Abstract
The expression of neurotensin (NT) and its receptor (NTR1) is up-regulated in experimental colitis and inflammatory bowel disease; NT/NTR1 interactions regulate gut inflammation. During active inflammation, metabolic shifts toward hypoxia lead to the activation of hypoxia-inducible factor (HIF)-1, which enhances vascular endothelial growth factor (VEGF) expression, promoting angiogenesis. We hypothesized that NT/NTR1 signaling regulates intestinal manifestations of hypoxia and angiogenesis by promoting HIF-1 transcriptional activity and VEGFα expression in experimental colitis. We studied NTR1 signaling in colitis-associated angiogenesis using 2,4,6-trinitrobenzenesulfonic acid–treated wild-type and NTR1-knockout mice. The effects of NT on HIF-1α and VEGFα were assessed on human colonic epithelial cells overexpressing NTR1 (NCM460-NTR1) and human intestinal microvascular-endothelial cells. NTR1-knockout mice had reduced microvascular density and mucosal integrity score compared with wild-type mice after 2,4,6-trinitrobenzenesulfonic acid treatment. VEGFα mRNA levels were increased in NCM460-NTR1 cells treated with 10−7 mol/L NT, at 1 and 6 hours post-treatment. NT exposure in NCM460-NTR1 cells caused stabilization, nuclear translocation, and transcriptional activity of HIF-1α in a diacylglycerol kinase–dependent manner. NT did not stimulate tube formation in isolated human intestinal macrovascular endothelial cells but did so in human intestinal macrovascular endothelial cells cocultured with NCM460-NTR1 cells. Our results demonstrate the importance of an NTR1–HIF-1α–VEGFα axis in intestinal angiogenic responses and in the pathophysiology of colitis and inflammatory bowel disease.
CME Accreditation Statement: This activity (“ASIP 2014 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2014 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
The term inflammatory bowel disease (IBD) is used to describe two chronic and debilitating inflammatory disorders, namely, Crohn disease (CD) and ulcerative colitis. Although genetic and environmental factors and microbes are involved in the development of both diseases,1 the etiology of IBD still remains largely unclear. The neuropeptide neurotensin (NT), via its high-affinity receptor, NTR1, is an important mediator of intestinal inflammation.2,3 NT, a 13–amino acid neuropeptide initially isolated from bovine hypothalamus,4 is expressed in the brain and the intestine.5 In the intestine, NT is expressed in the endocrine N cells, localized in the intestinal mucosa.6 Expression levels of NT and NTR1 are up-regulated in the inflamed colonic mucosa in animal models and IBD patients.2,3,7 Moreover, evidence from our and other laboratories suggests that NT/NTR-1 signaling is implicated in the pathophysiology of colitis by mechanisms involving the regulation of gene expression of inflammation-associated genes.7–11
It is well established that, during active inflammation such as IBD, there are severe metabolic shifts toward hypoxia,12–14 leading to the activation of the transcription factor hypoxia-inducible factors (HIF) 1 and 2.15,16 For example, surgical specimens of inflamed intestine contain elevated levels of HIF-1α and HIF-2α.17 Under normoxic conditions, HIF-1α is targeted for proteasomal degradation.18,19 HIF-1, as a key regulator of the physiological and pathophysiological responses to hypoxia, induces the expression of a broad genetic program, including angiogenic genes such as vascular endothelial growth factor (VEGF),20,21 by binding on the appropriate HIF-responsive elements (HREs) present on their promoter region. VEGFα is a major paracrine growth factor involved in blood vessel development as well as endothelial cell proliferation and migration, playing a central role in angiogenesis and neovascularization.22 Therefore, the regulation of HIF-1 is important for the reconstitution of the vasculature at sites of intestinal injury and inflammation. Several pro-inflammatory cytokines are able to activate HIF-1 in selected cell types in culture,23–25 whereas increased HIF signaling in mouse colon epithelium results in exacerbated colitis in vivo.26
Little is known about the ability of NT to modulate angiogenesis and neovascularization, whereas there is no evidence of a role for NT in these responses in the intestinal tract. NT and its analogue, TJN-950, induce neovascularization in the rat cornea in vivo.27 Moreover, liver tumor–initiating cells promote tumor angiogenesis via an NT/IL-8/CXCL1 signaling mechanism.28 We hypothesized that NT/NTR1 signaling promotes HIF-1 transcriptional activity in the inflamed gut, causing an increase in VEGFα expression, which may then play a role in intestinal manifestations of hypoxia and angiogenesis.
We show here that, during colitis, NTR1-deficient mice have reduced angiogenesis. We also show that NT/NTR1 signaling in human colonocytes induces HIF-1 activity in a diacylglycerol kinase (DGK)-dependent manner,29,30 causing increased expression of its downstream target VEGFα, which in turn triggers tube formation in human intestinal microvascular endothelial cells (HIMECs). We demonstrate, for the first time to our knowledge, that neuropeptide signaling can regulate the effects of the transcription factor HIF-1α, an event linked to NT-associated angiogenesis. Our results collectively demonstrate the importance of NTR1–HIF-1α–VEGFα interactions in the pathophysiology of colitis and the intestinal angiogenic response.
Materials and Methods
Antibodies and Reagents
We used the following antibodies and reagents: anti–HIF-1α polyclonal antibody (1:500, ab2185; Abcam plc, Cambridge, UK), β-actin monoclonal antibody (1:1000, 130065; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), β-tubulin polyclonal antibody (1:1000, PA1-41331; Thermo Fischer Scientific Inc., Rockford, IL), NT (048-03; Phoenix Biotechnology, Inc., San Antonio, TX), NTR-1 inhibitor SR-48692 (3721; Tocris Bioscience, Bristol, UK), HIF-1α inhibitor PX-478 (202350; MedKoo Biosciences, Inc., Chapel Hill, NC), DGK inhibitor II (266788; Calbiochem-Novabiochem Corp., San Diego, CA), VEGFα inhibitor CBO-P11 (676496; EMD Millipore, Billerica, MA), and basic fibroblast growth factor (13256-029; Life Technologies Corp, Carlsbad, CA).
Transduction of NCM460 Cell Line with NTR1
The human neurotensin receptor 1 gene (NTSR1) was isolated from pCR2.131 with EcoRV and inserted into lentiviral backbone CMV-IRES-GFP-PGK-Puro (cytomegalovirus, internal ribosome entry site, green fluorescent protein, phosphoglycerate kinase, puromycin resistance gene) at the EcoRV site, 5′ to internal ribosome entry site. Lentiviral particles expressing NTRS1 were generated by transient cotransfection of 293T cells with a three-plasmid combination, as previously described.32 After transduction, NCM460-NTR1 cells were maintained as described below in Cell Cultures.
