Abstract
Factor inhibiting hypoxia-inducible factor 1 (FIH-1; official symbol HIF1AN) is a hydroxylase that negatively regulates hypoxia-inducible factor 1α but also targets other ankyrin repeat domain–containing proteins such as Notch receptor to limit epithelial differentiation. We show that FIH-1 null mutant mice exhibit delayed wound healing. Importantly, in vitro scratch wound assays demonstrate that the positive role of FIH-1 in migration is independent of Notch signaling, suggesting that this hydroxylase targets another ankyrin repeat domain–containing protein to positively regulate motogenic signaling pathways. Accordingly, FIH-1 increases epidermal growth factor receptor (EGFR) signaling, which in turn enhances keratinocyte migration via mitogen-activated protein kinase pathway, leading to extracellular signal–regulated kinase 1/2 activation. Our studies identify leucine-rich repeat kinase 1 (LRRK1), a key regulator of the EGFR endosomal trafficking and signaling, as an FIH-1 binding partner. Such an interaction prevents the formation of an EGFR/LRRK1 complex, necessary for proper EGFR turnover. The identification of LRRK1 as a novel target for FIH-1 provides new insight into how FIH-1 functions as a positive regulator of epithelial migration.
CME Accreditation Statement: This activity (“ASIP 2014 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2014 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
The asparaginyl hydroxylase factor-inhibiting hypoxia-inducible factor 1α (FIH-1; official symbol HIF1AN) was originally identified as a protein that interacts with and inhibits the activity of hypoxia-inducible factor 1α (HIF-1α) in the C-terminal transactivation domain1,2 by coupling the oxidative decarboxylation of 2-oxoglutarate to the hydroxylation of HIF-1α.3 Significantly, proteins containing the ankyrin repeat domain, such as Notch, are other substrates for FIH-1.3 Only recently has FIH-1 been recognized to have pleiotropic roles in maintaining epithelial homeostasis.4,5 For example, FIH-1 negatively regulates glycogen metabolism in corneal epithelium in a HIF-1α–independent manner via the direct involvement of the Akt/glycogen synthase kinase 3β signaling pathway.4 Furthermore, in epidermal and corneal epithelial keratinocytes, FIH-1 was shown to act as a negative regulator of differentiation via a coordinate decrease in Notch signaling.5 What is not clear in these studies is whether FIH-1 affects other signaling pathways known to influence keratinocyte growth, differentiation, and migration. For example, the regulation of Notch 1 activity by FIH-15 raises the possibility of cross-talk with the epidermal growth factor receptor (EGFR)-signaling pathway, since EGFR signaling has been shown to be a negative regulator of Notch 1 gene expression and activity in keratinocytes.6
Once EGF binds to the EGFR, numerous signaling pathways are activated that impact on cell proliferation, migration, differentiation, and survival.7–9 With respect to the skin, EGFR impacts on epidermal and hair follicle development, keratinocyte proliferation, survival, cancer, and immune homeostasis.10 EGFR signaling also plays a prominent role in epidermal and corneal epithelial migration and wound repair. For example, in the epidermis, EGFR signaling has been shown to promote keratinocyte migration and wound repair.11 Likewise, corneal perturbations activate the EGFR and downstream Ras-Raf-Mek-Erk1/2 (Ras, Raf, mitogen-activated protein kinase kinase, extracellular signal–regulated kinase 1/2) and phosphoinositide 3 kinase–Akt signaling cascades, which are required for efficient wound healing and are attenuated in patients with diabetic keratopathies.12–14
The activation of EGFR also commences endocytic trafficking, whereby the receptor is either packaged in lysosomes for degradation or recycled to the cell surface.15,16 Endosomal trafficking is essential for establishing the extensiveness of the EGF-mediated signal, and thus much attention has been directed toward understanding the steps involved in the movement of the EGFR from the cell surface to cytoplasmic vesicles, such as the endosome, multivesicular body, and lysosome.16–18 Recently, leucine-rich repeat kinase 1 (LRRK1) was recognized as a key regulator of EGFR endosomal trafficking.19,20 Specifically, it is believed that LRRK1 forms a complex with activated EGFR through an interaction with growth factor receptor–bound protein 2 and that this complex is internalized in early endosomes.19 The mechanism by which LRRK1 regulates EGFR transport is from early to late endosomes.19
LRRK1 protein kinase is one of the ROCO proteins, which contain a GTPase-like domain [Ras of complex proteins (Roc)] and a C-terminal of Roc (COR) domain.21 ROCO proteins have a series of leucine-rich repeats and/or ankyrin repeats, with LRRK1 containing six N-terminal ankyrin repeats.22 This latter aspect of LRRK1 is noteworthy since, as mentioned above, proteins with ankyrin repeat domains are potential substrates for FIH-1.3 Thus, FIH-1 has the potential to directly interact with LRRK1, which could impact on EGFR signaling.
Here we show that ectopic expression of FIH-1 in keratinocytes increases phosphorylation of the EGFR, which positively affects keratinocyte migration via stimulation of the mitogen-activated protein kinase pathway, resulting in an increase in phosphorylated ERK1/2. Such enhanced migration is independent of Notch signaling. Moreover, our studies reveal that FIH-1 interacts with LRRK1 and prevents the formation of an EGFR/LRRK1 complex necessary for proper EGFR turnover.19 The identification of LRRK1 as a substrate for FIH-1 provides new insight into how FIH-1 functions as a positive regulator of epithelial migration. Thus, the breadth of FIH-1 epithelial biology is considerably larger than previously realized.
Material and Methods
Mice
The FIH-1 null mice were generated by breeding the FIHflox mouse with the Ella-Cre transgenic mouse.23 Wild-type (WT) mice (C57BL/6) were purchased from Charles River Laboratories. For skin wound healing assays, mice were first anesthetized, and the area assigned for wounding was shaved. Two 3-mm full-thickness punch wounds were generated on the dorsal skin. Wounds were imaged by a Nikon camera (Nikon Corp., Tokyo, Japan) at 3 and 5 days postwounding, and the sizes of wounds were analyzed using ImageJ software version 1.49d (NIH, Bethesda, MD). To monitor the histological process of wound healing, another cohort of mice was used and sacrificed at 2 days postwounding, and the wound was excised and fixed. A series of sections (N = 4; 50 microns away from each other) were stained with hematoxylin and eosin, and the distances were measured by ImageJ:
| (1) |
For corneal wounds, mice were first anesthetized, and the application of a rotating diamond burr to the surface of the central cornea resulted in the removal of the corneal epithelium, whereas the limbal epithelium remained intact and then tissues were embedded in paraffin blocks. Animal experiments were approved by the Northwestern University Animal Care and Use Committee.
Cell Culture, Transduction, and Transfection
Primary cultures of human epidermal keratinocytes (HEKs) were isolated from neonatal foreskin by Northwestern University Skin Disease Research Center keratinocyte core as described24 and maintained in medium 154 (Cascade BiologicsInc., Portland, OR) containing human keratinocyte growth supplements and 0.07 mmol/L CaCl2. Primary human corneal epithelial keratinocytes (HCEKs) were isolated from cadaver donor corneas provided by Midwest Eye Bank (Ann Arbor, MI) and cultured in CnT-20 media with supplements (CELLnTEC Advanced Cell Systems AG, Bern, Switzerland) on collagen IV–coated plates (BD Biosciences, San Jose, CA). The limbal derived corneal epithelial cell line, hTCEpi,25 was grown in keratinocyte serum-free medium with supplements (Invitrogen, Carlsbad, CA) and 0.15 mmol/L CaCl2.
For retroviral infections, keratinocytes were transduced with retroviral supernatants produced in Phoenix amphotropic packaging cells, as previously described.24 For lentiviral infections, keratinocytes were transduced with lentiviral supernatants (produced by the Northwestern University Skin Disease Research Center RNA/DNA Delivery Core Facility) for 6 hours and switched to fresh culture medium overnight.
