored in place with a permanent suture. All skin incisions were sutured closed using sterile 5-0 nylon suture (NLS Animal Health, Owings Mills, MD). Animals were administered subcutaneous fluids and standard analgesic agents (carprofen 5.0 mg/kg) as necessary, and were monitored carefully to avoid adverse effects. After recovery from anesthesia, the animals were housed individually for 7 days to permit adequate healing of sutures (housed in custom-designed divided cages during this time period, which permitted the siblings to interact with one another, while preventing the uninstrumented animals from disturbing the wounds of the instrumented animals). Animals were then returned to the standard-sized home cage (with the sibling) and allowed to recover for at least an additional 3 weeks before commencing experimentation for this study.
2.5. Radiotelemetry recordings and quantification of heart rate variables
ECG signals were recorded continuously with a radiotelemetry receiver (DSI, St. Paul, MN). The DSI system monitors several physiological signals, (i.e., ECG, locomotor activity, and body temperature) in conscious freely moving animals over an extended time period (several months) and provides the instrumentation necessary to study autonomic changes during critical periods (e.g., recovery from surgical procedures, social pairing and isolation, behavioral paradigms, drug administration, etc.).
Heart period (i.e., time between sequential R-waves of the ECG) was quantified from the continuous ECG recording using the software provided by DSI. A decrease in the duration of heart period is the equivalent to an increase in heart rate. Heart period instead of heart rate was used as the metric due to statistical features (i.e., distribution fits assumption for parametric analyses) and theoretical basis (i.e., increases in vagal influence to the heart are manifested in a direct lengthening of the heart period). The heart period time series output from the DSI software were visually inspected and edited for artifact with CardioEdit (Brain-Body Center, University of Illinois at Chicago). Respiratory sinus arrhythmia (RSA), a component of heart rate variability, was defined by the amplitude of the oscillations in the heart period time series in the frequencies of spontaneous breathing. RSA and heart period were quantified in sequential 5 s segments with CardioBatch (Brain-Body Center, University of Illinois at Chicago). RSA was quantified, consistent with the methods developed by Porges [23] and modified for the prairie vole [24]. The method applies time series procedures including polynomial and bandpass filters to extract the variance in the heart rate pattern in the frequency band defined by spontaneous respiration frequencies [25]. RSA was calculated and logarithmically transformed (ln) for sequential 5 s epochs in each minute of heart period data. The mean of the 5 s epochs within each minute was calculated and used in the data analyses. This method for quantifying RSA has unique advantages (see [26]), because it can estimate the amplitude of RSA during short time windows and provide an optimal procedure to study the dynamic shifts in vagal control of the heart during social and behavioral paradigms [26]. Reduced amplitude RSA is normally observed when heart rate increases.
2.6. Quantification of acoustic measures
Several variables were operationally defined to index features assumed to contribute to the prosodic features of mammalian vocalizations. Prosody, representing the modulation of vocalization frequencies within the frequency band (i.e., 20 – 40 kHz) used for social communication, was deconstructed into several quantifiable variables. First, the fundamental frequency of the vocalization was quantified. The fundamental is, when viewed in a spectrograph, the first energy peak. The fundamental frequency represents the perceptual attribute of vocal pitch. Second, the variance of the fundamental quantifies how much the fundamental pitch varies during the vocalization. Third, the 50% bandwidth reflects the frequency band in which the fundamental varies between 50% and 100% of its peak energy. When viewed as a spectral decomposition, the 50% bandwidth is the horizontal distance (i.e., band of frequencies) across the first energy peak (i.e., the fundamental) at 50% of the peak maximum (visually, the horizontal measurement of width is taken at half the peak’s maximum energy). Together, variance and 50% bandwidth are most closely related to pitch variability. Fourth, vocalization duration was calculated as the time in which the energy (amplitude) of the fundamental crossed a defined threshold demarcating vocalization energy from background noise.
