Abstract
Background
We tested the hypothesis that perinatal oxytocin, given to pregnant women to induce labor, is related to offspring bipolar disorder (BP) and worse childhood cognitive performance among offspring. We also tested the association between childhood cognition and later BP.
Methods
A population-based birth cohort derived from the Child Health and Development Study (CHDS) which included nearly all pregnant women receiving obstetric care from the Kaiser Permanente Medical Care Plan, Northern California Region (KPNC) between1959–1966. Prospectively obtained medical and offspring cognitive performance were used. Potential cases with BP from the cohort were identified by database linkages. This protocol identified 94 cases who were matched 1:8 to controls.
Results
Perinatal oxytocin was associated with a 2.4 times increased odds of later BP. Oxytocin was also associated with decreased performance on the Raven Matrices, but not on the Peabody Picture Vocabulary Test (PPVT). Childhood cognition was not associated with later BP.
Limitations
Loss to follow-up must be considered in all birth cohort studies. Additionally, the childhood cognitive battery did not include tests related to multiple domains of cognition which have been associated with later BP. A third limitation is the modest sample size of those exposed to oxytocin.
Conclusions
This study provides evidence for a potentially important perinatal risk factor for BP and cognitive impairment in childhood. While the association between perinatal oxytocin and offspring BP must be viewed cautiously until further studies can attempt to replicate the result, it lends support to the broader view that neurodevelopmental factors contribute to BP.
Keywords: Oxytocin, neurodevelopment, cognition, perinatal, Birth cohort, Raven Matrices, PPVT
Bipolar disorder (BP) has been thought to be related to schizophrenia (SZ) in certain respects, and as the neurodevelopmental hypothesis of SZ has become increasingly accepted, a similar hypothesis has been applied to BP (Bearden et al., 2001; Demjaha et al., 2012; Goodwin et al., 2008; Murray et al., 2004; Quraishi and Frangou, 2002; Sanches et al., 2008; Tamminga et al., 2013). The neurodevelopmental hypothesis posits that altered, pathological, or delayed maturation of the developing brain shifts the neurodevelopmental trajectory, followed by later onset of psychiatric illness (Fish et al., 1965; Meyer and Feldon, 2010; Millan, 2013; Murray and Lewis, 1987; Nasrallah and Weinberger, 1986; Oneal and Robins, 1958). Currently, evidence supporting the neurodevelopmental hypothesis of BP is less robust than the evidence supporting the corresponding hypothesis for SZ.
For instance, cognitive impairment during the premorbid and prodromal phases appears to be milder in BP compared with SZ (Seidman et al., 2013; Urfer-Parnas et al., 2010; Zanelli et al., 2010). Population based conscript studies have reported small but significant differences in overall premorbid cognitive performance in BP (Osler et al., 2007; Sorensen et al., 2012; Tiihonen et al., 2005; Urfer-Parnas et al., 2010), although some other studies have found better than average cognition (MacCabe et al., 2013). A review of longitudinal, family, and first episode neuropsychological studies found domain specific functions (executive and memory) are consistently impaired in those who later develop BP (Olvet et al., 2013). However, a review of population based studies concluded the evidence is not sufficient to determine whether premorbid cognitive impairment is a trait of later BP (Kravariti et al., 2009). In addition, fewer prenatal and perinatal exposures have been found to be associated with BP compared with SZ, though this may be because fewer studies have been performed (Sanches et al., 2008; Tsuchiya et al., 2003).
The neurodevelopmental approach to the origin and course of SZ and BP holds promise for improving outcomes because it searches for the causes and mechanisms which result in illness later in life, opening the potential for earlier and more effective intervention and prevention. As suggested by Insel, the neurodevelopmental approach identifies stages of disease progression, each of which may offer specific types of intervention and preventive strategies (Insel, 2010).
The rate of inducing labor has increased in recent decades, with oxytocin now being the most commonly used intervention (Mealing et al., 2009; Moleti, 2009). However, perinatal exogenous oxytocin has been associated with health risks for the neonate (Buchanan et al., 2012). These include lower Apgar scores and a greater need for neonatal intensive care (Oscarsson et al., 2006; Selo-Ojeme et al., 2011); and worse infant pre-feeding behaviors immediately following birth (Bell et al., 2013). As observed in clinical studies of adults, and consistent with animal models, oxytocin is associated with affective regulation and mood disorders (Demitrack and Gold, 1988; Lucht et al., 2009; Scantamburlo et al., 2007). In animal models, postnatal administration of oxytocin results in long-term maladaptive behavior and dysregulation of the HPA axis, as well as impairment in social and cognitive function (Carter, 2003; Engelmann et al., 1996; Rault et al., 2013). In clinical studies of healthy adults, oxytocin is associated with alterations in a number of cognitive domains including worse learning, attention, memory, and adaptive behaviors, but also with improving social and affiliative behaviors (Cardoso et al., 2014; Demitrack and Gold, 1988; Ellenbogen et al., 2012; Herzmann et al., 2012; Lerer et al., 2008).
Although oxytocin is associated with neurodevelopmental illnesses (Gregory et al., 2013; Kurth and Haussmann, 2011), no previous studies have examined the association of exogenous perinatal oxytocin and the risk of BP. Additionally, while some obstetric complications have been associated with childhood cognitive deficits (Leitner et al., 2007; Seidman et al., 2000), no studies of maternally administered exogenous oxytocin in relation to cognitive performance in childhood have been conducted.
Perinatal oxytocin to induce labor, therefore, merits further investigation for its association with BP and cognitive impairment, given the possible mechanisms by which it alters neurophysiology and its use during birth when the developing brain is at increased risk. The Child Health and Development Study (CHDS) is a large, representative birth cohort and the current nested case-control study identified BP cases and matched controls to assess prenatal and perinatal risks for later onset BP by longitudinal follow-up. In this study, we tested the following hypotheses: 1) the offspring of mothers who received oxytocin to induce labor are at greater risk for later BP; 2) the offspring of mothers who received oxytocin perform worse on childhood cognitive testing; and 3) predicated on substantiating the first two hypotheses, cognitive performance mediates the association between oxytocin and BP.
Secondary analyses were also conducted to attempt to determine whether gestational age, prolonged labor, analgesics, or delivery type confounded the relationship between oxytocin and BP. Gestational age and prolonged labor could each share a common cause with perinatal oxytocin induction if the pregnancy or labor is longer than expected, or could be antecedents leading to oxytocin administration. Analgesics during labor, reflecting the possibility that any medication intervention could alter the risk for later illness, and delivery type, which may indicate conditions of labor progress, could also confound or mediate an association between oxytocin and BP.
Methods
BP cases and matched controls were drawn from the Child Health and Development Study (CHDS) birth cohort. The CHDS recruited nearly all pregnant women receiving obstetric care (N=19,004) from the Kaiser Permanente Medical Care Plan, Northern California Region (KPNC) in Alameda County, California between 1959 and 1966 and followed them prospectively (van den Berg et al., 1988). Comprehensive data were collected from maternal medical records and interviews, child assessments, and other sources. KPNC enrolled approximately 30% of the population of the Bay Area of California at the time. This birth cohort has been extensively studied for prenatal and other early developmental risk factors for SZ (Brown et al., 2004; Brown et al., 2005; Brown et al., 2009; Freedman et al., 2011; Perrin et al., 2007).
