Abstract
The course of sugar fluxes into and out of protoplasts isolated from the mesophyll of Pisum sativum L. has been followed over brief time intervals (minutes). Light strongly stimulated net sugar influx at pH 8 as well as at pH 5.5. The proton conductor carbonyl cyanide m-chlorophenylhydrazone (CCCP) inhibited initial influx in the light, both at pH 8.0 and at pH 5.5. CCCP was without effect in the dark at either pH. All these results applied both to sucrose and to the nonmetabolizable glucose analog 3-O-methyl-d-glucose.
When protoplasts at pH 5.5 were transferred from light to darkness, “stored” light driving force maintained uptake in the dark at the full light rate for the first 7 minutes. At pH 8, however, even 4 minutes after transfer to dark, uptake was well below the light rate. Initial uptake rates over a range of external concentrations were derived from progress curves obtained in the light and in the dark, both at pH 5.5 and at 7.7. When initial rate was plotted against concentration, simple Michaelis-Menten kinetics were observed only under the condition pH 5.5, light. In the dark at both pH values, and in the light at pH 7.7, complex curves with intermediate plateaus were obtained, strongly resembling curves reported for systems where mixed negative and positive cooperativity is operating.
The same “Km for protons” was observed in the dark and in the light (10−7 molar). Switching protoplasts in the dark from pH 8 to 5.5 failed to drive sugar transport by imposed protonmotive force, as judged by lack of sensitivity to CCCP. Switching protoplasts which had taken up sugar in the dark at pH 5.5 to pH 7 induced net efflux of sugar. Flux analysis showed that this effect was entirely due to the prompt fall in influx.
It is concluded from the kinetic experiments that protonation alone is not sufficient to convert the sugar transport system to its fully activated high affinity form. A further light-dependent factor which acts synergistically with protonation is required.
Full text
PDF
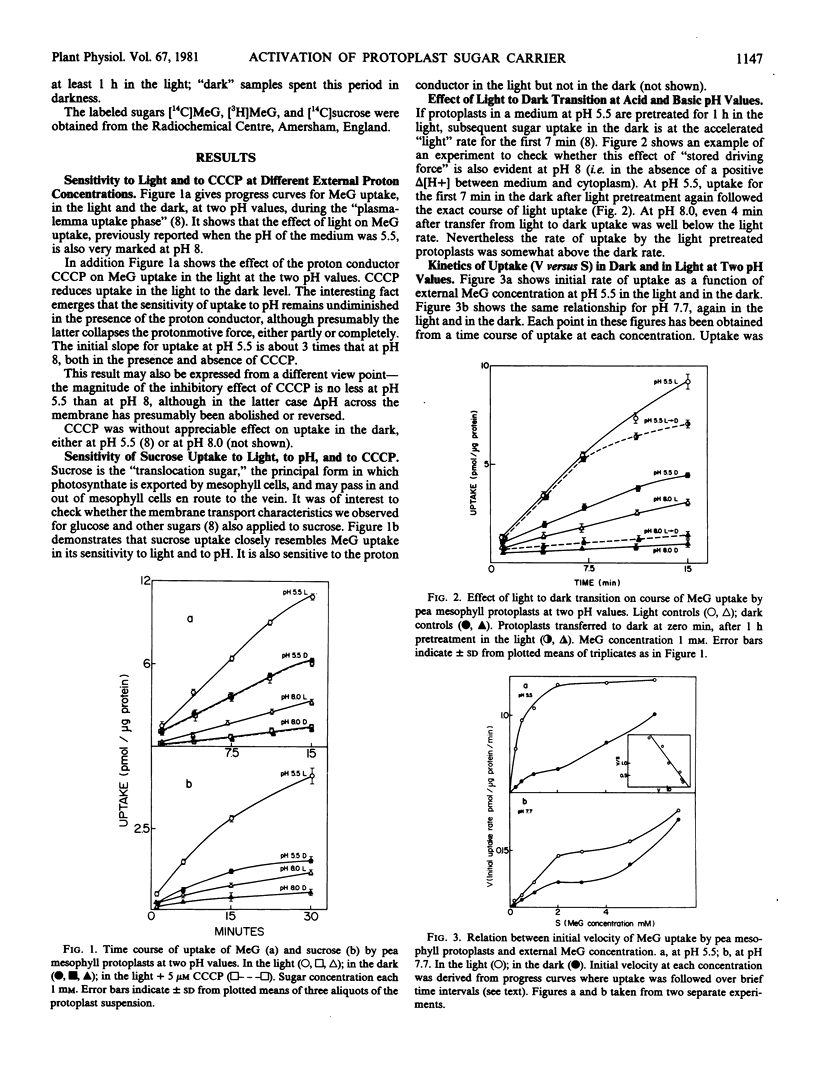


Selected References
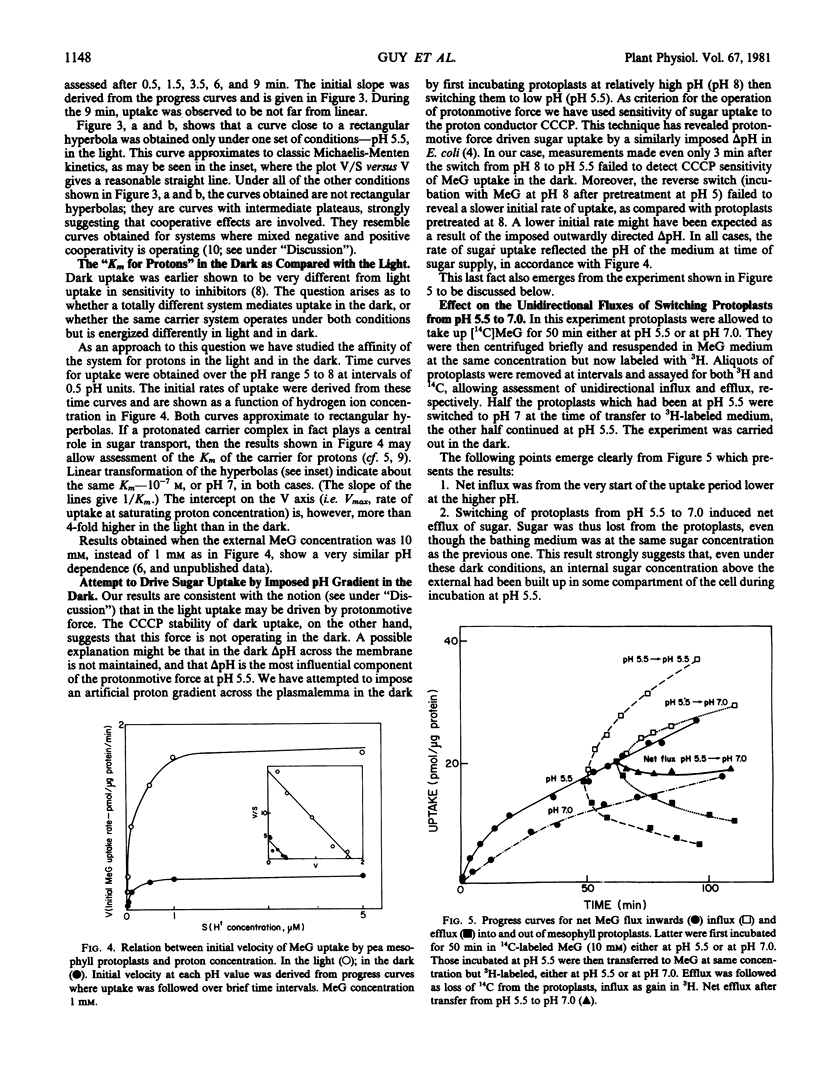
These references are in PubMed. This may not be the complete list of references from this article.
