Abstract
Previous studies have demonstrated the existence of a subset of B lymphocytes, regulatory B-cells (Bregs), which modulate immune function. Here, in vivo and in vitro experiments were undertaken to elucidate the role of these Bregs in controlling neuroinflammation following viral brain infection. We used multi-color flow cytometry to phenotype lymphocyte subpopulations infiltrating the brain, along with in vitro co-cultures to assess their anti-inflammatory and immunoregulatory roles. This distinctive subset of CD19+CD1dhiCD5+ B-cells was found to infiltrate the brains of chronically infected animals, reaching highest levels at the latest time point tested (30 d p.i.). B-cell-deficient Jh−/− mice were found to develop exacerbated neuroimmune responses as measured by enhanced accumulation and/or retention of CD8+ T-cells within the brain, as well as increased levels of microglial activation (MHC class II). Conversely, levels of Foxp3+ regulatory T-cells (Tregs) were found to be significantly lower in Jh−/− mice when compared to wild-type (Wt) animals. Further experiments showed that in vitro generated IL-10-secreting regulatory B-cells (B10) were able to inhibit cytokine responses from microglia following stimulation with viral antigens. These in vitro generated B10 cells were also found to promote proliferation of regulatory T-cells in co-culture studies. Finally, gain of function experiments demonstrated that reconstitution of Wt B-cells into Jh−/− mice restored neuroimmune responses to levels exhibited by infected Wt mice. Taken together, these results demonstrate that regulatory B-cells modulate T lymphocyte as well as microglial cell responses within the infected brain and promote CD4+Foxp3+ T-cell proliferation in vitro.
Introduction
The central nervous system (CNS) is a target for acute viral infection, as well as a reservoir of latent and persisting viruses (1, 2). The prevailing paradigm has been that the inert immunological status of the CNS parenchyma is maintained through exclusion of key components of the immune system. However, it is now known that systemic T lymphocytes (3) normally transit into the CNS to participate in immune surveillance (4). Proinflammatory cytokines, chemokines, and other mediators produced by resident glial cells, both astrocytes and microglia, induce activation and recruitment of immune cells in response to viral infection (5–8). However, a balance is required between the neuroimmune responses that are necessary to deal with invading pathogens and those that prevent these responses from harming surrounding brain tissue. Long-term persistence of immune cells that infiltrate the CNS in several viral infections demonstrates that inflammation needs to be controlled to prevent immune-driven pathology, through either native or external anti-inflammatory mechanisms. Various regulatory cell types and anti-inflammatory mediators have been shown to play critical roles in counteracting these overzealous neuroinflammatory responses and preventing immune-mediated neuropathology (9–11).
A number of anti-inflammatory mechanisms have been demonstrated to control excess neuroinflammation including the infiltration of inflammation-dampening regulatory T-cells (Tregs) (12). Interleukin (IL)-10 is the prototypical anti-inflammatory cytokine that suppresses cellular immunity and inhibits synthesis and release of proinflammatory mediators from activated microglia, (13, 14). Tregs have been identified as a predominant source of IL-10, but studies have shown that this cytokine can also be produced by many other types of immune cells including B-cells (15–17).
B lymphocytes have been traditionally considered antigen-presenting and antibody-producing cells. However, a number of studies in recent years have demonstrated the existence of a regulatory subset of B-cells (Bregs) that exhibit immunosuppressive functions (reviewed in (18). An early study by Fillatreau et al. (19), using a chimeric model where B-cells were specifically deleted of IL-10 production, demonstrated that these cells inhibited immune-mediated pathology in murine experimental autoimmune encephalomyelitis (EAE). Further studies went on to reveal that Bregs play important roles in control of immunopathology during autoimmune diseases (16), cancer (20), and organ transplantation (21). Breg cells have also been shown to modulate immune responses and immunopathology in infectious diseases (22, 23). Different phenotypes of Bregs have been described. These cells have been detected within splenic marginal zone (MZ) populations (24). IL-10 producing B-cells are found in the spleens of naive mice at low frequencies (1–5%), where they represent a subset of the CD19+CD1dhiCD5+ B-cell subpopulation (15, 16, 25). These CD1dhiCD5+ B-cells were found to be more enriched for IL-10–producing cells (9%–15%) than other B-cell subsets. Similar IL-10-producing B-cell subsets have been identified in healthy and autoimmune individuals. The capacity of human and mouse Breg cells to express IL-10 is central to their negative regulation of inflammation, autoimmunity, and adaptive as well as innate immune responses (26–29). B-cell expression of cytoplasmic IL-10 protein parallels both their expression of IL-10 transcripts and secretion of IL-10, as measured by ELISA (26–29). In this manuscript we use the term Breg to identify cells expressing the CD19+CD1dhiCD5+ surface markers, while the term B10 cells is used to describe B-lineage cells known to produce IL-10.
Our previous studies have demonstrated that viral brain infection with murine cytomegalovirus (MCMV) triggers accumulation and long-term persistence of both T lymphocyte- and B lymphocyte-lineage cells within the brain (30, 31). While CD8+ T-cells were found to be required for viral clearance and control of brain infection (32), persisting B-lineage cells were found to produce MCMV-specific antibodies and played a significant role in controlling spread of reactivated virus (31). In the present study, we assessed the presence of CD19+CD1dhiCD5+ Breg cells within the infected brain and investigated their anti-inflammatory and immunoregulatory roles.
Materials and Methods
Ethical statement
This study was carried out in strict accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (Protocol Number: 1105A99534) of the University of Minnesota. All surgery was performed under Ketamine anesthesia, and all efforts were made to minimize suffering.
Virus and animals
RM461, a MCMV expressing Escherichia coli β-galactosidase under the control of the human ie1/ie2 promoter/enhancer (33) was kindly provided by Edward S. Mocarski. The virus was maintained by passage in weanling female BALB/c mice. Salivary gland-passed virus was then grown in NIH 3T3 cells for 2 passages, which minimized any carryover of salivary gland tissue. Infected 3T3 cultures were harvested at 80% to 100% cytopathic effect and subjected to three freeze–thaw cycles. Cellular debris was removed by centrifugation (1000 ×g) at 4 °C, and the virus was pelleted through a 35% sucrose cushion (in Tris-buffered saline [50 mM Tris–HCl, 150 mM NaCl, pH 7.4]) at 23,000 ×g for 2 h at 4 °C. The pellet was resuspended in Tris buffered saline containing heat-inactivated fetal bovine serum (FBS, Sigma, St. Louis, MO). Viral stock titers were determined on 3T3 cells as 50% tissue culture infective doses (TCID50) per milliliter. Six to eight weeks old BALB/c mice were obtained from Charles River Laboratories (Wilmington, MA), while B-cell deficient mice (Jh−/−) were a kind gift from Dr. Steven McSorley (University of California, Davis) (34, 35). In Jh−/− mice, T-lymphocyte development proceeds normally, based on surface phenotype and quantity of cells in the spleen; splenic lymphocytes are enriched for T-cells due to B-cell deficiency (35). IL-10-GFP knock-in mice (B6.129S6-Il10tm1Flv/J) (28) were kindly provided by Dr. Sing Sing Way (Cincinnati Children’s Hospital, Cincinnati, OH). Foxp3EGFP mice (B6.Cg-Foxp3tm2Tch/J) were obtained from Jackson Laboratories, Bar Harbor, ME.
Intracerebroventricular infection
Infection of mice with MCMV was performed as previously described (36). Briefly, female mice (6–8 week old) were anesthetized using a combination of Ketamine and Xylazine (100 mg and 10 mg/kg body weight, respectively) and immobilized on a small animal stereotactic instrument equipped with a Cunningham mouse adapter (Stoelting Co., Wood Dale, IL). The skin and underlying connective tissue were reflected to expose reference sutures (sagittal and coronal) on the skull. The sagittal plane was adjusted such that the bregma and lambda were positioned at the same coordinates on the vertical plane. Virulent, salivary gland-passaged MCMV RM461 (1.5 × 105 TCID50 units in 10 μl), was injected into the right lateral ventricle at 0.9 mm lateral, 0.5 mm caudal to the bregma and 3.0 mm ventral to the skull surface using a Hamilton syringe (10 μl) fitted to a 27 G needle. The injection was delivered over a period of 3–5 min. The opening in the skull was sealed with bone wax and the skin was closed using 9 mm wound clips (Stoelting Co., Wood Dale, IL).
