Abstract
Attention-Deficit/Hyperactivity Disorder (ADHD) is highly prevalent among adolescents enrolled in behavioral health services but remains undertreated in this age group. Also the first-line treatment for adolescent ADHD, stimulant medication, is underutilized in routine practice. This article briefly describes three behavioral interventions designed to promote stronger integration of medication interventions into treatment planning for adolescent ADHD: family ADHD psychoeducation, family-based medication decision-making, and behavior therapist leadership in coordinating medication integration. It then introduces the Medication Integration Protocol (MIP), which incorporates all three interventions into a five-task protocol: ADHD Assessment and Medication Consult; ADHD Psychoeducation and Client Acceptance; ADHD Symptoms and Family Relations; ADHD Medication and Family Decision-Making; and Medication Management and Integration Planning. The article concludes by highlighting what behavior therapists should know about best practices for medication integration across diverse settings and populations: integrating medication interventions into primary care, managing medication priorities and polypharmacy issues for adolescents with multiple diagnoses, providing ADHD medications to adolescent substance users, and the compatibility of MIP intervention strategies with everyday practice conditions.
Keywords: adolescent ADHD, behavioral health, integrated care, medication
This article introduces a clinical resource, the Medication Integration Protocol (MIP), designed to assist behavior therapists in promoting stronger integration of pharmacological interventions for Attention-Deficit/Hyperactivity Disorder (ADHD) into behavioral health services for adolescents. MIP contains evidence-based clinical strategies and techniques that incorporate family ADHD psychoeducation, family-based medication decision-making, and coordinated medication management into behavioral treatment planning for adolescents with ADHD. MIP is a clinically flexible protocol that can be implemented by behavior therapists in diverse treatment settings, and it can serve as stand-alone intervention for ADHD or as one component in a multicomponent treatment plan for adolescents with co-occurring disorders.
Overview of ADHD-Related Morbidity in Adolescents Enrolled in Behavioral Care
National and Clinical Prevalence Estimates for Adolescent ADHD
Knowledge about the prevalence of ADHD in adolescent populations has grown enormously over the past decade. There is now consensus that ADHD is a chronic childhood mental health condition that persists across the developmental span of adolescence and into young adulthood (Taylor, 2009). Recent national prevalence data gathered by the Centers for Disease Control and Prevention indicate that among children aged 14–17, 19% of boys and 10% of girls have received an ADHD diagnosis at some point in their lives (Schwarz & Cohen, 2013). These data align with results from other national epidemiological surveys (e.g., Merikangas et al., 2011) to suggest that ADHD is the most prevalent behavioral health disorder among adolescents. Moreover, prevalence estimates are likely to increase in the near future as ADHD diagnostic criteria (American Psychiatric Association, 2000) are developmentally revised to support more accurate assessment and diagnostic practices for teens (Sibley et al., 2012).
With regard to youth seeking behavioral treatment, not only is ADHD a leading reason for referral among children age 3–12 (Pelham & Fabiano, 2008), it is also highly prevalent among adolescents, affecting between 18–48% of those enrolled in mental health and substance use services (Tims et al., 2002; Turner et al., 2004; Wu et al., 2011). These are conservative estimates of clinical prevalence given that ADHD is significantly underdiagnosed in adolescent samples (Sibley et al., 2012; Todd, Huang, & Henderson, 2008) and is frequently undetected when it co-occurs with other chronic psychiatric disorders for which teens are typically referred: oppositional defiant and conduct disorder, anxiety, depression, and substance use (see Merikangas et al., 2011). Rates of ADHD comorbidity with disruptive behavior disorders and substance use problems among teenagers participating in behavioral treatment typically exceed 70% (e.g., Chan, Dennis, & Funk, 2008; Thompson, Whitmore, Raymond, & Crowley, 2006). Based on these figures, of the 2.8 million adolescents enrolled annually in outpatient mental health care (Substance Abuse and Mental Health Services Administration, 2009), along with 125,000 in outpatient substance use treatment (Substance Abuse and Mental Health Services Administration, 2007), approximately 20–50% have ADHD. These data suggest that the behavioral healthcare system houses between 730,000 and 1.4 million teenagers with ADHD on a yearly basis.
