Abstract
Inhalational anthrax is caused by inhalation of Bacillus anthracis spores. The ability of B. anthracis to cause anthrax is attributed to the plasmid-encoded A/B-type toxins, edema toxin (edema factor and protective antigen) and lethal toxin (lethal factor and protective antigen), and a poly-d-glutamic acid capsule. To better understand the contribution of these toxins to the disease pathophysiology in vivo, we used B. anthracis Ames strain and isogenic toxin deletion mutants derived from the Ames strain to examine the role of lethal toxin and edema toxin after pulmonary spore challenge of cynomolgus macaques. Lethal toxin, but not edema toxin, was required to induce sustained bacteremia and death after pulmonary challenge with spores delivered via bronchoscopy. After intravenous challenge with bacilli to model the systemic phase of infection, lethal toxin contributed to bacterial proliferation and subsequent host death to a greater extent than edema toxin. Deletion of protective antigen resulted in greater loss of virulence after intravenous challenge with bacilli than deletion of lethal toxin or edema toxin alone. These findings are consistent with the ability of anti–protective antigen antibodies to prevent anthrax and suggest that lethal factor is the dominant toxin that contributes to the escape of significant numbers of bacilli from the thoracic cavity to cause anthrax after inhalation challenge with spores.
Inhalational anthrax, caused by inhalation of Bacillus anthracis spores, is the most lethal form of anthrax, often causing death within days of exposure. After pulmonary spore challenge, infection occurs in three phases: an invasion phase, in which lung and lymphatic vessel invasion is mediated by spore-laden phagocytes and possibly free spores; followed by a proliferation phase, in which bacilli proliferate in the draining lymphatic vessels and lymph nodes; and finally a terminal septicemic phase, in which bacteria disseminate hematogenously and proliferate in the blood and other organs.1 Death frequently occurs with massive bacteremia without the development of primary pneumonia.
The ability of B. anthracis to cause anthrax has been attributed primarily to plasmid-encoded virulence factors that consist of a poly-d-glutamic acid capsule (plasmid pX02) and two A/B-type toxins, lethal toxin (LT) and edema toxin (ET) (plasmid pX01). The capsule inhibits macrophage phagocytosis of vegetative bacilli in vitro and may inhibit the humoral immune response in vivo.2–4 The toxins are thought to exert their effects locally within phagosomes soon after spore germination, as well as at a distance in the systemic circulation.5–9 Protective antigen (PA), the toxin component common to both LT and ET, is the cell-binding subunit that binds to ubiquitous receptors on multiple cell types. LT is composed of PA and lethal factor (LF), whereas ET is composed of PA and edema factor (EF). Proteolytic cleavage of PA (83 kb) into 20-kb and 63-kb fragments, either on the cell surface or in body fluids, leads to heptamerization of the 63-kb PA fragment and formation of a prepore on the cell surface, with subsequent competitive binding to three molecules of LF, EF, or both.10–17 Endocytosis of the receptor complexes followed by acidification within the endosome results in a conformational change in the prepore, with subsequent delivery of the enzymatic moieties into the cytoplasm, where they exert their effects on the cell.18 LT is a zinc metalloproteinase that cleaves mitogen-activated protein kinases, and ET is an adenylate cyclase that increases cytoplasmic cAMP.19–21
Much of our current knowledge about the effects of B. anthracis toxins is derived from in vitro experiments or from challenge of animals with purified toxins (reviewed by Moayeri and Leppla22). In rodents challenged with lethal doses of purified toxins, evidence increasingly points to the systemic effects of LT and ET on the heart and vasculature with subsequent alterations in hemodynamic parameters as a primary pathogenic mechanism that leads to toxin-induced death in susceptible strains.23–26 However, interactions between the host and the infectious organism are more complex than what occurs after challenge with purified toxin. The outcome after pulmonary challenge with spores ultimately depends on host susceptibility to all of the virulence factors and their expression and activity at the appropriate stage of infection. Thus, in vitro experiments or experiments in animals using purified toxins may not accurately represent the role of the toxins after challenge with fully virulent spores.
To examine the role of anthrax toxins after pulmonary challenge with B. anthracis spores, we initially examined the virulence of isogenic toxin deletion mutants (PA−, LF−, and EF−) of a fully virulent strain of B. anthracis in BALB/c mice after intratracheal inoculation with spores. Systemic dissemination and lethality of the toxin deletion mutants in BALB/c mice were similar to the parental strain because of the high susceptibility of mice to capsule.27,28 Next we examined the virulence of isogenic capsule and toxin deletion mutants of B. anthracis, Ames strain, in New Zealand white rabbits and found that intrapulmonary delivery of capsule-negative or PA deletion mutant spores resulted in complete loss of virulence.29 Furthermore, elimination of each toxin alone by deletion of EF or LF individually resulted in a decrease in virulence for each of the mutants as indicated by an increase of ≥100-fold in the LD50 compared with the Ames strain. Absence of either LT or ET resulted in reduced dissemination and/or survival of vegetative bacilli outside the thoracic cavity. A similar reduction in virulence was observed after i.v. injection of vegetative bacilli of the PA, EF, and LF deletion mutants compared with the Ames strain. Dependence on PA for virulence after subcutaneous and intranasal spore challenge in New Zealand white rabbits was also recently reported using toxin deletion mutants of the virulent Vollum strain.30,31 In guinea pigs, however, deletion of PA, LF, and EF was not sufficient to abolish virulence. Thus, the importance of particular B. anthracis virulence factors appears to vary among host species.
Nonhuman primates (NHPs) are increasingly used as animal models to evaluate new vaccines and therapeutics for inhalational anthrax, many of which target specific B. anthracis virulence factors. Therefore, understanding the effect of these virulence factors on B. anthracis pathogenesis in NHPs is essential. B. anthracis, Ames strain, and isogenic toxin deletion mutants were used to examine, for the first time in an NHP model, the role of each of the individual toxin components in cynomolgus macaques after pulmonary challenge with spores. Previous studies found that cynomolgus macaques challenged with aerosolized B. anthracis spores are an appropriate model of human inhalational anthrax.32,33 In addition, cynomolgus macaques are increasingly being used to test vaccines and therapeutics against anthrax. Therefore, using the cynomolgus macaque NHP model, we examined the role of the toxins after pulmonary spore challenge. We also studied the role of toxins during a synchronized systemic phase of infection, bypassing the lung and draining lymph nodes, by intravenously infecting cynomolgus macaques with vegetative bacilli.
Materials and Methods
B. anthracis Strains
The Ames strain of B. anthracis was obtained from the US Army Medical Research Institute of Infectious Diseases (Frederick, MD). Isogenic toxin–deficient mutants for EF, LF, and PA were constructed on the Ames parental strain by replacing the cya coding sequence (62 bp upstream from the translational start site to 104 bp downstream from the translational stop site), the lef coding sequence (167 bp upstream from the translational start site to 59 bp upstream from the translational stop site), or the pagA coding sequence (87 bp downstream from the translational start site to 1383 bp upstream from the translational stop site) (http://www.ncbi.nlm.nih.gov/nuccore, accession number NC_001496.1), respectively, with an omega-kanamycin resistance gene cassette to help with screening of the mutants.28,34 The toxin-deficient phenotypes were confirmed by Western blot analyses of supernatants from cultures grown under conditions that promote toxin and capsule synthesis. Deletions of toxin component genes were confirmed using quantitative RT-PCR assay (TaqMan assay; Applied Biosystems, Carlsbad, CA). Growth curves for cultures of the mutant strains were similar to the Ames strain.
Spore Preparation
Spore stocks were streaked onto a nutrient broth yeast agar plate supplemented with sodium bicarbonate (NBY-NaCO3) plates and incubated overnight at 37°C. A few single colonies were then added to 50 mL of phage assay medium in a 500-mL flask. The culture was shaken at 250 rpm and incubated for 5 to 7 days at 30°C. Samples were viewed with a 100× objective to determine the proportion of phase-bright spores. The culture was then heated at 68°C for 40 minutes to kill any remaining vegetative cells. Spores were collected by centrifugation for 30 minutes at 16,000 × g and washed three times before being resuspended in sterile phosphate-buffered saline (PBS). The suspensions were aliquoted and frozen at −80°C. The titers of individual aliquots were determined by serial dilution and plating using an Autoplate 4000 (Spiral Biotech, Bethesda, MD). Challenge material was prepared by diluting the working stock spores in sterile PBS to provide the desired target concentration. All dilutions were performed in sterile PBS/0.01% Triton X-100 to minimize spore clumping.