Isolation of brain leukocytes and flow cytometry analysis
Leukocytes were isolated from MCMV-infected murine brains using a previously described procedure with minor modifications (37–40). In brief, brain tissues harvested from four to six animals were minced finely in RPMI 1640 (2 g/L D-glucose and 10 mM HEPES, Sigma) and digested in 0.0625% trypsin (in Ca/Mg-free HBSS, Sigma) at room temperature for 20 min. Single cell preparations from infected brains were resuspended in 30% Percoll (Sigma) and banded on a 70% Percoll cushion at 900 × g for 30 min at 15°C. Brain leukocytes obtained from the 30–70% Percoll interface were treated with Fc block (anti-CD32/CD16 in the form of 2.4G2 hybridoma culture supernatant with 2% normal rat and 2% normal mouse serum) to inhibit nonspecific Ab binding and were stained with anti-mouse immune cell surface markers for 45 min at 4°C and analyzed by flowcytometry. Bregs from brain-infiltrating leukocytes were identified by flowcytometry using Breg staining kit from BioLegend, San Diego, CA. Control isotype Abs were used for all isotype and fluorochrome combinations to assess nonspecific Ab binding. Live leukocytes were gated using forward scatter and side scatter parameters on a BD FACSCanto flow cytometer (BD Biosciences, San Jose, CA). Data was analyzed using FlowJo software (TreeStar, Ashland, OR).
B-cell isolation and stimulation
We employed two different protocols to generate B10 cells in vitro. B10 cells generated with the first protocol were used in microglia and Breg co-culture studies. In these experiments, B10 cells were prepared following published protocols with minor modifications. Briefly, CD19+ lymphocytes from IL-10-GFP knock-in mice were enriched by negative selection using a CD19+ cell purification kit, as per the manufacturer’s instructions (R&D Systems, Minneapolis, MN). For preparation of B10 cells, 1 × 106 purified B-cells were cultured with agonistic CD40 mAB (1 μg/ml, Miltenyi Biotec, Auburn, CA) for 48 h. For the last 5 h, cells were treated with LPS (10 μg/ml, Sigma), PMA and Ionomycin (eBioscience San Diego, CA). Post-stimulation, cells were subjected to FACS using FACSAria (BD Biosciences). GFP expression indicated that the cells were IL-10 competent. Post-sorting, the B10 cells were co-cultured with microglial cells at a ratio of 5:1, respectively. Microglial cells were stimulated with MCMV with an MOI of 5. Culture supernatants were collected after 24 h from the various treatment groups to assess cytokine production by ELISA. Additional protocols for generating B10 cells that avoid the use of LPS have been described (41). We employed B10 cells generated through this protocol in B10 and CD4+ T-cells co-culture studies at a ratio of 1:1. Briefly, splenic CD19+ lymphocytes from BALB/c mice were enriched by negative selection using a CD19+ cell purification kit, as per the manufacturer’s instructions (R&D Systems, Minneapolis, MN). Purity of the CD19+ cells was found to be >98.8%. For preparation of B10 cells, purified B-cells were cultured with IL-4 (10 ng/ml, eBioscience San Diego CA), with agonistic CD40 mAB (1 μg/ml, Miltenyi Biotec, Auburn CA) and BAFF (20 ng/ml, eBioscience) for 4 d. Approximately 45% of the B10 cells thus produced were found to be positive for intracellular IL-10, as measured by flow cytometry. Positive control cells, designated iTregs in Fig 6, were generated using previously published protocols with minor modifications (42). Briefly, CD4+ T-cells were isolated from spleens of Foxp3EGFP reporter mice by negative selection, according to manufacturer’s instructions (R&D Systems). Cells were cultured at a density of 2×106 cells/ml in 24-well plates for 5 days in RPMI medium supplemented with plate-bound anti-CD28 (1 μg/ml), anti-CD3 (10 μg/ml) (Bio X cell, West Lebanon, NH), IL-2 (10 ng/ml) and TGF-β1 (5 ng/ml) (eBioscience) to induce differentiation of Foxp3+ CD4+ T regulatory cells.
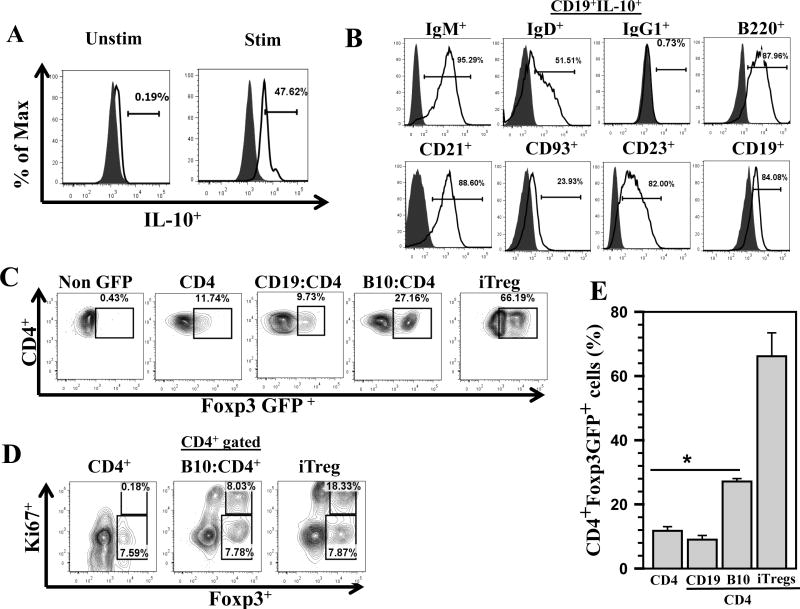
Figure 6. Induced B10 cells promote proliferation of Foxp3+ Treg cells.
Regulatory B10 cells were prepared from CD19+ cells as described and CD4+ T-cells were isolated from MHC-matched donors, Foxp3EGFP+, using a negative selection kit. Induced B10 cells were added to the CD4+ T-cell cultures at a 1:1 ratio. The co-cultures were then incubated for 72 h and Foxp3EGFP+ expression within the CD4+ T-cell population was assessed by flow cytometry for GFP expression to assess Treg phenotype. A. Flow cytometry histogram overlays show staining for isotype (grey, shaded) and intracellular IL-10 (solid line) levels in the induced B10 cells. B. B10 cells generated through this protocol were subjected to phenotyping to determine expression of various B-cell surface markers. Representative histogram overlays, gated from the CD19+IL-10+ population, which include isotype (grey, shaded) and specific markers (solid line) are shown. C. Representative flow cytometry contour plots of Foxp3EGFP+ within the CD4+ T-cell population are shown: non-GFP, CD4+ T-cells only (CD4), total B-cells with CD4+ T-cells (CD19+:CD4+), and induced B10 cells with CD4+ T-cells (B10:CD4+); along with a standard protocol used to generate in vitro Treg cells as a positive control (iTregs). D. Representative flow cytometry analysis of Foxp3+Ki67+ expression within CD4+ T-cells co-cultured with CD4 only, CD4:B10 and positive control iTreg are shown. E. The percentages of CD4+Foxp3+ in the various treatment groups from three independent experiments are shown. *P <0.01 versus CD4+T-cells alone and total B-cells+CD4+ T-cells
Murine microglial cell cultures
Microglial cells were isolated from cerebral cortices of 1-day-old mice as previously described (43, 44). Briefly, after a 30-min trypsinization (0.25%), dispersed cortical cells were plated in Falcon 75 cm2 culture flasks in DMEM (Sigma) containing 10% heat inactivated fetal bovine serum (FBS; Hyclone, Logan UT), penicillin (100 U/ml) and streptomycin (100 μg/ml) (Sigma). Medium was changed at days 1 and 4 after plating. On day 8 of culture, flasks were shaken for 20 min in an orbital shaker at 180–200 rpm to remove oligodendrocytes. On days 12–14 of culture, floating microglia were harvested by gentle shaking and seeded into 24- or 48-well culture plates (1–2 × 104 cells/well) and washed after 60 min. Adherent microglial cells were ≥ 95% pure, as determined by MAC-1 (microglial cell marker; Roche, Indianapolis, IN) and glial fibrillary acidic protein (GFAP-marker for astrocytes; Sigma) antibody staining (45, 46). All in vitro experiments used primary cell types which were genetically matched.
Adoptive transfer
Spleens from MCMV primed (1 × 105 TCID50/mouse, i.p. injection) donor animals were collected aseptically at 7 d post-priming. Single cell suspensions of immunocytes were depleted of RBC by treatment with 0.87% ammonium chloride, washed twice, and cell viability was confirmed using trypan blue. CD19+ lymphocytes were enriched by negative selection using a CD19+ cell purification kit, as per the manufacturer’s instructions (R&D systems). Immune cells were transferred (5 × 106 cells/mouse) via tail vein 1 d prior to infection with MCMV into syngenic (Jh−/−) recipients.