ADHD Clinical Symptoms and Multidomain Impairment in Adolescents
Adolescents who meet full diagnostic criteria for ADHD present well-documented behavioral deficits in attention, self-regulation, and social competence (Barkley, 2006; Wolraich et al., 2005). If untreated, ADHD symptoms remain relatively stable across adolescence and young adulthood and negatively impact multiple areas of development (Barkley, 2006). These behavioral symptoms typically precipitate school behavioral problems that include inconsistent attendance, poor grades, disruptive classroom behavior, poor time management and planning, and a disorganized approach to academics (Raggi & Chronis, 2006). Youth with ADHD also suffer a high rate of learning difficulties of several kinds (Cutting & Denckla, 2003), which creates an additional barrier to academic achievement (Bussing et al., 2012); and like ADHD, learning disabilities are considered chronic conditions that require intensive intervention and ongoing management (Fletcher, Lyon, Fuchs, & Barnes, 2007). These ADHD-related behavioral and learning problems together lead to greater incidence of grade retention and dropout and incur enormous costs in educational support services (Robb et al., 2011).
Additionally, clinical neuroscience has begun to map neurocognitive risk factors associated with childhood ADHD that routinely persist into adolescence, particularly executive functioning deficits in planning, cognitive flexibility, working memory, and processing speed (e.g., Coolidge, Thede, & Young, 2000). Executive dysfunction exacerbates, and may underlie, behavioral and learning problems experienced by youth with ADHD (Barkley, 2006), and it affects social as well as academic functioning (Raggi & Chronis, 2006). Thus the confluence of poor attention and self-regulation, learning difficulties, and for many, executive functioning deficits creates a profile of compounded impairment that compromises quality of life and complicates treatment planning for adolescents with ADHD.
Stimulant Medications Are Empirically Supported but Underutilized for Adolescent ADHD
Stimulant Medications are Empirically Supported for Treating ADHD in Adolescents
There is a strong evidence base supporting stimulant medications for adolescent ADHD. Traditional rapid-acting stimulants such as methylphenidate (Ritalin) have proven effective in reducing ADHD symptoms and improving cognitive and social functioning in teens (Smith, Waschbusch, Willoughby, & Evans, 2000). Recent research suggests that extended-release stimulants such as OROS-MPH (Concerta), which are FDA-approved for use with adolescents, are safe, well tolerated, and effective in reducing ADHD symptoms for this age group (e.g., McGough et al., 2006). They also help improve neuropsychological functioning (Wilson et al., 2006) and school performance (Langberg & Becker, 2012). Importantly, long-acting formulations lead to greater medication compliance in teenagers compared to rapid-acting stimulants (Sanchez et al., 2005). Effect sizes for ADHD symptom reduction in recent trials of OROS-MPH (e.g., Wilens et al., 2006) as well as other once-daily ADHD medications (e.g., Spencer et al., 2006) are in the medium-to-large range among teens, consistent with meta-analytic findings for immediate-release stimulants (Smith et al., 2000). Altogether, the best available evidence indicates that stimulants have significant benefits in multiple domains of functioning for adolescents.
Benefits of Integrated Care for Adolescent ADHD
Medication is the first-line option for treating adolescent ADHD (Faraone & Buitelaar, 2010) and is considered an essential component of effective treatment planning for this population, for two main reasons. First, so-called “combined therapies” that package pharmacological and behavioral interventions typically outperform “monotherapies” for complex disease states. Combined treatments are especially effective for health problems with heterogeneous patient profiles (e.g., heart disease) or with multifactorial etiologies (e.g., AIDS) (Hosking et al., 2005). Both types of challenges are present for ADHD. Combined treatments have generated the strongest empirical support for treating ADHD in children (Smith, Barkley, & Shapiro, 2006), outperforming medication-only and psychotherapy-only conditions across a broad range of outcomes (see Jensen et al., 2001; MTA Cooperative Group, 1999). Thus a multimodal approach that integrates medication with behavioral treatment is universally recommended for childhood ADHD (Wolraich et al., 2005). This endorsement of integrated care lines up with research findings for other youth disorders whose core symptoms are responsive to medication. For example, combined treatments have proven superior to single treatments for adolescent depression (Reinecke, Curry, & March, 2009) and youth anxiety (Piacentini et al., in press), among others.
