Abstract
Functional brain imaging studies with positron emission tomography (PET) have identified blood flow changes in widely separated areas of the brain during the performance of word-related tasks. In the present study, we have utilized event-related electrical potentials (ERPs) to investigate the temporal relationships among cortical areas previously identified by PET to be differentially activated when performing a task involving generating the uses of visually presented nouns versus reading aloud. ERPs showed strong task-related differences over left and middle inferior frontal and left parietotemporal regions. Frontal and left parietotemporal channels revealed these differences around 200 and 700 msec, respectively, after word presentation. These results provide the time course for parts of the anatomical circuit involved in generating the meaning of a word. Our results also demonstrate how combining the spatial localization of PET with the temporal resolution of ERPs greatly enhances the capacity to understand the mechanisms involved in human cognition.
Full text
PDF


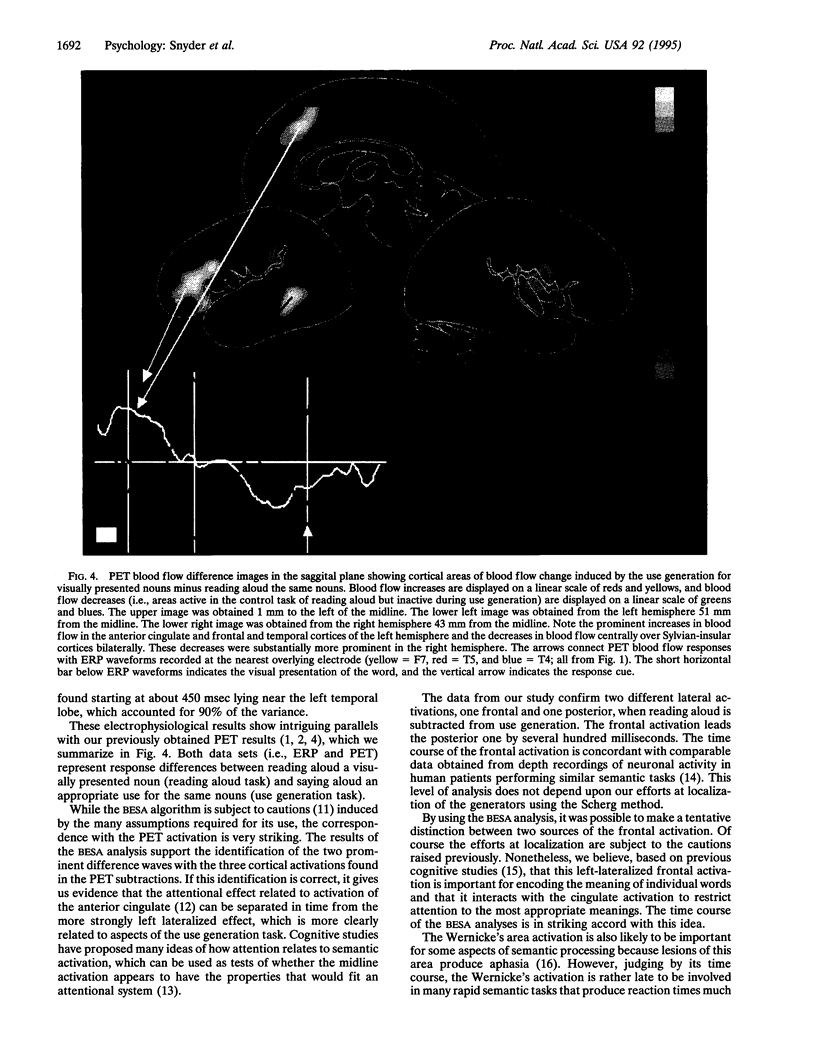

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdullaev Y. G., Bechtereva N. P. Neuronal correlate of the higher-order semantic code in human prefrontal cortex in language tasks. Int J Psychophysiol. 1993 May;14(3):167–177. doi: 10.1016/0167-8760(93)90031-j. [DOI] [PubMed] [Google Scholar]
- Damasio A. R. Aphasia. N Engl J Med. 1992 Feb 20;326(8):531–539. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- Frostig R. D., Lieke E. E., Ts'o D. Y., Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D., Patterson K., Wise R., Brown W. D., Friston K., Weiller C., Frackowiak R. The cortical localization of the lexicons. Positron emission tomography evidence. Brain. 1992 Dec;115(Pt 6):1769–1782. doi: 10.1093/brain/115.6.1769. [DOI] [PubMed] [Google Scholar]
- Kwong K. K., Belliveau J. W., Chesler D. A., Goldberg I. E., Weisskoff R. M., Poncelet B. P., Kennedy D. N., Hoppel B. E., Cohen M. S., Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G., Blamire A. M., Rothman D. L., Gruetter R., Shulman R. G. Echo-planar magnetic resonance imaging studies of frontal cortex activation during word generation in humans. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4952–4956. doi: 10.1073/pnas.90.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Tank D. W., Menon R., Ellermann J. M., Kim S. G., Merkle H., Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S. E., Fox P. T., Posner M. I., Mintun M., Raichle M. E. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988 Feb 18;331(6157):585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Posner M. I., Petersen S. E. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Raczkowski D., Kalat J. W., Nebes R. Reliability and validity of some handedness questionnaire items. Neuropsychologia. 1974 Jan;12(1):43–47. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- Raichle M. E., Fiez J. A., Videen T. O., MacLeod A. M., Pardo J. V., Fox P. T., Petersen S. E. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex. 1994 Jan-Feb;4(1):8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Snyder A. Z. Steady-state vibration evoked potentials: descriptions of technique and characterization of responses. Electroencephalogr Clin Neurophysiol. 1992 May-Jun;84(3):257–268. doi: 10.1016/0168-5597(92)90007-x. [DOI] [PubMed] [Google Scholar]
- Tucker D. M. Spatial sampling of head electrical fields: the geodesic sensor net. Electroencephalogr Clin Neurophysiol. 1993 Sep;87(3):154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]