Results
Bregs persist within chronically-infected brains
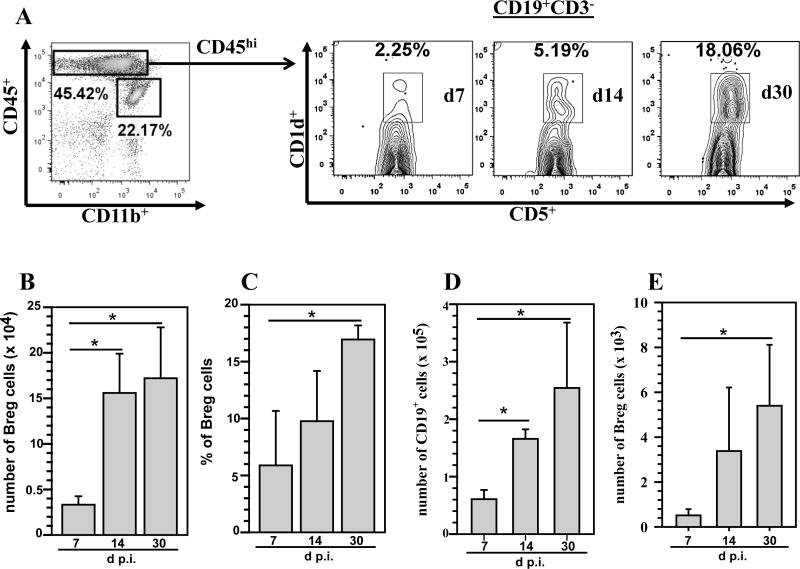
MCMV brain infection models have provided tremendous insights into viral neuropathogenesis and the role of immune responses in controlling infection (32, 47). In previous studies using our murine CMV brain infection model, we have identified brain-infiltrating B lymphocyte lineage cells, identified as CD19+ B-cells and CD138+ plasma cells (31). In this study, we also detected a B-cell subset expressing CD19+CD1dhiCD5+ phenotype which possesses negative regulatory functions within the brains of MCMV-infected mice. We used a multi-color flow cytometry approach to phenotype lymphocyte subpopulations infiltrating the brain at various time points p.i. Brain-infiltrating mononuclear cells were isolated on a Percoll gradient as described in the methods. These isolated cells were subsequently immunostained for expression of the markers CD45, CD11b, CD3, CD19, CD1d and CD5. Breg cells expressing the CD19+CD1dhiCD5+ cell surface markers were identified among the CD45hi population, which represent cells of lymphoid origin. At 7 d p.i., 2.25% of the cells among the CD19+ population were reactive to CD1dhiCD5+ and this subset was found to increase to 5.19% and 18.06% at 14 and 30 d p.i., respectively (Fig 1A). The absolute number of CD19+CD1dhiCD5+ cells was also determined. Presence of Breg cells was apparent within the infected brain starting at 7 d p.i. (3.3 ± 1.0 ×104), they were present at 14 d p.i. (1.5 ± 0.4 ×105), and persisted at least until 30 d p.i. (1.7 ± 0.5 × 105). There was a significant increase in the accumulation of Breg cells at both 14 and 30 d p.i. when compared to 7 d p.i. (1.5 ± 0.4 ×105 and 1.7 ± 0.5 × 105 versus 3.3 ± 1.0 ×104, respectively, p < 0.05 Student’s t test) (Fig 1B). We determined the percentage of CD19+CD1dhiCD5+ expressing cells at the indicated times p.i. from 3 independent experiments. Pooled data presented show that at 30 d p.i. there was a significant increase in the percentage of these cells infiltrating the brain when compared to 7 d p.i. (16.94 ± 1.2% versus 5.9 ± 4.8%, p < 0.05 Student’s t test) (Fig 1C). In order to investigate the infiltration kinetics of Bregs (CD1dhiCD5+) relative to total B-cells, we determined the absolute number of CD19+ cells among the total infiltrating CD45hi population (i.e., including CD3+ cells). The presence of CD19+ cells was observed as early as 7 d p.i. and there was a significant increase in their numbers at 14 and 30 d p.i. when compared to 7 d p.i. (1.6 ± 0.16 ×105 and 2.5 ± 1.1 × 105 versus 6.0 ± 1.6 ×104, respectively, p < 0.05 Student’s t test) (Fig 1D). The absolute number of Bregs (CD1dhiCD5+) within total infiltrating CD45hi population was then determined. We observed that there was a significant increase in the presence of Breg cells at 30 d p.i. when compared to 7 d p.i. (5.3 ± 2.7 ×103 versus 5.1 ± 2.7 ×102, respectively, p < 0.05 Student’s t test) (Fig 1E).
Figure 1. B regulatory cells (Bregs) persist in the brain during chronic viral infection.
Single cell suspensions of brain tissue obtained from MCMV-infected mice (3–5 animals) per time point were banded on a 70% Percoll cushion. Brain leukocytes were collected and labeled with PE-Cy5-conjugated Abs specific for CD45, AF700-labeled anti-CD11b Abs, and APC-Cy7 anti-CD19 Abs; and analyzed using flow cytometry. Breg cells were identified within the CD45hi cell population by using a Breg staining kit (BioLegend, San Diego, CA). The data obtained were processed using FlowJo software. A. Representative flow cytometric plots show the percentages of CD45hiCD19+CD1dhiCD5+ Breg cells within the infiltrating CD45hiCD3− population obtained from infected brains at 7, 14, and 30 d p.i. B. CD19+ CD1dhiCd5+ Abs were used to determine the total number of Breg cells within the infiltrating CD45hiCD3− population. Data shown are mean (±SEM) absolute number of infiltrating cells pooled from 3 independent experiments (n = 3–5). C. Percentage of Bregs within the infiltrating CD45hiCD3− cell population at indicated time points from three independent experiments are shown (n = 3–5). *P <0.05, 30 d p.i. versus 7 and 14 d p.i. D. Absolute numbers of CD19+ cells determined from the total CD45hi population including CD3+ cells. E. Absolute numbers of CD1dhiCD5+ cells determined within the infiltrating CD45hi population including CD3+ cells. *P <0.05, 30 d p.i. versus 7 and or 14 d p.i.
B-cell deficiency alters long-term T-cell persistence within the infected brain
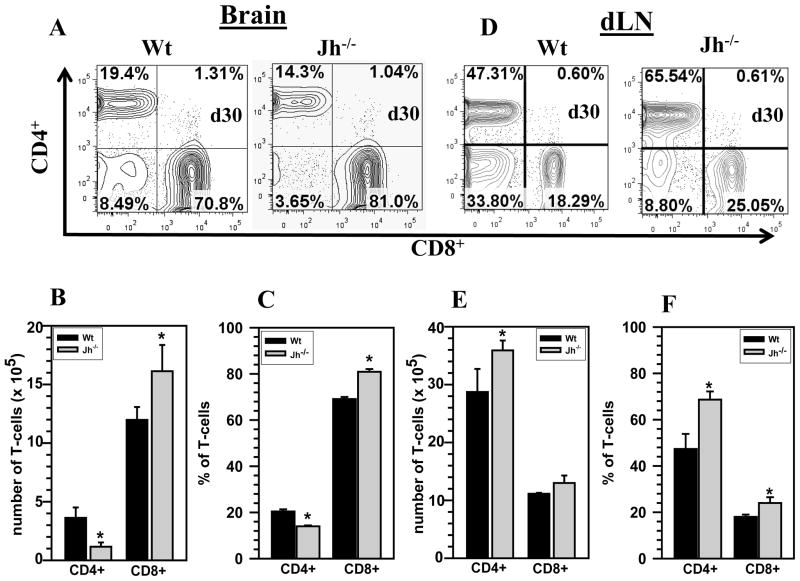
Previous studies from our laboratory have shown that CD8+ T-cells are essential in resolving MCMV brain infection, through a perforin-mediated mechanism (32). In subsequent studies we showed that memory CD8+ T-cells persisting in the brain drive chronic microglial activation through an interferon (IFN)-γ-dependent manner, despite the absence of active viral replication, suggesting that long-term persisting CD8+ T-cells may contribute to immunopathology in the CNS (30). In the current study, we found a correlation between the absence of B-cells and an increased presence of CD8+ T-cells along with decreased CD4+ T-cell numbers within infected brains. MCMV-infected Wt as well as B-cell deficient (Jh−/−) mice were analyzed for a number of neuroimmune responses which included phenotyping of the brain-infiltrating cell populations. The representative contour plots shown in Fig. 2 were obtained from isolated brain infiltrating cells, gated on CD45hi, and subsequently analyzed for the presence of CD4+ and CD8+ T-cells (Fig 2A). A significant increase in the absolute number of infiltrating CD8+ T-cells was observed in the Jh−/− mice (1.6 ± 0.2 × 106 versus 1.1 ± 0.11 × 106, p < 0.05), when compared to Wt animals (Fig 2B). On the contrary, a significant decrease in the absolute numbers of infiltrating CD4+ T-cells was observed in these animals (3.6 ±0.1× 105 versus 1.1 ± 0.3 ×105, p < 0.05) (Fig 2B). Analysis of pooled data from 3 independent experiments showed a significantly elevated percentage of CD8+ T-cells in the brains of B-cell deficient mice when compared to Wt controls, (80.9 ± 1.2% versus 69.1 ± 0.9%, respectively, p < 0.05 Student’s t test) (Fig 2C). Additionally, the CD4+ T-cell percentage was also found to be significantly lower in Jh−/− mice when compared with Wt mice (13.9 ± 0.4% versus 20.4 ± 0.9%, p < 0.05 Student’s t test) (Fig 2C). We also performed flow cytometry assays on single cell suspensions prepared from draining lymph nodes (dLN) of MCMV-infected Wt and Jh−/− animals at 30 d p.i. Interestingly, impaired T-cell recruitment into the CNS during B-cell deficiency was observed despite elevated levels of T-cells within the draining lymph nodes of Jh−/− mice. The flow cytometric analysis shown in Fig 2D indicated that there were elevated levels of both CD4+ and CD8+ T-cells in the dLN of Jh−/− mice at 30 d p.i. We determined absolute numbers and percentages of both CD4+ and CD8+ T-cells in the periphery (dLN) of Wt and Jh−/− animals and observed that there was a significant increase in the total number and percentage of CD4+ T-cells Jh−/− mice when compared to Wt (3.5 ± 0.1× 106 versus 2.8 ± 0.3 ×106 and 68.6 ± 3.5% versus 47.3 ± 6.5%, respectively, p < 0.05). On the contrary, we observed there was no significant increase in the total number of CD8+ T-cells in dLN of Jh−/− animals when compared to Wt mice at 30 d p.i, however, statistical analysis of percentage data showed a significant increase in CD8+ T-cells in the Jh−/− animals in the dLN at 30 d p.i., when compared Wt mice. (18.0 ± 0.5% versus 24.6 ± 2.3%, p < 0.05) (Figs 2E and F).