Second, the evidence base for single behavioral treatment of adolescent ADHD remains modest. Behavioral interventions for ADHD can be separated into two broad approaches: behavior management (BM) and training interventions (TIs) (Evans, Owens, & Bunford, in press). BM, which induces behavior change by manipulating contingencies in the target environment, is a well-established approach for childhood ADHD in both home and school settings (Pelham & Fabiano, 2008). However, no randomized trials of BM have focused on adolescents, and few have included teenage participants (Evans et al., in press). This creates doubt about the developmental translatability of BM for older youth, given that teens are monitored by adults less closely, are motivated by more diverse and less accessible rewards, and encounter numerous teachers and classrooms during the school day (Evans et al., in press; Fabiano et al., 2009). The TI category, which induces change by improving the skill set of the child, includes neurofeedback, cognitive training of executive functions, and organization skills training (Evans et al., in press). TIs have been tested in a few studies focused on adolescents, with mostly positive results. However, almost all TI studies have occurred in school settings, a natural choice given that teens are difficult to engage in clinic settings (Merikangas et al., 2011) and benefit substantially from having services in easy reach as school-based programming (Schultz, Storer, Watabe, Sadler, & Evans, 2011). Still, the potency of TIs for adolescents being treated in clinical care is not yet proven.
ADHD Medication is Vastly Underutilized among Adolescents in Routine Behavioral Care
Despite the well-established benefits of stimulant medications for adolescent ADHD, and the uncertain effectiveness of single behavioral treatments, medications remain under-prescribed for this clinical population (Zuvekas & Vitiello, 2012). It is true that medication prescriptions for adolescents have increased in recent years, in large measure due to growing recognition of ADHD prevalence in this age group (Swanson, Baler, & Volkow, 2010). Nevertheless, among adolescents who meet full diagnostic criteria for ADHD, medications remain vastly underutilized, with estimates that only about half of teenagers who would benefit from ADHD medications actually receive them (Poulin, 2007), compared with greater than two-thirds of younger children (Visser et al., 2010). This age-based disparity in quality care is even more pronounced among racial/ethnic minorities (Zuvekas & Vitiello, 2012).
There appear to be several reasons for this disparity in providing medication interventions for adolescent ADHD. As mentioned previously, ADHD is significantly under-diagnosed among adolescent clinical populations in everyday practice. In addition, providers in specialty mental health and addiction treatment settings are rarely trained to intervene effectively with comorbid ADHD diagnoses (Sobell & Sobell, 2007), and to date there are no established protocols that specifically promote the utilization of ADHD medications among teens in behavioral treatment. This is not surprising given that routine clinical care typically provides “fragmented” services in which pharmacological interventions are separate from, rather than coordinated with, behavioral interventions (Institute of Medicine, 2006). Therefore families of teenagers diagnosed with ADHD are often poorly informed about the choices of available medications and the risk and benefits of each, especially when treated by behavior therapists who have limited knowledge or confidence in discussing medications (see Murphy, 2005). For all these reasons, families in behavioral health settings do not routinely receive the information they need to make informed choices about ADHD medication use.
Furthermore, even among teens accurately diagnosed with ADHD and offered the option of initiating a medication regimen, medication acceptance and compliance are extremely difficult to achieve. ADHD medication compliance declines precipitously from childhood through adolescence (Sanchez et al., 2005), likely due to the inconvenience, stigma, and side effects of medication, combined with decreases in adult monitoring and increases in adolescent autonomy and self-care (Sanchez et al., 2005; Smith et al., 2000). Also families (especially caregivers) generally prefer behavioral interventions to medication as a primary treatment option (Pelham & Fabiano, 2008). And a recent study found that adolescents could not reliably discern whether they were taking active ADHD medication or placebo and rarely attributed behavioral effects to the ingested pill (Pelham et al., 2013), suggesting that teens are not generally disposed to advocate for their own medication.