Case Identification
Subjects with potential DSM-IV BP, which included BP I, BP II, BP NOS, and BP with psychotic features, were ascertained from three sources: the KPNC electronic medical records database, the Alameda County Behavioral Health Care Services (ABHCS) database, and a mailed survey to the entire living CHDS birth cohort (mothers and children). All CHDS cohort members who belonged to KPNC when first treated would have been ascertained from this source. Subjects who left KPNC prior to the first treatment of BP and who did not have other health insurance, but who still lived in Alameda County, would likely have been treated by ABHCS, and therefore ascertained. Subjects who were not ascertained by these two approaches were identified by the mailed survey.
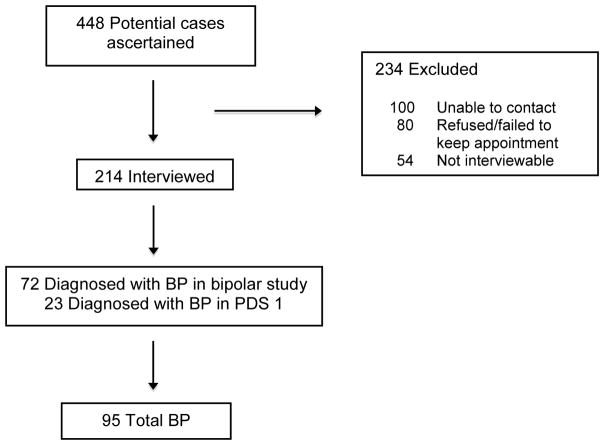
The ascertainment process identified 448 subjects who potentially met the criteria for BP and/or other psychotic disorders.
Ascertainment of KPNC subjects
Subjects with potential BP (and other psychotic disorders) were identified by screening KPNC inpatient and outpatient databases. Computerized record linkages between CHDS and KPNC identifiers were conducted on these databases. The inpatient database included all psychiatric hospitalizations of KPNC members regardless of the hospital at which treatment is received. This covered the period from 1981–2010. Those with discharge diagnoses of ICD-9 295–298 were considered as potential BP subjects. A database of outpatient treatment was introduced in 1981, but did not contain searchable codes for diagnoses until 1995. Potential BP cases from the outpatient database were considered potential cases if they received ICD-9 diagnoses of 295–298 excluding unipolar major depressive disorder. The outpatient pharmacy database, which commenced in 1992 was also used, with potential cases identified from prescriptions for mood stabilizing medications (lithium, carbamazepine, valproic acid). For subjects enrolled in KPNC at the time of ascertainment, the subject’s treating psychiatrist was contacted, informed about the study, and asked to approve contact with the subject to seek his/her consent to participate.
Subjects identified by these methods were invited to participate in the study, receiving a letter to the most recent address. Those who did not refuse contact by returning a postcard, were contacted to arrange a diagnostic interview. Several repeat appointments were scheduled for subjects who failed to attend the interview. Extensive efforts were made to locate individuals who were no longer living at the most recent listed address, including Department of Motor Vehicles (DMV) records, telephone directories, and contacting the subjects’ parents or siblings from CHDS or KPNC files. Mortality records, reverse directories, jail searches, and visits to previous addresses were also used as necessary.
Ascertainment by Alameda County Behavioral Health Care Services (ABHCS)
Outpatients with potential BP were also ascertained by electronic record linkage between the CHDS and ABHCS identifiers. The ABHCS database included treatment from 1993–2009. These subjects screened positive based on ICD-9 outpatient diagnoses of 295–298, excluding unipolar major depressive disorder. Procedures for finding and recruiting these potential subjects were similar to those used for ascertainment by KPNC.
Ascertainment of CHDS birth cohort by mailed questionnaire and follow-up
Third, ascertainment was initiated by letters mailed to all living mothers (N=6,971) and cohort members (N=13,009) with known addresses in the entire CHDS cohort (excluding families in which potential cases had already) along with a questionnaire on mental and physical health. This was conducted from 2009–11. Questionnaire respondents who reported “mental health problems” in an eligible cohort member (including the respondent him or herself) were contacted by a trained KPNC study interviewer who administered the Family Interview for Genetic Studies (FIGS) to screen for possible BP or psychotic illness in the cohort member. If the FIGS indicated at least one bipolar and/or psychotic symptom (delusions/hallucinations), then the cohort member was considered to have screened positive, and was invited to participate in the diagnostic interview. If the respondent (mother or sibling) described symptoms in a birth cohort member, the respondent was asked if he or she would be willing to have the study contact the affected family member about participation in the study. If the respondent agreed, the affected cohort member was contacted by letter and invited to participate.
Diagnostic protocol
A total of 214 subjects (48% of those ascertained) were interviewed using the Structured Clinical Interview for DSM-IV TR (SCID). The reasons that some subjects were not interviewed were: 100 could not be contacted, 80 refused or failed to keep the appointment, and 54 could not be interviewed because he/she had died, was incarcerated, was too psychotic or mentally disabled, or permission from the physician could not be obtained.
Study interviewers had a minimum of a master’s degree in a mental health field and were trained to reliably administer the SCID. DSM-IV-TR diagnoses including diagnostic qualifiers representing subtypes of BP were systematically assigned by consensus of three experienced clinicians (psychiatrists/Ph.D. psychologist), based on review of the SCID and medical records. This yielded 72 total BP cases. Among those interviewed, consensus diagnoses of non-BP disorders included SZ (n=61); major depressive disorder (n=62); and other diagnoses (n=19). These non-BP categories were not included in the present study. Although unipolar major depressive disorder was not included in the screening procedure, the diagnostic protocol enabled us to exclude subjects with database diagnoses of BP and/or psychotic disorders who were found instead to have unipolar depressive disorder in accord with structured research criteria.
Ascertainment from PDS I study
Additional cases of BP who had been ascertained through KPNC records by an earlier study (Prenatal Determinants of Schizophrenia I, PDS I) were included in the present study. Although the purpose of PDS I was to identify SZ and other schizophrenia spectrum disorder cases, BP cases were also diagnosed by interview in that study. The protocol for the PDS I included the same electronic linkages with the KPNC inpatient, outpatient, and pharmacy databases, and utilized the same ICD-9 diagnostic codes (295–298). Ascertainment covered the period from 1981–1998. The only other differences in the screening methods are that the PDS I did not include review of pharmacy records for treatment with mood stabilizers, and the PDS I included a second screening step, which involved psychiatrist review of abstracted data from inpatient/outpatient records for symptoms of psychosis. The Diagnostic Interview for Genetic Studies (DIGS), rather than the SCID, was used for interviewing potential subjects in the PDS I. There were 23 BP cases diagnosed in the PDS I study.