Cell Cultures
Human colonic epithelial cells (NCM460-NTR1) were cultivated in M3D medium (Incell Corporation, LLC, San Antonio, TX) supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 1% L-glutamine, 10 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in air supplemented with 5% CO2. Where indicated, cells were treated with 10−7 mol/L NT (30 minutes or 6 hours), in the presence of 7 × 10−7 VEGFα inhibitor CBO-P11, 30 minutes of pretreatment with 10−7 mol/L NTR1 antagonist SR-48692, 30 minutes of pretreatment with 3 × 10−6 mol/L DGK inhibitor II, or 18 hours of pretreatment with 40 × 10−6 mol/L HIF-1α inhibitor PX-478. Fractionation into cytoplasmic and nuclear preparations was performed where indicated using the Nuclei EZ Prep nuclear isolation kit (Sigma-Aldrich, St. Louis, MO).
HIMECs were isolated as previously described.33 Briefly, HIMECs were obtained from normal areas of the intestine from patients admitted for bowel resection. HIMECs were isolated by enzymatic digestion and subsequently cultured in MCDB131 medium (Sigma-Aldrich) supplemented with 20% fetal bovine serum (BioWhittaker, Inc., Walkersville, MD), antibiotics (BioWhittaker), heparin (Sigma-Aldrich), and endothelial cell growth factor (Hybridoma Core Facility, Cleveland, OH). Cultures of HIMECs were maintained at 37°C in 5% CO2. HIMECs were used between passages 7 and 12.
Gel Electrophoresis and Immunoblot Analysis
Protein samples were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis according to Laemmli34 and were transferred to polyvinylidene difluoride membranes in 25 mmol/L Tris, 192 mmol/L glycine. Membranes were blocked (phosphate-buffered saline, 10% nonfat dry milk, 0.05% Tween 20) and probed with antibodies, followed by corresponding horseradish peroxidase–labeled secondary antibodies (1:1000). Blots were developed with enhanced chemiluminescence reagent (PerkinElmer Inc., Waltham, MA). Western blot bands were quantified using the LAS-4000 mini image analyzer (Fujifilm Medical Systems USA, Inc., Stamford, CT). Data are represented by cropped images from the original membranes. Loading controls (β-actin or β-tubulin) were derived from the same original membranes.
Luciferase Assays Using Reporters Based on HRE Promoter Fragments
NCM460-NTR1 cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with HRE-luciferase, a pGL2 vector containing three hypoxia response elements from the Pgk-1 gene upstream of firefly luciferase (26731; Addgene, Inc., Cambridge, MA). Thymidine kinase (TK)-Renilla luciferase was cotransfected to control for transfection efficiency. Forty-eight hours after transfection, cells were pretreated with 3 × 10−6 mol/L DGK inhibitor II (30 minutes), followed by 10−7 mol/L NT, and cell extracts were prepared for luciferase activity to be measured using the Dual Luciferase Reporter Assay System (Promega Corp., Madison, WI).
Enzyme-Linked Immunosorbent Assay
Immunoassays of NCM460-NTR1 cell levels of VEGFα and HIF-1α (DY293B-05 and DYC1935-5, respectively; R&D Systems Inc., Minneapolis, MN) were performed according to the manufacturer's instructions.
Tube-Formation Assay
HIMECs (4 × 104 cells/well) were plated on 400 μL of Matrigel-coated (BD Biosciences, San Jose, CA), 24-well plate and supplemented with complete MCDB131 medium. In coculture experiments, NCM460-NTR1 1 × 103 cells per well was added to the HIMEC cultures. HIMECs or HIMEC/NCM460-NTR1 cocultures were serum-starved for 2 hours and then supplemented with M3D/MCDB131 medium mix (supplemented as indicated with 10−7 mol/L NT and/or 7 × 10−7 mol/L VEGF inhibitor CBO-P11). After 6 hours, cells were labeled with calcein (Life Technologies, Grand Island, NY) for 30 minutes according to the manufacturer's instructions and were visualized under a Leica DMI6000 microscope (Leica Microsystems Wetzlar GmbH, Wetzlar, Germany). Images were captured with an Orca-R2 ER-150 CCD camera (Hamamatsu Photonics K.K., Bridgewater, NJ) and processed with Photoshop CS5 version 12 (Adobe Systems Inc., San Jose, CA), as previously described.35 Relative total tube length present in each entire field (pixels/pixels2) was measured using ImageJ version 1.46d (NIH, Bethesda, MD).
Colitis Mouse Model
Animal studies were approved by the institutional Animal Care and Use Committee. Animals were maintained at the UCLA Animal Research Facility and received standard pelleted chow and water ad libitum. 2,4,6-Trinitrobenzenesulfonic acid (TNBS) colitis was induced as previously described36 in 8- to 12-week-old male homozygous NTR1-deficient (NTR1-KO) mice and control littermates (n = 8 per group) by 100-μL intracolonic injection of 250 mg/kg TNBS (Fluka Chemical Corp., Ronkonkoma, NY). Control groups were injected with 100 μL of 30% ethanol intracolonically. Mice were returned to their cages and sacrificed 48 hours post colitis induction by carbon dioxide. Mice that received intracolonic administration of 300 μg/kg NT (twice a day for 4 days post colitis induction) were sacrificed 6 days after colitis induction. Colon tissues were isolated and dissected for further analysis.
Quantitative RT-PCR
Total RNA from mouse or human colons was isolated using standard TRIzol reagent protocol (Life Technologies), and cDNA was prepared using the miRCURY LNA Universal RT miRNA PCR cDNA kit (Exiqon, Woburn, MA). Quantitative RT-PCR (qPCR) for mRNAs of interest was performed using the specific primers obtained (Applied Biosystems Inc., Foster City, CA) according to the manufacturer's instructions.