Figure 2. T-cell responses are altered within the brains of B-cell deficient mice.
Wt and B-cell deficient (Jh−/−) mice were infected with MCMV and brain-infiltrating leukocytes were collected at 30 d p.i. and labeled with PE-Cy5-conjugated Abs specific for CD45, AF700-labeled anti-CD11b, eFlour 450-CD4, and PE-Cy7-CD8; and analyzed using flow cytometry. A. Representative contour plots showing the percentages of CD4+ and CD8+ T-cells within the brain-infiltrating CD45hi population at 30 d p.i. are shown. B. Specific fluorescent-tagged mAB were used to stain CD4+ and CD8+ cells and determine total T-cell numbers within the infiltrating CD45hi population. Data shown are mean (±SEM) absolute number of infiltrating cells pooled from 3 independent experiments (n=5). C. The percentage of CD4+ and CD8+ T-cell subsets at 30 d p.i. from three independent experiments are shown (n=5). D. Representative contour plots from dLN of Wt and Jh−/− mice infected with MCMV at 30 d p.i. E. The absolute number of CD4+ and CD8+ T-cells from the dLN of Wt and Jh−/− animals was determined and presented as pooled data from 3 independent experiments (n=5). F. The percentage of CD4+ and CD8+ T-cell subsets in dLN from three independent experiments are shown (n=5). *P <0.05 versus infected Wt.
Frequencies of CD4+Foxp3+ regulatory T-cells were reduced in the brains of Jh−/− mice
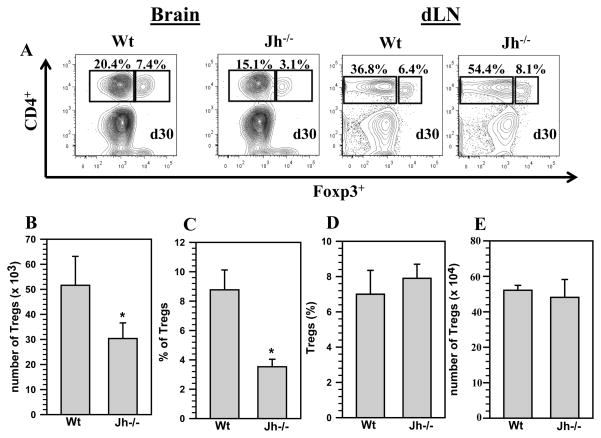
Tregs are critical for normal functioning immune responses by providing a mechanism to maintain self-tolerance, while still allowing for protective responses against microbes. Several studies have investigated the role of Treg cells, defined as CD4+Foxp3+, in regulation of immune responses to infectious diseases (48–50). Since we observed a significant decrease in the presence of CD4+ T-cells within the brains of Jh−/− mice at 30 d p.i., despite higher levels in the dLN, we next examined whether other CD4+ T-cell subsets, particularly anti-inflammatory cells such as regulatory T-cells, were altered. In these experiments we assessed the presence of regulatory T-cells in the brains of chronically infected Wt and Jh−/− mice. At 30 d p.i., we collected brain tissues and processed them for isolation of infiltrating mononuclear cells. The cells were subjected to staining with cell surface markers CD45, CD11b, and CD4 followed by intranuclear staining for Foxp3. Representative contour plots from Wt and Jh−/− mice for CD4+Foxp3+ T-cells, from brain and dLN, that were gated from the CD45hi cell population are shown (Fig 3A). Analysis of pooled data showed a significant decrease in the absolute numbers of CD4+Foxp3+ T-cells recruitment within the infected brains in Jh−/− mice (5.1 ± 1.1 × 104 versus 3.0 ± 0.6 × 104, p <0.05) (Fig 3B). Pooled data from 3 independent experiments also showed a significant decrease in the percentage of CD4+Foxp3+ T-cells in Jh−/− mice when compared to MCMV infected Wt animals, 3.5 ± 0.5% versus 8.77 ± 0.5%, p < 0.05 Student’s t test (Fig 3C). We also determined the percentage and absolute numbers for Tregs in the dLN from Wt and Jh−/− animals at 30 d p.i., and the data presented show that there were no significant differences in the presence of Tregs in the dLN of Jh−/− mice when compared with Wt animals (Figs 3D and E).
Figure 3. Absence of B-cells leads to decreased numbers of regulatory T-cells within the brain.
Wt and Jh−/− mice were infected with MCMV and brain tissue samples were obtained at 30 d p.i. Brain leukocytes were collected and labeled with PE-Cy5-conjugated Abs specific for CD45, AF700-labeled anti-CD11b, eFlour450-CD4, and FITC-Foxp3; and analyzed using flow cytometry. A. Representative contour plots show the percentages of CD4+Foxp3+ Tregs within the infiltrating CD45hi population in the infected brains at 30 d p.i. Also, representative contour plots are shown from dLN of Wt and Jh−/− mice collected at 30 d p.i. B. FITC labeled anti-Foxp3 Abs were used to determine the total number of Tregs within the infiltrating CD45hi population (n=5). Data shown are mean (±SEM) absolute number of infiltrating cells pooled from 3 independent experiments. C. The percentage of CD4+Foxp3+ Tregs within CD45hi population from three independent experiments are also shown (n=5). D. The absolute number of CD4+Foxp3+ T-cells was also determined from the dLN of Wt and Jh−/− animals and presented as pooled data from 3 independent experiments (n=5). E. The percentage of CD4+Foxp3+ T-cell subsets in dLN from three independent experiments are shown (n=5). *P <0.05 versus infected Wt.