Preparation of Vegetative Cells for Intravenous Infections
Vegetative cultures were prepared as previously described.28 Briefly, a B. anthracis spore stock was used to streak NBY-NaHCO3 to a final concentration of 0.8% and kanamycin (50 μg/mL), when appropriate, and incubated in 5% CO2 at 37°C for 24 hours. Next, a culture was prepared by incubating colonies in 40 mL of Luria-Bertani broth that contained 0.5% glycerol (and antibiotics when appropriate) and shaken at 200 rpm overnight at 27°C. A subculture was then prepared by transferring an aliquot of the overnight culture to fresh NBY-NaHCO3 at a starting OD600 of 0.1 and placed in a shaking incubator of 10% CO2 at 37°C for approximately 3 to 4 hours until reaching an OD600 of 1.2 and then centrifuged, washed with Dulbecco's PBS, and diluted with Dulbecco's PBS to the final inoculum concentration. Inoculums were verified by diluting and plating on sheep blood agar plates to obtain the actual concentration.
Animals
All animal work was performed at the Lovelace Respiratory Research Institute (LRRI) in a facility fully accredited by the International Association for the Assessment and Accreditation of Laboratory Animal Care. All procedures were performed in accordance with the guidelines promulgated in the Guide for the Care and Use of Laboratory Animals (National Research Council, Washington, DC) and approved by the Institutional Animal Care and Use Committee at the LRRI. All animals were provided with approved environmental enrichment during the study. All animals were determined to be healthy before initiation of experimental procedures based on physical examination and evaluation of prestudy hematologic and clinical chemical test results.
Juvenile to adult (1.3 to 7.3 kg; males and females) Vietnamese cynomolgus macaques were obtained from Covance Research Products (Alice, TX). All animals were tuberculin skin test negative and seronegative for Cercopithecine herpesvirus 1 (B virus), simian retrovirus, simian immunodeficiency virus, simian T-lymphotropic virus, and simian foamy virus. On arrival at the LRRI, animals were quarantined for 30 days before housing in individual cages in the biosafety level 3 facility. Animals had contact with cohorts through allogrooming panels in cages and were fed a commercial primate diet (Global Primate Diet 2050; Harlan Teklad, Madison, WI) supplemented with fresh fruits and vegetables. Animals were maintained on a 12:12-hour light:dark cycle and provided with a variety of enrichment devices to promote psychological well-being. Details regarding individual animals are given in Supplemental Table S1.
Anthrax Challenge
Exposures were performed for several months by trained personnel in an animal biosafety level 3 containment area. Animals were typically challenged serially in small groups (1 to 3 per strain per dose), and lethality data from the preceding challenge with a given strain and dose route were used to determine the doses for subsequent challenges to minimize the total number of animals used. For intrabronchial challenges, anesthesia was induced using 10 mg/kg of intramuscular ketamine, followed by intubation and maintenance of anesthesia with 3% isoflurane. A pediatric bronchoscope (BFXP40; Olympus America, Center Valley, PA) was passed through the endotracheal tube into the right mainstem bronchus, just distal to the carina. A 1-mL spore aliquot was introduced through the bronchoscope into the lung, followed by rinsing with a 1-mL aliquot of sterile PBS and injection of 10 mL of air. The bronchoscope was removed from the right lung and repositioned in the left mainstem bronchus, just distal to the carina, and the procedure was repeated. Depositions of the aliquots into the lungs were verified by visual confirmation using the bronchoscope video camera feature. The actual dose delivered for each spore preparation was calculated by averaging the doses delivered in three mock inoculations, whereby the spore aliquots were collected and cultured after passing through the bronchoscope. Animals were challenged with the different strains of B. anthracis on different days, and the bronchoscope was sterilized between strains. For i.v. challenges, animals were anesthetized with 10 mg/kg of intramuscular ketamine, and 1-mL aliquots of vegetative bacilli were delivered slowly (for 1 minute) by i.v. injection into the cephalic vein.
Postchallenge Monitoring and Determination of Lethality
Animals were monitored after challenge by trained technicians in consultation with the clinical veterinary staff. Monitoring included at least twice-daily evaluation of activity level, mentation, appetite, and temperature measurement using subcutaneous microchips. During the first 7 days after challenge, blood was collected daily from animals conditioned to primate chairs for qualitative bacterial culture, hematologic analysis, and limited clinical chemistry assays (alanine aminotransferase, total protein, blood urea nitrogen, lactate dehydrogenase, and C-reactive protein). Hematology measurements were performed using an Advia120 (Bayer Corporation, Diagnostic Division, Tarrytown, NY), and clinical chemistry measurements were performed using a Hitachi 911 chemistry analyzer (Roche Diagnostics, Indianapolis, IN). Thereafter, blood was collected weekly for the same assays until study termination on day 14 or 21. For the animals dosed with spores by bronchoscopy, survival was monitored for 21 days with the exception of the three animals challenged with the PA deletion mutant, which were euthanized on day 14. The animals given vegetative bacilli by the i.v. route were monitored for 14 days. Animals euthanized at study termination and animals determined to be moribund during the clinical observation period were euthanized by i.v. overdose of sodium pentobarbital. The LD50 values were determined using logistic regression analysis (SAS statistical software version 9.3; SAS Institute Inc, Cary, NC) with 95% confidence intervals.
Necropsy and Histologic Analysis
Necropsies were performed on all animals immediately after euthanasia or within 12 hours of death. Gross lesions were recorded, and samples were collected for bacteriologic and histopathologic analysis. Samples collected for bacteriologic analysis included blood (before moribund euthanasia only), tracheobronchial lymph node (TBLN), right middle lung lobe, spleen, and liver. Samples placed in 10% neutral-buffered formalin for histologic analysis included TBLN, spleen, liver, brain, and formalin-infused lungs. Tissues were fixed for approximately 14 days, trimmed, and processed routinely, and 5-μm tissue sections were stained with H&E and/or Brown and Brenn stain for histopathologic analysis by a board-certified veterinary pathologist (J.A.H.).
Qualitative Blood Culture
Shortly after collection, blood collected into EDTA tubes was plated onto tryptic soy agar (TSA) plus sheep blood using a standard quadrant streak method. To culture any bacteria potentially present in low numbers and not detectable using the quadrant streak method, an aliquot (250 μL) was removed and placed into 5 mL of sterile tryptic soy broth. Tryptic soy broth samples were allowed to incubate at 37°C ± 2°C for 24 to 48 hours, after which the broth was thoroughly mixed and qualitatively processed as described for the EDTA blood samples above. TSA plus sheep blood plates were incubated at 37°C ± 2°C for 18 to 24 hours, after which B. anthracis presence and purity were visually assessed.
Quantitative Tissue Culture
Tissue collected at necropsy was placed in 5 mL of sterile 1% peptone in tared 50-mL vials and weighed. Tissue samples were individually processed using sterile disposable tissue grinders. Processed tissue suspensions were serially diluted in sterile 1% peptone. From select dilutions, 100-μL aliquots were removed and spread onto sterile 90-mm TSA plates in triplicate (300 μL total for each dilution). TSA plates were incubated at 37°C ± 2°C for 18 to 24 hours, after which purity was visually assessed and B. anthracis colonies were counted. In some instances after standard culture, aliquots of processed lung and TBLN samples were heat shocked in a 65°C water bath for 30 minutes, diluted, and plated as already described to quantify spores. For coinfection studies that used a combination of wild-type (WT) and PA− strains, aliquots of organ homogenates were plated on agar plates both with and without 50 μg/mL of kanamycin to distinguish the number of PA− (kanamycin resistant) organisms from WT organisms.