In total, then, 95 cases with BP were diagnosed following ascertainment from all sources and clinical interview (Figure 1).
Figure 1.
Ascertainment of bipolar disorder cases
After a complete description of the study was provided to the subjects, written informed consent was obtained. The study protocol was approved by the Institutional Review Boards of the New York State Psychiatric Institute and KPNC.
Control Selection
In order to ensure that controls would have been equally likely (as their matched cases) to be ascertained if they had been treated for BP in KPNC or ABHCS, controls were matched to cases on membership in KPNC (for cases ascertained through KPNC records) or residence in Alameda County (for cases ascertained through ABHCS or by CHDS mailing survey) in the year the case was first treated as reported in the SCID. For KPNC, membership in the plan at first treatment of the case was used for control matching, since they would have been in KPNC databases if they sought care for BP. For ABHCS and mailed survey cases, DMV records indicating residence in Alameda County at the time of case diagnosis was used, since these subjects would have represented the population at risk for treatment at same time.
Birth cohort members who screened positive for potential BP or psychotic disorders but did not have BP (N=376) were excluded as potential control subjects. Siblings of selected controls were excluded from further control selection, so that controls were independent observations, each representing a single family or pregnant woman. Control matching criteria included: date of birth (+/− 30 days), sex, and availability of maternal archived sera. A ratio of 1:8 case to controls was achieved.
This protocol yielded 754 matched controls.
Definition of exposure
Oxytocin administration to induce labor was contemporaneously documented in medical charts in the CHDS and systematically abstracted by the CHDS. The oxytocin analyses include 93 cases, matched up to 1:8 with controls (total N controls=738).
Childhood cognitive measures
Prospective cognitive testing of randomly selected, large subsets of this birth cohort was conducted at ages 5 and ages 9–11. Childhood cognitive measures included the Peabody Picture Vocabulary Test (PPVT) and the Raven Progressive Matrices and Raven Coloured Matrices (Raven). Fifty cases and 215 matched controls received the cognitive tests in childhood. None of those tested at age 5 were also tested at ages 9–11 in this subset of the birth cohort.
The PPVT is a test of receptive verbal ability (Strauss et al., 2006). The examinee is shown a plate with four pictures, the examiner speaks a word describing one of the four, and the examinee selects the correct picture either by speaking or pointing. In the CHDS, the PPVT was given to 3,413 children at age 5 and 3,737 at ages 9–11. Standardization of the PPVT for this sample was performed by converting raw scores to standard scores (z scores) by using the mean and standard deviation observed in each tested sample by age group. For the PPVT, the mean was set at 100, standard deviation of 15. Once standardized, testing for ages 5 and 9–11 were combined to gain statistical power.
The Raven instruments are tests of visual-spatial processing, inductive reasoning, relational reasoning, and problem solving (Strauss et al., 2006). Each question displays a pattern with a block missing and four choices, one of which accurately completes the pattern. In the CHDS, a randomly selected subset of children was tested at age 5 with the Raven Coloured Matrices, which consists of 21 plates. At age 9–11, another randomly selected subset was tested with the Raven Progressive Matrices, which consists of 60 plates. A total of 3,412 five year olds were assessed with the Raven, and 3,737 children at ages 9–11. For these analyses, cohort norms for the Raven were calculated with mean set at 0 and a standard deviation of 1. After converting the scores into standard units based on the proportion correct, the results were combined for age 5 and ages 9–11 to gain statistical power.
As a result of standardizing the scoring for both the PPVT and the Raven, differences between groups are measured and reported in standard deviation units.
Analytic method
Potential confounders were identified based on literature review of associations with BP, oxytocin, or cognition. These potential confounders included maternal age, race, educational level, maternal psychiatric history, and gestational age at birth. Data on these factors were included in the CHDS database; all maternal variables were obtained from maternal interview, and other potential confounders were abstracted from obstetric/delivery records into the CHDS. Based on differences between cases and controls in this sample, and the potential for an effect on child cognition, the following potential confounders were considered: maternal psychiatric history, maternal age (<35 [reference], ≥35), maternal ethnicity (Caucasian [reference], African American, other), and maternal educational achievement (defined as maternal education: <high school, high school only [reference], some college/college graduate) (Lundborg et al., 2014; Manly and Echemendia, 2007; Park et al., 2013). Gestational age (number of days after last menstrual period) was also considered as a potential confounding variable because longer gestation could increase the chance of induction. These potential confounders were entered into the adjusted models when associated with exposure and outcome at a probability less than 0.1.
As noted above, length of labor, analgesics, and delivery type were analyzed as potential confounders. Length of labor, contemporaneously documented in the CHDS, was calculating as the time from onset of labor to delivery and the time from second stage to delivery. Prolonged labor was defined as 1) equal to or greater than six hours for first stage, 2) equal to or greater than two hours for second stage for first births, and 3) equal to or greater than one hour for second stage for additional births (ACOG, 2003; Spong et al., 2012; Zhang et al., 2010). This variable was dichotomized to “prolonged” versus “not prolonged.” In addition, labor time was examined as a continuous variable. Analgesics were coded by drug name in the CHDS. A dichotomous variable for any “analgesic” versus “none” was constructed as the purpose was to assess any medication intervention compared to no intervention. Third, delivery type was coded at birth and treated here as a dichotomous variable: caesarean or vaginal delivery.
Conditional logistic regression models were used for the matched case-control analyses assessing BP as an outcome. This included the association between oxytocin and BP, and childhood cognition and BP. The GEE exchangeable correlation structure, which produced the smallest QIC value, was used for the analyses of cognitive performance included all children in the birth cohort who were tested (including siblings), since the aim of these analyses was to relate oxytocin to cognitive performance regardless of a BP diagnosis.
Results
Demographics
As seen in Table 1, only maternal race and maternal psychiatric history potentially differed by case status. However, maternal race was not associated with oxytocin (for African-Americans, p = 0.90; for “other,” p = 0.94) nor with BP (for African-Americans, p = 0.70; for “other,” p = 0.2). Maternal psychiatric history also was not associated with oxytocin (p = 0.46) nor with BP (p = 0.43). Therefore, none of the demographic covariates were treated as confounders in analyzing the association between oxytocin and BP.
Table 1.