Increased CD8+ T-cell presence and reduced regulatory T-cells promote enhanced chronic microglial activation with B-cell deficiency
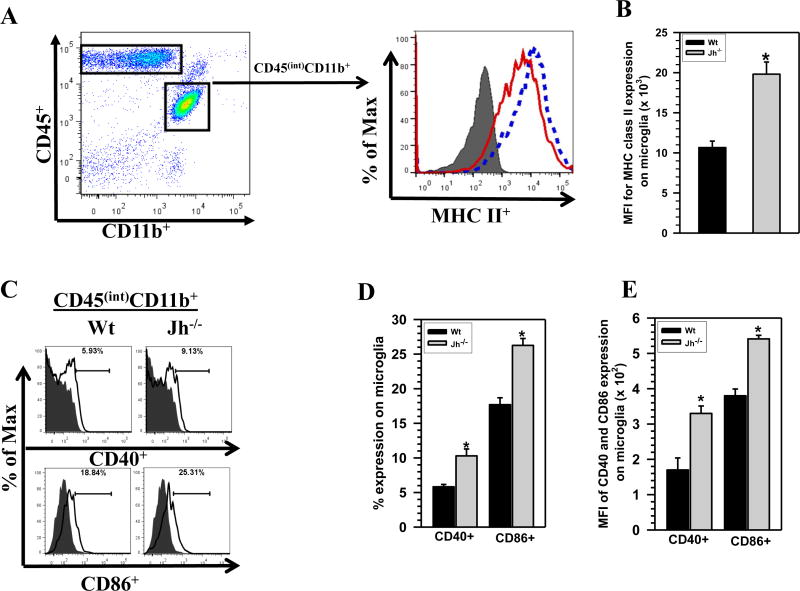
To compare chronic activation of brain-resident microglia between MCMV-infected Wt versus Jh−/− animals, these cells were assessed by first gating on the CD45intCD11b+ population, isolated at 30 d p.i., followed by evaluation of MHC class II, CD40, and CD86 expression using flow cytometry. Representative histogram overlays demonstrate a shift in the expression levels of MHC class II on microglia from Jh−/− mice (dotted line) when compared with Wt animals (solid line), (Fig 4A). This shift indicated an increased level of microglial activation within the brains of B-cell-deficient animals. In addition, the mean fluorescence intensity (MFI) of MHC II expression was also measured between groups and was found to be significantly higher among microglia isolated from Jh−/− mice (1.0 ± 0.1 ×104 versus 1.9 ± 0.1 ×104, p < 0.01 Student’s t test) (Fig 4B). CD40 and CD86 expression on microglial cells was also assessed and the flow cytometry histogram data showed increased expression of these molecules on microglia derived from Jh−/− animals at 30 d p.i. (Fig 4C). Statistical analysis of percent expression of CD40 and CD86 molecules showed a significant increase in their expression on cells isolated from Jh−/− mice when compared with Wt animals (10.2 ± 0.9% versus 5.8 ± 0.3% and 26.2 ± 1.0 % versus 17.6 ± 1.0%, respectively, p < 0.05 Student’s t test) (Fig 4D). In addition, mean fluorescence intensity for CD40 and CD86 expression on microglial cells was measured and was found to be significantly higher among those isolated from Jh−/− mice (170 ± 30 versus 380 ± 19 for CD40 and 330 ± 23 versus 540 ± 13 for CD86, p < 0.05 Student’s t test) (Fig 4E).
Figure 4. B-cell deficient mice display increased levels of chronic microglial cell activation.
Brain mononuclear cells isolated from animals at 30 d p.i. were stained using anti-CD45, anti-CD11b, and anti-MHC-II surface marker Abs. A. Up-regulation of MHC class II on the CD45intCD11b+ resident microglial cells from Wt and Jh−/− mice in response to viral brain infection was compared. An overlay of histograms from isotype (grey, shaded), Wt (red, solid line) mice and from Jh−/− (blue, dotted line) animals is shown. B. Data presented show mean fluorescent intensity (MFI) of MHC-II binding to microglia from Wt versus Jh−/− mice (n=5). C. Representative histogram overlays for CD40 and CD86 expression on microglial cells isolated from Wt and Jh−/− mice are shown. The overlay of histograms from isotype (grey, shaded) and specific marker (solid line) are shown for the respective groups. D. Data presented in the bar graph shows percentage of CD40 and CD86 expression on microglia from Wt versus Jh−/− mice (n=5). *p < 0.01 versus infected Wt for Fig 4B and *p < 0.05 versus infected Wt for Fig 4D. E. Mean fluorescent intensity (MFI) of CD40 and CD86 expression on microglia obtained from Wt versus Jh−/− mice. *p < 0.01 versus infected Wt for Fig 4B; and *p < 0.05 versus infected Wt for Fig 4D and E.
In-vitro generated B10 cells modulate microglial cell responses through IL-10 production
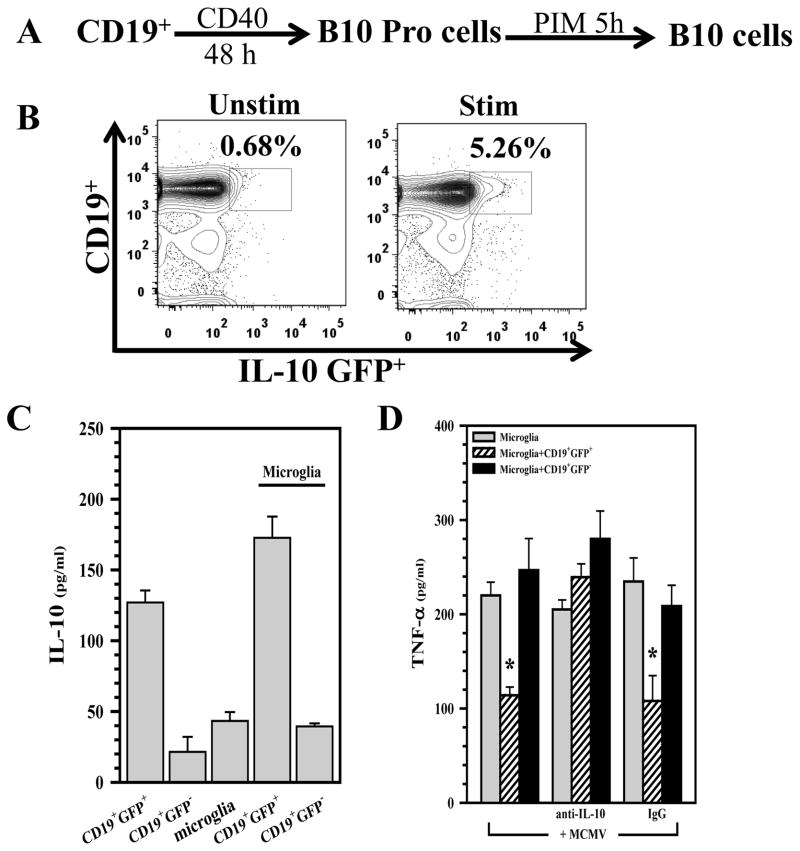
Previous studies have shown that the anti-inflammatory cytokine IL-10 inhibits secretion of proinflammatory mediators from macrophages (51). In this study, we examined the effect of IL-10, produced by B10 cells, on microglial cells stimulated with MCMV. To evaluate the effects of Bregs on cytokine production by microglia, we focused on the subset of IL-10-producing Bregs, B10 cells, for which we can generate enriched populations in vitro. Tumor necrosis factor (TNF)-α levels were quantified from supernatants of purified microglial cell cultures following stimulation with MCMV in either the presence or absence of B10 cells. Fig 5A shows the protocol used for in vitro generation of B10 cells from naïve B-cells of MHC-matched IL-10-GFP knock-in donor mice. Representative contour plots of B-cells that were treated with the above protocol and analyzed for GFP+ cells, indicative of IL-10 synthesis, are shown in Fig 5B. The GFP+ cells were then enriched by fluorescent activated cell sorting (FACS) and these enriched cells were used in subsequent co-culture experiments. In the first set of experiments, we detected IL-10 production in supernatants from the B10: microglial cell co-cultures. Data demonstrate that IL-10 was produced in the co-culture system that included CD19+GFP+ cells, but it was not detected when CD19+GFP− cells were used to reconstitute the co-cultures (Fig 5C). Further studies using this co-culture system revealed that B10 cells had a suppressive effect on microglial cells that were stimulated with MCMV for 24 h. Microglia which were co-cultured with CD19+GFP+ B10 cells produced significantly lower levels of TNF-α in response to stimulation with MCMV when compared to microglial cells co-cultured with CD19+GFP− cells (Fig 5D). We went on to demonstrate that addition of anti-IL-10 neutralizing antibody to the co-culture system resulted in an abrogation of the suppressive effect of B10 cells on microglia, demonstrating an inhibitory role for B10-produced IL-10 (Fig 5D).
Figure 5. Induced B10 cells modulate microglial cell activation.