Following the recording of the vocalizations, the 16 bit audio .wav files (mono; 250 kHz) were imported into Audacity 1.3 Beta (Audacity, Pittsburgh, PA) and visualized as a spectrogram to verify the presence of USVs as well as the proper drift correction and subsequent .wav file merging performed in Adobe Audition. Playback rate was also slowed by a factor of 10 (i.e., 25 kHz) to transform the ultrasound vocalizations into an audible range for the experimenter. The audio files were transferred to a MATLAB program (MathWorks, Natick, MA), which identified, isolated, and extracted the individual USVs through the use of a graphical user interface (GUI). The program applied the following processes to the audio file: 1) partitioned the file into 100 ms (25000 samples) segments of audio data, 2) passed data through a Butterworth 10th order bandpass filter for a maximally flat passband (e.g., 20-35 kHz for prairie voles) with a sharp cut-off frequency and minimum ringing in the time domain, and 3) bandpassed audio data were normalized, transformed into the frequency domain with an FFT, and the spectra were smoothed using a Hanning window (max side lobe level: −31 dB; side lobe roll-off rate: 60 dB/decade). An amplitude threshold was then applied to classify each segment as either a USV or not. Segments classified as USVs were automatically exported as individual .wav files to a user-specified folder location.
The .wav files were exported into a LabView-based user interface (National Instruments, Austin, TX) for quantification of the prosodic features. Following initiation of the automated analysis, a fast Fourier transform (FFT) was performed on each USV, followed by a peak detection process to isolate the fundamental (first energy peak) and harmonics (subsequent energy peaks) from the resulting FFT. The 50% bandwidth of the fundamental was calculated by isolating the fundamental’s FFT peak, measuring the maximum value of the peak’s amplitude, calculating half the vertical distance of this value, and quantifying the horizontal distance (or width) across the peak at this point. Additionally, the spectrogram for each USV was further converted into two-dimension array of coordinates representing the frequency and time domains to quantify the variance and duration of the fundamental frequency. An output of all quantified acoustic variables was generated in both the user-interface as well as a .csv file for further processing in Excel (Microsoft, Redmond, WA).
Following quantification with the LabView-based interface, the prosodic data for each minute of the 30 min trial was quantified for each of the three voles (i.e., 90 minutes of data). If no measurable USV was identified in a minute epoch, the epoch was dropped from further analyses. If more than one vocalization was emitted within a specific minute, acoustic features were pooled across the vocalizations and highest values (i.e., highest fundamental frequency, longest duration, widest bandwidth, and greatest variability of the fundamental) were used in the analyses. The natural log of the 50% bandwidth and variance of the fundamental was used to ensure that distributional features conformed to assumptions necessary for parametric analyses.
2.7. Statistical analysis
USVs from each of the three radiotelemetry implanted voles were analyzed minute-by-minute across a 30 min trial. Sixty-five quantifiable USVs were identified. These USVs occurred in 23 min of the 90 min of data collected. RSA and heart period were quantified and matched to the minutes containing USVs. Based on the per-min analyses, a total of 23 minutes of data were used in the statistical analyses (10 minutes for vole 1, 5 minutes for vole 2, and 8 minutes for vole 3). Data pooled across the three voles were used to evaluate the relationships among the vocalization features and between vocalization features measures of cardiac vagal tone (i.e., heart period, RSA).
3. Results
The descriptive statistics of the acoustic and autonomic variables are listed in Table 1. Correlations among the acoustic variables are listed in Table 2. As fundamental frequency increases there were significant increases in the duration of the vocalization, in the number of frequencies used to express 50% of the acoustic energy, and in the variability of the fundamental within the vocalization.
Table 1.
Mean and +/− range for acoustic variables (n=66) and autonomic measures (n=23). Data for 50% bandwidth, variance, and RSA were logarithmically transformed. Both untransformed and transformed (ln) values for 50% bandwidth are presented.
| All Vocalizations | |
|---|---|
| Fundamental Frequency (Hz) | 34067.46 +/− 27744.01 |
| 50% Bandwidth (Hz) | 4933.18 +/− 8819.48 |
| 50% Bandwidth (ln; Hz) | 8.41 +/− 1.54 |
| Variance (ln; Hz) | 13.19 +/− 7.61 |
| Duration (ms) | 1.20 +/− 4.21 |
| RSA (ms2) | 3.32 +/− 3.11 |
| Heart Period (ms) | 131.24 +/− 54.81 |
Table 2.