IHC Analysis
Colon tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Tissue sections were prepared by the Translational Pathology Core Laboratory (UCLA). Sections were blocked and incubated with either rabbit polyclonal von Willebrand Factor (vWF) antibody (AB7356; EMD Millipore) or mouse monoclonal CD-31 antibody (550274; BD Pharmingen; BD Biosciences, San Jose, CA) overnight at 4°C. After washing, sections were incubated with donkey anti-rabbit IgG, and slides were stained with an ABC kit for color development (sc-2018; Santa Cruz Biotechnology, Inc.). Sections were photographed under the microscope, and computerized vWF-stained or CD-31–stained image analysis was performed using the Scion Image Software (Scion Corp., Frederick, MD) as previously described.37–39
Histological Scoring
Colon tissues of mice were sectioned and stained with hematoxylin and eosin. Microphotographs (original magnification, ×20) were recorded at multiple locations of colons and analyzed in a blinded manner (K.B., I.K.M.L.), with specimens scored on a scale of 0 to 6 for mucosal integrity, 0 to 3 for neutrophil infiltration, and 0 to 1 for mucosal edema, according to a previously described scoring system for acute colitis.40
Results
NT/NTR1 Signaling Is Required for Colitis-Associated Neovascularization
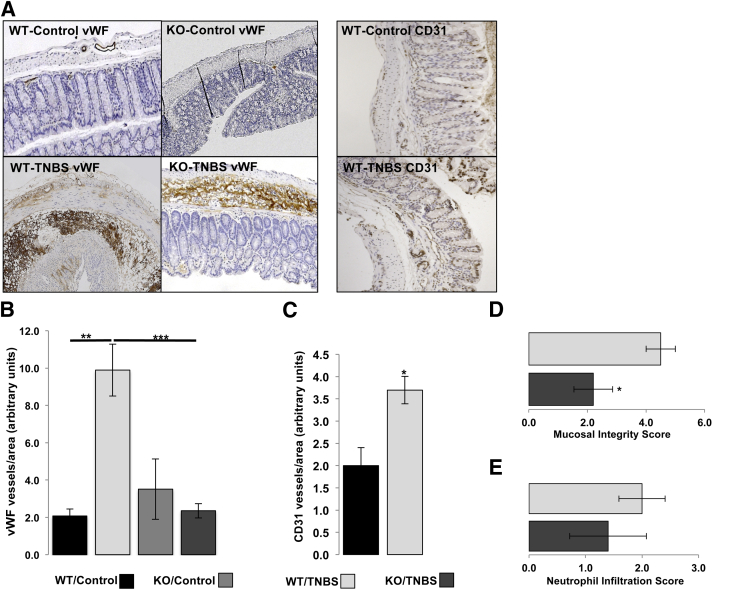
It has been previously shown that angiogenesis is an integral component of IBD pathogenesis, with vascular density being increased in the intestine of IBD patients.37,41 NT signaling has been connected to tumor angiogenesis in the liver via NT/IL-8/CXCL1 signaling pathway.28 To assess the potential significance of NT/NTR1 signaling in IBD with regard to angiogenesis, we compared the vascular density between WT and NTR1-KO mice after 2 days' treatment with TNBS. Immunohistochemistry analysis using an anti-vWF antibody showed that while colonic angiogenesis was increased significantly in TNBS-treated WT mice compared with that in untreated animals (P = 0.0055), TNBS-treated NTR1-KO mice showed significantly less vWF-positive cells (P = 0.0007), at levels comparable to untreated NTR1-KO controls (Figure 1, A and B). To verify the increased angiogenic response after 2 days of TNBS-induced colitis, we also performed immunohistochemistry analysis using an anti–CD-31 antibody and found increased CD-31–positive cells in the colonic mucosa of TNBS-exposed versus control mice (P = 0.02785) (Figure 1, A and C). Moreover, histological scoring of TNBS-treated mice showed that TNBS-associated mucosal integrity score was significantly improved in NTR1-KO mice compared with that in TNBS-exposed WT mice (P = 0.0334) (Figure 1D). Neutrophil infiltration was increased in the TNBS-exposed colon of WT mice, with numerically lower levels in NTR1-KO mice, but the results did not reach statistical significance (Figure 1E). These results suggest that NTR1 is important for colitis outcome, consistent with findings from a prior study using a different colitis model,42 and that it is required for inflammation-driven angiogenesis.
Figure 1.
NTR1-KO mice or WT littermates received (or did not receive) intracolonic enema of TNBS (250 mg/kg). Forty-eight hours later. mice were sacrificed; colon tissues were collected, fixed in formalin, and embedded in paraffin; and endothelial cells were stained with von Willebrand factor (vWF) antibody or CD31 antibody. A: Representative microphotographs of vWF- and CD31-stained colon tissues. B: Relative vWF+ cells (endothelial cells/mm2) in colon tissues. C: Relative CD31+ cells (endothelial cells/mm2) in colon tissues. Comparison between mucosal integrity (D) and neutrophil infiltration scores40 (E) given for TNBS-treated NTR1-KO mice or WT littermates. Results are representative or two separate experiments. Data are expressed as means ± SEM (B–E). n = 8 mice per group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (Student's t-test). Original magnification, ×5 (A).
NT Stimulates VEGFα Expression in Vitro and in Vivo
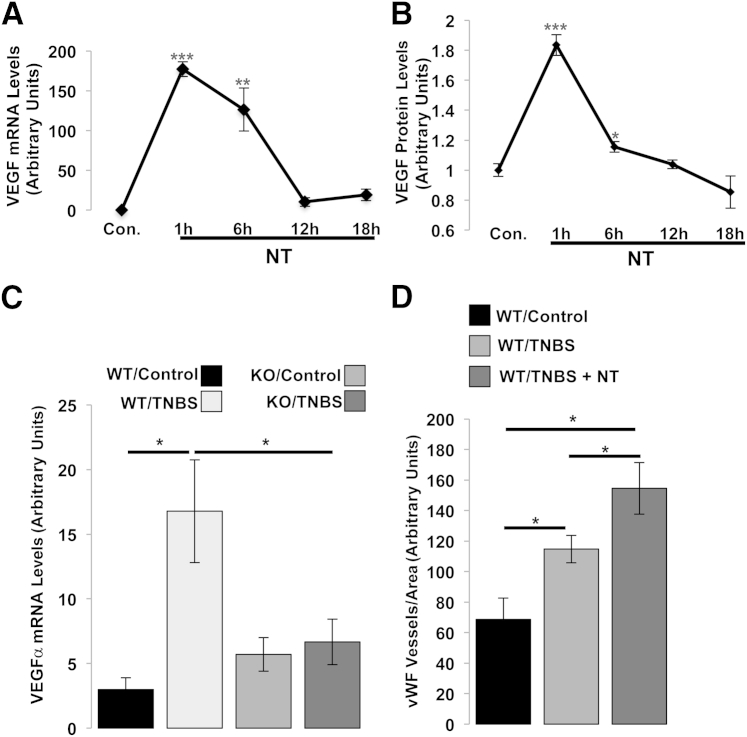
NT and its cognate receptor, NTR1, are involved in intestinal inflammation and experimental colitis.2,3,42 The results shown in Figure 1, A and B also indicate that NT/NTR1 activation is involved in colitis-associated angiogenesis. Since VEGFα is a major angiogenic factor linking angiogenesis and IBD pathogenesis,43 we examined the association of NTR1 signaling with VEGFα expression. Human colonic epithelial cells stably expressing NTR1 (NCM460-NTR1) were exposed to 10−7 mol/L NT, total RNA was isolated, and qPCR was performed to assess VEGFα levels of expression. We found that VEGFα expression was significantly increased at 1 and 6 hours after NT treatment (P < 0.0001 and P = 0.0082, respectively), whereas after 12 and 18 hours' incubation, VEGFα levels were statistically indistinguishable from controls (Figure 2A). We further examined the protein level of VEGF using enzyme-linked immunosorbent assay and found significantly increased VEGF expression in NCM460-NTR1 cell lysates after 1 and 6 hours of treatment with 10−7 mol/L NT (P < 0.0001 and P = 0.0176, respectively) (Figure 2B). These results indicate that NT/NTR1 signaling increases VEGF at both the transcriptional and the translational levels.
Figure 2.