To silence gene expression, 20 nmol/L siRNA smart pools targeting at least two different sequences in the FIH-1 and LRRK1 genes or a scrambled negative control (Dharmacon, Inc., Lafayette, CO) were transiently transfected into cells using siRNA transfection reagent (RNAiMAX; Invitrogen), as described.26
Immunoprecipitation and Western Blot Analysis
For immunoprecipitation, protein lysates were prepared in radioimmunoprecipitation assay buffer [50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 0.25% deoxycholic acid, 1% NP-40, 1 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, phosphatase inhibitor cocktail (Thermo Fisher Scientific Inc., Rockford, IL), and protease inhibitor cocktail (Thermo Fisher Scientific Inc.)] and subjected to immunoprecipitation, as previously described.27 The supernatant was added with 20 μL of protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and the indicated antibodies and rotated for 2 hours at 4°C. The beads were washed three times with ice-cold phosphate-buffered saline and subjected to immunoblot analysis.
Western blot analyses were performed as described previously.5 The following antibodies were used: FIH-1 (sc-271780), α-tubulin (sc-23948), EGFR (sc-373746), glyceraldehyde-3-phosphate dehydrogenase (sc-25778; Santa Cruz Biotechnology), LRRK1 (PA5-13868; Thermo Fisher Scientific Inc.), p-tyrosine (05-321; EMD Millipore, Billerica, MA), p-EGFR (2234 and 2237), and p-ERK (4370; Cell Signaling Technology, Inc., Beverly, MA).
IHC Analysis and Immunofluorescence
Paraffin sections were processed for immunohistochemical (IHC) analysis or hematoxylin and eosin staining, as described previously.5 For FIH-1 staining, sections were incubated for 1 hour with FIH-1 rabbit polyclonal antibody (1:300; sc-48813; Santa Cruz Biotechnology). Sections were counterstained with hematoxylin to visualize morphology and mounted in Permount (Thermo Fisher Scientific Inc.). Images were obtained using a Zeiss AxioCam HR digital camera mounted on a Zeiss Axioplan 2 bright field microscope system with a Plan-Neofluar 40×/0.75 objective (Carl Zeiss AG, Oberkochen, Germany). AxioVision software version 4.8 (Carl Zeiss AG) was used to acquire the images.
Early endosome abundance (EEA) 1 staining was performed as previously described.19 Briefly, cells grown on culture slides were incubated in the medium without supplements overnight and then treated with Alexa Fluor 647 EGF complex (Life Technologies Corp., Carlsbad, CA) (100 ng/mL) at 4°C for 30 minutes. After washing with phosphate-buffered saline, cells were incubated in the medium without supplements and EGF for the indicated time at 37°C and fixed with 4% paraformaldehyde for 10 minutes. Slides were incubated overnight with an antibody recognizing EEA1 (1:100; 610457; BD Transduction Laboratories, San Jose, CA). After washing, slides were incubated with Alexa 488-linked secondary IgG (Vector Laboratories, Inc, Burlingame, CA). Images were acquired using a laser-scanning confocal microscope imaging system (UV LSM 510 META; Carl Zeiss) with a Plan Apochromat63 chroma/1.4 oil immersion objective. The Pearson coefficient was analyzed by ImageJ.
Inhibitors
For pharmacological inhibition of FIH-1 activity, Mek-ERK1/2, EGFR, Notch signaling, and endocytosis, the cells were pretreated with 1 mmol/L dimethyloxalylglycine (Santa Cruz Biotechnology) for 2 hours, 10 μmol/L U0126 (Sigma-Aldrich Corp., St. Louis, MO) for 2 hours, 0.1 μmol/L AG1478 (Sigma-Aldrich Corp.) for 2 hours, 10 μmol/L N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) (Sigma-Aldrich Corp.) for 16 hours, and 20 μM chloroquine overnight, respectively.
Scratch Wound Assay
Cells were grown to confluence on 12-well plastic dishes, and linear scratch wounds (in triplicate) were generated on the confluent monolayers using a 200-μL pipette tip. Images were obtained at room temperature using a Zeiss AxioCam MR digital camera mounted on a Zeiss Axiovert 40CFL inverted light microscope with an A-plan 10×/0.25 Ph1Var objective (Carl Zeiss AG). AxioVision software was used to acquire the images. The percentage decrease in the wound gaps were calculated using AxioVision computer-assisted image analysis and normalized to the time-0 wounds. To rule out the contribution of proliferation in the sealing of linear scratch wounds, cells were pretreated with 5 μg/mL mitomycin C (EMD BioSciences, Inc., San Diego, CA).
Statistical Analysis
All values are expressed as means ± SD. The significance of the differences between two groups was evaluated by an unpaired Student's t-test and two-way analysis of variance test.
Results
FIH-1 Is a Positive Regulator of Keratinocyte Migration and Wound Healing
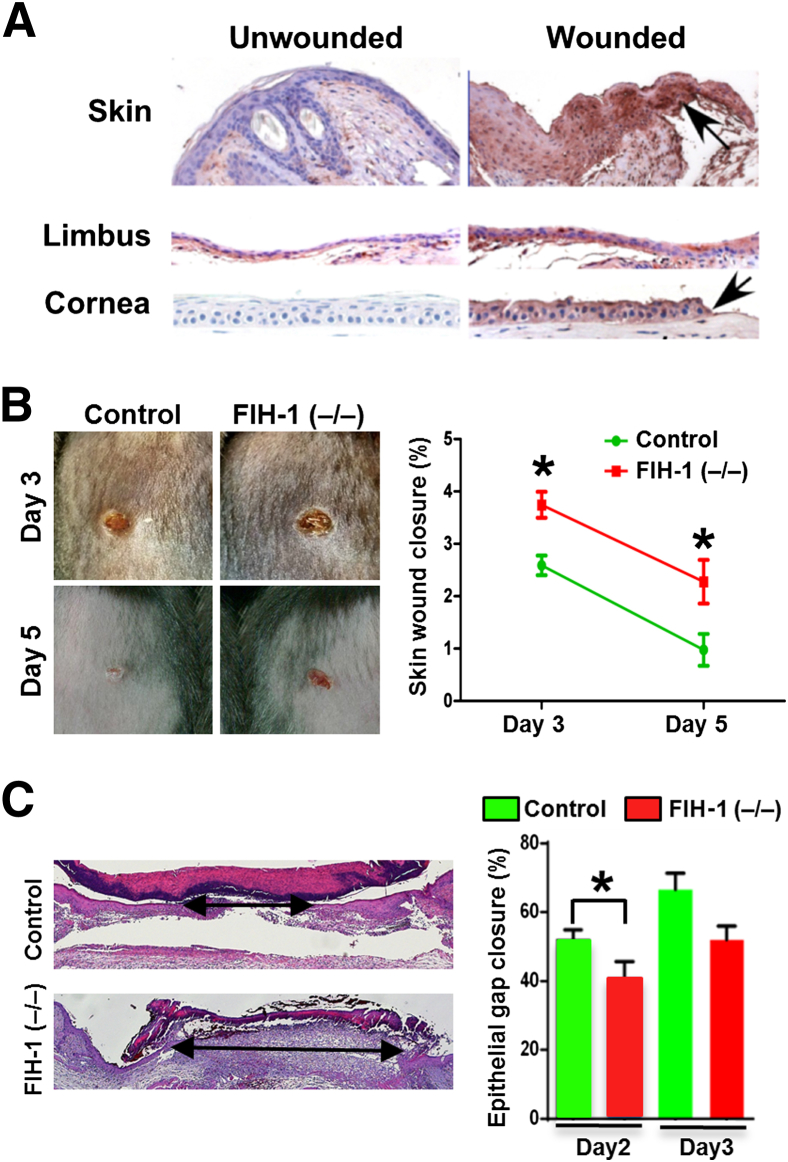
Increased FIH-1 levels are associated with defects in keratinocyte differentiation and glycogen metabolism.4,5,28 Not surprisingly, FIH-1 is normally undetectable in mouse epidermis and corneal epithelium (Figure 1A). In contrast, there is a marked up-regulation of FIH on wounding, particularly in cells at the leading edge of the wound (Figure 1A). This led us to hypothesize that FIH-1 plays a role in epithelial cell migration.