Correlations among maximum acoustic variables for vole vocalization measured following cage relocation challenge. Correlation coefficients (n=66) are shown below.
| Fundamental Frequency |
50% Bandwidth |
Variance | Duration | |
|---|---|---|---|---|
| Fundamental Frequency | 1 | .479* | .527* | .434* |
| 50% Bandwidth | .479* | 1 | .320 | −.431* |
| Variance | .527* | .320 | 1 | .277 |
| Duration | .434* | −.431* | .277 | 1 |
Pearson correlation, p < 0.05 (2 tailed)
Correlations between autonomic and acoustic variables are listed in Table 3. Heart period was significantly correlated with fundamental frequency and duration. Shorter heart periods (i.e., faster heart rate) were associated with vocalizations characterized by a higher fundamental (i.e., higher pitch) and shorter duration. There was a trend towards a greater variability of the fundamental within each vocalization being related to shorter heart periods. RSA was not related to any acoustic variable. Moreover, during the social stress challenge of being separated from the cage mate, heart period and RSA were not significantly correlated (r (23) = .281).
Table 3.
Correlations between maximum acoustic variables and heart period following cage relocation. Correlations are calculated on the 23 minutes of heart period during which vocalizations occurred. Correlation coefficients (n=23) are shown below.
| RSA | Heart Period | |
|---|---|---|
| Fundamental Frequency | .009 | − 571*** |
| 50% Bandwidth | −.223 | −.144 |
| Variance | .180 | −.347* |
| Duration | .116 | −.505** |
Pearson correlation, p < 0.10
Pearson correlation, p < 0.05
Pearson correlation, p < 0.01 (2-tailed).
Similar observations with human infants have associated short shrill vocalizations with faster heart rate (i.e., shorter heart periods). In a previous study (see [27]), we reported that the same variables collected from the vole vocalizations were correlated with heart period in the same direction with similar magnitudes when infant cries were elicited with a social challenge (i.e., the face-to-face still face procedure). Table 4 provides the comparison of correlations between acoustic variables and heart period for the voles and previously reported infants. In addition, the relationship among the acoustic variables in the infant and vole were similar (see Table 5).
Table 4.
Comparison of correlations between acoustic variables and heart period for vole vocalizations (n=23) and 6 month human infants (n=75; see [27]). Correlation coefficients are shown below.
| Acoustic variable | Infant | Vole |
|---|---|---|
| Fundamental Frequency | −.368** | − 571*** |
| 50% Bandwidth | −.334** | −.144 |
| Variance | −.610** | −.347* |
| Duration | −.402** | −.505** |
Pearson correlation, p < 0.10
Pearson correlation, p < 0.05
Pearson correlation, p < 0.01 (2-tailed).
Table 5.
Comparison of correlations between maximum fundamental frequency and other maximum acoustic features for vole vocalizations (n=23) and 6 month human infants (n=75). Correlation coefficients are shown below.
Pearson correlation, p < 0.05
Pearson correlation, p < 0.01 (2 tailed).
4. Discussion
This is the first study demonstrating the covariation of acoustic features of vocalizations and autonomic state in the prairie vole. The data support the hypothesized convergence between heart rate (heart period) and the acoustic features related to prosody in vole vocalizations. The data confirm the utility of monitoring acoustic features of vocalizations as an index of autonomic activity in voles. Moreover, the data collected in the vole are convergent with our previous report demonstrating the covariation of acoustic features and heart rate in the human infant and the covariation of fundamental frequency with the other acoustic variables [27]. The vole data, similar to infant data, illustrate a common mammalian adaptive biobehavioral response system to challenges in which the vagal pathways involved in regulating the acoustic features of vocalizations are synchronized with the vagal pathways involved in regulating hearts [28-30].