Results
Bronchoscopic delivery of B. anthracis spores to cynomolgus macaques results in lethality and target organ disease comparable to aerosol delivery. Bronchoscopy was used to deliver different doses of B. anthracis spores directly into the lungs of cynomolgus macaques. Bronchoscopic delivery of spores was used instead of aerosol delivery to better control the challenge doses and exclude the confounding effects of spore uptake and germination in the nasal cavity and gastrointestinal tract, thereby facilitating a more precise comparison of Ames parent strain with each of the toxin deletion mutants. The survival of cynomolgus macaques challenged with varying doses of B. anthracis Ames strain spores is shown in Figure 1, and details regarding individual animals are provided in Supplemental Table S1. All animals challenged with >105 spores died, and all animals challenged with <104 spores survived until study termination on day 21 after challenge. Between 104 and 105 spores, 2 of 5 animals died, which is consistent with the reported LD50 of approximately 6 × 104 spores for cynomolgus macaques after aerosol challenge with Ames spores.32 Animals receiving higher doses of Ames spores succumbed to anthrax more rapidly than those receiving lower doses (Figure 1). Overall, there were no apparent differences in survival days between males and females.
Figure 1.
Survival of cynomolgus macaques after bronchoscopic delivery of varying doses of Ames strain Bacillus anthracis spores. Animals were monitored for 21 days after challenge, and animals still alive at study termination on day 21 were considered to be survivors. Individual animal identification numbers are provided to the right of each bar, and the details regarding individual animals are listed in Supplemental Table S1.
Lesions in target organs (lung, TBLNs, liver, spleen, and brain) were evaluated in animals that succumbed to anthrax after bronchoscopic delivery of Ames strain spores. Lesions were comparable to those reported after aerosol challenge,32 and there was no gross or microscopic evidence of bronchoscope-induced trauma to the conducting airways or lungs. Large bacilli were detected within the vessels of many organs (Figure 2). Lung lesions consisted of widespread edema and hemorrhage with infrequent small foci of neutrophilic alveolitis. The TBLN lesions consisted of multifocal, fibrinosuppurative to necrotizing inflammation in the cortex, paracortex, and medullary cords with variable lymphocytolysis (Figure 2B). Splenic lesions consisted of suppurative to necrotizing inflammation in the red pulp with lymphocytolysis in the white pulp (Figure 2C). Liver lesions primarily consisted of accumulation of neutrophils and necrotic or apoptotic cell debris within sinusoids (Figure 2D), although occasional foci of coagulation necrosis were also observed in a few animals. Other lesions included hemorrhage, edema, and mild neutrophilic inflammation within the mediastinum and hemorrhage within the meninges (Figure 3) and adrenal cortex. Target tissues from animals that survived until study termination on day 21 were also cultured and examined histologically and found to be without detectable bacteria or lesions.
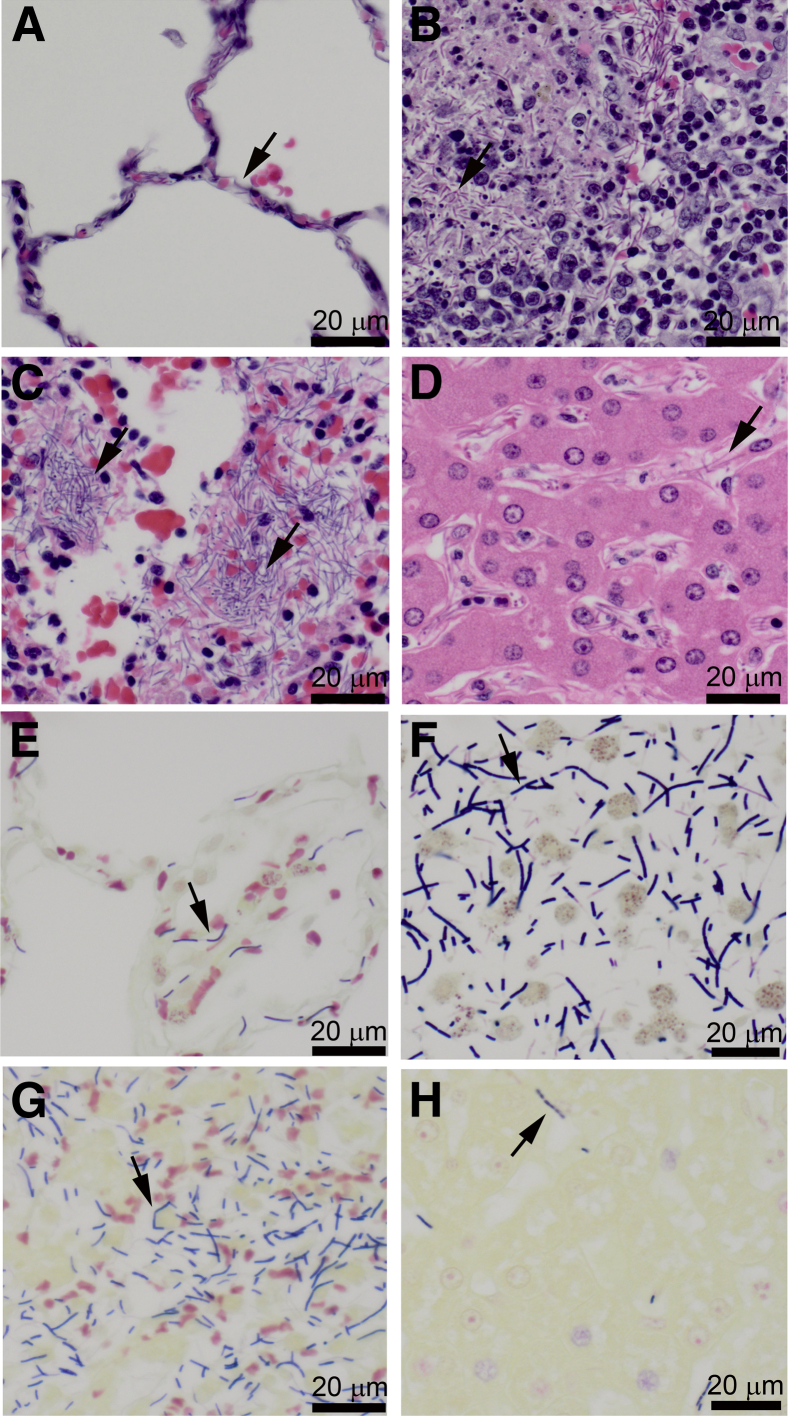
Figure 2.
Terminal target organ lesions in cynomolgus macaques challenged with Ames strain Bacillus anthracis by bronchoscopy are similar to those observed after inhalation of aerosolized spores. Terminal lesions were similar regardless of inoculum dose or time to death. Sections were stained with H&E (A–D) and Brown and Brenn stain (E–H). A and E: Alveolar septa in the lungs are distended with bacilli. B and F: Bacilli in the subcapsular sinus and cortex of a tracheobronchial lymph node, with cortical lymphocytolysis. C and G: Numerous bacilli in the splenic red pulp. D and H: Bacilli with scattered neutrophils in the hepatic sinusoids. Arrows point to bacilli. Original magnification: ×400 (A–H).
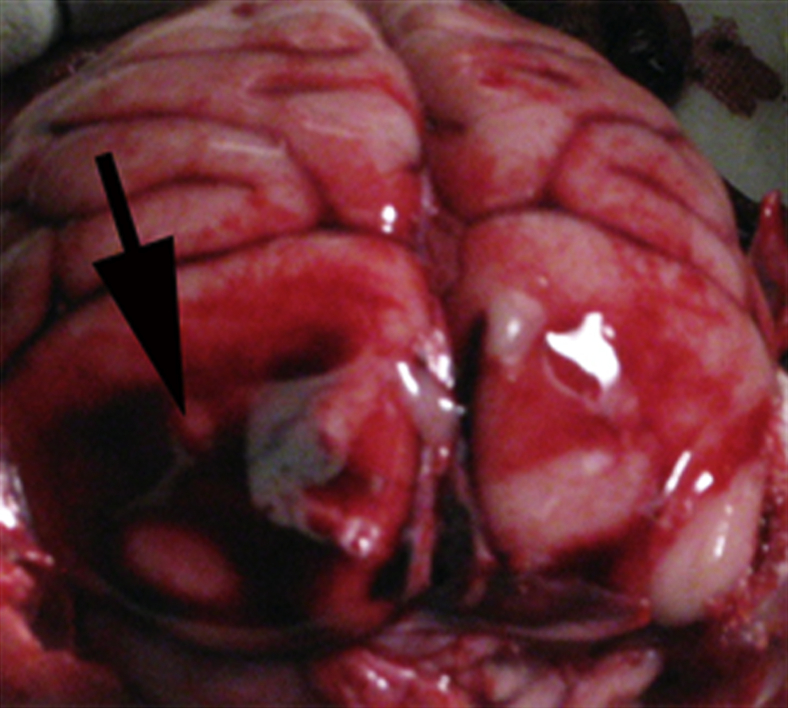
Figure 3.