A: NT/NTR1 signaling promotes the expression of VEGF. NCM460-NTR1 cells were treated as indicated with 10−7-mol/L NT. VEGF mRNA levels were assessed by qPCR. Results are representative of two separate experiments. B: VEGF protein levels were assessed at indicated time points by enzyme-linked immunosorbent assay. C: NTR1-KO mice or WT littermates received as indicated intracolonic enema of TNBS (250 mg/kg). Forty-eight hours later, mice were sacrificed and colon tissues were collected for RNA isolation. The levels of VEGFα expression were detected by qPCR. Results are representative of two separate experiments. D: C57BL6J mice received intracolonic enema of 250 mg/kg TNBS, and 48 hours later mice received intracolonic enema of 300 μg/kg NT twice a day (8 hours apart) for the following 4 days. Mice were sacrificed on the 6th day, colon tissues were isolated, and VEGF mRNA levels in colon samples were assessed by qPCR. Results are representative of two separate experiments. Data are expressed as means ± SEM. n = 3 (A); n = 6 (B); n = 8 per group (C and D). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (Student's t-test).
Increased VEGFα mRNA Expression during TNBS-Induced Colitis Is NTR1 Dependent
To confirm the in vivo significance of NTR1-induced VEGFα mRNA expression in NCM460-NTR1 cells, we compared VEGFα mRNA levels between WT and NTR1-KO mice after 2 days of intracolonic TNBS exposure. Real-time PCR showed that, compared with vehicle administration, colonic VEGFα mRNA levels were increased in response to TNBS administration in WT mice (P = 0.0131) (Figure 2C). Interestingly, TNBS-treated NTR1-KO mice showed no significant increase in VEGFα mRNA levels compared with vehicle-treated NTR1-KO animals and had significantly lower VEGFα levels compared with TNBS-treated WT mice (P = 0.0423). On the other hand, the significant increase in vWF staining in TNBS-treated mouse colons compared with controls (P = 0.0375) was further increased after intracolonic administration of NT (P = 0.0273 versus controls and P = 0.0419 versus TNBS-treated colons) (Figure 2D). These data collectively suggest that NT/NTR1 signaling has a role in colitis-induced angiogenesis.
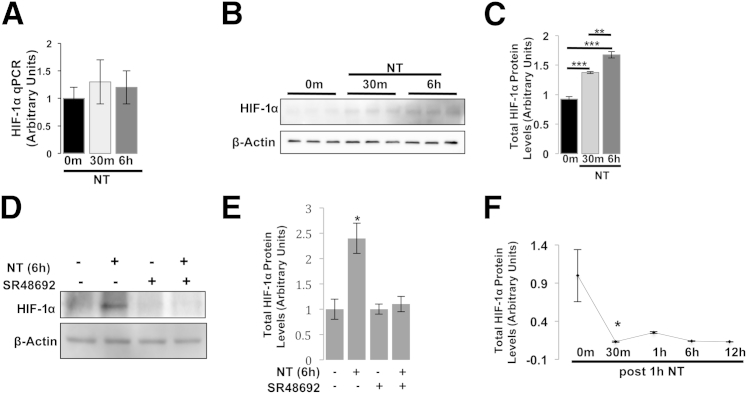
NT Signaling Promotes HIF-1α Stabilization in NCM460-NTR1 Cells
Active inflammation such as IBD is characterized by metabolic shifts toward hypoxia.12–14 Under these conditions, the HIF, a transcription factor promoting VEGFα expression,20,21 is activated. We therefore hypothesized that the induction of VEGFα expression in NCM460-NTR1 cells after NT treatment involves NTR1-induced HIF-1α activation. To address this hypothesis, NCM460-NTR1 cells were treated with 10−7 mol/L NT for 30 minutes or 6 hours, and total cell lysates were collected for mRNA and protein analyses. Using qPCR, we showed that HIF-1α levels were not increased after NT treatment (Figure 3A). However, Western blot analysis revealed that HIF-1α protein accumulates in the cytosol (30 minutes, P = 0.0006; 6 hours, P = 0.0004; and P = 0.0063 for 30 minutes versus 6 hours) (Figure 3, B and C). Importantly, this NT response was reversed when cells were pretreated with the specific NTR1 antagonist SR48692 (Figure 3, D and E). To further examine this response, we performed a pulse-chase enzyme-linked immunosorbent assay for HIF-1α. In short, NCM460-NTR1 cells were treated with 10−7 mol/L NT for 1 hour. Cells were then put in NT-free media, and cell lysates were prepared at the indicated time points. Our results showed that the increase in HIF-1α total levels significantly subsided as soon as 30 minutes after the removal of NT from the culture medium (P = 0.0351) (Figure 3F). These results suggest that NT signaling inhibits HIF-1α degradation in NCM460-NTR1 cells in an NTR1-dependent manner.
Figure 3.
NT promotes HIF-1α protein stabilization. NCM460-NTR1 cells were treated as indicated with 10−7-mol/L NT. A: HIF-1α mRNA levels were assessed by qPCR. HIF-1α protein levels were assessed by Western blot analysis (B) and band densitometry (C). D: NCM460-NTR1 cells were pretreated as indicated with 10−7 mol/L NTR1 inhibitor SR48692 (30 minutes) and/or 10−7 mol/L NT, and HIF-1α protein levels were assessed using WB analysis and band densitometry (E). F: NCM460-NTR1 cells were treated with 10−7 mol/L NT for 1 hour, stimulus was removed by switching to complete media without NT, and HIF-1α protein levels were assessed at indicated time points by enzyme-linked immunosorbent assay. Results are representative of two separate experiments. Data are expressed as means ± SEM. n = 3. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (Student's t-test).
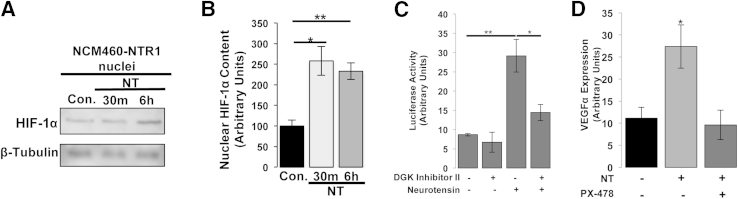
NT Signaling Promotes HIF-1α Nuclear Translocation and Transcriptional Activity in NCM460-NTR1 Cells
We next investigated whether NT signaling promotes activation, nuclear translocation, and transcriptional activity of HIF-1α. Western blot analysis performed on isolated nuclei and cytoplasmic cell fractions in NCM460-NTR1 cells incubated with NT revealed that NT promotes nuclear translocation of HIF-1α (Figure 4, A and B). We next assessed transcriptional activity of HIF-1 using an HRE-luciferase reporter plasmid. NCM460-NTR1 cells were cotransfected with HRE-luciferase and TK-Renilla reporter plasmids, and 48 hours post transfection, cells were treated with 10−7 mol/L NT or vehicle for 6 hours. We found that luciferase activity was significantly increased after 6 hours of NT treatment (P = 0.0085) (Figure 4C). Previous reports indicated that hypoxia results in the accumulation of the intracellular phosphatidic acid levels through the action of DGK, whereas the pharmacological inhibition of DGK prevents HIF-1–dependent transcription.29,30 We found that pretreatment of cells with 3 × 10−6 mol/L DGK inhibitor II (30 minutes) significantly attenuated HIF-1α–induced luciferase activity in response to NT (P = 0.0356), indicating that NT/NTR1 signaling promotes HIF-1α stabilization and subsequent transcriptional activity in a DGK-dependent manner (Figure 4C). To link NT-dependent VEGFα expression to HIF-1α transcriptional activity, we pretreated NCM460-NTR1 cells with the HIF-1α inhibitor PX-478. Our results show that pharmacological inhibition of HIF-1α diminished NT-dependent VEGFα expression (P = 0.0257) (Figure 4D). Thus, NTR1 activation results in stabilization/activation, nuclear translocation, and transcriptional activity of HIF-1α, leading to increased VEGF expression in a HIF-1α–dependent manner.