Figure 1.
FIH-1 promotes wound healing in mouse skin and eyes. A: Full-thickness skin wounding by 3-mm punch or removal of the corneal epithelium by application of a rotating diamond burr to the surface of the central cornea was performed. Immunohistochemical analysis of FIH-1 in unperturbed and wounded epithelia was conducted. Note perturbation increases FIH-1 expression in epidermal, limbal, and corneal epithelia. B: Representative skin wound images from control and FIH-1–deficient [FIH-1 (−/−)] mice at different time points after full-thickness skin wounding (left panel). Quantitative analysis of wound closure obtained from four different mice (right panel). C: Hematoxylin and eosin–stained sections of control and FIH-1–deficient mice show the gaps between the leading edges of wounds 2 days postwounding (arrows; left panel). Degrees of re-epithelialization of the wound at days 2 and 3 were calculated based on the percentages of original wound diameter (right panel). Data are expressed as means ± SD (B). n = 4 (B). ∗P < 0.05. Original magnification: ×400 (A).
To determine whether FIH-1 is required for normal wound healing, we examined the ability of mice with a null mutation in the FIH gene (FIH-1−/−) to heal full-thickness skin wounds. Wound closure was visibly delayed in FIH-1−/− mice beginning 3 days after wound initiation (P < 0.001) (Figure 1B). Wounds were clinically visible in the FIH-1−/− mice 5 days postwounding, whereas wounds were virtually closed in the control mice at this time (Figure 1B). Re-epithelialization, a histological measure of epidermal closure, was less in control mice versus the FIH-1−/− mice on day 2 (P < 0.05) and on day 3 (P = 0.057) after wound initiation (Figure 1C). Taken together, these findings support the idea that FIH-1 enhances re-epithelialization, which contributes, in part, to efficient wound healing.
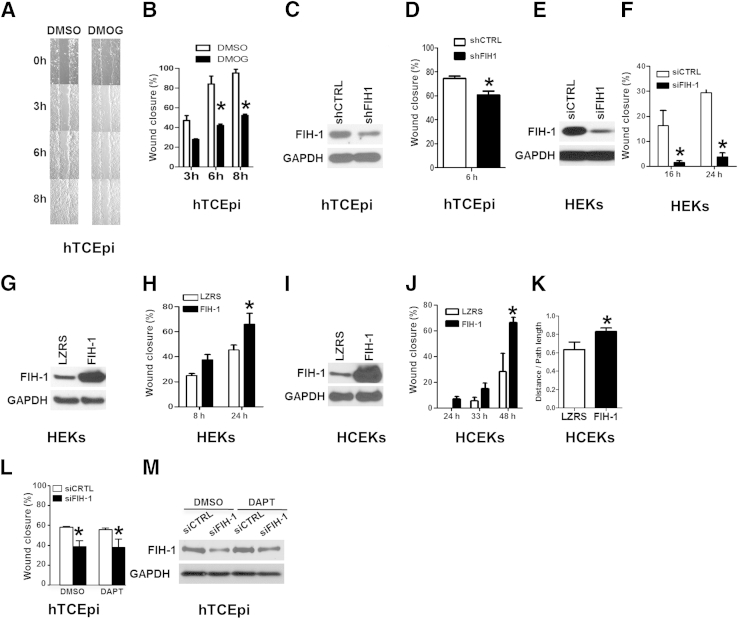
To examine whether FIH-1 has a direct effect on epithelial cell migration, we used a scratch wound assay in a telomerase immortalized human corneal epithelial cell line,25 which has high endogenous levels of FIH-1.5 Treatment of hTCEpi cells with the cell-permeable FIH inhibitor dimethyloxalylglycine2 to inhibit hydroxylase activity resulted in a 50% delay in sealing the linear scratch wounds (Figure 2, A and B). This finding suggests that the hydroxylase activity of FIH-1 is important in normal wound healing.
Figure 2.
FIH-1 positively regulates keratinocyte cell migration. A and B: The limbal-derived corneal epithelial hTCEpi cells were treated with the cell-permeable FIH inhibitor dimethyloxalylglycine (DMOG) or dimethyl sulfoxide (DMSO) for 2 hours. At this time, confluent hTCEpi cells were scratch-wounded and then allowed to migrate until wounds were closed (8 hours; vehicle). C and D: hTCEpi cells were lentivirally transduced with an shRNA against either FIH-1 (shFIH-1) or sh control (shCTRL). These transduced cells were used for immunoblot analysis of FIH-1 and GAPDH (C) and scratch-wound–healing assays (D). E and F: HEKs were transfected with an siRNA pool against either FIH-1 (siFIH-1) or scrambled control (siCTRL) and then used for immunoblot analysis of FIH-1 and GAPDH (E) and scratch-wound–healing assays (F). G–J: HEKs and HCEKs were retrovirally transduced with FIH-1 or empty vector (LZRS) and then immunoblot analyses of FIH-1 and GAPDH were performed in HEKs (G) and HCEKs (I) and scratch-wound–healing assays were conducted in HEKs (H) and HCEKs (J). The percentage of wound closure from a representative experiment was measured temporally using AxioVision software. K: Single-cell migration assays were performed in HCEKs transduced with either FIH-1 or empty vector (LZRS) using a Nikon Biostation system. The persistency of migration path was calculated based on the ratios of the distance between the starting and ending points and the total path length. L: Scratch-wound assays were performed on hTCEpi cells transfected with either siFIH-1 or siCTRL after 16-hour pretreatment with 10-μmol/L DAPT. DAPT is a γ-secretase inhibitor that blocks Notch signaling. M: Immunoblot analyses of FIH-1 and GAPDH were performed after scratch-wounding. Data are means ± SD (B, D, F, H, J, K, and L). n = 3 (B, D, F, H, J, and L); n = 8 (K). ∗P < 0.05.
As dimethyloxalylglycine is a general hydroxylase inhibitor and could have off-target effects, we also used shFIH-1 to knock down FIH-1 in hTCEpi cells (Figure 2C), and with such treatment, the healed distance was 20% less at 6 hours (Figure 2D). To exclude the possible nonspecific effect of RNA interference, we also used a siRNA smart pool, targeting two different sequences in FIH-1 mRNA. In hTECpi cells, knocking down FIH-1 using this siRNA smart pool also led to a significantly slower wound closure (Figure 2L). To confirm the siRNA results in primary keratinocytes, we used HEKs and noted an even greater delay in sealing scratch wounds when FIH-1 was decreased in these cells (Figure 2, E and F). Notably, HEKs and HCEKs express relatively low levels of FIH-1 and seal wounds slower compared with hTCEpi cell lines. We reasoned that elevating the levels of FIH-1 in HEKs and HCEKs would enhance wound sealing if this hydroxylase were a positive regulator of epithelial cell migration. To overexpress FIH-1, we retrovirally transduced submerged primary cultures of HEKs and HCEKs. With FIH-1 transduction in HEKs and HCEKs, sealing of linear scratch wounds was increased (Figure 2, G–J). In all studies, proliferation was blocked using mitomycin C. To complement these studies, we also looked at the effects of FIH-1 on the migratory ability of single cells using live-cell imaging and observed a 50% enhancement in the directional migration (path length/distance) of FIH-1–transduced HCEKs compared with empty vector (LZRS)-transduced HCEKs (Figure 2K).