- Flagg J. L., Wilson T. H. Galactoside accumulation by Escherichia coli, driven by a pH gradient. J Bacteriol. 1976 Mar;125(3):1235–1239. doi: 10.1128/jb.125.3.1235-1236.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Phloem Loading of Sucrose: pH Dependence and Selectivity. Plant Physiol. 1977 Apr;59(4):750–755. doi: 10.1104/pp.59.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy M., Reinhold L. Membrane transport of sugars and amino acids in isolated protoplasts. Plant Physiol. 1978 Apr;61(4):593–596. doi: 10.1104/pp.61.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy M., Reinhold L., Michaeli D. Direct evidence for a sugar transport mechanism in isolated vacuoles. Plant Physiol. 1979 Jul;64(1):61–64. doi: 10.1104/pp.64.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy M., Reinhold L., Rahat M. Energization of the sugar transport mechanism in the plasmalemma of isolated mesophyll protoplasts. Plant Physiol. 1980 Mar;65(3):550–553. doi: 10.1104/pp.65.3.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton cotransport system of chlorella. pH-dependent change in Km values and translocation constants of the uptake system. J Gen Physiol. 1974 Nov;64(5):568–581. doi: 10.1085/jgp.64.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc G., Rimon G., Kaback H. R. Glucose 6-phosphate transport in membrane vesicles isolated from Escherichia coli: effect of imposed electrical potential and pH gradient. Biochemistry. 1980 May 27;19(11):2522–2528. doi: 10.1021/bi00552a034. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr The role of negative cooperativity and half-of-the-sites reactivity in enzyme regulation. Curr Top Cell Regul. 1976;10:1–40. doi: 10.1016/b978-0-12-152810-2.50008-5. [DOI] [PubMed] [Google Scholar]
- Linask J., Laties G. G. Multiphasic absorption of glucose and 3-o-methyl glucose by aged potato slices. Plant Physiol. 1973 Feb;51(2):289–294. doi: 10.1104/pp.51.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab W. G., Komor E. A possible mechanistic role of the membrane potential in proton-sugar cotransport of Chlorella. FEBS Lett. 1978 Mar 1;87(1):157–160. doi: 10.1016/0014-5793(78)80156-2. [DOI] [PubMed] [Google Scholar]
- Spanswick R. M. Evidence for an electrogenic ion pump in Nitella translucens. I. The effects of pH, K + , Na + , light and temperature on the membrane potential and resistance. Biochim Biophys Acta. 1972 Oct 23;288(1):73–89. doi: 10.1016/0005-2736(72)90224-6. [DOI] [PubMed] [Google Scholar]
- Teipel J., Koshland D. E., Jr The significance of intermediary plateau regions in enzyme saturation curves. Biochemistry. 1969 Nov;8(11):4656–4663. doi: 10.1021/bi00839a064. [DOI] [PubMed] [Google Scholar]
- Wilson D. B. Source of energy for the Escherichia coli galactose transport systems induced by galactose. J Bacteriol. 1974 Nov;120(2):866–871. doi: 10.1128/jb.120.2.866-871.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