Figure 4.
NT promotes HIF-1α protein activation, nuclear translocation, and transcriptional activity. NCM460-NTR1 cells were treated as indicated with 10−7 mol/L NT, and HIF-1α protein levels in isolated nuclei were assessed by WB analysis (A) and band densitometry (B). C: NCM460-NTR1 cells were transfected with HRE-luciferase reporter plasmid, treated as indicated with 3 × 10−6 DGK inhibitor II and/or 10−7 mol/L NT (6 hours), and luciferase activity was assessed using the Dual Luciferase Reporter Assay System. D: NCM460-NTR1 cells were treated as indicated with 10−7 mol/L NT (6 hours), with or without 18hour pretreatment with 40 × 10−6 mol/L HIF-1α inhibitor PX-478, and the expression of VEGFα was assessed by qPCR. Results are representative of three separate experiments. Data are expressed as means ± SEM (B–D). n = 3. ∗P < 0.05 and ∗∗P < 0.01 (Student's t-test).
NT Signaling Is Pro-Angiogenic
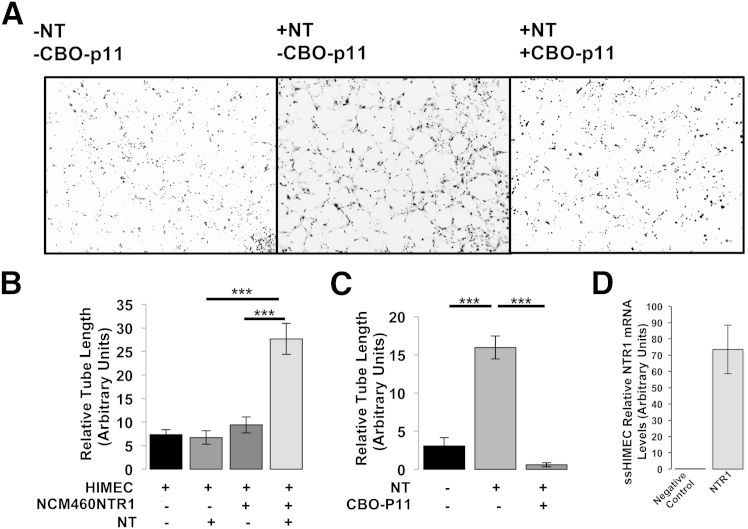
We finally tested whether the NT-dependent induction of VEGFα expression in NCM460-NTR1 cells promotes angiogenesis in vitro by measuring tube formation of HIMECs. We first measured NTR1 mRNA and found that HIMECs express detectable levels of NTR1 (Figure 5D). However, direct exposure of HIMECs to 10−7 mol/L NT (6 hours) did not result in increased tube formation when these cells were cultured alone on Matrigel (Figure 5B), whereas exposure of the same cells to basic fibroblast growth factor resulted in increased tube formation serving as a positive control (data not shown). In contrast, NT administration to HIMECs cocultured with NCM460-NTR1 cells showed a significant increase in tube formation compared with HIMECs exposed to NT or HIMECs co-cultured with NCM460-NTR1 cells in the absence of NT exposure (P < 0.0001) (Figure 5, A and B). Importantly, with the exposure of HIMEC/NCM460-NTR1 coculture to 10−7 mol/L NT (6 hours) in the presence of 7 × 10−7 mol/L VEGFα inhibitor CBO-P11, this effect was reversed (P < 0.0001) (Figure 5, A and C). These results indicate that NT increases angiogenesis in HIMECs by promoting VEGFα expression in intestinal epithelial cells.
Figure 5.
Human intestinal microvascular-endothelial cells (HIMECs) were subjected to Matrigel tube formation assay in coculture with NCM460-NTR1 cells and treated as indicated with 10−7 mol/L NT and 7 × 10−7 mol/L VEGFα inhibitor CBO-P11. Representative microphotographs of randomly selected fields (A) were used to calculate total tube length per area unit (pixel/pixel2) using ImageJ software (B and C). D: Negative control (adiponectin) and NTR1 mRNA levels were assessed by qPCR. Results are representative of four separate experiments. Data are expressed as means ± SEM (B–D). n = 3. ∗∗∗P < 0.001 (Student's t-test). Original magnification, ×10 (A).
Discussion
Previous studies have indicated that the expression of the neuropeptide NT and its high-affinity receptor, NTR1, is upregulated in the colonic mucosa of animal models of intestinal inflammation and in colon tissue samples from IBD patients.2,3,7 We and others also reported that NT/NTR1 interactions promote acute intestinal inflammation of different etiologies.2,3,7,42 Here we show that, compared with WT, animals lacking NTR1 have significantly lower intestinal vascularity during colitis and that this effect is associated with reduced histological damage during acute, TNBS-induced colitis. Our in vitro and in vivo evidence also indicates that NT influences angiogenesis by stimulating increased expression of the proangiogenic factor VEGFα in colonocytes, which in turn stimulates tube formation by acting on HIMECs. We also present evidence that the mechanism of VEGFα activation in colonocytes in response to NT involves the activation of the transcription factor HIF-1α. This HIF-1α NT response was rather specific since NT failed to stabilize HIF-2 (data not shown), another VEGF regulator in our experimental model. To our knowledge, this is the first report to indicate that NT regulates angiogenesis in the inflamed intestine.