Since Notch signaling has been suggested to play a role in keratinocyte migration,29 we examined whether a FIH-1/Notch interaction affects the ability of keratinocytes to seal scratch wounds. We performed scratch wound assays after pharmacological blockade of Notch signaling with DAPT. With the inhibition of Notch signaling, the delayed wound healing observed in keratinocytes treated with siFIH-1 was not rescued (Figure 2, L and M). Collectively, these results suggest that FIH-1 is a positive regulator of keratinocyte migration, independent of Notch signaling.
FIH-1 Activates EGFR Signaling in Human Keratinocytes
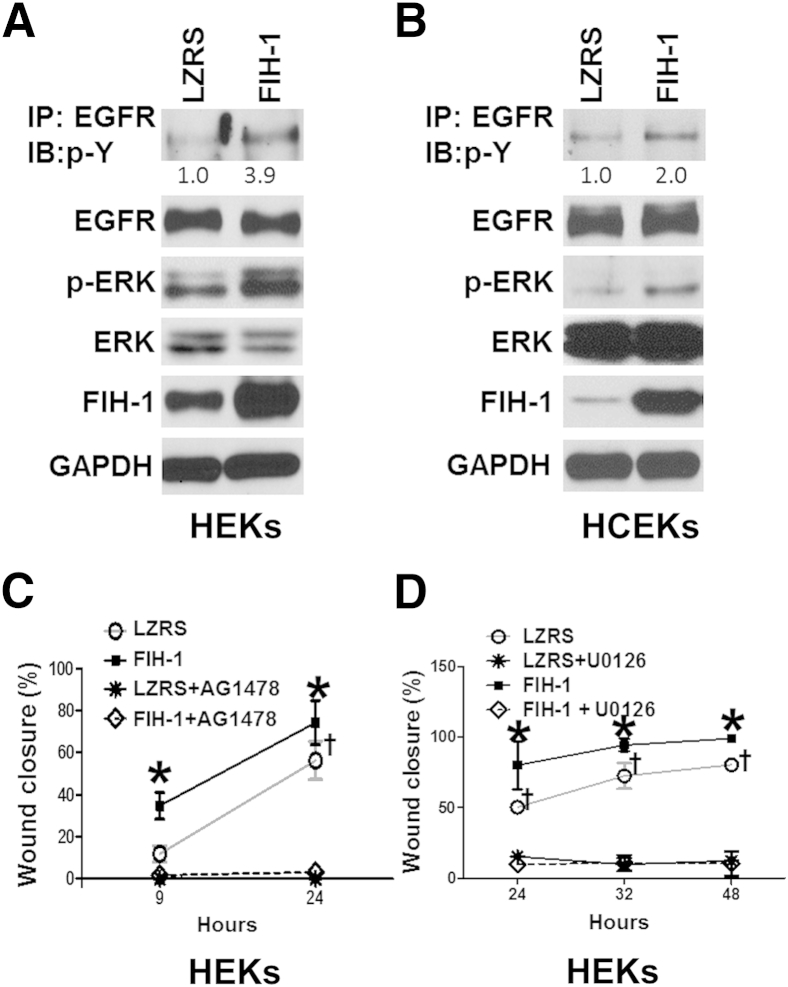
Given that EGFR signaling plays a crucial role in keratinocyte migration,11–13,27,30,31 we asked whether FIH-1 directly affects EGFR signaling. We retrovirally transduced submerged primary cultures of HEKs and HCEKs maintained in low calcium (Figure 3, A and B). Cell extracts were subjected to immunoprecipitation with anti-EGFR antibody, followed by immunoblot analysis with anti-phosphotyrosine antibody. Immunoblot analysis confirmed that EGFR phosphorylation was increased in the FIH-1–transduced HEKs and HCEKs compared with the LZRS controls (Figure 3, A and B). With FIH-1, the amount of pERK1/2 in the HEKs and HCEKs was also increased (Figure 3, A and B). To determine whether FIH-1 accelerates wound healing in the absence of EGFR or ERK1/2 signaling, we performed scratch wound assays after pharmacological blockade of the upstream activator of EGFR (AG1478) or MEK1/2 (U0126). As expected, after interference with EGFR (Figure 3C) or ERK1/2 (Figure 3D) signaling, scratch wound sealing was inhibited in both control and FIH-1–transduced keratinocytes compared with untreated and FIH-1 transduced keratinocytes. This finding indicates that the positive effect that FIH-1 exerts on keratinocyte migration is primarily working through the EGFR/ERK1/2 signaling axis.
Figure 3.
FIH-1 enhances EGFR signaling to promote keratinocyte cell migration. HEKs (A) and HCEKs (B) were retrovirally transduced with either FIH-1 or empty vector (LZRS). Protein lysates from HEKs or HCEKs were harvested for immunoprecipitation (IP) or Western blot analysis (IB). Immunoprecipitated EGFR was probed for phosphotyrosine (p-Y). Radioimmunoprecipitation assay–soluble lysates were also immunoblot-analyzed for total EGFR, p-ERK, ERK, FIH-1, and GAPDH. C and D: Results from scratch-wound assays on HEKs retrovirally transduced with either FIH-1 or empty vector (LZRS) after 2-hour pretreatment with 10-μmol/L U0126 (a Mek1/2 inhibitor) and 0.1-μmol/L AG1478 (EGFR inhibitor) to inhibit the Mek1/2-ERK1/2 and EGFR pathways, respectively. Data are expressed as means ± SD (C and D). n = 3. ∗P < 0.05 versus empty vector (LZRS); †P < 0.05 versus FIH-1 with U0126 or AG1478.
FIH-1 Interacts with LRRK1 to Alter EGFR Signaling
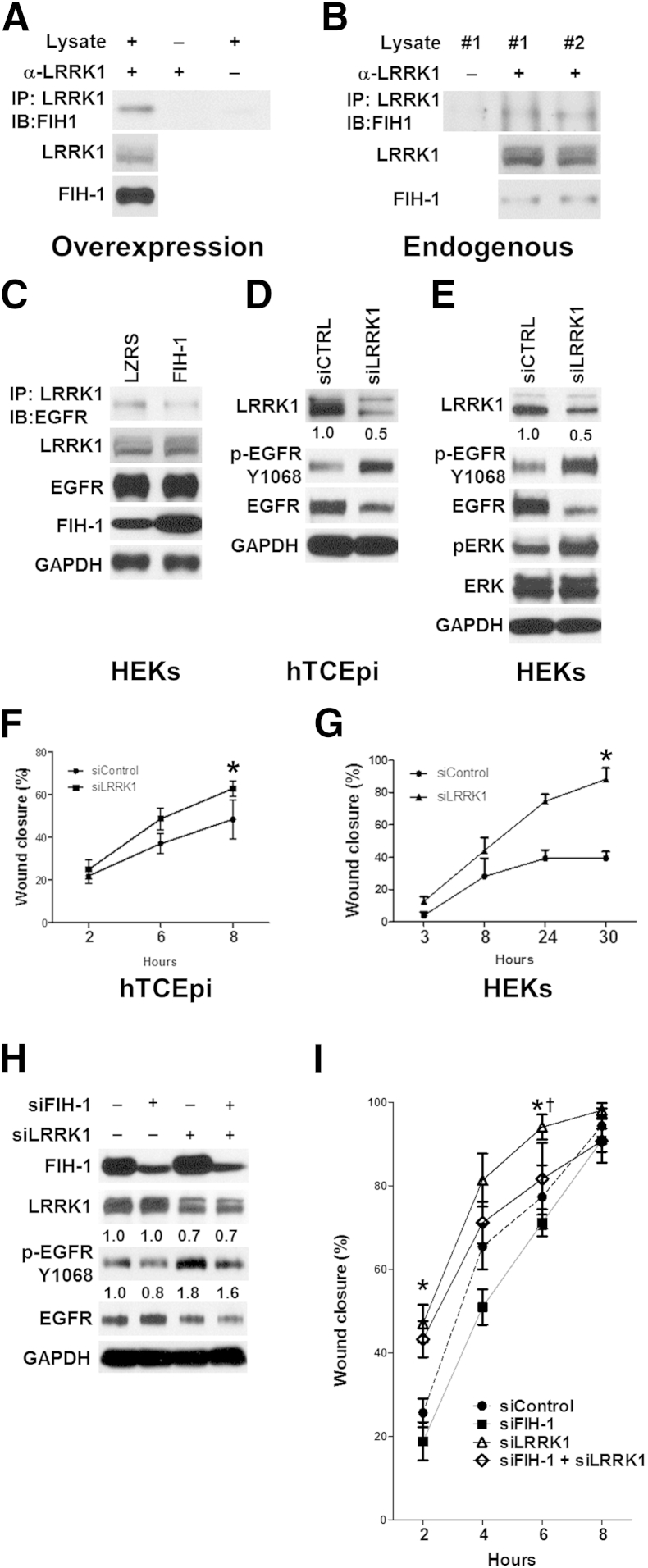
A major determinant of the intensity and duration of EGFR signaling is the trafficking event that relocalizes the receptors from the cell surface to intracellular endocytic compartments.19,32,33 To address FIH-1 and EGFR signaling in this context, we looked at the proteins involved in endosomal trafficking that were potential substrates for FIH-1. LRRK1 regulates endosomal trafficking of the EGFR,19,20 and this kinase contains six N-terminal ankryin repeats,22 which could serve as a substrate for FIH-1.3 LRRK1 appears to be expressed in many tissues; however, its expression in skin and/or epithelia has not been reported.22 Immunoblot analysis showed that LRRK1 was expressed in primary cultures of HEKs cultured in low-calcium conditions (Figure 4E). To determine whether FIH-1 forms a complex with LRRK1 in these cells, FIH-1 was ectopically expressed in HEKs, and cell lysates were immunoprecipitated with an anti-LRRK1 antibody, followed by immunoblot analysis with anti–FIH-1 antibody. Such experiments demonstrate that LRRK1 associates with FIH-1 when overexpressed in keratinocytes (Figure 4A). Consistent with this association, endogenous FIH-1 immunoprecipitated with LRRK1 (Figure 4B).