Demographic comparison of bipolar disorder cases and controls
| Bipolar Cases N=94 |
Controls N=746 |
P value | |
|---|---|---|---|
| Maternal age at child’s birth Mean (SD) |
27.3 (6) | 28.0 (6) | 0.32 |
| Maternal Education, N (%) | 0.85 | ||
| < High school | 18 (21) | 128 (19) | |
| High school graduate | 32 (37) | 271 (39) | |
| Some college or college graduate | 36 (42) | 293 (42) | |
| Maternal Race, N (%) | 0.07 | ||
| White | 64 (69) | 435 (58) | |
| African-American | 24 (26) | 215 (29) | |
| Other | 5 (5) | 92 (13) | |
| Paternal Race, N (%) | 0.14 | ||
| White | 55 (70) | 387 (59) | |
| African-American | 19 (24) | 189 (29) | |
| Other | 5 (6) | 79 (12) | |
| Maternal psychiatric history (any), N (%) | 24 (25) | 132 (18) | 0.07 |
| Birthweight in grams, Mean (SD) | 3374 (21) | 3289 (17) | 0.17 |
| Low Birthweight <2500g, N (%) |
7 (7) | 43 (6) | 0.52 |
| Gestational age in days, Mean (SD) | 281 (16) | 280 (14) | 0.33 |
Oxytocin and BP
Of 831 subjects in the nested case-control study, 34 received oxytocin to induce labor: 8 cases and 26 controls. As shown in Table 2, oxytocin was associated with a 2.45-fold increased risk of BP in adulthood (HR = 2.45; CI: 1.08, 5.58; p = 0.032).
Table 2.
Oxytocin and risk for later bipolar disorder (N=840)
| Number exposed | Hazard Ratio | CI | p value | |
|---|---|---|---|---|
| Oxytocin* | Exposed cases: 8 Exposed controls: 26 |
2.45 | (1.08, 5.58) | 0.03 |
| Controlling for gestational age** | Exposed: 34 | 2.44 | (1.07, 5.55) | 0.03 |
| Controlling for maternal psychiatric history*** | Exposed: 34 | 2.99 | (1.08, 8.3) | 0.04 |
9 subjects missing data
13 subjects missing data
11 subjects missing data
Confounding of the oxytocin association with BP
None of the additional potential confounders/antecedents were associated with BP. Gestational age was not associated with either BP (p = 0.38) or oxytocin (p = 0.93). Prolonged labor, from active onset to birth, was not associated with BP (p = 0.41); prolonged first stage was not associated with BP (p = 0.1); prolonged second stage was marginally associated with BP (p = 0.064). However, no subject who had prolonged second stage also received oxytocin. Nearly all of the mothers of both cases and controls received an analgesic during pregnancy. Analgesics were not associated with BP (p = 0.65). Regarding delivery type, neither cesarean nor vaginal births were associated with BP (for both variables, p = 0.99).
Childhood cognition and BP
Fifty cases who later developed BP and 215 matched controls underwent cognitive assessments with the Raven and PPVT during childhood. Table 3 presents the data on the childhood testing. Cases had a higher mean PPVT score than controls, but also had a lower mean Raven score. The range of scores for cases on the PPVT was much wider than that for controls. In contrast, the range of scores of the Raven for cases was narrower compared to controls. Childhood cognition, as measured by both tests, was not associated with later BP (Table 3: PPVT: p = 0.19; Raven: p = 0.93).
Table 3.
Summary data and conditional logistic regression for childhood cognitive testing and BP
| Summary distributions | Regression | |||||
|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Minimum | Maximum | Hazard ratio (CI) | ||
| PPVT | Case (N = 50) | 103.1 | 14.69 | 53.93 | 145.47 | 1.016 (0.992, 1.041) |
| Control (N = 213) | 100.1 | 12.45 | 73.78 | 127.8 | ||
| Raven | Case (N = 50) | 0.0237 | 0.85 | −2.327 | 1.62 | 1.015 (0.732, 1.408) |
| Control (N = 214) | 0.0254 | 0.996 | −2.327 | 2.33 | ||
PPVT: Peabody Picture Vocabulary Test. Raven: Raven Progressive Matrices and Raven Coloured Matrices
Oxytocin and childhood cognition
In bivariate analyses to test for potential confounders (Table 4), gestational age, maternal education, and maternal race were each significantly associated with oxytocin and cognition. As a result, each of these covariates was included in the final model.
Table 4.
Bivariate analyses of covariates and perinatal oxytocin and Raven Progressive Matrices in the full birth cohort
| Oxytocin | Cognition | |||||
|---|---|---|---|---|---|---|
| Parameter Estimate | 95% CI | Pr > |Z| | Parameter Estimate | 95% CI | Pr > |Z| | |
| Maternal race: African-American | −0.01 | (−0.02, −0.0001) | <.0001 | −0.65 | (0.14, 0.20) | <.0001 |
| Maternal race: other | −0.03 | (−0.04, −0.02) | 0.047 | −0.02 | (−0.7, −0.59) | 0.638 |
| Maternal education: HS graduate | −0.00 | (−0.01, 0.01) | 0.99 | −0.287 | (−0.35, −0.22) | <.0001 |
| Maternal education: some college or more | −0.01 | (−0.02, −0.001) | 0.03 | 0.396 | (0.34, 0.45) | <.0001 |
| Gestational age | 0.002 | (−0.001, 0.006) | 0.14 | 0.004 | (0.003, 0.006) | <.0001 |
Notes: White is the reference group for maternal race; less than high school is the reference group for maternal education. Gestational age, maternal race, and maternal education were tested in bivariate models with only oxytocin and separately with only Raven score.
On the Raven, oxytocin and childhood cognitive performance were significantly associated, with a reduced score of .14 (CI: −0.26, −0.03, p = .02) standard deviation units. Table 5 presents the fully adjusted model. Controlling for a number of maternal and family demographic variables, oxytocin remained significantly related to worse Raven performance. The effect size for the reduced score on the Raven was relatively the same in both the unadjusted and fully adjusted models. On the PPVT, oxytocin was associated with a reduced score of .45 (CI: −2.12, 1.23, p = 0.6) standard deviation units, but the result fell short of statistical significance.
Table 5.
Fully adjusted model of perinatal oxytocin and childhood Raven standardized score
| Parameter Estimate | 95% CI | Pr > |Z| | |
|---|---|---|---|
| Intercept | −0.55 | (−0.96, −0.14) | 0.0088 |
| Oxytocin | −0.14 | (−0.26, −0.03) | 0.0141 |
| Gestational age | 0.002 | (0.001, 0.004) | 0.004 |
| Maternal psychiatric history | −0.019 | (−0.09, 0.06) | 0.618 |
| Maternal education: HS graduate | −0.20 | (−0.27, −0.14) | <.0001 |
| Maternal education: some college or more | 0.38 | (0.33, 0.43) | <.0001 |
| Maternal race: African-American | −0.61 | (−0.66, −0.55) | <.0001 |
| Maternal race: other | 0.02 | (−0.06, 0.11) | 0.6477 |
Discussion
Perinatal oxytocin administration was associated with a greater than twofold increased odds of later BP. In an effort to more clearly test oxytocin’s association with BP, gestational age, delivery type, analgesics, and duration of labor were each tested as potential confounders. The lack of association of each supports the conclusion that the effect of oxytocin is not a generic proxy for labor complications and is not confounded, at least by these measured variables. Although too few mothers received only analgesics in this study to permit testing for interaction, a prior study found no interaction effect between analgesics and oxytocin (Oscarsson et al., 2006).