Evidence Base on Three Innovations for Supporting the Integration of Medication Interventions into Behavioral Services for Adolescent ADHD
In order to reduce the quality-of-care gap for adolescents with ADHD, it is essential to conduct valid, multimodal, multidomain assessments of ADHD symptomatology and related areas of functioning (Wolraich et al., 2005) and develop innovative clinical procedures designed to support the integration of ADHD medication into behavioral care (see Bukstein & Cornelius, 2006; Robin, 2006). Such procedures would enhance therapist confidence in addressing ADHD problems within their caseloads as well as supply basic clinical tools for effective intervention. Specifically, new procedures are needed to (1) increase opportunities for adolescents and caregivers to make informed decisions about ADHD medication acceptance and (2) support family participation and compliance in medication regimens. Below we briefly describe three evidence-based strategies that can advance ADHD medication integration efforts in this manner; in the section that follows, we introduce a conceptual model that incorporates all three strategies within a unified protocol for integrating medication into behavioral treatment planning for adolescent ADHD.
Family Psychoeducation in Adolescent ADHD
ADHD psychoeducation refers to a set of well-developed interventions that provide structured information about signature symptoms, course of the disorder, impacts on multiple domains of functioning (family, school, peers), individual differences associated with ADHD, and best treatment practices that include the combination of medication, behavior therapy, and school-based interventions (Montoya, Colom, & Ferrin, 2011). This information is packaged in an easy-to-digest format and sets the stage for developing a unique profile of ADHD-related symptoms and problems for each client (e.g., Lopez et al., 2005). Psychoeducation in mental health disorders has been shown to enhance medication and behavioral treatment effects (e.g., Fristad, 2006) and to improve treatment adherence (Vieta, 2005) and medication compliance (Cummings & Fristad, 2007) for clients with a variety of behavioral problems. Moreover, family psychoeducation targeted to caregivers and youth conjointly is especially helpful for youth receiving psychiatric medication (Fristad, 2006). Several research-based protocols exist for childhood disorders in addition to ADHD, including depression (Sanford et al., 2006), bipolar disorder (Fristad, 2006), and eating disorders (Geist et al., 2000).
Family-based Medication Decision-Making
Family-based medication decision-making interventions, in which family history and attitudes about psychiatric medication are systematically processed in the context of current options and benefit-cost decisions about adolescent ADHD medication, appear to be prerequisite for safe and consistent medication use in teenagers with ADHD. There are two compelling reasons to favor family-based interventions for facilitating decisions about medication. First, family factors play a lead role in predicting medication acceptance and compliance among teenagers (Smith et al., 2000) as well as affecting safety, tolerance, polypharmacy, and liability issues associated with prescribing stimulants to at-risk youth (Kollins, 2007). Second, evidence-based family therapy models for adolescent behavior problems (Henggeler & Sheidow, 2012; Hogue & Liddle, 2009) feature interventions that specifically boost adolescent investment in therapy activities by (a) developing a personally meaningful treatment agenda in which the teen can be a motivated participant and (b) (re)moralizing the teen by generating hope that self-defined problems can and will improve (Diamond, Liddle, Hogue, & Dakof, 1999; Liddle, 1995; Robbins et al., 2006). Thus, because family-focused interventions are well suited to address parental monitoring and family relational processes that influence ADHD medication usage, and also to meaningfully engage teens as well as caregivers in the decision-making process, they are ideal candidates for promoting effective medication decision-making among adolescents with ADHD (see Robin, 2006).