The covariation between heart rate and vocal prosody are based on the shared neuromodulation of the heart and laryngeal and pharyngeal muscles via different efferent pathways through the myelinated branch of the vagus that originates in the nucleus ambiguus. The findings are consistent with the Polyvagal Theory [28, 29, 31]. Specifically, increasing vagal efferent activity through pathways imbedded in the myelinated vagus supports a physiological state associated with calmness and shifts vocalizations towards longer durations characterized by lower and more variable fundamental frequencies that signal conspecifics that they are safe to approach. In parallel with the increased vagal efferent influence on the heart, there is increased neural tone to the laryngeal and pharyngeal muscles (via contraction of the TA muscle). However, although we had anticipated a relation between RSA and prosodic features, and RSA and heart rate, none were observed. Similarly, significant correlations between heart period and acoustic features, but not between RSA and acoustic features were observed in an infant study quantifying the same acoustic variables (see [27]). Thus, it is possible that during severely negative challenges, such as emotional distress in the infants or cage relocation in the vole, that there is both a vagal withdrawal and an excitation of sympathetic nervous system influences to the heart may have a role in heart rate and vocalization responses. Although the mechanisms mediating the covariation of the acoustic features of vocalization have been assumed to be mediated via the vagus, the sympathetic nervous system could contribute to the heart rate response when the vagal system is depressed.
Contraction of the TA muscle shifts production of acoustic energy to lower frequencies with increased frequency modulation within the band of acoustic frequencies used in social communication (see [1]). Prairie voles, due to the physics of the middle ear transfer function, have evolved structures to detect acoustic features that signal that the vole producing these vocalizations is in a physiological state that is safe to approach. In contrast, retraction of this circuit (decreased vagal output) shifts production of acoustic energy to higher frequencies (via contraction of the CT muscle, anatomically antagonistic to the TA) with decreased frequency modulation that signal that the vole producing these vocalizations is in a physiological state (i.e., low vagal tone and often high sympathetic tone) that could easily support aggressive behavior and misread “social” approach behaviors as intrusive.
Spectrographic analyses, performed as part of the detection and quantification programs (see below), identified patterns in the vole USV paralleled observations of other rodent USVs monitored in other laboratories [2, 32, 33]. However, detailed analyses of USVs are difficult and time consuming. The identification of USVs in the complex acoustic background of a laboratory is especially difficult. To identify and to describe vole USVs required the development of an automated computation-based system capable of isolating, extracting, and quantifying the prosodic features of USV vocalizations. The system developed has two software modules: a MATLAB module that identifies and extracts rodent USVs and a LabView module that quantifies the prosodic features of vocalizations including fundamental frequency, duration, 50% bandwidth, and variance of the fundamental.
While the present study demonstrates an overall covariance of acoustic features with heart rate, the specific acoustic features significantly correlated with heart rate could conceivably vary in future studies. Challenges may require communication, via variations in acoustic features, of specific signals often related to visceral states (e.g., hunger, illness, positive engagement, mate solicitation, pup retrieval, or predator intrusion). Since voles were relatively silent in their home cages, it was not possible to index a base level with which to compare the response to the disruptions. In future research, attempts will be made to design paradigms that reliably elicit positive social cuing during which the relation between the acoustic parameters and physiological parameters may differ from the findings in this study. It is possible that increased modulation of acoustic frequencies, cuing more positive social behaviors, may be related to increases in RSA, since they share common neural pathways.
Future research should evaluate the status of vagal regulation as a variable that might moderate the relation between acoustic features of vocalizations and heart rate parameters. Although the voles in this study were selected for inclusion based on having high vagal regulation of heart rate, voles with less vagal regulation of heart rate might have a weaker covariation between acoustic features of vocalizations and heart rate. This rule of inclusion excluded voles that did not express an optimal physiological state following surgery (see [14, 34]). Interestingly, the cage relocation challenge was sufficiently stressing to disrupt the vagal dominance in heart rate regulation, which was observed by low nonsignificant correlations between heart period and RSA.
The study has limitations. First, due to the small sample size, the inference from these data is limited. Thus, the findings need to be viewed in terms of a demonstration project evaluating the feasibility of extracting USVs in the vole, while synchronously monitoring heart rate. Second, the analyses are limited due to the confounding of sources of variance, since the dataset used for the correlations include data collected both within and between voles. Nevertheless, there is justification for such analyses given the adaptive function of communicating visceral state to both heterospecifics as well unfamiliar conspecifics, which may not be familiar with the vole that is vocalizing. Third, the paradigms in both the infant and vole studies focus on the covariation of heart rate and acoustic variables in response to signals of danger and uncertainty. Neither study provides an opportunity to observe the covariation of these variables during positive interactions. During more positive social engagement settings, when infants and voles use vocalizations to signal safety and not uncertainty or danger, a different profile of responses might be observed. It is possible that in this more positive and safe context, systematic increases in vagal activity may result in a covariation of increases in RSA and an array of acoustic features that may include lower fundamental and increases in the bandwidth and variance measures. However, given these vulnerabilities, the consistency between the findings in the vole and human infant are remarkable. Thus, the data seem to demonstrate a robust relation between acoustic features of vocalizations and heart rate in two disparate mammals living in very different environments.