Childhood cognition was not associated with BP. This is consistent with other research which suggests that the cognitive impairments are more modest in BP compared with SZ and are domain-specific rather than generalized (Bearden et al., 2001; Bearden et al., 2010; Daban et al., 2006; Goodwin et al., 2008; Harvey et al., 2010; Lim et al., 2013; Quraishi and Frangou, 2002; Savitz et al., 2005; Stefanopoulou et al., 2009). Oxytocin was associated with worse performance on the Raven, but not on the PPVT. This suggests that perinatal oxytocin may impair offspring neurodevelopment, although caution must be exercised with regard to causal inference. To our knowledge, this is the first evidence that oxytocin to induce labor is related to childhood cognition in a longitudinal prospective birth cohort. The significantly reduced performance on the Raven compared to the non-significantly reduced score on the PPVT may reflect a neurodevelopmental effect of oxytocin on specific brain regions. Although correlated, the test instruments measure different functional abilities and implicate different brain regions, with the Raven being associated with executive abilities and prefrontal cortical functioning (Crone et al., 2009; Gray et al., 2003; Zanelli et al., 2010). The PPVT is a test of receptive language, a complex cognitive domain but one more closely associated with temporal lobe functioning than executive functioning (Levin et al., 2001; Strauss et al., 2006; Wells et al., 2008; Weyandt and Willis, 1994). However, further testing would be needed to assess the hypothesis that perinatal oxytocin differentially affects cognitive domains. In spite of the observed association between oxytocin and Raven performance, the latter could not have been a mediator of the relationship between oxytocin and BP, given that Raven performance was not associated with BP.
Endogenous oxytocin
Endogenous oxytocin plays an important role at birth for both the mother and infant (Bell et al., 2014; Maggi et al., 1994). Uterine contractions during parturition are prompted by endogenous maternal oxytocin, released from the hypothalamus and pituitary (Brunton et al., 2013; Maggi et al., 1994). Endogenous maternal oxytocin also mediates a temporary GABA switch in the fetus, from excitatory to inhibitory, in preparation for labor (Ben-Ari et al., 2012; Ceanga et al., 2010; Khazipov et al., 2008; Tyzio et al., 2006). This switch is neuroprotective for the fetal brain, reducing anoxic episodes and providing an analgesic effect by reducing pain signaling (Ben-Ari et al., 2012; Ceanga et al., 2010; Khazipov et al., 2008; Mazzuca et al., 2011). Too little endogenous oxytocin risks fetal hypoxic-ischemic insult during the birthing process. However, too much endogenous oxytocin, also increases the risk for fetal hypoxia-ischemia (Ceanga et al., 2010), which in turn has been associated with cognitive impairment and psychiatric illness later in life (Buka et al., 1993; Li et al., 2012; Zornberg et al., 2000). The neuroprotective benefit of oxytocin against fetal hypoxic-ischemic insult appears to occur within a narrow range of its expression, at least in hippocampal cell cultures (Ceanga et al., 2010), with both high and low concentrations failing to protect the fetal brain during critical periods of neurodevelopment.
Exogenous oxytocin
Exogenous maternal oxytocin has been associated with lower Apgar scores and with increased need for neonatal intensive care (Oscarsson et al., 2006; Selo-Ojeme et al., 2011). Convergent evidence supports a potential role for oxytocin in other psychiatric illnesses, including an increased risk of ADHD (Kurth and Haussmann, 2011), which is occasionally comorbid with BP, as well as autism (Gregory et al., 2013). Further, exogenous oxytocin to induce labor could plausibly disrupt the maternal stress response system which has been shown to affect fetal neuronal development, neural plasticity, and myelination (Brunton and Russell, 2011; Duthie and Reynolds, 2013).
While the specific mechanism by which these outcomes are associated with oxytocin remains to be more fully studied, the effect on GABA is a promising hypothesis. Disruption of GABA by exogenous oxytocin during a sensitive developmental period could have long-term effects on this neurotransmitter’s role in functioning of the prefrontal and limbic regions, brain areas which are impaired in BP as well as other psychiatric illnesses (Brooks et al., 2008; Cerullo et al., 2012; Strakowski et al., 2005; Wessa et al., 2014). Disruptions in early development of the GABA system are reported to have long-term consequences (Ben-Ari et al., 2012; Le Magueresse and Monyer, 2013). Because GABA, and its precursor glutamate, plays an essential role in early neuronal development, affecting neuronal migration, dendritic arborization, and circuit formation (Le Magueresse and Monyer, 2013), it is possible that exogenous oxytocin during a critical developmental window could disrupt the protective switch in GABA function. Moreover, GABA maturation continues through adolescence in normal development (Catts et al., 2013); and, alterations of the GABA neurotransmitter system have been found in subjects with BP (Benes et al., 2007; Gigante et al., 2012). Thus, it is possible that oxytocin’s disruption of GABA during a critical neurodevelopmental window explains at least some of the results reported here.
Strengths and limitations
There are several strengths of the study. First, the longitudinal design of the birth cohort, the long follow-up period (1981–2009), and the diagnostic procedures, all strengthen the validity of the exposure measures and case ascertainment. Second, the data were based on prospectively collected measures of oxytocin administration, in addition to the other prenatal and perinatal exposures as well as premorbid cognitive testing in childhood.
The study also has certain limitations. As with any prospective longitudinal study, loss to follow-up needs to be addressed. It is possible that people with BP were more likely to drop out of the study or to refuse to participate. However, the overall prevalence of BP in this study of 0.08 percent of the 12,000 KPNC members still enrolled in 1981 is similar to the findings in most international prevalence studies (Ferrari et al., 2011). Additionally, no evidence has suggested that loss to follow-up is associated with the exposures or outcomes of this study, and prior research in this birth cohort has found such an effect to be very unlikely (Susser et al., 2000). A second limitation is the narrowness of the cognitive battery. The two test instruments do not measure the breadth of cognitive domains which have been found to be impaired in BP: executive functioning, memory, learning and memory. A third limitation is the modest sample size of those exposed to oxytocin.
Conclusion
This study provides evidence for a potentially important perinatal risk factor for BP and for cognitive impairment in childhood. While the association between perinatal oxytocin and offspring BP must be viewed cautiously until further studies can attempt to replicate the result, it lends support to the broader view that neurodevelopmental factors contribute to BP. Specifically, the findings of this study support the neurodevelopmental hypothesis in the sense that a specific early life exposure was found to be associated with an increased risk for later BP and a particular cognitive outcome. We hypothesize a pathway from exogenous oxytocin to GABA dysfunction, an altered developmental trajectory, and later life BP. However, further work on each part of that pathway is essential. The association appears not to be confounded by key associated risk factors. Moreover, this work should support further research on oxytocin and childhood cognitive functioning, as well as on prenatal and perinatal risks for BP.
Perinatal oxytocin is associated with an increased risk of offspring bipolar
Perinatal oxytocin is association with impaired cognition in childhood
Childhood cognition was not associated with later onset bipolar
Study was drawn from a prospective, longitudinally followed birth cohort
Study case ascertainment based on a rigorous diagnostic research protocol
Acknowledgments
Supported by NIMH grants 2T32-MH-13043 to DF; 5R01-MH073080 and 5K02-MH65422 to ASB; 5R01 MH069819 to CS; and National Institute on Child Health and Development grants N01-HD-1-3334 and NO1-HD-6-3258.