Meningeal hemorrhage in a cynomolgus macaque that succumbed after bronchoscopic delivery of Ames strain Bacillus anthracis. Arrow points to a focus of meningeal hemorrhage in the occipital cortex.
LT, but Not ET, Is Essential for Development of Fatal Anthrax after Bronchoscopic Spore Delivery
The LD50 values for spores of the WT Ames strain and each of the toxin deletion mutants after bronchoscopic challenge of cynomolgus macaques are given in Table 1. Delivery of up to 9 × 107 spores of the LF and PA deletion mutants was insufficient to induce mortality in cynomolgus macaques. In contrast, animals dosed with the EF deletion mutant succumbed in an inoculum dose-dependent manner (Figure 4). At the lower end of the dosing range (4 to 7 × 103 spores), spores of the EF deletion mutant were more lethal than spores of the Ames parental strain. Lesions in target organs of the animals that succumbed after challenge with the EF deletion mutant were similar to those observed in the animals challenged with the parental Ames strain spores with bacilli detected in multiple organs (data not shown). Target tissues from animals that survived until study termination on day 21 after bronchoscopic challenge with the EF deletion mutant were cultured and examined histologically and found to be without detectable bacteria or lesions.
Table 1.
LD50 Values for Ames and Isogenic Mutant Strains After Pulmonary Challenge with Spores in Cynomolgus Macaques
| Strain | Genotype | Means ± SEM LD50∗ (log10) | 95% CI (log10) |
|---|---|---|---|
| Ames | WT | 4.13 ± 0.02 | 3.83–4.42 |
| EF− | cya− | 3.40 ± 0.18 | 2.56–4.23 |
| LF− | lef− | >7.9† | NA |
| PA− | pagAR− | >7.9† | NA |
Groups of NHPs were challenged by bronchoscopy with Bacillus anthracis spores of either the Ames strain (WT) or the EF−, LF−, or PA− strain at doses ranging from 102 to 108 spores per NHP for the WT and EF− strains (n = 3 to 5 NHP per dose per strain) and 105 to 108 spores per NHP for the LF− and PA− strain (n = 3 to 6 NHP per dose per strain). The NHPs were monitored twice daily for survival and clinical observations during infection.
EF, edema factor; LF, lethal factor; NA, not applicable; NHP, nonhuman primate; PA, protective antigen; WT, wild type.
The LD50 values for the Ames or the EF− strains in the NHPs were determined using logistic regression analysis and were found to be significantly different (z = −4.03, P < 0.0001).
All animals inoculated with the highest challenge dose tested of 9 × 107 spores per NHP of the LF− or PA− strains survived.
Figure 4.
Survival of cynomolgus macaques after bronchoscopic delivery of varying doses of Bacillus anthracis edema factor deletion mutant spores. Animals were monitored for 21 days after challenge, and animals still alive at study termination on day 21 were considered to be survivors. Individual animal identification numbers are provided to the right of each bar, and the details regarding individual animals are listed in Supplemental Table S1.
Although the animals challenged with the LF and PA deletion mutants survived the challenge, they developed mild clinical disease, characterized by decreased appetite and activity level and development of a mild, dry cough during the first week to 10 days after challenge. Elevations in C-reactive protein and peripheral blood neutrophil counts were detected during a 2- to 3-week period of observation, indicating the development of an inflammatory response to the LF and PA deletion mutants (Figure 5). A low-level transient bacteremia was detected in a few animals during the first 7 days after challenge (Figure 5), indicating that the spores were able to germinate and bacilli were able to escape from the thoracic cavity at low levels despite the deletion of LF and PA. At the time of study termination, however, the lungs, TBLNs, liver, and spleen from several of these animals were cultured, and B. anthracis was detected only in the lungs (approximately 105 CFU/g in animals challenged with >107 spores and <103 CFU/g in animals challenged with 105 spores) and TBLNs (approximately 103 CFU/g in animals challenged with >107 spores and <102 CFU/g in animals challenged with 105 spores).
Figure 5.
Inflammatory response in cynomolgus macaques after bronchoscopic delivery of spores of Bacillus anthracis lethal factor (LF−, two dose levels) and protective antigen (PA−, one dose level) deletion mutants. Elevations in C-reactive protein (CRP) (A and C) and peripheral blood neutrophil counts (B, D, and E) were identified after challenges. At each dose level, the CRP levels and neutrophil counts are given for the same three animals. For the animals challenged with spores of the PA deletion mutant, there was insufficient serum to assay CRP on each day after challenge. The mean CRP level on day 4 is reported below the neutrophil graph for these three animals. Asterisks (B, D and E) indicate the days on which bacteremia was detected in specific animals.
In six of the animals challenged with the LF deletion mutant, aliquots of lung and TBLN culture samples were heat shocked in a 65°C water bath for 30 minutes before culture initiation. Essentially all the bacteria cultured from these samples were heat resistant, suggesting that the residual bacteria were present as spores. Microscopic evaluation of lungs collected at study termination from the animals challenged with the LF and PA deletion mutants revealed the presence of a dose-dependent chronic granulomatous pneumonia on days 14 (PA deletion mutant) and 21 (LF deletion mutant) after challenge, presumably in response to the presence of residual spores in the lungs (Figure 6). The draining lymph nodes of the animals challenged with the LF and PA deletion mutant spores exhibited lymphoid hyperplasia with prominent germinal center formation. No significant lesions were detected in the liver, spleen, or brain of any of these animals, and there were no detectable bacteria in these tissues.
Figure 6.
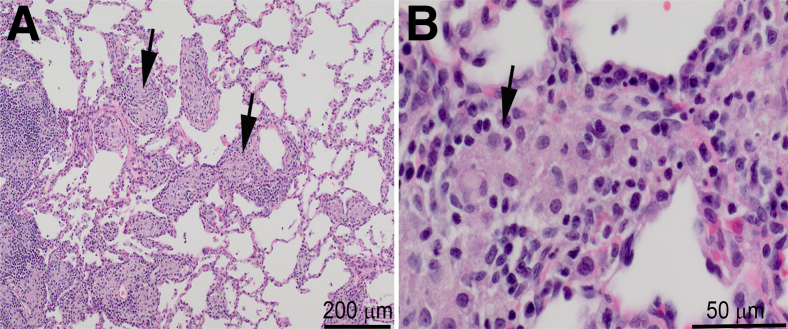
Chronic granulomatous pneumonia in a cynomolgus macaque 21 days after bronchoscopic challenge with 107 spores of the lethal factor deletion mutant. Sections were stained with H&E. Lesion severity was dose dependent and also present in the animals challenged with the PA deletion mutant. Arrows point to foci of granulomatous inflammation. Original magnification: ×40 (A); ×200 (B).
LT Contributes More to B. anthracis Virulence than ET in a Systemic Model of Infection with Vegetative Bacilli
B. anthracis spores that lack LT were unable to cause high levels of bacteremia and death in cynomolgus macaques after bronchoscopic delivery. To bypass the lungs and draining lymph nodes and evaluate the role of toxins during the systemic phase of anthrax, cynomolgus macaques were challenged by i.v. injection (cephalic vein) with vegetative forms of the Ames strain and each of the respective toxin deletion mutants. The survival of cynomolgus macaques after i.v. challenge with different doses of each of the strains is given in Table 2. The Ames and EF deletion mutants were uniformly lethal within 2 to 3 days after challenge at all i.v. doses examined. Cynomolgus macaques survived i.v. doses of up to 5.8 × 104 CFU (target dose was 1 × 105 CFU) of the LF deletion mutant bacilli and up to 1.3 × 106 CFU of the PA deletion mutant bacilli. The i.v. doses of ≥106 CFU of the LF deletion mutant bacilli and 107 CFU of the PA deletion mutant bacilli were required to cause lethality in cynomolgus macaques, again within 2 to 3 days after challenge.