A. Splenic CD19+ B-cells from IL-10-GFP knock-in mice were cultured with agonistic CD40 mAB (1 μg/ml, eBioscience San Diego, CA) for 48 h. For the last 5 h, cells were treated with LPS (10 μg/ml, Sigma), PMA (50 ng/ml, eBioscience San Diego, CA), ionomycin (100 ng/ml, eBioscience San Diego, CA), and monensin (PIM) (eBioscience San Diego, CA) for 5 h to induce IL-10-producing B10 cells. B. Representative flow cytometry contour plots showing IL-10 induction in CD19+ cells that were stimulated with the above protocol. C. The induced B10 cells were then added to purified cultures of primary murine microglia (1:1 ratio) and IL-10 production in these co-cultures was assessed using ELISA. D. CD19+IL-10-GFP+ B10, as well as CD19+IL-10-GFP−, cells were enriched using FACS and then added to primary microglial cell cultures. Co-cultures were incubated for 5 h, followed by stimulation with MCMV (MOI=5), and assessment of TNF-α production (at 24h) using ELISA. *p < 0.05 CD19+GFP+ versus microglia alone; *p < 0.05 anti-IL-10 versus IgG isotype
B10 cells promote proliferation of CD4+Foxp3+ Tregs
Our data show that the absence of B-cells resulted in reduced numbers of Tregs persisting within the brain following viral infection. To address whether B10 cells promote conversion of CD4+ T-cells into Tregs or induce their proliferation, we utilized an in vitro generated B10 and CD4+ T-cell co-culture system. B10 cells were generated following the protocol noted in the methods section. To confirm the successful generation of B10 cells using the specified stimulation protocol, we first examined the cells for their ability to produce IL-10 by intracellular staining and flow cytometry. In these experiments, IL-10 production was detected in approximately 47% of the B-cells. Correspondingly, these in vitro generated B10 cells were subsequently phenotyped for typical B-cell markers such as IgM+ (95%), IgD+ (51%), IgG1+ (66%), B220+ (87%), CD21+ (88%), CD93+ (23%), CD23+ (82%) and CD19+ (84%) prior to their use in co-culture studies. Representative histogram plots show IL-10 producing CD19+ cells as well as the expression of various surface markers following treatment (Fig 6A and B, respectively). These B10 cells were subsequently co-cultured with CD4+ T-cells from Foxp3EGFP+ mice for 72 h, followed by analysis of Foxp3EGFP expression using flow cytometry. Representative contour plots shown are from: CD4+ T-cells from non-GFP animals for gating purposes, CD4+ T-cells alone, CD19+: CD4+ T-cell co-cultures, B10: CD4+ T-cell co-cultures, and induced Tregs (iTreg) as a positive control (using a standard protocol to generate iTregs from naïve CD4+ T-cells) (Fig 6C). The ability of B-cells to induce Treg proliferation, both in vitro and in vivo, has been previously reported (52). In order to differentiate between conversion and proliferation of Tregs we performed intranuclear staining for Ki67 expression in CD4+ T-cells co-cultured with B10 cells. These experiments showed that approximately 8% of the Foxp3+ cells also expressed Ki67, suggesting that the Tregs proliferated in presence of B10 cells. Representative contour plots, gated on CD4+ cells, are shown from CD4+ only, B10:CD4+, and iTregs (Fig. 6D). The amount of CD4+Foxp3+ T-cells present was found to be significantly higher in B10:CD4+ T-cell co-cultures when compared with the other treatment groups. (11 ± 1.3% (CD4+ only), and 9 ± 1.3% (CD19+:CD4+) versus 27 ± 0.9% (B10:CD4+), p < 0.01 Student’s t test). Data shown in the bar graph are from three independent co-culture experiments (Fig 6E).
Transfer of B-cells into MCMV-infected Jh−/− mice restores T-cell levels within chronically-infected brains
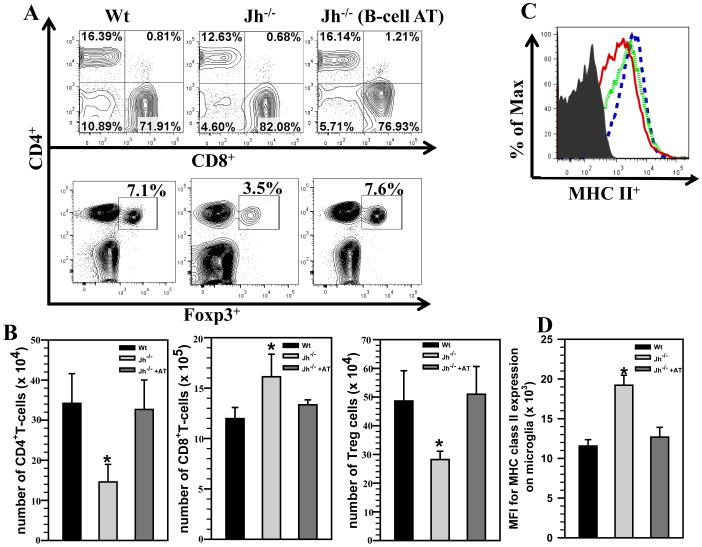
To evaluate the contributions of B-cells in regulating neuroimmune responses during the chronic phase of disease, we went on to perform adoptive transfer experiments. Using this gain-of-function approach, we transferred MCMV-primed CD19+ B-cells, isolated from MHC matched donors, into Jh−/− mice. These B-cell deficient Jh−/− mice received 5 × 106 primed CD19+ B-cells 1 d prior to infection. At 30 days p.i., we harvested brains from Wt, Jh−/−, and reconstituted (B-cell AT) mice, and brain-infiltrating leukocytes were purified using a Percoll gradient. These cells were then analyzed by flow cytometry following the appropriate immunostaining. Representative contour plots for CD4+, CD8+ (upper panel) and CD4+ Foxp3+ (lower panel) are shown from the various groups of animals at 30 d p.i. (Fig 7A). Adoptive transfer of B-cells into Jh−/− mice restored the levels of CD4+, CD8+ and Foxp3+CD4+ T-cells to those observed in infected Wt mice. We determined absolute numbers for CD4+ T-cells (3.4 ± 0.7 ×105 (Wt), 3.2 ± 0.7 ×105 (Jh−/− +AT) versus 1.4 ± 0.4 ×105 (Jh−/−), p < 0.05 Student’s t test), CD8+ T-cells (1.1 ± 0.1 ×106 (Wt), 1.3 ± 0.4 ×106 (Jh−/− +AT) versus 1.6 ± 2.2 ×106 (Jh−/−), p < 0.05 Student’s t test), and CD4+Foxp3+ T-cells (4.8 ± 1.0 ×104 (Wt), 5.0 ± 0.9 ×104 (Jh−/− +AT) versus 2.8 ± 0.2 ×104 (Jh−/−), p < 0.05 Student’s t test) within the brains of Wt and Jh−/− animals. These data indicated that replenishing B-cells into Jh−/− animals restored neuroimmune responses to levels exhibited by Wt animals (Fig 7B). Similarly, MHC class II expression on microglial cells isolated from Jh−/− mice receiving B-cell transfer was also restored to the levels exhibited by Wt animals. Representative histogram overlays are shown from the various groups of animals (Fig 7C). Mean fluorescence intensity of MHC II expression was also measured among the groups and was found to be significantly higher on microglia isolated from Jh−/− mice (1.1 ± 0.1 ×104 (Wt), 1.2 ± 0.1 ×104 (Jh−/− +AT) versus 1.9 ± 0.1 ×104 (Jh−/−), p < 0.01 Student’s t test) (Fig 7D).
Figure 7. B-cell replenishment restores T-cell levels within chronically-infected brains.
MCMV-primed splenocytes and lymph node cells from donor BALB/c mice were enriched for CD19+ cells using negative selection. These B-cells were then adoptively transferred via tail vein injection into MHC-matched Jh−/− recipients 1 d prior to the infection with MCMV. Wt and Jh−/− mice served as appropriate controls and brain tissue samples were obtained from each group at 30 d p.i. Brain leukocytes were collected and labeled with PE-Cy5-conjugated Abs specific for PE-Cy5-labeled anti-CD45, AF700-labeled anti-CD11b, eFlour450-labeled anti-CD4, PE-Cy7-labeled anti-CD8, FITC-labeled anti-Foxp3 and APC-Cy7-labeled anti-MHC II and analyzed using flow cytometry. A. Representative contour plots showing the percentages of CD4+, CD8+ (upper panel), and CD4+Foxp3+ T-cells (lower panel) in the infiltrating CD45hi population within the infected brains from each group. B. The absolute numbers of CD4+, CD8+ and CD4+Foxp3+ T-cells were also determined among the brain-infiltrating CD45hi cells from Wt, Jh−/−, and Jh−/− with B-cell AT animals. Pooled data obtained from 3 independent experiments are presented (n=5 per group). *p < 0.05 Jh−/−+AT versus Jh−/− C. Histogram overlays from isotype (grey, shaded), Wt (red, solid), Jh−/− (blue, dashed), and Jh−/− mice that received CD19+ cells (green, dotted) are shown for MHC class II up-regulation on CD45intCD11b+ resident microglia. D. Data presented show mean fluorescent intensity (MFI) of MHC-II binding from Wt, Jh−/− and Jh−/−+AT mice (n=5 per group).*p < 0.01 Jh−/−+AT versus Jh−/−
Discussion
Neuroimmune responses elicited during CNS infections are critical in controlling viral replication and promoting successful resolution; however, certain aspects of both innate and adaptive responses, if not appropriately regulated, may induce neuropathology. It is clear that excessive production of inflammatory mediators induced during various stages is a primary mechanism responsible for pathogenesis during many viral infections. Therefore, adequate function of the immune regulation arm, mediated by immunosuppressive cytokines and regulatory immune cells, plays a critical role in controlling excess inflammatory responses and their associated damage (53).