Behavior Therapist Leadership in Coordinating Medication Integration
Most practicing behavior therapists are non-medical professionals—social workers, psychologists, and counselors of other kinds—who are not certified to prescribe and monitor psychiatric medications. Nevertheless, in order to integrate ADHD medication interventions more fully into behavioral services for adolescents, behavior therapists need to play a lead role in coordinating the pharmacological and behavioral aspects of treatment for each client. For behavior therapists who are not certified prescribers, this means expanding their traditional clinical duties in two ways. First, they should acquire reasonable fluency in the types, dosing algorithms, anticipated effects, and potential side effects of ADHD medications for teenagers. This will afford them information about ADHD medication that is required to consult effectively with prescribing providers and work effectively with families in making medication decisions and assessing medication impacts across multiple domains of functioning. Numerous educational resources on adolescent ADHD medication are targeted directly at non-medical counselors to facilitate self-education in this area (e.g., Dendy, 2000, 2006). Therapists can also access a wealth of continuing education materials, including on-line distance learning courses (e.g., www.dcplearner.com/chadd_ceu), that provide structured education in this area along with assessment and certification of knowledge gained.
Second, non-prescribing behavior therapists should assume leadership for coordinating the case activities shared among the family, therapist, and prescriber (who may be a physician, nurse, or psychologist). This may include gaining family consent to consult directly with the prescriber about family issues that arise week-to-week in therapy sessions and bear upon medication titration, compliance, and liability; working with the prescriber to establish a coordinated service plan to support medication maintenance; helping to monitor ongoing effectiveness and side effects, especially when medication management sessions with the prescriber become less frequent; minimizing client burden related to regular appointments with multiple providers; and including the prescriber as indicated in behavioral treatment sessions. For guidance in this heightened liaison role, behavior therapists can rely on evidence-based protocols for therapist-family-school collaboration for ADHD youth (Power et al., 2012), or other case coordination models for multidisciplinary clinical teams (e.g., Mitchell, Tieman, & Shelby-James, 2008), adapting relevant principles for the purpose of managing therapist-family-prescriber activities related to ADHD medication.
A Family-Based Approach to Promoting Adolescent ADHD Medication Interventions in Behavioral Care: Medication Integration Protocol (MIP)
Numerous clinician resources are available to support implementation of each of the three practice innovations described above. Moreover, there are myriad ways in which to incorporate these innovations into existing clinical frameworks and practices. This article introduces a model that combines all three innovations into a single clinical protocol, the Medication Integration Protocol (MIP; Hogue & Bobek, 2013). MIP is a family-based protocol for integrating pharmacological and behavioral interventions for adolescents diagnosed with ADHD as either a single or co-occurring disorder. Although intended for use with adolescents not currently taking ADHD medication—for whom medication decision-making is a primary concern—certain MIP interventions may be appropriate for medicated clients who would nonetheless benefit from more extensive ADHD psychoeducation and more efficient coordination of medication management and behavioral services.
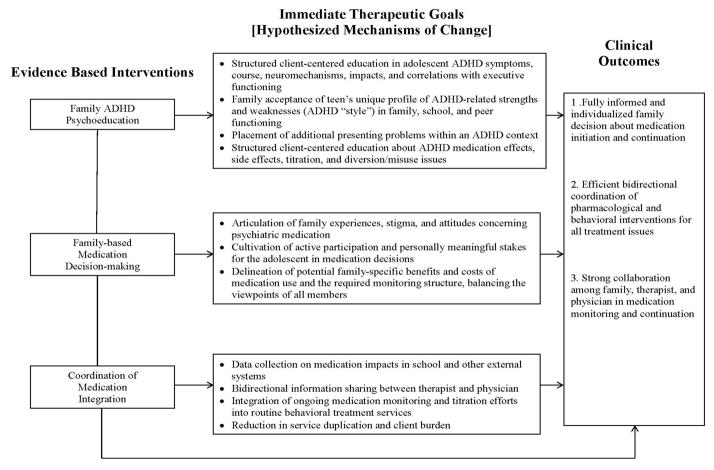
Figure 1 depicts the MIP conceptual model. The figure links the three practice innovations—family ADHD psychoeducation, family-based medication decision-making, and behavioral therapist leadership in coordinating integration efforts—to hypothesized mechanisms of change (i.e., immediate outcomes) targeted in the adolescent and family during protocol implementation, and ultimately to the desired case outcomes. MIP is a modular protocol (see next paragraph) composed of five Tasks, each of which is briefly described below. The MIP Tasks and their constituent subtasks are listed in the MIP Fidelity Monitoring Checklist contained in Figure 2. The MIP fidelity checklist functions as a clinical log that can be appended to standard case notes and used by the therapist to support MIP case planning and Task implementation throughout treatment.