Footnotes
Disclosures: The authors report no financial relationships with commercial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David Freedman, CUNY Institute for State and Local Governance, New York.
Alan S. Brown, Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York; and Department of Epidemiology, Mailman School of Public Health, Columbia University, New York
Ling Shen, KPNC Permanente Division of Research, Oakland, California.
Catherine A. Schaefer, KPNC Permanente Division of Research, Oakland, California
References
- ACOG. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstetrics and gynecology. 2003;102:1445–1454. doi: 10.1016/j.obstetgynecol.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001;3:106–150. doi: 10.1034/j.1399-5618.2001.030302.x. discussion 151–103. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Woogen M, Glahn DC. Neurocognitive and neuroimaging predictors of clinical outcome in bipolar disorder. Current psychiatry reports. 2010;12:499–504. doi: 10.1007/s11920-010-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AF, Erickson EN, Carter CS. Beyond Labor: The Role of Natural and Synthetic Oxytocin in the Transition to Motherhood. Journal of Midwifery & Womens Health. 2014;59:35–42. doi: 10.1111/jmwh.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AF, White-Traut R, Rankin K. Fetal exposure to synthetic oxytocin and the relationship with prefeeding cues within one hour postbirth. Early Human Development. 2013;89:137–143. doi: 10.1016/j.earlhumdev.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. The Neuroscientist (Baltimore, Md) 2012;18:467–486. doi: 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JO, Wang PW, Bonner JC, Rosen AC, Hoblyn JC, Hill SJ, Ketter TA. Decreased prefrontal, anterior cingulate, insula, and ventral striatal metabolism in medication-free depressed outpatients with bipolar disorder. Journal of psychiatric research. 2008;43:181–188. doi: 10.1016/j.jpsychires.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. American Journal of Psychiatry. 2004;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Brown AS, Schaefer CA, Quesenberry CP, Liu LY, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. American Journal of Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD, McKeague IW, Kochetkova A, Kern D, Schaefer CA. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. The American journal of psychiatry. 2009;166:683–690. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Neuroendocrine control of maternal stress responses and fetal programming by stress in pregnancy. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:1178–1191. doi: 10.1016/j.pnpbp.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA, Hirst JJ. Allopregnanolone in the brain: Protecting pregnancy and birth outcomes. Prog Neurobiol. 2013 doi: 10.1016/j.pneurobio.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Buchanan SL, Patterson JA, Roberts CL, Morris JM, Ford JB. Trends and morbidity associated with oxytocin use in labour in nulliparas at term. Aust N Z J Obstet Gynaecol. 2012;52:173–178. doi: 10.1111/j.1479-828X.2011.01403.x. [DOI] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Lipsitt LP. PREGNANCY DELIVERY COMPLICATIONS AND PSYCHIATRIC-DIAGNOSIS - A PROSPECTIVE-STUDY. Archives of General Psychiatry. 1993;50:151–156. doi: 10.1001/archpsyc.1993.01820140077009. [DOI] [PubMed] [Google Scholar]
- Cardoso C, Orlando MA, Brown CA, Ellenbogen MA. Oxytocin and enhancement of the positive valence of social affiliation memories: An autobiographical memory study. Social neuroscience. 2014;9:186–195. doi: 10.1080/17470919.2013.873079. [DOI] [PubMed] [Google Scholar]
- Carter CS. Developmental consequences of oxytocin. Physiology & behavior. 2003;79:383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Catts VS, Fung SJ, Long LE, Joshi D, Vercammen A, Allen KM, Fillman SG, Rothmond DA, Sinclair D, Tiwari Y, Tsai SY, Weickert TW, Weickert CS. Rethinking schizophrenia in the context of normal neurodevelopment. Frontiers in Cellular Neuroscience. 2013;7 doi: 10.3389/fncel.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceanga M, Spataru A, Zagrean AM. Oxytocin is neuroprotective against oxygen-glucose deprivation and reoxygenation in immature hippocampal cultures. Neuroscience letters. 2010;477:15–18. doi: 10.1016/j.neulet.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Cerullo MA, Fleck DE, Eliassen JC, Smith MS, DelBello MP, Adler CM, Strakowski SM. A longitudinal functional connectivity analysis of the amygdala in bipolar I disorder across mood states. Bipolar Disorders. 2012;14:175–184. doi: 10.1111/j.1399-5618.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, van Leijenhorst L, Honomichl RD, Christoff K, Bunge SA. Neurocognitive development of relational reasoning. Developmental science. 2009;12:55–66. doi: 10.1111/j.1467-7687.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daban C, Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Balanza-Martinez V, Salazar-Fraile JS, Selva-Vera G, Vieta E. Specificity of cognitive deficits in bipolar disorder versus schizophrenia - A systematic review. Psychotherapy and Psychosomatics. 2006;75:72–84. doi: 10.1159/000090891. [DOI] [PubMed] [Google Scholar]
- Demitrack MA, Gold PW. Oxytocin: neurobiologic considerations and their implications for affective illness. Progress in neuro-psychopharmacology & biological psychiatry. 1988;12(Suppl):S23–51. doi: 10.1016/0278-5846(88)90072-3. [DOI] [PubMed] [Google Scholar]
- Demjaha A, MacCabe JH, Murray RM. How genes and environmental factors determine the different neurodevelopmental trajectories of schizophrenia and bipolar disorder. Schizophr Bull. 2012;38:209–214. doi: 10.1093/schbul/sbr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie L, Reynolds RM. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology. 2013;98:106–115. doi: 10.1159/000354702. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Linnen AM, Grumet R, Cardoso C, Joober R. The acute effects of intranasal oxytocin on automatic and effortful attentional shifting to emotional faces. Psychophysiology. 2012;49:128–137. doi: 10.1111/j.1469-8986.2011.01278.x. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: Focus on learning and memory. Neuroscience and biobehavioral reviews. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Baxter AJ, Whiteford HA. A systematic review of the global distribution and availability of prevalence data for bipolar disorder. Journal of affective disorders. 2011;134:1–13. doi: 10.1016/j.jad.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Fish B, Shapiro T, Halpern F, Wile R. THE PREDICTION OF SCHIZOPHRENIA IN INFANCY .3. A 10-YEAR FOLLOW-UP REPORT OF NEUROLOGICAL AND PSYCHOLOGICAL-DEVELOPMENT. American Journal of Psychiatry. 1965;121:768–775. doi: 10.1176/ajp.121.8.768. [DOI] [PubMed] [Google Scholar]