Table 2.
Survival of Cynomolgus Macaques after Intravenous Challenge with Vegetative Bacilli of the Ames Strain and Each of the Toxin Deletion Mutants
| Log10 dose (range) | Surviving animals by strain (no. surviving/no. challenged) |
|||
|---|---|---|---|---|
| Ames | EF− | LF− | PA− | |
| 3–4 | ND | 0/1 | ND | ND |
| 4–5 | 0/1 | 0/2 | 3/3 | ND |
| 5–6 | 0/1 | ND | ND | ND |
| 6–7 | 0/1 | ND | 0/2 | 2/2 |
| 7–8 | ND | ND | 0/1 | 0/1 |
ND, not done.
It was possible that the mechanism of death after i.v. challenge might be different in the animals that died from the LT-producing (Ames and EF deletion mutant) virulent organisms compared with the high doses of markedly attenuated LT-nonproducing strains (LF and PA deletion mutants). In general, the macroscopic and microscopic findings in the monkeys that died after i.v. challenge with the EF and LF deletion mutant bacilli were similar to those in monkeys that died after intrabronchial and i.v. challenge with the Ames strain. This finding suggests that the pathologic findings associated with terminal anthrax does not depend on the presence of a specific toxin. However, one of the monkeys that succumbed to the LF deletion mutant exhibited extensive suppurative meningoencephalitis microscopically. Likewise, the single monkey that succumbed to the PA deletion mutant also exhibited extensive suppurative meningoencephalitis (Figure 7). This lesion was not observed in any of the other monkeys described in this study. There were no other significant macroscopic or microscopic findings in the single monkey that died from the high challenge with the PA deletion mutant (Figure 7). It is also noteworthy that the terminal bacterial burdens in the lungs, liver, spleen, and TBLNs were similar in monkeys that succumbed after lethal i.v. doses of Ames strain and the EF and LF deletion mutants, but the terminal tissue burdens for the single animal that succumbed to the highest i.v. dose of the PA deletion mutant were 103 to 105 lower in each of the tissues that were cultured (Table 3). This latter animal that succumbed to the highest i.v. dose of the PA deletion mutant, but had the lowest bacterial burden in the lungs, liver, spleen, and TBLNs, was one of the two monkeys that developed suppurative meningoencephalitis.
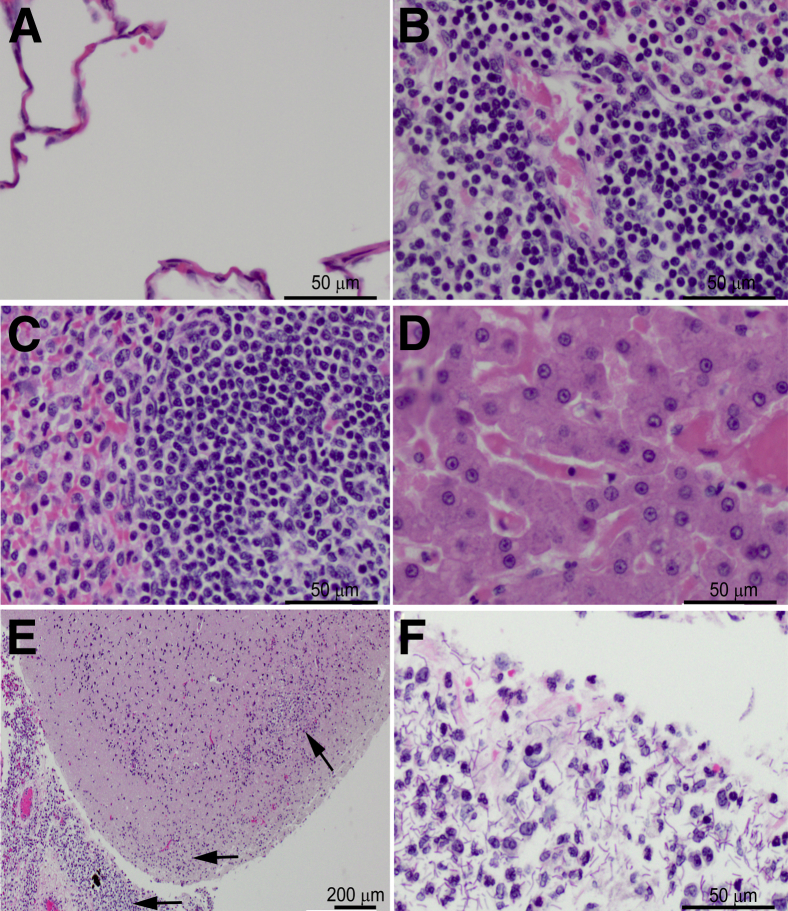
Figure 7.
Suppurative meningoencephalitis in a cynomolgus macaque succumbing to i.v. delivery of 107 CFU of protective antigen deletion mutant bacilli. Sections were stained with H&E. Lesions were not apparent in lungs (A), tracheobronchial lymph nodes (B), spleen (C), or liver (D). E and F: The brain and meninges exhibited marked suppurative meningoencephalitis (arrows) with abundant bacilli. Original magnification: ×200 (A–D and F); ×20 (E).
Table 3.
Quantitative Bacterial Cultures from Tissues of Cynomolgus Macaques Lethally Challenged by the I.V. Route with Bacilli of the Ames Strain and Each of the Toxin Deletion Mutants
| Group | Animal no. | Death day | I.V. dose (CFU) | Concentration (CFU/g) |
|||
|---|---|---|---|---|---|---|---|
| Spleen | Liver | TBLN | Lung | ||||
| Ames | V1 | 2 | 2.2 × 104 | 5.0 × 108 | 3.1 × 108 | 1.4 × 108 | 7.1 × 108 |
| V2 | 2 | 2.1 × 105 | 1.2 × 108 | 1.5 × 108 | 3.8 × 107 | 4.2 × 108 | |
| V3 | 2 | 2.2 × 106 | 4.3 × 107 | 9.4 × 107 | 1.9 × 107 | 3.6 × 107 | |
| EF− | V4 | 1 | 2.2 × 103 | 3.0 × 107 | 1.2 × 108 | 4.4 × 106 | 3.2 × 108 |
| V5 | 3 | 2.7 × 104 | 2.8 × 107 | 2.1 × 107 | 1.3 × 106 | 3.1 × 107 | |
| V6 | 3 | 2.7 × 104 | 4.9 × 108 | 2.1 × 108 | 1.1 × 108 | 2.5 × 108 | |
| LF− | V10 | 3 | 1.4 × 106 | 2.0 × 108 | 2.7 × 107 | 3.0 × 106 | 3.5 × 108 |
| V11 | 2 | 1.4 × 106 | 2.2 × 107 | 1.8 × 105 | 1.3 × 107 | 2.8 × 107 | |
| V12 | 2 | 1.4 × 107 | 9.6 × 107 | 9.7 × 106 | 5.2 × 107 | 2.3 × 107 | |
| PA− | V15 | 2 | 1.3 × 107 | 2.7 × 104 | 3.3 × 102 | 1.0 × 102 | 1.2 × 105 |
Cultures were initiated at the time of necropsy, between 2 and 12 hours after death.
EF, edema factor; LF, lethal factor; PA, protective antigen; TBLN, tracheobronchial lymph node.