Our results indicate that NT does not increase tube formation when exposed to HIMEC in vitro, despite the presence of NTR1 in these cells. A low level of NTR1 expression may be involved in this lack of response. Previous results have indicated limited NT-induced activation of human colonic NCM460 epithelial cells, which express low levels of endogenous NTR1, but increased NTR1-associated NF-κB and mitogen-activated protein kinase signaling in NCM460 overexpressing this receptor.9,11,31 Similarly, there is increased expression of NTR1 in the colonic mucosa in colitis and IBD compared with levels of expression in normal colon.3,7,44 Previous studies have also demonstrated the possibility that NT is involved in angiogenic responses.27,28 The administration of an NT analogue enhanced the migration of human umbilical vein endothelial cells and induced neovascularization in the rat cornea in vivo.27 Moreover, liver tumor–initiating cells promoted tumor angiogenesis in vivo via an NT/IL-8/CXCL1 signaling mechanism.28
We found that, in colonic epithelial cells, NT activates HIF-1α by a mechanism that involves stabilization, nuclear translocation, and transcriptional activity of HIF-1α. With pretreatment of cells with DGK inhibitor II, the effect of NT/NTR1 signaling on HIF-1α transcriptional activity was partially reversed. This finding suggests that HIF-1α activation in our system is driven, at least in part, by previously described molecular pathways involving the activation of a G-protein–coupled receptor and the subsequent intracellular accumulation of phosphatidic acid, through the action of DGK.29 Phosphatidic acid is a known oxygen sensor since it accumulates during hypoxic conditions, leading to the activation of HIF-1α.30 With pretreatment of cells with the specific HIF-1α inhibitor PX-478, VEGFα expression in response to NT was substantially reduced, suggesting that NT/NTR1-induced VEGFα expression involves, at least in part, the activation of HIF-1α. This idea is consistent with molecular evidence demonstrating that the promoter region of VEGFα contains HREs.20,21 However, several other transcription factors have binding sites on the VEGFα promoter, including early growth response factor 1,45 a factor activated by NTR1 signaling,10,46 whereas insulin-like growth factor I receptor, which is transactivated by NTR1,11 also regulates VEGFα transcription in human colonic epithelial cells.47
During the clinical course of IBD, there is extensive tissue injury caused by tissue edema, increased inflammatory cell infiltrates, and increased angiogenesis, characteristic of the severe metabolic shifts toward hypoxia, a process known to promote inflammation.12–14,48 Hypoxic conditions evident in the colonic mucosa in colitis play a central role in the pathophysiology of intestinal inflammation and IBD,49,50 in part by regulating HIF-1α activation and inflammation-dependent angiogenesis in colitis.49,50 Our evidence suggests that NT can influence angiogenesis by promoting intestinal inflammation, which leads to hypoxia and HIF-1α–driven increased angiogenesis, as well as by directly activating HIF-1α on intestinal epithelial cells, leading to increased VEGFα expression and increased angiogenesis. Moreover, increased intestinal angiogenesis, by itself, regulates the development and progress of colitis,51 in part by facilitating the entry and distribution of immune cells in the colonic mucosa.52
A key molecule in the induction of angiogenesis is VEGFα, a paracrine growth factor involved in blood vessel development as well as endothelial cell migration. Up-regulated angiogenesis in the colon during active disease is a key component of IBD driven to a large extent by VEGFα expression,43,53 which represents a target for IBD treatment.46 Accordingly, VEGFα expression is elevated in the serum and colon of IBD patients.43,54 Our results show that NT treatment of NCM460-NTR1 colonic epithelial cells was associated with increased VEGFα mRNA and protein levels, whereas the in vivo increase in VEGFα expression after TNBS treatment was lower in NTR1-KO mice. The source of NT in this pathway includes neither human colonic epithelial cells nor HIMECs since these cells do not express NT mRNA (data not shown), excluding the possibility of an autocrine signaling axis. Collectively, our results underline an important role for NT in colitis-associated angiogenesis by an NTR1-driven signaling network that involves HIF-1–regulated VEGFα expression (Figure 6).
Figure 6.
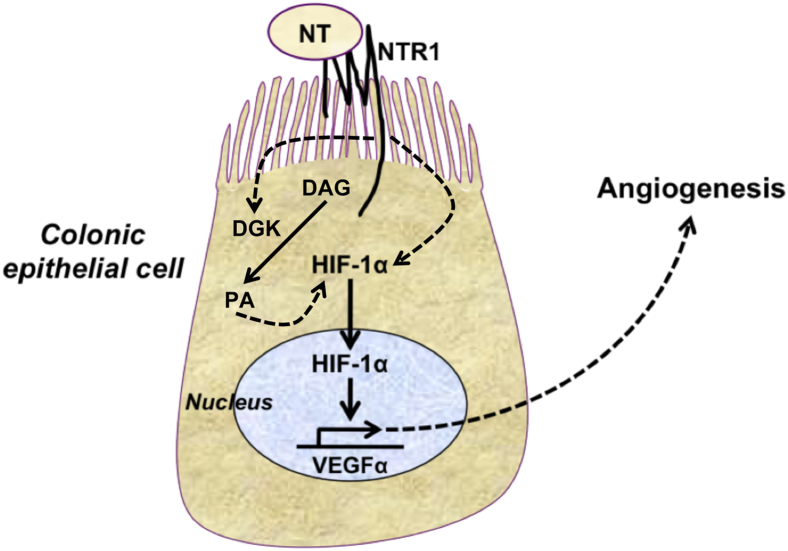
Schematic representation of NT-NTR1 interactions in intestinal epithelial cells leading to stimulation of intracellular signaling pathways during colitis and involving the transcription factor HIF-1α and VEGFα and angiogenesis. NTR1 signaling activates HIF-1α transcriptional activity via pathways that at least in part involve the activation of DGK, an enzyme that catalyzes the conversion of diacylglycerol (DAG) to phosphatidic acid (PA). The accumulation of PA leads to increased expression of HIF-1α, promoting HIF-1α–dependent pathways. Solid arrows represent direct interactions. Dotted arrows represent indirect and hypothetical interactions based on the available evidence.
Acknowledgments
We thank the Morphology and Cell Imaging Core of the CURE Center for assisting with the imaging studies and the Translational Pathology Core Laboratory at UCLA for processing colonic tissue sections.
Overall project design and hypotheses were developed by K.B. and C.P. K.B. conducted experiments, analyzed the data, and wrote the manuscript. I.K.M.L. performed some of the signaling cellular experiments. G.W. and C.F. contributed to the design and execution of the angiogenesis-related studies. D.I. contributed to experimental design and data interpretation. All authors participated in revising the manuscript and agreed to the final version. C.P. supervised the overall project.
Footnotes
Supported by grants from the NIH (DK60729, 1P50 DK64539, and DK 47373 to C.P.; DK50984 and DK69854 to C.F.), a fellowship grant award from the Crohn's and Colitis Foundation of America, Inc. (K.B.), the Blinder Research Foundation for Crohn's Disease (I.K.M.L.), and the Morphology and Cell Imaging Core of the CURE Center (CURE:DDRCC DK41301).
Disclosures: None declared.