5. Conclusions
Through the application of software modules designed to identify the occurrence of a USV, relatively long segments of acoustic recordings could be scanned. In this study 90 minutes of data were processed from three voles. From these 90 minutes of recordings, the program identified 65 USVs that were occurring in 23 minutes. Synchronized measures of heart period were linked to the times when the USVs were detected and enabled the evaluation of the covariation between acoustic features of USVs and heart rate. Similar to human infants [27], the prairie vole data demonstrate the utility of prosodic features as a sensitive index of heart rate. Consistent with the Polyvagal Theory, the study supports the hypothesis that vocalizations convey information related to visceral state. Specifically, due to the common neural pathways regulating the heart and acoustic features of vocalizations, shorter heart periods (i.e., faster heart rate) were associated with increases in fundamental frequency and shorter duration vocalizations – features of vocalizations that humans perceive as anxiety. These findings parallel clinical observations in humans in which higher pitch (i.e., fundamental frequency) and less variability in acoustic features of vocalizations (i.e., less vocal prosody) convey a physiological state (increases in heart rate) consistent with “mobilization” (i.e., fight or flight behaviors) or pain.
Highlights.
Muscles involved in vocal prosody and the heart share a neural pathway
An automated procedure detected prosodic features in prairie vole vocalizations
Features of vocal prosody were correlated with heart rate in prairie voles
Similar acoustic features covary with heart rate in vole and human infant
Acknowledgements
The project described was conducted as part of the doctoral dissertation of AMS. This study was supported, in part, by R01HD053570 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development awarded to SWP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Porges SW, Lewis GF. The polyvagal hypothesis: common mechanisms mediating autonomic regulation, vocalizations and listening. In: Brudzynski SM, editor. Handbook of Mammalian Vocalization: An Integrative Neuroscience Approach. Academic Press; London: 2009. pp. 255–264. [Google Scholar]
- 2.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46(1):28–34. [PubMed] [Google Scholar]
- 3.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychological Bulletin. 2002;128(6):961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 4.Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33(4):508–15. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Field T, Diego M. Vagal activity, early growth and emotional development. Infant Behav Dev. 2008;31(3):361–73. doi: 10.1016/j.infbeh.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porges SW. Social engagement and attachment: a phylogenetic perspective. Ann N Y Acad Sci. 2003;1008:31–47. doi: 10.1196/annals.1301.004. [DOI] [PubMed] [Google Scholar]
- 7.Porges SW. The Polyvagal Theory: phylogenetic contributions to social behavior. Physiol Behav. 2003;79(3):503–13. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 8.Larson SK, Porges SW. The ontogeny of heart period patterning in the rat. Dev Psychobiol. 1982;15(6):519–28. doi: 10.1002/dev.420150604. [DOI] [PubMed] [Google Scholar]
- 9.Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol. 1987;262(4):546–62. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- 10.Ayres JG, Gabbott PLA. Vocal cord dysfunction and laryngeal hyperresponsiveness: a function of altered autonomic balance? Thorax. 2002;57(4):284–285. doi: 10.1136/thorax.57.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirano M, Ohala J, Vennard W. The function of laryngeal muscles in regulating fundamental frequency and intensity of phonation. J Speech Hear Res. 1969;12(3):616–28. doi: 10.1044/jshr.1203.616. [DOI] [PubMed] [Google Scholar]
- 12.Ohala JJ. How is Pitch Lowered? J. Acoust. Soc. Am. 1972;52(1A):124. [Google Scholar]
- 13.Hoh JFY. Laryngeal muscles as highly specialized organs in airway protection, respiration and phonation. In: Brudzynski SM, editor. Handbook of Mammalian Vocalization: An Integrative Neuroscience Approach. Academic Press; London: 2009. pp. 13–22. [Google Scholar]