Figure 4.
FIH-1 forms a complex with LRRK1 that affects EGFR activation and keratinocyte migration. A and B: Interaction of LRRK1 with FIH-1. Complex formation of ectopically overexpressed (A) or endogenous (B) FIH-1 with endogenous LRRK1 in HEKs was detected by immunoprecipitation with anti-LRRK1 antibodies, followed by immunoblot analysis with anti–FIH-1 antibodies. C: Interaction of endogenous LRRK1 with endogenous EGFR. Complex formation in HEKs retrovirally transduced with either FIH-1 or empty vector (LZRS) was detected by IP with anti-LRRK1 antibodies, followed by immunoblot analysis with anti-EGFR antibodies. Radioimmunoprecipitation assay–soluble lysates were also immunoblot-analyzed for total EGFR, FIH-1, and GAPDH. D–G: Limbal-derived corneal epithelial hTCEpi cells (D and F) and HEKs (E and G) were transfected with an siRNA pool against either LRRK1 (siLRRK1) or a scrambled control (siCTRL). Seventy-two hours after transfection, immunoblot analysis was performed using antibodies against LRRK1, p-EGFR, GAPDH, and p-ERK (D and E) and scratch-wound–healing assays were conducted (F and G). H and I: hTCEpi cells were transfected with siRNA pools against siFIH-1, siLRRK1, siFIH-1+siLRRK1, or scrambled control (siControl). Seventy-two hours after transfection, immunoblot analyses were performed using antibodies against LRRK1, p-EGFR, GAPDH, and FIH-1 (H) and scratch-wound–healing assays were performed (I). Data are means ± SD (F, G, and I). n = 3 (F, G, and I). ∗P < 0.05 (F and G); ∗P < 0.05 versus siControl (I); †P < 0.05 versus siFIH-1+siLRRK1.
Recently, it was reported that LRRK1 can form a complex with phosphorylated EGFR to promote the delivery of p-EGFR from early to late endosomes.19 These investigations were performed in cell lines (eg, HeLa, COS-7, and HEK 293), and it was not clear whether an EGFR/LRRK1 interaction existed in primary cultures of human keratinocytes. We were able to detect such an interaction in HEKs (Figure 4C).
To investigate the relationship between FIH-1 and this EGFR/LRRK1 interaction, we ectopically expressed FIH-1 in HEKs, and cell lysates were immunoprecipitated with anti-LRRK1 antibody, followed by immunoblot analysis with anti-EGFR antibody. We observed a marked decrease in the EGFR/LRRK1 interaction in the FIH-1–transduced HEKs compared with the LZRS controls (Figure 4C), suggesting that FIH-1 inhibits the LRRK1/EGFR interaction.
Next, we asked whether FIH-1 deficiency delays keratinocyte migration via LRRK1-mediated inhibition of EGFR. Knockdown of LRRK1 in a corneal epithelial cell line (Figure 4D) and in HEKs (Figure 4E) resulted in an increase in p-EGFR compared with that in controls, suggesting that interfering with LRRK1 sustains EGFR signaling. As a result of the increase in p-EGFR signaling due to the knockdown of LRRK1 (Figure 4, D and E), the corneal epithelial cell line (Figure 4F) and HEKs (Figure 4G) that were deficient in LRRK1 sealed scratch wounds faster than did control cells (Figure 4, F and G). With the loss of LRRK1 in the corneal epithelial cell line, p-EGFR was markedly up-regulated (Figure 4H), and such cells sealed wounds extremely fast (Figure 4I). Conversely, loss of FIH-1 in these cells led to a down-regulation in p-EGFR (Figure 4H) and a slowed rate of cell migration (Figure 4I). Importantly, preventing the up-regulation of LRRK1 by siRNA-mediated knockdown in FIH-1–deficient cells normalized p-EGFR and migration (Figure 4, H and I). In keratinocytes in which FIH-1 and LRRK1 were silenced, there was residual LRRK1, which could mediate EGFR endosomal trafficking. Collectively, these findings indicate that FIH-1 interacts with LRRK1, which increases EGFR signaling and accelerates keratinocyte migration.