References
- 1.Sands B.E. Inflammatory bowel disease: past, present, and future. J Gastroenterol. 2007;42:16–25. doi: 10.1007/s00535-006-1995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castagliuolo I., Wang C.C., Valenick L., Pasha A., Nikulasson S., Carraway R.E., Pothoulakis C. Neurotensin is a proinflammatory neuropeptide in colonic inflammation. J Clin Invest. 1999;103:843–849. doi: 10.1172/JCI4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koon H.W., Kim Y.S., Xu H., Kumar A., Zhao D., Karagiannides I., Dobner P.R., Pothoulakis C. Neurotensin induces IL-6 secretion in mouse preadipocytes and adipose tissues during 2,4,6,-trinitrobenzensulphonic acid-induced colitis. Proc Natl Acad Sci U S A. 2009;106:8766–8771. doi: 10.1073/pnas.0903499106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carraway R., Leeman S.E. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- 5.Uhl G.R., Goodman R.R., Snyder S.H. Neurotensin-containing cell bodies, fibers and nerve terminals in the brain stem of the rat: immunohistochemical mapping. Brain Res. 1979;167:77–91. doi: 10.1016/0006-8993(79)90264-6. [DOI] [PubMed] [Google Scholar]
- 6.Helmstaedter V., Feurle G.E., Forssmann W.G. Ultrastructural identification of a new cell type–the N-cell as the source of neurotensin in the gut mucosa. Cell Tissue Res. 1977;184:445–452. doi: 10.1007/BF00220968. [DOI] [PubMed] [Google Scholar]
- 7.Brun P., Mastrotto C., Beggiao E., Stefani A., Barzon L., Sturniolo G.C., Palù G., Castagliuolo I. Neuropeptide neurotensin stimulates intestinal wound healing after chronic intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G621–G629. doi: 10.1152/ajpgi.00140.2004. [DOI] [PubMed] [Google Scholar]
- 8.Bakirtzi K., Hatziapostolou M., Karagiannides I., Polytarchou C., Jaeger S., Iliopoulos D., Pothoulakis C. Neurotensin signaling activates microRNAs-21 and -155 and Akt, promotes tumor growth in mice, and is increased in human colon tumors. Gastroenterology. 2011;141:1749–1761.e1. doi: 10.1053/j.gastro.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao D., Kuhnt-Moore S., Zeng H., Wu J.S., Moyer M.P., Pothoulakis C. Neurotensin stimulates IL-8 expression in human colonic epithelial cells through Rho GTPase-mediated NF-kappa B pathways. Am J Physiol Cell Physiol. 2003;284:C1397–C1404. doi: 10.1152/ajpcell.00328.2002. [DOI] [PubMed] [Google Scholar]
- 10.Zhao D., Zhan Y., Zeng H., Koon H.W., Moyer M.P., Pothoulakis C. Neurotensin stimulates expression of early growth response gene-1 and EGF receptor through MAP kinase activation in human colonic epithelial cells. Int J Cancer. 2007;120:1652–1656. doi: 10.1002/ijc.22407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao D., Bakirtzi K., Zhan Y., Zeng H., Koon H.W., Pothoulakis C. Insulin-like growth factor-1 receptor transactivation modulates the inflammatory and proliferative responses of neurotensin in human colonic epithelial cells. J Biol Chem. 2011;286:6092–6099. doi: 10.1074/jbc.M110.192534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuta G.T., Turner J.R., Taylor C.T., Hershberg R.M., Comerford K., Narravula S., Podolsky D.K., Colgan S.P. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokura S., Yoshida N., Yoshikawa T. Anoxia/reoxygenation-induced leukocyte-endothelial cell interactions. Free Radic Biol Med. 2002;33:427–432. doi: 10.1016/s0891-5849(02)00852-3. [DOI] [PubMed] [Google Scholar]
- 14.Saadi S., Wrenshall L.E., Platt J.L. Regional manifestations and control of the immune system. FASEB J. 2002;16:849–856. doi: 10.1096/fj.01-0690hyp. [DOI] [PubMed] [Google Scholar]
- 15.Semenza G.L. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 16.Pugh C.W., Ratcliffe P.J. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 17.Giatromanolaki A., Sivridis E., Maltezos E., Papazoglou D., Simopoulos C., Gatter K.C., Harris A.L., Koukourakis M.I. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003;56:209–213. doi: 10.1136/jcp.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maxwell P.H., Wiesener M.S., Chang G.W., Clifford S.C., Vaux E.C., Cockman M.E., Wykoff C.C., Pugh C.W., Maher E.R., Ratcliffe P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 19.Tanimoto K., Makino Y., Pereira T., Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semenza G.L. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 2003;54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- 21.Feinman R., Deitch E.A., Watkins A.C., Abungu B., Colorado I., Kannan K.B., Sheth S.U., Caputo F.J., Lu Q., Ramanathan M., Attan S., Badami C.D., Doucet D., Barlos D., Bosch-Marce M., Semenza G.L., Xu D.Z. HIF-1 mediates pathogenic inflammatory responses to intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2010;299:G833–G843. doi: 10.1152/ajpgi.00065.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoeben A., Landuyt B., Highley M.S., Wildiers H., Van Oosterom A.T., De Bruijn E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 23.Hellwig-Bürgel T., Rutkowski K., Metzen E., Fandrey J., Jelkmann W. Interleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1. Blood. 1999;94:1561–1567. [PubMed] [Google Scholar]
- 24.Thornton R.D., Lane P., Borghaei R.C., Pease E.A., Caro J., Mochan E. Interleukin 1 induces hypoxia-inducible factor 1 in human gingival and synovial fibroblasts. Biochem J. 2000;350 Pt 1:307–312. [PMC free article] [PubMed] [Google Scholar]
- 25.Albina J.E., Mastrofrancesco B., Vessella J.A., Louis C.A., Henry W.L., Jr., Reichner J.S. HIF-1 expression in healing wounds: HIF-1alpha induction in primary inflammatory cells by TNF-alpha. Am J Physiol Cell Physiol. 2001;281:C1971–C1977. doi: 10.1152/ajpcell.2001.281.6.C1971. [DOI] [PubMed] [Google Scholar]
- 26.Shah Y.M., Ito S., Morimura K., Chen C., Yim S.H., Haase V.H., Gonzalez F.J. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134:2036–2048. doi: 10.1053/j.gastro.2008.03.009. 2048.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ushiro S., Mizoguchi K., Yoshida S., Jimi S., Fujiwara T., Yoshida M., Wei E.T., Kitabgi P., Amagaya S., Ono M., Kuwano M. Stimulation of cell-surface urokinase-type plasminogen activator activity and cell migration in vascular endothelial cells by a novel hexapeptide analogue of neurotensin. FEBS Lett. 1997;418:341–345. doi: 10.1016/s0014-5793(97)01403-8. [DOI] [PubMed] [Google Scholar]
- 28.Tang K.H., Ma S., Lee T.K., Chan Y.P., Kwan P.S., Tong C.M., Ng I.O., Man K., To K.F., Lai P.B., Lo C.M., Guan X.Y., Chan K.W. CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology. 2012;55:807–820. doi: 10.1002/hep.24739. [DOI] [PubMed] [Google Scholar]