- Freedman D, Deicken R, Kegeles LS, Vinogradov S, Bao Y, Brown AS. Maternal-fetal blood incompatibility and neuromorphologic anomalies in schizophrenia: Preliminary findings. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:1525–1529. doi: 10.1016/j.pnpbp.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigante AD, Bond DJ, Lafer B, Lam RW, Young LT, Yatham LN. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar Disorders. 2012;14:478–487. doi: 10.1111/j.1399-5618.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Martinez-Aran A, Glahn DC, Vieta E. Cognitive impairment in bipolar disorder: Neurodevelopment or neurodegeneration? An ECNP expert meeting report. Eur Neuropsychopharmacol. 2008;18:787–793. doi: 10.1016/j.euroneuro.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Anthopolos R, Osgood CE, Grotegut CA, Miranda ML. Association of autism with induced or augmented childbirth in North Carolina Birth Record (1990–1998) and Education Research (1997–2007) databases. JAMA Pediatr. 2013;167:959–966. doi: 10.1001/jamapediatrics.2013.2904. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Wingo AP, Burdick KE, Baldessarini RJ. Cognition and disability in bipolar disorder: lessons from schizophrenia research. Bipolar Disord. 2010;12:364–375. doi: 10.1111/j.1399-5618.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- Herzmann G, Young B, Bird CW, Curran T. Oxytocin can impair memory for social and non-social visual objects: A within-subject investigation of oxytocin’s effects on human memory. Brain research. 2012;1451:65–73. doi: 10.1016/j.brainres.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Tyzio R, Ben-Ari Y. Effects of oxytocin on GABA signalling in the foetal brain during delivery. Progress in brain research. 2008;170:243–257. doi: 10.1016/S0079-6123(08)00421-4. [DOI] [PubMed] [Google Scholar]
- Kravariti E, Kane F, Murray RM. Neurocognitive Endophenotypes for Bipolar Disorder: Evidence from Case-Control, Family and Twin Studies. Springer; Dordrecht: 2009. [Google Scholar]
- Kurth L, Haussmann R. Perinatal Pitocin as an Early ADHD Biomarker: Neurodevelopmental Risk? Journal of Attention Disorders. 2011;15:423–431. doi: 10.1177/1087054710397800. [DOI] [PubMed] [Google Scholar]
- Le Magueresse C, Monyer H. GABAergic Interneurons Shape the Functional Maturation of the Cortex. Neuron. 2013;77:388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Leitner Y, Fattal-Valevski A, Geva R, Eshel R, Toledano-Alhadef H, Rotstein M, Bassan H, Radianu B, Bitchonsky O, Jaffa AJ, Harel S. Neurodevelopmental outcome of children with intrauterine growth retardation: A longitudinal, 10-year prospective study. Journal of child neurology. 2007;22:580–587. doi: 10.1177/0883073807302605. [DOI] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Molecular psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Levin HS, Song J, Ewing-Cobbs L, Chapman SB, Mendelsohn D. Word fluency in relation to severity of closed head injury, associated frontal brain lesions, and age at injury in children. Neuropsychologia. 2001;39:122–131. doi: 10.1016/s0028-3932(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Gonzalez P, Zhang L. Fetal stress and programming of hypoxic/ischemic-sensitive phenotype in the neonatal brain: Mechanisms and possible interventions. Prog Neurobiol. 2012;98:145–165. doi: 10.1016/j.pneurobio.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CS, Baldessarini RJ, Vieta E, Yucel M, Bora E, Sim K. Longitudinal neuroimaging and neuropsychological changes in bipolar disorder patients: Review of the evidence. Neuroscience and biobehavioral reviews. 2013;37:418–435. doi: 10.1016/j.neubiorev.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, Rosenberger A, Grabe HJ, Schroeder W, Voelzke H, Freyberger HJ, Herrmann FH, Kroemer H, Rosskopf D. Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33:860–866. doi: 10.1016/j.pnpbp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Lundborg P, Nilsson A, Rooth DO. Parental Education and Offspring Outcomes: Evidence from the Swedish Compulsory School Reform. American Economic Journal-Applied Economics. 2014;6:253–278. [Google Scholar]
- MacCabe JH, Wicks S, Lofving S, David AS, Berndtsson A, Gustafsson JE, Allebeck P, Dalman C. Decline in Cognitive Performance Between Ages 13 and 18 Years and the Risk for Psychosis in Adulthood A Swedish Longitudinal Cohort Study in Males. Jama Psychiatry. 2013;70:261–270. doi: 10.1001/2013.jamapsychiatry.43. [DOI] [PubMed] [Google Scholar]
- Maggi M, Baldi E, Susini T. Hormonal and local regulation of uterine activity during parturition: Part I--The oxytocin system. Journal of endocrinological investigation. 1994;17:739–756. doi: 10.1007/BF03347771. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Echemendia RJ. Race-specific norms: Using the model of hypertension to understand issues of race, culture, and education in neuropsychology. Archives of Clinical Neuropsychology. 2007;22:319–325. doi: 10.1016/j.acn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Mazzuca M, Minlebaev M, Shakirzyanova A, Tyzio R, Taccola G, Janackova S, Gataullina S, Ben-Ari Y, Giniatullin R, Khazipov R. Newborn analgesia mediated by oxytocin during delivery. Frontiers in Cellular Neuroscience. 2011;5 doi: 10.3389/fncel.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mealing NM, Roberts CL, Ford JB, Simpson JM, Morris JM. Trends in induction of labour, 1998–2007: A population-based study. Aust N Z J Obstet Gynaecol. 2009;49:599–605. doi: 10.1111/j.1479-828X.2009.01086.x. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90:285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Millan MJ. An epigenetic framework for neurodevelopmental disorders: From pathogenesis to potential therapy. Neuropharmacology. 2013;68:2–82. doi: 10.1016/j.neuropharm.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Moleti CA. Trends and Controversies in Labor Induction. MCN-Am J Matern-Child Nurs. 2009;34:40–47. doi: 10.1097/01.NMC.0000343864.49366.66. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 1987;295:681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophrenia Research. 2004;71:405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, Weinberger DR. The Neurology of schizophrenia. Elsevier Science Pub. Co; New York: 1986. [Google Scholar]
- Olvet DM, Burdick KE, Cornblatt BA. Assessing the potential to use neurocognition to predict who is at risk for developing bipolar disorder: a review of the literature. Cogn Neuropsychiatry. 2013;18:129–145. doi: 10.1080/13546805.2012.724193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oneal P, Robins LN. CHILDHOOD PATTERNS PREDICTIVE OF ADULT SCHIZOPHRENIA - A 30-YEAR FOLLOW-UP-STUDY. American Journal of Psychiatry. 1958;115:385–391. doi: 10.1176/ajp.115.5.385. [DOI] [PubMed] [Google Scholar]
- Oscarsson ME, Amer-Wahlin I, Rydhstroem H, Kallen K. Outcome in obstetric care related to oxytocin use. A population-based study. Acta obstetricia et gynecologica Scandinavica. 2006;85:1094–1098. doi: 10.1080/00016340600804530. [DOI] [PubMed] [Google Scholar]