- 14.Grippo AJ, Lamb DG, Carter CS, Porges SW. Cardiac regulation in the socially monogamous prairie vole. Physiol Behav. 2007;90(2-3):386–93. doi: 10.1016/j.physbeh.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grippo AJ, Carter CS, McNeal N, Chandler DL, Larocca MA, Bates SL, et al. 24-hour autonomic dysfunction and depressive behaviors in an animal model of social isolation: implications for the study of depression and cardiovascular disease. Psychosom Med. 2011;73(1):59–66. doi: 10.1097/PSY.0b013e31820019e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blake BH. Ultrasonic calling in isolated infant prairie voles (Microtus ochrogaster) and montane voles (M. montanus) J Mammalogy. 2002;83(2):536–545. [Google Scholar]
- 17.Lepri JJ, Theodorides M, Wysocki CJ. Ultrasonic vocalizations by adult prairie voles, Microtus ochrogaster. Cellular and Molecular Life Sciences. 1988;44(3):271–273. doi: 10.1007/BF01941736. [DOI] [PubMed] [Google Scholar]
- 18.Campi KL, Karlen SJ, Bales KL, Krubitzer L. Organization of sensory neocortex in prairie voles (Microtus ochrogaster) J Comp Neurol. 2007;502(3):414–26. doi: 10.1002/cne.21314. [DOI] [PubMed] [Google Scholar]
- 19.Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol Psychiatry. 2007;62(10):1162–70. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yee JR, Cavigelli SA, Delgado B, McClintock MK. Reciprocal Affiliation Among Adolescent Rats During a Mild Group Stressor Predicts Mammary Tumors and Lifespan. Psych Med. 2008;70:1050–1059. doi: 10.1097/PSY.0b013e31818425fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. Phenotypic screening for heart rate variability in the mouse. American Journal of Physiology-Heart and Circulatory Physiology. 2000;279:H733–H740. doi: 10.1152/ajpheart.2000.279.2.H733. [DOI] [PubMed] [Google Scholar]
- 22.Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular status and neuroendocrine responses to stress in rats. Journal of Nutrition. 2003;133:1921–1929. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- 23.Porges SW. Method and Apparatus for Evaluating Rhythmic Oscillations in Aperiodic Physiological Response Systems. 1985 [Google Scholar]
- 24.Yongue BG, McCabe PM, Porges SW, Rivera M, Kelley SL, Ackles PK. The effects of pharmacological manipulations that influence vagal control of the heart on heart period, heart-period variability and respiration in rats. Psychophysiology. 1982;19(4):426–32. doi: 10.1111/j.1469-8986.1982.tb02499.x. [DOI] [PubMed] [Google Scholar]
- 25.Porges SW. Method and apparatus for evaluating rhythmic oscillations in aperiodic physiological response systems. USA: 1985. [Google Scholar]
- 26.Lewis GF, Furman SA, McCool MF, Porges SW. Statistical strategies to quantify respiratory sinus arrhythmia: Are commonly used metrics equivalent? Biol Psychol. 2012;89(2):349–64. doi: 10.1016/j.biopsycho.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart AM, Lewis GF, Heilman KJ, Davila MI, Coleman DD, Aylward SA, et al. The covariation of acoustic features of infant cries and autonomic state. Physiol Behav. 2013;120C:203–210. doi: 10.1016/j.physbeh.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med. 2009;76(Suppl 2):S86–90. doi: 10.3949/ccjm.76.s2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42(2):123–46. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 30.Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev. 1995;19(2):225–33. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- 31.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chabout J, Serreau P, Ey E, Bellier L, Aubin T, Bourgeron T, et al. Adult Male Mice Emit Context-Specific Ultrasonic Vocalizations That Are Modulated by Prior Isolation or Group Rearing Environment. PLoS One. 2012;7(1):e29401. doi: 10.1371/journal.pone.0029401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual Repertoire of Vocalizations in the BTBR T+tf/J Mouse Model of Autism. PLoS One. 2008;3(8):e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williamson JB, Lewis G, Grippo AJ, Lamb D, Harden E, Handleman M, et al. Autonomic predictors of recovery following surgery: a comparative study. Auton Neurosci. 2010;156(1-2):60–6. doi: 10.1016/j.autneu.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]