In addition to antibody production, specific subsets of B-cells are also known to regulate T-cell- and macrophage-driven immune responses and over the last decade the concept of anti-inflammatory Breg cells has emerged (18). In the present study, our first experiments analyzed the presence of these Breg cells during chronic viral brain infection with MCMV. Results obtained showed that there was a sustained presence of Breg cells within the brains of mice following viral infection. While assessing function we found that B-cell deficient animals developed exacerbated neuroimmune responses, as measured by enhanced accumulation of CD8+ T-cells and increased levels of microglial activation. Additional experiments showed that the absence of B-cells led to a significant reduction in accumulation of regulatory T-cells within the brains of infected Jh−/− mice when compared with Wt animals. Furthermore, in vitro generated B10 cells were able to inhibit cytokine responses from MCMV-stimulated microglial cells. We went on to show that these in vitro generated B10 cells also promoted proliferation of Foxp3-expressing regulatory T-cells. In the final experiments, adoptive transfer of B-cells into MCMV-infected Jh−/− mice was found to restore T-cell levels to those of Wt animals within chronically-infected brains.
A number of cell surface molecules have been described as markers for Bregs, although complete elucidation of these regulatory cells remains to be achieved. It has been proposed that B-cells with immunoregulatory function may represent a transient stage of activation and maturation (54). In this study, we phenotyped Bregs based on the expression of specific cell surface markers that constituted reactivity to CD19+CD1dhiCD5+. B-cells, Breg and B10 cells are known to regulate adaptive immune responses through multiple mechanisms which include: secretion of IL-10 (55) and promoting proliferation of CD4+Foxp3+ Tregs (52, 56), as well as through inhibitory B7 family molecules which promote Treg and IL-10 production (56). Previous studies have shown that IL-10 competent B-cells (B10 cells) are predominantly found within this Breg subset, particularly in the spleen (41).
It has been shown that responses of Bregs can be induced by stimulation with TLR4 and TLR9 ligands, as well as signaling through the B-cell receptor (BCR) (15, 57). Krug A., et al (58) reported that MCMV engages with TLR9 to induce dendritic and interferon-producing cell activation and enhance immune responses to infection. It is also possible that the Breg response observed in this study results from direct stimulation of B-cells by MCMV or its antigens. Although a number of studies involving various infectious agents have demonstrated the expansion of Bregs in peripheral immune appendages, to our knowledge, this is the first demonstration of the chronic presence of Bregs within the brain following MCMV infection.
We previously demonstrated that CD8+ T-cells control replication and persist within the brain even after active viral replication ceases (32, 59). In addition, our studies and others have shown that IFN-γ produced from infiltrating T-cells drives microglial activation (30, 60). Persistence of CD8+ T-cells is particularly important because they constitute the major brain-infiltrating immune cell type during the chronic phase of infection; they are retained at a ratio of 3:1 to CD4+ T-cells. In the present study, we observed that there were significantly higher numbers of CD8+ T-cells present in the chronically-infected brain (i.e., 30 d p.i.) in the absence of B-cells. Conversely, we observed a lower frequency of CD4+ and CD4+Foxp3+ T-cells than in Wt animals. Similar findings have been reported using an EAE model in which the authors noted that depletion of B-cells during disease development results in significant reduction in total CD4+ T-cell, myelin oligodendrocyte glycoprotein (MOG)-specific T-effector, and Treg numbers within the CNS. However, these authors also state that before disease induction there was significant increase in the accumulation of all the above three cell types, suggesting that depletion of B-cells at specific stages of the disease may have had different outcomes on encephalitogenic T-cells (16).
Bregs have been shown to control macrophage-driven pro-inflammatory responses. Previous studies have revealed that B-cell depletion causes enhanced macrophage activation and cytokine production during Listeria infection (61). Similarly, in this study we reported increased activation of brain resident microglia as assessed by MHC class II expression perhaps in the context of reduced Treg presence and in turn insufficient control of CD8+ T-cells influx. This increased activation of microglia could be due to enhanced production of IFN-γ. IFN-γ has been shown to drive microglial activation (60). There was also a small increase in the expression levels of CD40 and CD86 molecules on the microglial cells at 30 d p.i., which confirmed increased activation of these cells in B-cell deficient animals. Our in vitro studies provide a mechanistic explanation for the inhibition of microglial cell activation through B10 cell-produced IL-10. The data presented in this study are in agreement with findings that have demonstrated that B10 cells down-regulate macrophage activation following LPS stimulation (61).
Bregs have also been shown to promote conversion of CD4+ T-cells into a regulatory T-cell phenotype. A recent study, using a collagen-induced arthritis (CIA) model, demonstrated that B10 cells promoted Treg conversion when co-cultured with naïve CD4+ T-cells (62). T-cell plasticity has been well recognized, and many factors are known to influence plasticity including: cellular conditions, transcriptional circuitry, and chromatin modifications (63). Our in vitro studies using B10:CD4+ T-cell co-cultures found that these cells increased Treg proliferation that is demonstrated by Ki67 expression by Foxp3+ cells. Utilizing both in vivo and in vitro approaches a recent elegant study by Ray A. et al (52) has shown that Treg proliferation in the presence of B-cells is attributed to the interaction of glucocorticoid-induced TNFR family-related protein (GITR) with its ligand, the study also demonstrated that Treg proliferation was IL-10 independent. Although Tregs are not the main focus of this study, their role in controlling neuroinflammation is well established. Likewise, the presence of B10 cells during chronic phase of the disease could be beneficial as they are capable of driving proliferation of Tregs and in turn may aid in controlling excessive neuroinflammation.
In animal models of allergic disease, Amu and colleagues (64) suggested that Bregs may have a more systemic effect on Treg expansion. Other studies demonstrated that IL-10-producing B10 cells function to down-regulate autoimmunity and allergic disorders by inducing Tregs (15, 64, 65). These findings can be extended to our chronic viral brain infection model. In particular, adoptive transfer of B-cells in this animal model resulted in enhanced amounts of Tregs, to a level exhibited by B-cell-sufficient animals. Thus, our data demonstrate that transfer of B-cells inhibits the exacerbated neuroimmune responses that are observed in B-cell-deficient mice.
Results generated from these studies demonstrate that the absence of B-cells leads to exacerbated microglial cell- and T-cell-mediated neuroinflammation following viral infection, suggesting the importance of these cells in controlling chronic and persistent immune activation in the brain. This knowledge may be useful in the development of therapeutic applications to modulate chronic neuroimmune activation and its associated neuropathology.
Acknowledgments
Funding: This project was supported by Award Number R01NS-038836 from the National Institute of Neurological Disorders and Stroke.