PA Deletion Mutants Are Cleared More Rapidly than Ames in a Systemic Model of Infection with Vegetative Bacilli
Previously, we co-infected New Zealand white rabbits with approximately equal inoculums of Ames and the PA deletion mutant with spores via bronchoscopy or with vegetative bacilli via i.v. injection and found that the toxins produced by the Ames strain organisms did not increase dissemination or systemic survival of the PA deletion mutant organisms compared with levels seen in rabbits challenged with the PA deletion mutant alone.29 To begin to examine whether the same was true in monkeys, we intravenously injected three cynomolgus macaques with a mixture of bacilli that contained approximately equal inoculums of Ames and PA deletion mutant bacilli and euthanized the animals after 24 to 25 hours to quantitatively culture the lungs, spleen, and liver. The results are given in Table 4. Of the two monkeys that received 104 CFU of each strain, both appeared to be clearing or controlling the proliferation of the PA deletion mutant bacilli better than the Ames bacilli. A third monkey dosed intravenously with approximately 106 CFU of each strain and euthanized at 24 to 25 hours after infection had a similar pattern to the first two monkeys. Of note, none of these monkeys were clinically ill at the time of euthanasia. A fourth monkey was dosed intravenously with 105 CFU each of Ames and PA deletion mutant bacilli and monitored until clinical signs of disease developed. This monkey was euthanized at 30.5 hours after infection after exhibiting reduced activity, increased respiratory rate, and discolored red urine consistent with being near death. The tissue burdens of both Ames and the PA deletion mutant were higher than their initially injected inoculums. However, the tissue bacterial burdens of the Ames strain were still several orders of magnitude higher than for the PA deletion mutant and were similar to the terminal organ bacterial burdens found post mortem in monkeys previously challenged with the Ames strain alone (Table 3).
Table 4.
Quantitative Bacterial Cultures from Tissues of Cynomolgus Macaques Challenged by the I.V. Route with Mixtures of Bacilli of the Ames Strain and the PA Deletion Mutant
| Animal no. | Time to euthanasia (h) | I.V. dose (CFU) |
Concentration (CFU/g) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Lung |
Spleen |
Liver |
|||||||
| Ames | PA− | WT | PA− | WT | PA− | WT | PA− | ||
| V16 | 24.5 | 6.0 × 104 | 4.0 × 104 | 1.5 × 104 | 4.1 × 103 | 4.2 × 105 | 2.4 × 103 | 1.4 × 104 | BLD |
| V17 | 25.0 | 6.0 × 104 | 4.0 × 104 | BLD | BLD | 6.0 × 103 | 5.8 × 102 | 3.0 × 103 | BLD |
| V18 | 24.5 | 8.0 × 105 | 1.8 × 106 | BLD | BLD | 8.5 × 103 | 8.2 × 102 | BLD | BLD |
| V19∗ | 30.5 | 1.0 × 105 | 1.0 × 105 | 5.3 × 108 | 6.0 × 105 | >1.5 × 109 | 4.5 × 107 | 1.2 × 109 | 2.2 × 107 |
BLD, below limit of detection; PA, protective antigen; WT, wild type.
V19 was the only monkey of this group with clinical signs related to anthrax at the time of euthanasia.
Discussion
Understanding the roles of LT and ET in the species that are used to model inhalational anthrax in humans is important for developing new vaccines and therapeutic countermeasures against anthrax. Thus, we assessed the role of B. anthracis LT and ET in cynomolgus macaques using specific toxin deletion mutants created from the pX01+ and pX02+ Ames strain of B. anthracis. In cynomolgus macaques, LT was required for sustained bacteremia and death after pulmonary challenge with spores and was the major toxin required to cause a lethal infection after i.v. delivery of vegetative bacilli. This dependence on LT for developing disseminated anthrax after respiratory tract challenge with spores is similar to what has been reported in A/J mice challenged by aerosol with toxin deletion mutants of the a capsular Sterne strain of anthrax35 but is in contrast to the rabbit model where the Ames mutant expressing ET alone is as capable of causing disseminated anthrax as the mutant expressing LT alone, albeit at higher doses than the WT parental strain.29 In cynomolgus macaques, deletion of EF in the presence of LT resulted in even greater lethality than the Ames strain spores after pulmonary challenge. This unexpected result may be due to greater production of LF in the absence of EF or may indicate that the absence of EF permits greater binding of LF to PA and hence greater LT activity in the early stages after spore germination. Interestingly, the greater lethality of mutants expressing LT alone was not apparent in the rabbit model.29 Deletion of PA eliminated the effects of both ET and LT and resulted in complete loss of lethality after bronchoscopic challenge of cynomolgus macaques with spores and markedly reduced virulence in the i.v. bacilli model. In contrast to LT, ET expression alone did not allow for sustained bacteremia and death after pulmonary spore challenge, and it contributed only minimally to lethality after i.v. challenge with high doses of bacilli.
Importantly, both the LF and PA deletion mutants were capable of causing infection after bronchoscopic challenge even though the infection was not fatal. Cynomolgus macaques challenged with >6 × 105 spores of the LF and >9 × 107 spores of the PA deletion mutants exhibited mild clinical signs of illness and dose-dependent, chronic lung inflammation at the time of study termination 14 to 21 days after challenge. Cultures revealed that some bacteria, presumably spores, were present in the lungs and TBLNs at study termination. Peripheral blood neutrophil counts and serum C-reactive protein levels measured at several time points after challenge indicated an inflammatory response to the bronchoscopically delivered LF and PA deletion mutant spores, and blood cultures performed daily during the first week after challenge revealed transient, low-level bacteremia in several animals. The enlarged TBLNs with prominent germinal centers identified at study termination suggested that an adaptive immune response developed after challenge, and it is possible that these animals might be immune to a subsequent challenge with virulent spores. Indeed, one might predict that the LF deletion mutant capable of making PA would be more protective than the PA deletion mutant.
On the basis of available data about anthrax and the B. anthracis toxins, Tournier et al1 proposed a model of inhalational anthrax pathogenesis in which the toxins exert local effects on lung macrophages and dendritic cells during the invasion and proliferation phases in the lungs and draining lymph nodes and systemic effects on endothelial cells and the cardiovascular system during the terminal bacteremic phase of the disease. In agreement with this proposed model, our data suggest that LT functions as a virulence factor, facilitating proliferation and/or survival of germinated bacilli in the pulmonary lymphatics and draining lymph nodes after pulmonary challenge. In addition, LT, and to a lesser extent ET, contributes to bacillus survival and proliferation systemically in cynomolgus macaques after i.v. challenge. The question then arises of whether LT plays an additional, more direct role in killing the host, as has been suggested by studies using animal models challenged with purified toxins.
Despite clear evidence of the ability of both purified LT and ET to cause death in rats and mice,8,9,23,36,37 the relevance of experimental animal exposures to a parenteral bolus of purified toxins for studying the effects of toxins during the terminal, septicemic phase of anthrax is uncertain. Clearly, the use of purified toxins disregards the complex interplay between the bacilli and the host immune response. In addition, there are uncertainties about the relevance of the dose of purified toxins used in the rodent studies and whether a bolus dosing protocol better enables the toxins to overwhelm potential detoxification and excretion mechanisms of the host. Recently, in studies using New Zealand rabbits and rhesus macaques, the serum concentration of toxin components was found to increase gradually between 2 and 4 days after spore challenge.38–40 Paradoxically, in both species the individual animals with the highest serum toxin concentrations on day 2 were the animals that survived the longest, indicating that the toxin levels during a natural infection are controlled by complex mechanisms. Furthermore, the recent identification of a putative serum protease that cleaves PA and results in loss of LT activity highlights the complexities and potential for species differences in susceptibility to anthrax toxins.39 Although identified in both mouse and human sera, the human protease had greater activity than the mouse protease, and the amount of protease activity in human sera varied for different individuals, leading the authors to speculate that variations in protease activity may contribute to individual susceptibility to systemic anthrax toxins in humans.
The role of anthrax toxin in killing rhesus macaques was previously investigated.41 Injection of in vitro produced toxin revealed that anthrax toxin alone was sufficient to kill rhesus macaques. However, the amount of toxin used was specified in terms of minutes to death of a rat, a species highly susceptible to anthrax toxins but highly resistant to anthrax, so the precise amount of LT and ET delivered is unknown. In addition, considering the likely existence of a toxin-neutralizing serum protease in NHP serum, a bolus dose of toxin might be expected to overwhelm the protease activity more so than the gradual increase in toxin observed during the septicemic phase of anthrax. Interestingly, in rhesus macaques dying of anthrax after challenge with spores, not all animals had detectable toxemia near the time of death.