FIH-1 Regulates EGFR Endosomal Trafficking
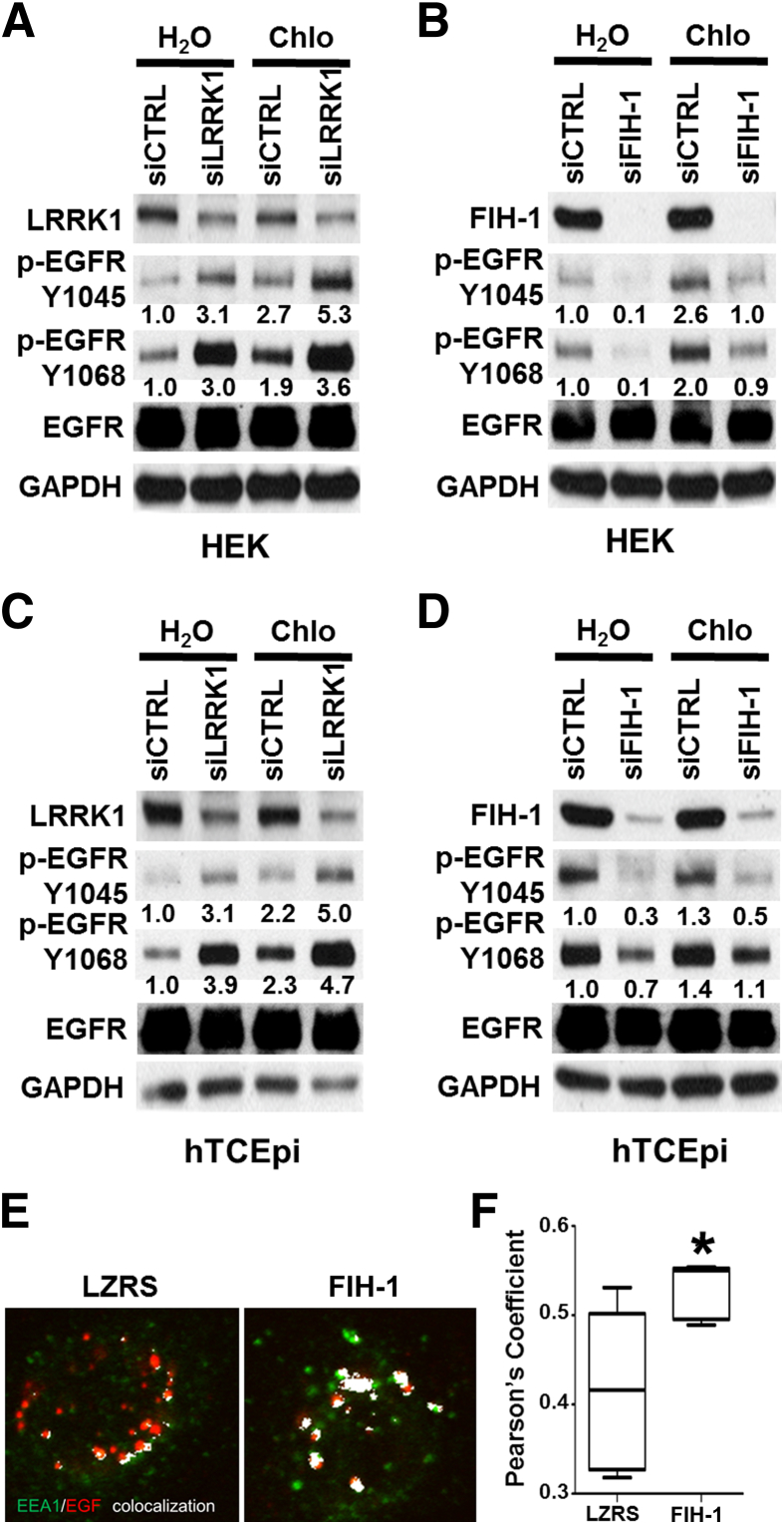
In other cell types, LRRK1 has been shown to play a role in EGFR endosomal trafficking.19,20 Therefore, we wondered whether endosomal trafficking is involved in the regulation of EGFR signaling by FIH-1/LRRK1. We treated HEKs and a corneal epithelial cell line with chloroquine, which is a well-accepted means of blocking lysosomal/endosomal fusion and thus of interrupting trafficking.34–36 Consistent with a negative role of LRRK1 on EGFR activation (Figure 4), EGFR activation was increased with the genetic loss of LRRK1 (Figure 5, A and C), which could be enhanced by chloroquine treatment (Figure 5, A and C). This finding supports the idea that LRRK1 regulates EGFR signaling via altering endosomal trafficking.
Figure 5.
FIH-1/LRRK1 regulates EGFR signaling in part by interfering with endosomal trafficking. HEKs (A and B) and limbal-derived corneal epithelial hTCEpi cells (C and D) were transfected with siLRRK1 (A and C), siFIH-1 (B and D), or siCTRL. After transfection (72 hours), cells were treated with 20-μmol/L chloroquine (Chlo) for 2 hours or vehicle control (water), and lysates were harvested and immunoblot-analyzed with antibodies against FIH-1, LRRK1, p-EGFR (Y1045 or Y1068), and GAPDH. E and F: hTECpi cells transduced with an empty vector (LZRS) or FIH-1 were treated with fluorescence-labeled EGF (Alexa Fluor 647 EGF complex; red). Cells were harvested at 30 minutes after treatment and stained for EEA1 (an early endosome marker; green). Colocalization of EGF and EEA1 were highlighted as white pixels (E) and Pearson's coefficient was analyzed by ImageJ (F). The box-and-whiskers plots represent the median line; whiskers, minimum to maximum. n = 5 (F). ∗P < 0.05.
To examine whether the effect of FIH-1 on EGFR activation also involves the endosome, we conducted a similar experiment and observed that with the knockdown of FIH-1, phosphorylation of the EGFR was diminished, which could be restored by chloroquine treatment (Figure 5, B and D). These results suggest that FIH-1 maintains EGFR signaling via an endosomal trafficking pathway required for normal cell migration.
To test further the role of FIH-1 in EGFR endosomal trafficking, hTECpi cells transduced with an empty vector or FIH-1 were treated with fluorescence-labeled EGF. In this manner we were able to detect an activated EGF/EGFR complex. Cells were harvested at 30 minutes after treatment and stained for EEA1 (an early endosome marker). In control cells (Figure 5E), EGF colocalization with EEA1 was noted after 30 minutes treatment; however, in the FIH-1–overexpressing cells (Figure 5E), co-localization occurred to a significantly greater extent. This finding suggests that FIH-1 may induce an accumulation of EGF/EGFR complex in early endosomes. Taken together, these findings implicate a regulatory role for FIH-1/LRRK1 in the endosomal trafficking of EGFR, ultimately promoting the migration of keratinocytes.
Discussion
This article describes a novel mechanism by which the hydroxylase FIH-1 maintains EGFR signaling by interfering with LRRK1 and thus acts as a positive regulator of epithelial cell migration. The addition of migration to the portfolio of cellular processes that FIH-1 influences underscores the importance of this hydroxylase in epithelial biology. FIH-1 is not prominently expressed in unperturbed epithelia; however, it is up-regulated in conditions of abnormal differentiation, such as psoriasis and diabetic corneal keratopathies5 and wound healing (Figure 1). Interestingly, the limbal epithelium is one tissue in which FIH-1 is constitutively expressed.5 The limbal epithelium is unique in that it is the site of the corneal epithelial stem cells.37–45 Consequently, limbal epithelial basal cells are less differentiated than are corneal epithelial basal cells. Thus, the finding that FIH-1 is normally present in the limbal epithelium has been proposed as a mechanism that helps to maintain the relatively undifferentiated phenotype characteristic of this tissue, since FIH-1 attenuates Notch signaling, thereby inhibiting differentiation.5 Limbal epithelial basal cells are also considered more migratory than are corneal epithelial basal cells since there is a continuous centripetal migration of limbal basal cells into the corneal epithelium to maintain homeostasis,46,47 and after a central corneal epithelial wound is sustained, limbal epithelial basal cells rapidly migrate into the cornea to re-epithelialize the defect.37,48 Therefore, having a constant source of FIH-1 as a positive regulator of cell migration makes excellent biological sense from a limbal/corneal epithelial perspective.
miR-31 targets FIH-1 in epidermal and corneal/limbal keratinocytes.4 In the context of the present study, miR-31 can be thought of as a negative regulator of migration. This idea is entirely consistent with a large body of work that has resulted in the concept that miR-31 is the anti-metastatic miRNA.49–51 The anti-metastatic nature of miR-31 is attributed to the ability of this miRNA to simultaneously suppress integrin-α5, radixin, and RhoA.49–51 The consequence of such suppression was a 20-fold reduction in invasion and a 10-fold reduction in migration in breast cancer cell lines. Our finding that miR-31 targets FIH-1, which is a positive regulator of migration, raises the possibility that FIH1 may be yet another gene that contributes to the invasion/metastasis process. Interestingly, we and others have shown that FIH-1 is up-regulated in basal and squamous cell carcinomas5,52; however, the functional significance of such up-regulation is presently unknown. Even though we and others have shown that miR-31 is a crucial negative regulator of migration, it was shown recently that this miRNA may promote migration in an oral squamous carcinoma cell line.53 This finding suggests that the effects of miR-31/FIH-1 in cell migration could be tissue dependent.