- Osler M, Lawlor DA, Nordentoft M. Cognitive function in childhood and early adulthood and hospital admission for schizophrenia and bipolar disorders in Danish men born in 1953. Schizophrenia Research. 2007;92:132–141. doi: 10.1016/j.schres.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Park AL, Fuhrer R, Quesnel-Vallee A. Parents’ education and the risk of major depression in early adulthood. Social Psychiatry and Psychiatric Epidemiology. 2013;48:1829–1839. doi: 10.1007/s00127-013-0697-8. [DOI] [PubMed] [Google Scholar]
- Perrin MA, Chen H, Sandberg DE, Malaspina D, Brown AS. Growth trajectory during early life and risk of adult schizophrenia. British Journal of Psychiatry. 2007;191:512–520. doi: 10.1192/bjp.bp.106.034694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quraishi S, Frangou S. Neuropsychology of bipolar disorder: a review. Journal of affective disorders. 2002;72:209–226. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- Rault JL, Carter CS, Garner JP, Marchant-Forde JN, Richert BT, Lay DC., Jr Repeated intranasal oxytocin administration in early life dysregulates the HPA axis and alters social behavior. Physiology & behavior. 2013;112:40–48. doi: 10.1016/j.physbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Sanches M, Keshavan MS, Brambilla P, Soares JC. Neurodevelopmental basis of bipolar disorder: A critical appraisal. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32:1617–1627. doi: 10.1016/j.pnpbp.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. Neuropsychological dysfunction in bipolar affective disorder: a critical opinion. Bipolar Disorders. 2005;7:216–235. doi: 10.1111/j.1399-5618.2005.00203.x. [DOI] [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Marechal P, Pequeux C, Ansseau M, Legros JJ. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Buka SL, Goldstein JM, Horton NJ, Rieder RO, Tsuang MT. The relationship of prenatal and perinatal complications to cognitive functioning at age 7 in the New England Cohorts of the National Collaborative Perinatal Project. Schizophr Bull. 2000;26:309–321. doi: 10.1093/oxfordjournals.schbul.a033455. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Cherkerzian S, Goldstein JM, Agnew-Blais J, Tsuang MT, Buka SL. Neuropsychological performance and family history in children at age 7 who develop adult schizophrenia or bipolar psychosis in the New England Family Studies. Psychological Medicine. 2013;43:119–131. doi: 10.1017/S0033291712000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selo-Ojeme D, Rogers C, Mohanty A, Zaidi N, Villar R, Shangaris P. Is induced labour in the nullipara associated with more maternal and perinatal morbidity? Archives of gynecology and obstetrics. 2011;284:337–341. doi: 10.1007/s00404-010-1671-2. [DOI] [PubMed] [Google Scholar]
- Sorensen HJ, Saebye D, Urfer-Parnas A, Mortensen EL, Parnas J. Premorbid intelligence and educational level in bipolar and unipolar disorders: a Danish draft board study. Journal of affective disorders. 2012;136:1188–1191. doi: 10.1016/j.jad.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Spong CY, Berghella V, Wenstrom KD, Mercer BM, Saade GR. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstetrics and gynecology. 2012;120:1181–1193. doi: 10.1097/aog.0b013e3182704880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanopoulou E, Manoharan A, Landau S, Geddes JR, Goodwin G, Frangou S. Cognitive functioning in patients with affective disorders and schizophrenia: a meta-analysis. International review of psychiatry (Abingdon, England) 2009;21:336–356. doi: 10.1080/09540260902962149. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Molecular psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests : administration, norms, and commentary. 3. Oxford University Press; New York: 2006. [Google Scholar]
- Susser ES, Schaefer CA, Brown AS, Begg MD, Wyatt RJ. The design of the prenatal determinants of schizophrenia study. Schizophrenia Bulletin. 2000;26:257–273. doi: 10.1093/oxfordjournals.schbul.a033451. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA. Clinical Phenotypes of Psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) American Journal of Psychiatry. 2013;170:1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Haukka J, Henriksson M, Cannon M, Kieseppa T, Laaksonen I, Sinivuo J, Lonnqvist J. Premorbid intellectual functioning in bipolar disorder and schizophrenia: results from a cohort study of male conscripts. The American journal of psychiatry. 2005;162:1904–1910. doi: 10.1176/appi.ajp.162.10.1904. [DOI] [PubMed] [Google Scholar]
- Tsuchiya KJ, Byrne M, Mortensen PB. Risk factors in relation to an emergence of bipolar disorder: a systematic review. Bipolar Disord. 2003;5:231–242. doi: 10.1034/j.1399-5618.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Cossart R, Khalilov I, Minlebaev M, Hubner CA, Represa A, Ben-Ari Y, Khazipov R. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314:1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- Urfer-Parnas A, Mortensen EL, Saebye D, Parnas J. Pre-morbid IQ in mental disorders: a Danish draft-board study of 7486 psychiatric patients. Psychological Medicine. 2010;40:547–556. doi: 10.1017/S0033291709990754. [DOI] [PubMed] [Google Scholar]
- van den Berg BJ, Christianson RE, Oechsli FW. The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley. Paediatric and perinatal epidemiology. 1988;2:265–282. doi: 10.1111/j.1365-3016.1988.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Wells CT, Mahone EM, Matson MA, Kates WR, Hay T, Horska A. Relationship of temporal lobe volumes to neuropsychological test performance in healthy children. Brain and cognition. 2008;68:171–179. doi: 10.1016/j.bandc.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Kanske P, Linke J. Bipolar disorder: A neural network perspective on a disorder of emotion and motivation. Restorative Neurology and Neuroscience. 2014;32:51–62. doi: 10.3233/RNN-139007. [DOI] [PubMed] [Google Scholar]
- Weyandt LL, Willis WG. EXECUTIVE FUNCTIONS IN SCHOOL-AGED CHILDREN - POTENTIAL EFFICACY OF TASKS IN DISCRIMINATING CLINICAL GROUPS. Developmental Neuropsychology. 1994;10:27–38. [Google Scholar]
- Zanelli J, Reichenberg A, Morgan K, Fearon P, Kravariti E, Dazzan P, Morgan C, Zanelli C, Demjaha A, Jones PB, Doody GA, Kapur S, Murray RM. Specific and Generalized Neuropsychological Deficits: A Comparison of Patients With Various First-Episode Psychosis Presentations. American Journal of Psychiatry. 2010;167:78–85. doi: 10.1176/appi.ajp.2009.09010118. [DOI] [PubMed] [Google Scholar]
- Zhang J, Landy HJ, Branch DW, Burkman R, Haberman S, Gregory KD, Hatjis CG, Ramirez MM, Bailit JL, Gonzalez-Quintero VH, Hibbard JU, Hoffman MK, Kominiarek M, Learman LA, Van Veldhuisen P, Troendle J, Reddy UM. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstetrics and gynecology. 2010;116:1281–1287. doi: 10.1097/AOG.0b013e3181fdef6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornberg GL, Buka SL, Tsuang MT. Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. The American journal of psychiatry. 2000;157:196–202. doi: 10.1176/appi.ajp.157.2.196. [DOI] [PubMed] [Google Scholar]