Figure 1.
Medication Integration Protocol (MIP) Conceptual Model: Interventions, Mechanisms, and Outcomes
Figure 2.
MIP Fidelity Monitoring Checklist
MIP Tasks are considered modular (Chorpita, Daleiden, & Weisz, 2005) because they can be delivered in any order that is clinically indicated based on the status and progress of the case. The exception is Task 1, which should be prior to the initial therapy session. Tasks can also be staggered across several sessions and/or interspersed with interventions from other Tasks. It follows that the length of time needed to complete each Task will vary greatly depending on the profile of the given family, practice habits of the provider team, and progress of the case. Each MIP Task consists of several Therapeutic Goals and Completion Criteria (which are subject to clinician judgment) that might be achieved in a single session or as a continuous intervention sequence across multiple sessions. These are detailed below, followed by an expanded discussion of the key clinical interventions associated with each Task. Note that Task 5 is implemented only with families who decide to initiate medication.
Task 1: ADHD Assessment and Medication Consult
Therapeutic goals: Collect clinical data for the provider team on ADHD-related behavioral and school functioning to assist case planning. Completion criteria: Assess ADHD symptoms and impairments using evidence-based methods, including a continuous performance task (if available); Make a formal ADHD diagnosis and determine client eligibility for medication; Screen for executive functioning (EF) and learning deficits and, when indicated, make an appropriate educational services referral to the teen’s school or an independent tutoring service.
Standard or customized intake procedures can be used determine whether the adolescent meets diagnostic criteria for ADHD, taking into account the assessment challenges associated with obtaining divergent perspectives (caregiver, youth, school personnel) on the presence and severity of symptoms, developmental changes in symptom expression, and incomplete or skewed knowledge about symptom expression in various settings. Brief, computerized continuous performance tasks that assess sustained attention using age-based norms are available for clinical use. MIP also requires that clinicians gather assessment data directly from school personnel, for three reasons: to make a confident and specific diagnosis, obtain reliable data on the history and current standing of school performance indicators (including enrollment in educational support services), and set the stage for marking significant improvements in school functioning for clients that elect to start medication.
Task 2: ADHD Psychoeducation and Client Acceptance
Therapeutic goals: Educate the family about the clinical and developmental implications of the teen’s ADHD and EF assessment data; Define how the teen’s specific ADHD-related characteristics impact family, school, and peer functioning. Completion criteria: Family understands that ADHD is a prevalent, brain-based, and lifelong (i.e., medical/neuropsychiatric) condition; Family has articulated and accepted the particular ADHD profile of the teen, including personality strengths as well as symptoms; Family has defined the teen’s unique profile of EF deficits; Family understands that EF deficits are related to stress and performance challenges at home and school, and that relevant accommodations and supports may be needed to bring about improvement; Therapist and family have reviewed the client-specific profile of ADHD-related problems in family, school, and peer functioning; Family and therapist have linked significant ADHD-related problems to specific treatment goals.
Psychoeducation slides (contained in the MIP manual or available as a separate slideshow from the first author) are used as therapeutic prompts for interactive discussion in three domains: ADHD basic facts, executive skills, and ADHD medication facts. The first domain introduces data on ADHD prevalence rates, behavioral symptoms, and common impacts on developmental functioning; delivers a strong anti-stigma message while encouraging the teen to take ownership of ADHD-related characteristics; and educates the family about the neurobiology of ADHD (using accessible metaphors) to promote family acceptance, defuse moral attributions, and establish practical expectations for change. The second domain defines executive functioning (e.g., working memory, behavioral inhibition, emotional control, analysis and reconstitution, planning and organization); elaborates the relations among three main influences on academic achievement for teens with ADHD: intelligence, EF skills, and ADHD behavioral symptoms (especially inattention and impulsivity); and presents information on learning problems of various kinds. The third domain describes the common benefits, course, and side effects of ADHD medications; details the trial-and-error approach to appropriate dosing; and summarizes other key factors that inform decision-making about medication initiation.