We show here that FIH-1 complexes with LRRK1 to maintain EGFR signaling activity. An FIH-1/LRRK1 interaction is novel and can be explained by the presence of multiple N-terminal ankyrin repeats that comprise a portion of the LRRK1 protein. These ankyrin repeat domains can serve as substrate for FIH-1.3 LRRK1 and its homologue LRRK2 belong to the ROCO protein family, with LRRK1 containing six ankyrin repeats, seven leucine-rich repeats, a GRPase-like domain of Roc, a COR domain, and a serine/threonine kinase domain.21 LRRK2 contains most of the domains of the ROCO family.22 In terms of function, most attention has been directed toward LRRK2, as linkage studies have demonstrated that mutations in the LRRK2 gene in humans are linked to Parkinson's disease.54,55 Loss of LRRK2 protein in mice resulted in pathophysiological changes in kidneys.56 Furthermore, LRRK2 has been implicated in autophagy as well as in defective Wnt signaling, leading to impaired neurogenesis.57 Recently, a comparison of the disruption in the LRRK1 versus the LRRK2 gene in mice indicated that disruption of the LRRK2 gene failed to result in skeletal phenotypes; however, loss of LRRK1 resulted in defective osteoclast function and severe osteopetrosis.58 This finding suggests that LRRK1 and LRRK2 have distinct functions in certain tissues (eg, LRRK1 in bone and LRRK2 in the nervous system) and that LRRK2 cannot compensate for LRRK1 or vice versa.58 Other than a role for LRRK1 in skeletal muscle physiology, LRRK1 is best recognized for its regulation of EGFR transport from early to late endosomes, which is necessary for proper recycling of the EGFR.19,20 However, until now, the functional significance of the LRRK1/EGFR interaction was unclear. Our finding that the loss of LRRK1 in HEKs and HCEKs apparently enhanced the ability of these cells to seal linear scratch wounds due to increased EGFR signaling activity suggests that the LRRK1/EGFR complex is fundamental in the maintenance of proper epithelial cell migration. This function is novel for this kinase in epithelial biology.
Acknowledgments
We thank Jong Kook Park for helpful discussions, Dr. Randall S. Johnson (University of Cambridge, Cambridge, UK) for kindly giving FIH-1 null mice as a gift, the Northwestern University Skin Disease Research Center (NU-SDRC) Skin Tissue Engineering core facility for primary epidermal keratinocyte cultures, the NU-SDRC DNA/RNA delivery core facility for lentiviral constructs, the NU-SDRC Morphology and Phenotyping core facility for assistance in morphological analysis, and the Northwestern University Center for Advanced Microscopy for assistance in colocalization analysis.
Footnotes
Supported by NIH grants EY06769, EY017536, and EY019463 (R.M.L.) and AR062110 (S.G.), a Dermatology Foundation Career Development Award and research grant (H.P.), and grant AR057216 form the National Institute of Arthritis and Musculoskeletal and Skin Diseases (The Northwestern University Skin Disease Research Center).
Disclosures: None declared.
References
- 1.Mahon P.C., Hirota K., Semenza G.L. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lando D., Peet D.J., Gorman J.J., Whelan D.A., Whitelaw M.L., Bruick R.K. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cockman M.E., Webb J.D., Kramer H.B., Kessler B.M., Ratcliffe P.J. Proteomics-based identification of novel factor inhibiting hypoxia-inducible factor (FIH) substrates indicates widespread asparaginyl hydroxylation of ankyrin repeat domain-containing proteins. Mol Cell Proteomics. 2009;8:535–546. doi: 10.1074/mcp.M800340-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng H., Hamanaka R.B., Katsnelson J., Hao L.L., Yang W., Chandel, Lavker R.M. MicroRNA-31 targets FIH-1 to positively regulate corneal epithelial glycogen metabolism. FASEB J. 2012;26:3140–3147. doi: 10.1096/fj.11-198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng H., Kaplan N., Hamanaka R.B., Katsnelson J., Blatt H., Yang W., Hao L., Bryar P.J., Johnson R.S., Getsios S., Chandel, Lavker R.M. microRNA-31/factor-inhibiting hypoxia-inducible factor 1 nexus regulates keratinocyte differentiation. Proc Natl Acad Sci U S A. 2012;109:14030–14034. doi: 10.1073/pnas.1111292109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolev V., Mandinova A., Guinea-Viniegra J., Hu B., Lefort K., Lambertini C., Neel V., Dummer R., Wagner E.F., Dotto G.P. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nat Cell Biol. 2008;10:902–911. doi: 10.1038/ncb1750. [erratum in: Nat Cell Biol 2013;15:124] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olayioye M.A., Neve R.M., Lane H.A., Hynes N.E. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 9.Yarden Y., Sliwkowski M.X. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 10.Mascia F., Denning M., Kopan R., Yuspa S.H. The black box illuminated: signals and signaling. J Invest Dermatol. 2012;132:811–819. doi: 10.1038/jid.2011.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Repertinger S.K., Campagnaro E., Fuhrman J., El-Abaseri T., Yuspa S.H., Hansen L.A. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982–989. doi: 10.1111/j.0022-202X.2004.23478.x. [DOI] [PubMed] [Google Scholar]
- 12.Xu K., Yu F.S. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:3301–3308. doi: 10.1167/iovs.10-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu K.P., Li Y., Ljubimov A.V., Yu F.S. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes. 2009;58:1077–1085. doi: 10.2337/db08-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zieske J.D., Takahashi H., Hutcheon A.E., Dalbone A.C. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest Ophthalmol Vis Sci. 2000;41:1346–1355. [PubMed] [Google Scholar]
- 15.Maxfield F.R., McGraw T.E. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 16.Sorkin A., von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madshus I.H., Stang E. Internalization and intracellular sorting of the EGF receptor: a model for understanding the mechanisms of receptor trafficking. J Cell Sci. 2009;122:3433–3439. doi: 10.1242/jcs.050260. [DOI] [PubMed] [Google Scholar]
- 18.Rush J.S., Quinalty L.M., Engelman L., Sherry D.M., Ceresa B.P. Endosomal accumulation of the activated epidermal growth factor receptor (EGFR) induces apoptosis. J Biol Chem. 2012;287:712–722. doi: 10.1074/jbc.M111.294470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanafusa H., Ishikawa K., Kedashiro S., Saigo T., Iemura S., Natsume T., Komada M., Shibuya H., Nara A., Matsumoto K. Leucine-rich repeat kinase LRRK1 regulates endosomal trafficking of the EGF receptor. Nat Commun. 2011;2:158. doi: 10.1038/ncomms1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishikawa K., Nara A., Matsumoto K., Hanafusa H. EGFR-dependent phosphorylation of leucine-rich repeat kinase LRRK1 is important for proper endosomal trafficking of EGFR. Mol Biol Cell. 2012;23:1294–1306. doi: 10.1091/mbc.E11-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosgraaf L., Van Haastert P.J. Roc, a Ras/GTPase domain in complex proteins. Biochim Biophys Acta. 2003;1643:5–10. doi: 10.1016/j.bbamcr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Korr D., Toschi L., Donner P., Pohlenz H.D., Kreft B., Weiss B. LRRK1 protein kinase activity is stimulated upon binding of GTP to its Roc domain. Cell Signal. 2006;18:910–920. doi: 10.1016/j.cellsig.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Zhang N., Fu Z., Linke S., Chicher J., Gorman J.J., Visk D., Haddad G.G., Poellinger L., Peet D.J., Powell F., Johnson R.S. The asparaginyl hydroxylase factor inhibiting HIF-1alpha is an essential regulator of metabolism. Cell Metab. 