References
- 1.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 2.McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol. 2011;11:318–329. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kivisakk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, Ransohoff RM, Khoury SJ. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu S, Sheng WS, Schachtele SJ, Lokensgard JR. Reactive oxygen species drive herpes simplex virus (HSV)-1-induced proinflammatory cytokine production by murine microglia. J Neuroinflammation. 2011;8:123. doi: 10.1186/1742-2094-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aravalli RN, Hu S, Rowen TN, Palmquist JM, Lokensgard JR. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol. 2005;175:4189–4193. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- 7.Phares TW, Disano KD, Hinton DR, Hwang M, Zajac AJ, Stohlman SA, Bergmann CC. IL-21 optimizes T cell and humoral responses in the central nervous system during viral encephalitis. J Neuroimmunol. 2013;263:43–54. doi: 10.1016/j.jneuroim.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart BS, Demarest VL, Wong SJ, Green S, Bernard KA. Persistence of virus-specific immune responses in the central nervous system of mice after West Nile virus infection. BMC Immunol. 2011;12:6. doi: 10.1186/1471-2172-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anghelina D, Zhao J, Trandem K, Perlman S. Role of regulatory T cells in coronavirus-induced acute encephalitis. Virology. 2009;385:358–367. doi: 10.1016/j.virol.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reuter D, Sparwasser T, Hunig T, Schneider-Schaulies J. Foxp3+ regulatory T cells control persistence of viral CNS infection. PLoS One. 2012;7:e33989. doi: 10.1371/journal.pone.0033989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham JB, Da Costa A, Lund JM. Regulatory T cells shape the resident memory T cell response to virus infection in the tissues. J Immunol. 2014;192:683–690. doi: 10.4049/jimmunol.1202153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard M, O’Garra A. Biological properties of interleukin 10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 14.Marques CP, Hu S, Sheng W, Cheeran MC, Cox D, Lokensgard JR. Interleukin-10 attenuates production of HSV-induced inflammatory mediators by human microglia. Glia. 2004;47:358–366. doi: 10.1002/glia.20045. [DOI] [PubMed] [Google Scholar]
- 15.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 19.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 20.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM, Balkwill FR. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goenka R, Parent MA, Elzer PH, Baldwin CL. B cell-deficient mice display markedly enhanced resistance to the intracellular bacterium Brucella abortus. J Infect Dis. 2011;203:1136–1146. doi: 10.1093/infdis/jiq171. [DOI] [PubMed] [Google Scholar]
- 23.Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, Kuhl AA, Loddenkemper C, Haury M, Nedospasov SA, Kaufmann SH, Steinhoff U, Calado DP, Fillatreau S. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33:777–790. doi: 10.1016/j.immuni.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci U S A. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas KM, Watanabe R, Matsushita T, Nakashima H, Ishiura N, Okochi H, Fujimoto M, Tedder TF. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol. 2010;184:4789–4800. doi: 10.4049/jimmunol.0902391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K, Sato S, Tedder TF, Fujimoto M. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184:4801–4809. doi: 10.4049/jimmunol.0902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, Harhaj E, Flavell RA. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Maseda D, Smith SH, DiLillo DJ, Bryant JM, Candando KM, Weaver CT, Tedder TF. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol. 2012;188:1036–1048. doi: 10.4049/jimmunol.1102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutnal MB, Hu S, Little MR, Lokensgard JR. Memory T cells persisting in the brain following MCMV infection induce long-term microglial activation via interferon-gamma. J Neurovirol. 2011;17:424–437. doi: 10.1007/s13365-011-0042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mutnal MB, Hu S, Lokensgard JR. Persistent humoral immune responses in the CNS limit recovery of reactivated murine cytomegalovirus. PLoS One. 2012;7:e33143. doi: 10.1371/journal.pone.0033143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheeran MC, Gekker G, Hu S, Palmquist JM, Lokensgard JR. T cell-mediated restriction of intracerebral murine cytomegalovirus infection displays dependence upon perforin but not interferon-gamma. J Neurovirol. 2005;11:274–280. doi: 10.1080/13550280590952808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoddart CA, Cardin RD, Boname JM, Manning WC, Abenes GB, Mocarski ES. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J Virol. 1994;68:6243–6253. doi: 10.1128/jvi.68.10.6243-6253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanton MR, Way SS, Shlomchik MJ, McSorley SJ. Cutting edge: B cells are essential for protective immunity against Salmonella independent of antibody secretion. J Immunol. 2012;189:5503–5507. doi: 10.4049/jimmunol.1201413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, Huszar D. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 36.Cheeran MC, Gekker G, Hu S, Min X, Cox D, Lokensgard JR. Intracerebral infection with murine cytomegalovirus induces CXCL10 and is restricted by adoptive transfer of splenocytes. J Neurovirol. 2004;10:152–162. doi: 10.1080/13550280490441130. [DOI] [PubMed] [Google Scholar]
- 37.Cheeran MC, Hu S, Palmquist JM, Bakken T, Gekker G, Lokensgard JR. Dysregulated interferon-gamma responses during lethal cytomegalovirus brain infection of IL-10-deficient mice. Virus Res. 2007;130:96–102. doi: 10.1016/j.virusres.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine B, Hardwick JM, Trapp BD, Crawford TO, Bollinger RC, Griffin DE. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 39.Marten NW, Stohlman SA, Zhou J, Bergmann CC. Kinetics of virus-specific CD8+-T-cell expansion and trafficking following central nervous system infection. J Virol. 2003;77:2775–2778. doi: 10.1128/JVI.77.4.2775-2778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mutnal MB, Cheeran MC, Hu S, Little MR, Lokensgard JR. Excess neutrophil infiltration during cytomegalovirus brain infection of interleukin-10-deficient mice. J Neuroimmunol. 2010;227:101–110. doi: 10.1016/j.jneuroim.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard WJ, Tedder TF. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fantini MC, Dominitzki S, Rizzo A, Neurath MF, Becker C. In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat Protoc. 2007;2:1789–1794. doi: 10.1038/nprot.2007.258. [DOI] [PubMed] [Google Scholar]
- 43.Chao CC, Hu S, Sheng WS, Bu D, Bukrinsky MI, Peterson PK. Cytokine-stimulated astrocytes damage human neurons via a nitric oxide mechanism. Glia. 1996;16:276–284. doi: 10.1002/(SICI)1098-1136(199603)16:3<276::AID-GLIA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 44.Suzumura A, Mezitis SG, Gonatas NK, Silberberg DH. MHC antigen expression on bulk isolated macrophage-microglia from newborn mouse brain: induction of Ia antigen expression by gamma-interferon. J Neuroimmunol. 1987;15:263–278. doi: 10.1016/0165-5728(87)90121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giulian D, Baker TJ, Shih LC, Lachman LB. Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med. 1986;164:594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassan NF, Rifat S, Campbell DE, McCawley LJ, Douglas SD. Isolation and flow cytometric characterization of newborn mouse brain-derived microglia maintained in vitro. J Leukoc Biol. 1991;50:86–92. doi: 10.1002/jlb.50.1.86. [DOI] [PubMed] [Google Scholar]
- 47.Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22:99–126. doi: 10.1128/CMR.00023-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zelinskyy G, Werner T, Dittmer U. Natural regulatory T cells inhibit production of cytotoxic molecules in CD8+ T cells during low-level Friend retrovirus infection. Retrovirology. 2013;10:109. doi: 10.1186/1742-4690-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sungur CM, Tang-Feldman YJ, Ames E, Alvarez M, Chen M, Longo DL, Pomeroy C, Murphy WJ. Murine natural killer cell licensing and regulation by T regulatory cells in viral responses. Proc Natl Acad Sci U S A. 2013;110:7401–7406. doi: 10.1073/pnas.1218767110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qureshi MH, Garvy BA, Pomeroy C, Inayat MS, Oakley OR. A murine model of dual infection with cytomegalovirus and Pneumocystis carinii: effects of virus-induced immunomodulation on disease progression. Virus Res. 2005;114:35–44. doi: 10.1016/j.virusres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 52.Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol. 2012;188:3188–3198. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y, Chen Y, Li Z, Han Y, Sun Y, Wang Q, Liu B, Su Z. Role of IL-10-producing regulatory B cells in control of cerebral malaria in Plasmodium berghei infected mice. Eur J Immunol. 2013;43(11):2907–2918. doi: 10.1002/eji.201343512. [DOI] [PubMed] [Google Scholar]
- 54.Gray D, Gray M. What are regulatory B cells? Eur J Immunol. 2010;40:2677–2679. doi: 10.1002/eji.201040961. [DOI] [PubMed] [Google Scholar]
- 55.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 56.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 57.Miles K, Heaney J, Sibinska Z, Salter D, Savill J, Gray D, Gray M. A tolerogenic role for Toll-like receptor 9 is revealed by B-cell interaction with DNA complexes expressed on apoptotic cells. Proc Natl Acad Sci U S A. 2012;109:887–892. doi: 10.1073/pnas.1109173109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 59.Marques CP, Cheeran MC, Palmquist JM, Hu S, Urban SL, Lokensgard JR. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J Immunol. 2008;181:6417–6426. doi: 10.4049/jimmunol.181.9.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 61.Horikawa M, Weimer ET, DiLillo DJ, Venturi GM, Spolski R, Leonard WJ, Heise MT, Tedder TF. Regulatory B cell (B10 Cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J Immunol. 2013;190:1158–1168. doi: 10.4049/jimmunol.1201427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang M, Deng J, Liu Y, Ko KH, Wang X, Jiao Z, Wang S, Hua Z, Sun L, Srivastava G, Lau CS, Cao X, Lu L. IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am J Pathol. 2012;180:2375–2385. doi: 10.1016/j.ajpath.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 63.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125:1114–1124. e1118. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 65.Hussaarts L, van der Vlugt LE, Yazdanbakhsh M, Smits HH. Regulatory B-cell induction by helminths: implications for allergic disease. J Allergy Clin Immunol. 2011;128:733–739. doi: 10.1016/j.jaci.2011.05.012. [DOI] [PubMed] [Google Scholar]