To complete Task 2, therapist and family discuss a checklist of positive and negative personality and social characteristics associated with ADHD (ADHD Style Index) and three checklists of common ADHD-related clinical problems in the family, school, and peer domains (Problem Scorecards). These exercises anchor generic psychoeducation about ADHD symptoms and EF deficits to client-specific characteristics and priorities identified by the family; encourage further teen ownership and family acceptance of the ADHD condition, including identification of desirable social traits, while instilling a non-blaming explanatory narrative for ADHD-related difficulties in functioning; and identify the most troublesome problems to be targeted by family-endorsed treatment goals.
Task 3: ADHD Symptoms and Family Relations
Therapeutic goals: Utilize family-based interventions to explore ADHD impact on family functioning, moderate negative attributions about ADHD behaviors, and reframe ADHD-related symptoms as relational problems with both medication and behavioral solutions. Completion criteria: Family has adopted a non-blaming and cooperative attitude toward the teen’s ADHD characteristics; Family understands the relational impact of ADHD on all family members; Family understands how ADHD is related to the primary reason(s) for referral and accepts that addressing ADHD directly will improve coping among all members; Therapist and family have brainstormed specific behavioral accommodations in the home to support improvement in high-priority family and school problems.
Family members often enter therapy with strong negative attributions about the teen’s ADHD-related deficits. The therapist can facilitate more constructive family engagement in these areas by using relabeling: altering negative attributions about a given behavior by emphasizing an unrecognized or mislabeled cause, thereby casting it in a more benign light. One kind of relabeling, ADHD Acquittal, moves the family away from a disapproving attribution—ascribing personal/moral blame to an ADHD-related behavior presumed to be under the teen’s control—and toward a neutral attribution—accepting a common ADHD characteristic that arises from a neurobiological condition. For example, “lazy” is recast as inattentive or distractible, “irresponsible” as poor sense of time, “disruptive” as impulsive, and so on. A second kind of relabeling, ADHD Rewards, introduces rewarding or adaptive aspects of ADHD characteristics: the “flip side” of distractibility is alertness, of immaturity is youthful exuberance, of impulsivity is creativity, and so on. Both kinds of relabeling interventions are primed by psychoeducation activities completed in Task 2.
Reframing interventions seek to change the focus of discussion about ADHD-related deficits from “individual” adolescent problems to “family” problems that affect, and are affected by, the larger family environment. To accomplish this, the therapist engages the family in describing (a) how behavioral problems affect the emotional valence and everyday functioning of the home and (b) how the family responds to (and perhaps exacerbates) these problems on a regular basis. When successful, reframing helps relieve the teen from bearing the exclusive burden of the problems, lowers defensiveness and reduces the likelihood of hostile exchanges or escalating negativity in session, and prompts renewed investment from all members in changing how the family supports the teen’s developmental success.
Task 4: ADHD Medication and Family Decision-Making
Therapeutic goals: Review basic psychoeducation facts about ADHD medication and family attitudes about such; in collaboration with the prescribing provider, support family decision-making about medication initiation. Completion criteria: Therapist and family have reviewed all previous discussions with the prescriber on medication-related issues; Family understands the unique benefits of medication in general and its potential specific benefits for the teen in home, school, and peer contexts; Therapist and family have discussed medication stigma, side effects, trial-and-error titration, and substance use issues; Family has accepted, refused, deferred, or been declared ineligible for a medication regimen. For medication accepters only: Therapist introduced a weekly medication log that tracks adherence, effects, and side effects.