2010;11:364–378. doi: 10.1016/j.cmet.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Getsios S., Simpson C.L., Kojima S., Harmon R., Sheu L.J., Dusek R.L., Cornwell M., Green K.J. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J Cell Biol. 2009;185:1243–1258. doi: 10.1083/jcb.200809044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson D.M., Li L., Fisher S., Pearce V.P., Shay J.W., Wright W.E., Cavanagh H.D., Jester J.V. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005;46:470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- 26.Lin S., Gordon K., Kaplan N., Getsios S. Ligand targeting of EphA2 enhances keratinocyte adhesion and differentiation via desmoglein 1. Mol Biol Cell. 2010;21:3902–3914. doi: 10.1091/mbc.E10-03-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan N., Fatima A., Peng H., Bryar P.J., Lavker R.M., Getsios S. EphA2/Ephrin-A1 signaling complexes restrict corneal epithelial cell migration. Invest Ophthalmol Vis Sci. 2012;53:936–945. doi: 10.1167/iovs.11-8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng H., Katsnelson J., Yang W., Brown M.A., Lavker R.M. FIH-1/c-kit signaling: a novel contributor to corneal epithelial glycogen metabolism. Invest Ophthalmol Vis Sci. 2013;54:2781–2786. doi: 10.1167/iovs.12-11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Movahedan A., Majdi M., Afsharkhamseh N., Sagha H.M., Saadat, Shalileh K., Milani B.Y., Ying H., Djalilian A.R. Notch inhibition during corneal epithelial wound healing promotes migration. Invest Ophthalmol Vis Sci. 2012;53:7476–7483. doi: 10.1167/iovs.12-10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 31.Fang K.S., Ionides E., Oster G., Nuccitelli R., Isseroff R.R. Epidermal growth factor receptor relocalization and kinase activity are necessary for directional migration of keratinocytes in DC electric fields. J Cell Sci. 1999;112(Pt 12):1967–1978. doi: 10.1242/jcs.112.12.1967. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Pennock S., Chen X., Wang Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol Cell Biol. 2002;22:7279–7290. doi: 10.1128/MCB.22.20.7279-7290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pennock S., Wang Z. Stimulation of cell proliferation by endosomal epidermal growth factor receptor as revealed through two distinct phases of signaling. Mol Cell Biol. 2003;23:5803–5815. doi: 10.1128/MCB.23.16.5803-5815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubois L., Lecourtois M., Alexandre C., Hirst E., Vincent J.P. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell. 2001;105:613–624. doi: 10.1016/s0092-8674(01)00375-0. [DOI] [PubMed] [Google Scholar]
- 35.Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oksvold M.P., Skarpen E., Wierød L., Paulsen R.E., Huitfeldt H.S. Re-localization of activated EGF receptor and its signal transducers to multivesicular compartments downstream of early endosomes in response to EGF. Eur J Cell Biol. 2001;80:285–294. doi: 10.1078/0171-9335-00160. [DOI] [PubMed] [Google Scholar]
- 37.Cotsarelis G., Cheng S.Z., Dong G., Sun T.T., Lavker R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 38.Khan O.A., Rogers V., Sharma R., Ohri S.K. Lung cancer masquerading as prosthetic valve endocarditis. Heart Lung Circ. 2008;17:161–163. doi: 10.1016/j.hlc.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Lavker R.M., Sun T.T. Epithelial stem cells: the eye provides a vision. Eye (Lond) 2003;17:937–942. doi: 10.1038/sj.eye.6700575. [DOI] [PubMed] [Google Scholar]
- 40.Lavker R.M., Tseng S.C., Sun T.T. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Pellegrini G., Dellambra E., Golisano O., Martinelli E., Fantozzi I., Bondanza S., Ponzin D., McKeon F., De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schermer A., Galvin S., Sun T.T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stepp M.A., Zieske J.D. The corneal epithelial stem cell niche. Ocul Surf. 2005;3:15–26. doi: 10.1016/s1542-0124(12)70119-2. [DOI] [PubMed] [Google Scholar]
- 44.Tseng S.C.G. Concept and application of limbal stem cells. Eye (Lond) 1989;3:141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 45.Wolosin J.M., Budak M.T., Akinci M.A. Ocular surface epithelial and stem cell development. Int J Dev Biol. 2004;48:981–991. doi: 10.1387/ijdb.041876jw. [DOI] [PubMed] [Google Scholar]
- 46.Collinson J.M., Chanas S.A., Hill R.E., West J.D. Corneal development, limbal stem cell function, and corneal epithelial cell migration in the Pax6(+/-) mouse. Invest Ophthalmol Vis Sci. 2004;45:1101–1108. doi: 10.1167/iovs.03-1118. [DOI] [PubMed] [Google Scholar]
- 47.Collinson J.M., Morris L., Reid A.I., Ramaesh T., Keighren M.A., Flockhart J.H., Hill R.E., Tan S.S., Ramaesh K., Dhillon B., West J.D. Clonal analysis of patterns of growth, stem cell activity, and cell movement during the development and maintenance of the murine corneal epithelium. Dev Dyn. 2002;224:432–440. doi: 10.1002/dvdy.10124. [DOI] [PubMed] [Google Scholar]
- 48.Lehrer M.S., Sun T.T., Lavker R.M. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci. 1998;111(Pt 19):2867–2875. doi: 10.1242/jcs.111.19.2867. [DOI] [PubMed] [Google Scholar]
- 49.Valastyan S., Reinhardt F., Benaich N., Calogrias D., Szász A.M., Wang Z.C., Brock J.E., Richardson A.L., Weinberg R.A. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032–1046. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Valastyan S., Chang A., Benaich N., Reinhardt F., Weinberg R.A. Activation of miR-31 function in already-established metastases elicits metastatic regression. Genes Dev. 2011;25:646–659. doi: 10.1101/gad.2004211. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Valastyan S., Chang A., Benaich N., Reinhardt F., Weinberg R.A. Concurrent suppression of integrin alpha5, radixin, and RhoA phenocopies the effects of miR-31 on metastasis. Cancer Res. 2010;70:5147–5154. doi: 10.1158/0008-5472.CAN-10-0410. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Pelletier J., Dayan F., Durivault J., Ilc K., Pécou E., Pouysségur J., Mazure N.M. The asparaginyl hydroxylase factor-inhibiting HIF is essential for tumor growth through suppression of the p53-p21 axis. Oncogene. 2012;31:2989–3001. doi: 10.1038/onc.2011.471. [DOI] [PubMed] [Google Scholar]
- 53.Liu C.J., Tsai M.M., Hung P.S., Kao S.Y., Liu T.Y., Wu K.J., Chiou S.H., Lin S.C., Chang K.W. miR-31 ablates expression of the HIF regulatory factor FIH to activate the HIF pathway in head and neck carcinoma. Cancer Res. 2010;70:1635–1644. doi: 10.1158/0008-5472.CAN-09-2291. [DOI] [PubMed] [Google Scholar]
- 54.Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R.J., Calne D.B., Stoessl A.J., Pfeiffer R.F., Patenge N., Carbajal I.C., Vieregge P., Asmus F., Müller-Myhsok B., Dickson D.W., Meitinger T., Strom T.M., Wszolek Z.K., Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 55.Gilks W.P., Abou-Sleiman P.M., Gandhi S., Jain S., Singleton A., Lees A.J., Shaw K., Bhatia K.P., Bonifati V., Quinn N.P., Lynch J., Healy D.G., Holton J.L., Revesz T., Wood N.W. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 56.Tong Y., Yamaguchi H., Giaime E., Boyle S., Kopan R., Kelleher R.J., 3rd, Shen J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berwick D.C., Harvey K. LRRK2: an éminence grise of Wnt-mediated neurogenesis? Front Cell Neurosci. 2013;7:82. doi: 10.3389/fncel.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biskup S., Moore D.J., Rea A., Lorenz-Deperieux B., Coombes C.E., Dawson V.L., Dawson T.M., West A.B. Dynamic and redundant regulation of LRRK2 and LRRK1 expression. BMC Neurosci. 2007;8:102–113. doi: 10.1186/1471-2202-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]