Results from our study suggest that the primary effect of LT during the systemic bacteremic phase is to facilitate survival and proliferation of vegetative bacteria. In addition, the presence of similar pathologic findings and similar terminal bacterial burdens in the animals that died after i.v. challenge with Ames and the LF and EF deletion mutants suggests that death from anthrax is not toxin specific but may be due to the high level of bacteremia, which has recently been proposed in the literature.42 The absence of LT in the LF and PA deletion mutants presumably permits survival of the phagocytic cells that are required for clearance of the bacilli from the circulation. The low terminal bacterial burdens in the tissues of the animal that succumbed to challenge with a bolus i.v. dose of 107 CFU of the PA deletion mutant indicate that the host was still able to control bacterial proliferation to some extent, presumably because of the preserved viability of its phagocytic cells. This animal likely succumbed because of the inability of the liver and spleen to effectively remove such a large number of bacilli delivered as a single i.v. bolus. The bacilli that escaped clearance seeded the meninges and established a suppurative meningoencephalitis, explaining death in the absence of a substantial bacterial burden in other organs. Notably, neutrophilic meningoencephalitis was identified in one of the monkeys that succumbed to the LF deletion mutant after i.v. delivery of 106 CFU of bacilli. The recently identified plasmid-encoded (pX01) adhesion protein B. anthracis S-layer protein A, which has been found to mediate attachment to brain microvascular endothelial cells and disrupt the tight junction proteins, may have contributed to the development of meningoencephalitis in the absence of LF or PA.43,44 The slight difference in survival between the monkeys challenged with high i.v. doses of the LF deletion mutant compared with the PA deletion mutant (Table 2), along with the higher terminal tissue burdens for monkeys challenged with the LF deletion mutants (Table 3), suggests that ET alone has a slight role as a virulence factor during the systemic phase of infection, although the exact nature of that role was not clear in these experiments.
In the final experiment in which cynomolgus macaques were challenged with an i.v. mixture of approximately equal numbers of Ames and PA deletion mutant bacilli, the tissue bacterial burdens for the PA deletion mutant were substantially lower than for the Ames strain at 24 to 25 hours after challenge and immediately before the anticipated death of the animal. These data indicate that host defenses were better able to control the growth of the PA deletion mutant compared with the Ames bacilli and are similar to our results in rabbits in that regard.29 In addition, these results suggest that even during the disseminated stage of anthrax, the toxin may exert its function in close proximity to the bacillus that secreted it. However, in the monkey that was euthanized with clinical signs indicating impending death, there was clear evidence of proliferation of both the Ames and PA deletion mutants above the initial inoculum based on the bacterial tissue burdens detected. The proliferation of the PA deletion mutant above the initial inoculum levels in this monkey may have been the result of i) the very large numbers of Ames bacilli and hence the systemic presence of the Ames toxins, allowing survival and proliferation of the mutant bacilli; ii) phagocyte death and depletion induced by the Ames-derived LT; and iii) nonspecific Ames-induced clinical illness rather than a specific response to Ames toxin production.
Our data together with data from other studies are compatible with B. anthracis LT playing a major role in the pathogenesis of inhalational anthrax by suppressing the innate immune response, thereby facilitating the survival and proliferation of the organism within the lung and draining lymph nodes and ultimately in target organs and the systemic circulation. ET has a lesser role as a virulence factor, and its role in the cynomolgus macaque is limited to the systemic phase of anthrax. These results are significant in that they suggest LT is likely to be the more relevant toxin target for new vaccines and therapeutics against inhalational anthrax in humans. In addition, therapeutics targeting ET activity alone, particularly in the early stages after challenge with spores, may enhance bacterial virulence. Our results are also consistent with the sepsis model, suggesting the death in cases of inhalational anthrax is a result of the high-level bacteremia.
Acknowledgments
We thank Theresa M. Kohler (The University of Texas Houston Health Science Center, Houston, TX) for assistance with the mutant strain construction, the University of New Mexico Clinical and Translational Sciences Center Biomedical Informatics Core for their assistance with the statistical analyses, and the LRRI clinical veterinary staff and numerous technicians, in particular Katherine Morse and Lorenzo B. Epps, for excellent technical assistance in the performance of these studies.
Footnotes
Supported by National Institute of Allergy and Infectious Diseases grant P01 AI056295 (C.R.L., M.F.L., J.A.L., and J.A.H.).
Disclosures: None declared.
Current address of T.B., Department of Microbiology and Immunology, The University of Texas Medical Branch, Galveston, TX; of M.F.L. and C.R.L., Center for Infectious Disease, Colorado State University, Fort Collins, CO.
Supplemental Data
References
- 1.Tournier J.N., Quesnel-Hellmann A., Cleret A., Vidal D.R. Contribution of toxins to the pathogenesis of inhalational anthrax. Cell Microbiol. 2007;9:555–565. doi: 10.1111/j.1462-5822.2006.00866.x. [DOI] [PubMed] [Google Scholar]
- 2.Keppie J., Smith H., Harris-Smith P.W. The chemical basis of the virulence of bacillus anthracis. II. Some biological properties of bacterial products. Br J Exp Pathol. 1953;34:486–496. [PMC free article] [PubMed] [Google Scholar]
- 3.Makino S., Uchida I., Terakado N., Sasakawa C., Yoshikawa M. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J Bacteriol. 1989;171:722–730. doi: 10.1128/jb.171.2.722-730.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman J.W., Nitecki D.E. Studies on the relation of a prior immune response to immunogenicity. Immunology. 1967;13:577–583. [PMC free article] [PubMed] [Google Scholar]
- 5.Banks D.J., Barnajian M., Maldonado-Arocho F.J., Sanchez A.M., Bradley K.A. Anthrax toxin receptor 2 mediates Bacillus anthracis killing of macrophages following spore challenge. Cell Microbiol. 2005;7:1173–1185. doi: 10.1111/j.1462-5822.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 6.Guidi-Rontani C., Levy M., Ohayon H., Mock M. Fate of germinated Bacillus anthracis spores in primary murine macrophages. Mol Microbiol. 2001;42:931–938. doi: 10.1046/j.1365-2958.2001.02695.x. [DOI] [PubMed] [Google Scholar]
- 7.Guidi-Rontani C., Weber-Levy M., Labruyere E., Mock M. Germination of Bacillus anthracis spores within alveolar macrophages. Mol Microbiol. 1999;31:9–17. doi: 10.1046/j.1365-2958.1999.01137.x. [DOI] [PubMed] [Google Scholar]
- 8.Firoved A.M., Miller G.F., Moayeri M., Kakkar R., Shen Y., Wiggins J.F., McNally E.M., Tang W.J., Leppla S.H. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am J Pathol. 2005;167:1309–1320. doi: 10.1016/S0002-9440(10)61218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moayeri M., Haines D., Young H.A., Leppla S.H. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beauregard K.E., Collier R.J., Swanson J.A. Proteolytic activation of receptor-bound anthrax protective antigen on macrophages promotes its internalization. Cell Microbiol. 2000;2:251–258. doi: 10.1046/j.1462-5822.2000.00052.x. [DOI] [PubMed] [Google Scholar]
- 11.Bragg T.S., Robertson D.L. Nucleotide sequence and analysis of the lethal factor gene (lef) from Bacillus anthracis. Gene. 1989;81:45–54. doi: 10.1016/0378-1119(89)90335-1. [DOI] [PubMed] [Google Scholar]
- 12.Ezzell J.W., Jr., Abshire T.G. Serum protease cleavage of Bacillus anthracis protective antigen. J Gen Microbiol. 1992;138:543–549. doi: 10.1099/00221287-138-3-543. [DOI] [PubMed] [Google Scholar]