- 29.Temes E., Martín-Puig S., Aragonés J., Jones D.R., Olmos G., Mérida I., Landázuri M.O. Role of diacylglycerol induced by hypoxia in the regulation of HIF-1alpha activity. Biochem Biophys Res Commun. 2004;315:44–50. doi: 10.1016/j.bbrc.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Aragonés J., Jones D.R., Martin S., San Juan M.A., Alfranca A., Vidal F., Vara A., Mérida I., Landázuri M.O. Evidence for the involvement of diacylglycerol kinase in the activation of hypoxia-inducible transcription factor 1 by low oxygen tension. J Biol Chem. 2001;276:10548–10555. doi: 10.1074/jbc.M006180200. [DOI] [PubMed] [Google Scholar]
- 31.Zhao D., Keates A.C., Kuhnt-Moore S., Moyer M.P., Kelly C.P., Pothoulakis C. Signal transduction pathways mediating neurotensin-stimulated interleukin-8 expression in human colonocytes. J Biol Chem. 2001;276:44464–44471. doi: 10.1074/jbc.M104942200. [DOI] [PubMed] [Google Scholar]
- 32.Naldini L., Blömer U., Gage F.H., Trono D., Verma I.M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binion D.G., West G.A., Ina K., Ziats N.P., Emancipator S.N., Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology. 1997;112:1895–1907. doi: 10.1053/gast.1997.v112.pm9178682. [DOI] [PubMed] [Google Scholar]
- 34.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Im E., Venkatakrishnan A., Kazlauskas A. Cathepsin B regulates the intrinsic angiogenic threshold of endothelial cells. Mol Biol Cell. 2005;16:3488–3500. doi: 10.1091/mbc.E04-11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castagliuolo I., Morteau O., Keates A.C., Valenick L., Wang C.C., Zacks J., Lu B., Gerard N.P., Pothoulakis C. Protective effects of neurokinin-1 receptor during colitis in mice: role of the epidermal growth factor receptor. Br J Pharmacol. 2002;136:271–279. doi: 10.1038/sj.bjp.0704697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danese S., Sans M., de la Motte C., Graziani C., West G., Phillips M.H., Pola R., Rutella S., Willis J., Gasbarrini A., Fiocchi C. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 38.Vermeulen P.B., Gasparini G., Fox S.B., Toi M., Martin L., McCulloch P., Pezzella F., Viale G., Weidner N., Harris A.L., Dirix L.Y. Quantification of angiogenesis in solid human tumours: an international consensus on the methodology and criteria of evaluation. Eur J Cancer. 1996;32A:2474–2484. doi: 10.1016/s0959-8049(96)00379-6. [DOI] [PubMed] [Google Scholar]
- 39.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169–180. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 40.Kruschewski M., Foitzik T., Perez-Cantó A., Hübotter A., Buhr H.J. Changes of colonic mucosal microcirculation and histological examination in two colitis models: an experimental study using intravital microscopy and a new histological scoring system. Dig Dis Sci. 2001;46:2336–2343. doi: 10.1023/a:1012334727509. [DOI] [PubMed] [Google Scholar]
- 41.Rutella S., Fiorino G., Vetrano S., Correale C., Spinelli A., Pagano N., Arena V., Maggiano N., Repici A., Malesci A., Danese S. Infliximab therapy inhibits inflammation-induced angiogenesis in the mucosa of patients with Crohn's disease. Am J Gastroenterol. 2011;106:762–770. doi: 10.1038/ajg.2011.48. [DOI] [PubMed] [Google Scholar]
- 42.Bugni J.M., Rabadi L.A., Jubbal K., Karagiannides I., Lawson G., Pothoulakis C. The neurotensin receptor-1 promotes tumor development in a sporadic but not an inflammation-associated mouse model of colon cancer. Int J Cancer. 2012;130:1798–1805. doi: 10.1002/ijc.26208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scaldaferri F., Vetrano S., Sans M., Arena V., Straface G., Stigliano E., Repici A., Sturm A., Malesci A., Panes J., Yla-Herttuala S., Fiocchi C., Danese S. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136:585–595.e5. doi: 10.1053/j.gastro.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 44.Riegler M., Castagliuolo I., Wang C., Wlk M., Sogukoglu T., Wenzl E., Matthews J.B., Pothoulakis C. Neurotensin stimulates Cl(-) secretion in human colonic mucosa In vitro: role of adenosine. Gastroenterology. 2000;119:348–357. doi: 10.1053/gast.2000.9310. [DOI] [PubMed] [Google Scholar]
- 45.Pagès G., Pouysségur J. Transcriptional regulation of the Vascular Endothelial Growth Factor gene–a concert of activating factors. Cardiovasc Res. 2005;65:564–573. doi: 10.1016/j.cardiores.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 46.Gammie S.C., Lee G., Scotti M.A., Stevenson S.A., Gessay G.M. Neurotensin induced Egr-1 activity is altered in the postpartum period in mice. Brain Res. 2012;1433:47–55. doi: 10.1016/j.brainres.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 47.Akagi Y., Liu W., Zebrowski B., Xie K., Ellis L.M. Regulation of vascular endothelial growth factor expression in human colon cancer by insulin-like growth factor-I. Cancer Res. 1998;58:4008–4014. [PubMed] [Google Scholar]
- 48.Goggins B.J., Chaney C., Radford-Smith G.L., Horvat J.C., Keely S. Hypoxia and Integrin-Mediated Epithelial Restitution during Mucosal Inflammation. Front Immunol. 2013;4:272. doi: 10.3389/fimmu.2013.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colgan S.P., Taylor C.T. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glover L.E., Colgan S.P. Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology. 2011;140:1748–1755. doi: 10.1053/j.gastro.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chidlow J.H., Jr., Langston W., Greer J.J., Ostanin D., Abdelbaqi M., Houghton J., Senthilkumar A., Shukla D., Mazar A.P., Grisham M.B., Kevil C.G. Differential angiogenic regulation of experimental colitis. Am J Pathol. 2006;169:2014–2030. doi: 10.2353/ajpath.2006.051021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Alessio S., Tacconi C., Fiocchi C., Danese S. Advances in therapeutic interventions targeting the vascular and lymphatic endothelium in inflammatory bowel disease. Curr Opin Gastroenterol. 2013;29:608–613. doi: 10.1097/MOG.0b013e328365d37c. [DOI] [PubMed] [Google Scholar]
- 53.Cromer W.E., Ganta C.V., Patel M., Traylor J., Kevil C.G., Alexander J.S., Mathis J.M. VEGF-A isoform modulation in an preclinical TNBS model of ulcerative colitis: protective effects of a VEGF164b therapy. J Transl Med. 2013;11:207. doi: 10.1186/1479-5876-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanazawa S., Tsunoda T., Onuma E., Majima T., Kagiyama M., Kikuchi K. VEGF, basic-FGF, and TGF-beta in Crohn's disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. Am J Gastroenterol. 2001;96:822–828. doi: 10.1111/j.1572-0241.2001.03527.x. [DOI] [PubMed] [Google Scholar]