- 13.Milne J.C., Furlong D., Hanna P.C., Wall J.S., Collier R.J. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J Biol Chem. 1994;269:20607–20612. [PubMed] [Google Scholar]
- 14.Mogridge J., Cunningham K., Collier R.J. Stoichiometry of anthrax toxin complexes. Biochemistry. 2002;41:1079–1082. doi: 10.1021/bi015860m. [DOI] [PubMed] [Google Scholar]
- 15.Mogridge J., Cunningham K., Lacy D.B., Mourez M., Collier R.J. The lethal and edema factors of anthrax toxin bind only to oligomeric forms of the protective antigen. Proc Natl Acad Sci U S A. 2002;99:7045–7048. doi: 10.1073/pnas.052160199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panchal R.G., Halverson K.M., Ribot W., Lane D., Kenny T., Abshire T.G., Ezzell J.W., Hoover T.A., Powell B., Little S., Kasianowicz J.J., Bavari S. Purified Bacillus anthracis lethal toxin complex formed in vitro and during infection exhibits functional and biological activity. J Biol Chem. 2005;280:10834–10839. doi: 10.1074/jbc.M412210200. [DOI] [PubMed] [Google Scholar]
- 17.Klimpel K.R., Molloy S.S., Thomas G., Leppla S.H. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc Natl Acad Sci U S A. 1992;89:10277–10281. doi: 10.1073/pnas.89.21.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedlander A.M. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986;261:7123–7126. [PubMed] [Google Scholar]
- 19.Duesbery N.S., Webb C.P., Leppla S.H., Gordon V.M., Klimpel K.R., Copeland T.D., Ahn N.G., Oskarsson M.K., Fukasawa K., Paull K.D., Vande Woude G.F. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 20.Leppla S.H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982;79:3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitale G., Pellizzari R., Recchi C., Napolitani G., Mock M., Montecucco C. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- 22.Moayeri M., Leppla S.H. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med. 2009;30:439–455. doi: 10.1016/j.mam.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo S.R., Willingham M.C., Bour S.H., Andreas E.A., Park S.K., Jackson C., Duesbery N.S., Leppla S.H., Tang W.J., Frankel A.E. Anthrax toxin-induced shock in rats is associated with pulmonary edema and hemorrhage. Microb Pathog. 2008;44:467–472. doi: 10.1016/j.micpath.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Moayeri M., Crown D., Dorward D.W., Gardner D., Ward J.M., Li Y., Cui X., Eichacker P., Leppla S.H. The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS) PLoS Pathog. 2009;5:e1000456. doi: 10.1371/journal.ppat.1000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson L.E., Kuo S.R., Katki K., Dang T., Park S.K., Dostal D.E., Tang W.J., Leppla S.H., Frankel A.E. Anthrax toxins induce shock in rats by depressed cardiac ventricular function. PLoS One. 2007;2:e466. doi: 10.1371/journal.pone.0000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson L.E., Mock J., Lal H., Lu G., Bourdeau R.W., Tang W.J., Leppla S.H., Dostal D.E., Frankel A.E. Lethal and edema toxins of anthrax induce distinct hemodynamic dysfunction. Front Biosci. 2007;12:4670–4675. doi: 10.2741/2416. [DOI] [PubMed] [Google Scholar]
- 27.Drysdale M., Heninger S., Hutt J., Chen Y., Lyons C.R., Koehler T.M. Capsule synthesis by Bacillus anthracis is required for dissemination in murine inhalation anthrax. EMBO J. 2005;24:221–227. doi: 10.1038/sj.emboj.7600495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heninger S., Drysdale M., Lovchik J., Hutt J., Lipscomb M.F., Koehler T.M., Lyons C.R. Toxin-deficient mutants of Bacillus anthracis are lethal in a murine model for pulmonary anthrax. Infect Immun. 2006;74:6067–6074. doi: 10.1128/IAI.00719-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovchik J.A., Drysdale M., Koehler T.M., Hutt J.A., Lyons C.R. Expression of either lethal toxin or edema toxin by Bacillus anthracis is sufficient for virulence in a rabbit model of inhalational anthrax. Infect Immun. 2012;80:2414–2425. doi: 10.1128/IAI.06340-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy H., Weiss S., Altboum Z., Schlomovitz J., Glinert I., Sittner A., Shafferman A., Kobiler D. Differential contribution of Bacillus anthracis toxins to pathogenicity in two animal models. Infect Immun. 2012;80:2623–2631. doi: 10.1128/IAI.00244-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy H., Weiss S., Altboum Z., Schlomovitz J., Rothschild N., Blachinsky E., Kobiler D. Lethal factor is not required for Bacillus anthracis virulence in guinea pigs and rabbits. Microb Pathog. 2012;51:345–351. doi: 10.1016/j.micpath.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Vasconcelos D., Barnewall R., Babin M., Hunt R., Estep J., Nielsen C., Carnes R., Carney J. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis) Lab Invest. 2003;83:1201–1209. doi: 10.1097/01.lab.0000080599.43791.01. [DOI] [PubMed] [Google Scholar]
- 33.Henning L.N., Comer J.E., Stark G.V., Ray B.D., Tordoff K.P., Knostman K.A., Meister G.T. Development of an inhalational Bacillus anthracis exposure therapeutic model in cynomolgus macaques. Clin Vaccine Immunol. 2012;19:1765–1775. doi: 10.1128/CVI.00288-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chand H.S., Drysdale M., Lovchik J., Koehler T.M., Lipscomb M.F., Lyons C.R. Discriminating virulence mechanisms among Bacillus anthracis strains by using a murine subcutaneous infection model. Infect Immun. 2009;77:429–435. doi: 10.1128/IAI.00647-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loving C.L., Khurana T., Osorio M., Lee G.M., Kelly V.K., Stibitz S., Merkel T.J. Role of anthrax toxins in dissemination, disease progression, and induction of protective adaptive immunity in the mouse aerosol challenge model. Infect Immun. 2009;77:255–265. doi: 10.1128/IAI.00633-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui X., Moayeri M., Li Y., Li X., Haley M., Fitz Y., Correa-Araujo R., Banks S.M., Leppla S.H., Eichacker P.Q. Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R699–R709. doi: 10.1152/ajpregu.00593.2003. [DOI] [PubMed] [Google Scholar]
- 37.Cui X., Li Y., Li X., Laird M.W., Subramanian M., Moayeri M., Leppla S.H., Fitz Y., Su J., Sherer K., Eichacker P.Q. Bacillus anthracis edema and lethal toxin have different hemodynamic effects but function together to worsen shock and outcome in a rat model. J Infect Dis. 2007;195:572–580. doi: 10.1086/510856. [DOI] [PubMed] [Google Scholar]
- 38.Boyer A.E., Quinn C.P., Woolfitt A.R., Pirkle J.L., McWilliams L.G., Stamey K.L., Bagarozzi D.A., Hart J.C., Jr., Barr J.R. Detection and quantification of anthrax lethal factor in serum by mass spectrometry. Anal Chem. 2007;79:8463–8470. doi: 10.1021/ac701741s. [DOI] [PubMed] [Google Scholar]
- 39.Goldman D.L., Zeng W., Rivera J., Nakouzzi A., Casadevall A. Human serum contains a protease that protects against cytotoxic activity of Bacillus anthracis lethal toxin in vitro. Clin Vaccine Immunol. 2008;15:970–973. doi: 10.1128/CVI.00064-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molin F.D., Fasanella A., Simonato M., Garofolo G., Montecucco C., Tonello F. Ratio of lethal and edema factors in rabbit systemic anthrax. Toxicon. 2008;52:824–828. doi: 10.1016/j.toxicon.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Klein F., Hodges D.R., Mahlandt B.G., Jones W.I., Haines B.W., Lincoln R.E. Anthrax toxin: causative agent in the death of rhesus monkeys. Science. 1962;138:1331–1333. doi: 10.1126/science.138.3547.1331. [DOI] [PubMed] [Google Scholar]
- 42.Coggeshall K.M., Lupu F., Ballard J., Metcalf J.P., James J.A., Farris D., Kurosawa S. The sepsis model: an emerging hypothesis for the lethality of inhalation anthrax. J Cell Mol Med. 2013;17:914–920. doi: 10.1111/jcmm.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebrahimi C.M., Sheen T.R., Renken C.W., Gottlieb R.A., Doran K.S. Contribution of lethal toxin and edema toxin to the pathogenesis of anthrax meningitis. Infect Immun. 2011;79:2510–2518. doi: 10.1128/IAI.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kern J.W., Schneewind O. BslA, a pXO1-encoded adhesin of Bacillus anthracis. Mol Microbiol. 2008;68:504–515. doi: 10.1111/j.1365-2958.2008.06169.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.