To be successful in the decision-making process, it is essential to cultivate the teen’s active involvement. Often this is not an easy task: Counseling for adolescents is invariably initiated by caregivers or other authorities, leaving many teenagers insufficiently informed, resentful, and/or reluctant about participating. Due to developmental limitations some teens may be unable to recognize the degree to which their ADHD-related behaviors are problematic. To promote adolescent investment in making a considered decision, therapists should explore whether and how ADHD medication can have personally meaningful benefit for the teen. First, the therapist should clearly establish that the teen’s participation and viewpoints are as important as anyone’s, which may be a (relatively) new mindset for some families. Second, the therapist should use examples drawn from the teen’s own depiction of his/her daily life to formulate individualized benefits, in active collaboration with the teen. When indicated, the therapist might gently challenge the teen to adopt new perspectives or consider the upside of trying new approaches, in the service of engaging in thoughtful and context-specific decision-making. These interventions can also engender sturdier teen commitment to starting medication (if so decided) and adhering to the prescribed regimen on a daily basis.
For the vast majority of families, the most important characteristic influencing readiness to initiate ADHD medication during the teenage years is school achievement. Alarming school problems frequently emerge in late middle school and early high school as academic curricula require increasingly greater levels of organization, long-term planning, and academic self-direction. These new demands can be extremely difficult for students with ADHD to satisfy, even among those who maintained steady progress in earlier years due to intelligence, creativity, home and school academic supports, and motivation to achieve. New organizational demands can quickly outstrip the teen’s ability to adjust and cope, resulting in a pile-up of disappointments and failures that inevitably undermine academic confidence and drive. For such students, medication offers the compelling possibility of immediate improvement in several facets of school functioning, making it the prime lever for moving family members toward medication acceptance.
Task 5: Medication Management and Integration Planning
Therapeutic goals: Formulate a case coordination framework for the prescribed medication regimen, with therapist and prescriber working in integrated fashion to support compliance and monitor effects. Completion criteria: Prescriber initiated a medication plan for the client, including ongoing medication management visits; Therapist and family established routine discussion of medication issues during behavioral treatment sessions, including compliance, benefits and side effects, and medication management visits; Prescriber, therapist, and family created a working arrangement for regular communication and integrated case planning about medication for the duration of pharmacological intervention.
Protocol Resources
The MIP provider manual (Hogue & Bobek, 2013; available from the first author upon request) contains a complete description of the implementation goals, strategies, and completion criteria described above for each respective Task. It also includes 29 psychoeducation slides to review with families in session, divided into three core areas: (a) Basic Facts about Adolescent ADHD, (b) ADHD and Executive Functioning, and (c) ADHD Medication Facts and Decision-Making. The provider manual also contains the MIP Fidelity Monitoring Checklist (see Figure 2); the ADHD Style Index and Problem Scorecards (see Task 2) for logging client-specific profiles of ADHD-related characteristics and problem areas; and a medication tracking log.
ADHD Medication in Usual Care: What Behavior Therapists Should Know About Medication Integration Across Diverse Settings and Populations
In order to be fully informed clinicians and advocates for their adolescent clients with ADHD, behavior therapists should be aware of important recommendations and caveats related to best practices for adolescent ADHD medication prescription. Although decisions about prescription are firmly in the hands of credentialed prescribers, non-prescribing therapists who treat teens with ADHD are encouraged to become knowledgeable about several key topics pertaining to medication integration strategies in diverse settings and with diverse populations. This will position them to better counsel their clients and better inform collaborating prescribers about behavioral issues that influence, and are influenced by, medication issues during treatment.
Medication Integration in Primary Medical Care
The MIP strategies described above are designed to facilitate better integration of pharmacological interventions into existing behavioral care services. Therefore they can be readily used by psychiatrists, pediatricians, nursing staff, and other prescribers in primary medical settings who desire to enhance their medication interventions with ADHD teens by adopting a more systematic approach to educating adolescents and families about the disorder and engaging them in family-tailored decision-making (e.g., Lopez et al., 2005). Because ADHD medications are intended not only to reduce ADHD symptoms but also to improve ADHD-related quality of life indicators, particularly executive functioning and school performance, there are potentially multidomain benefits to increasing ADHD medication acceptance and compliance among youth in primary settings. Also, MIP strategies can be efficiently translated across disease states to improve the processes of integrated care for other adolescent disorders for which evidence-based medications are available but underutilized, such as anxiety, depression, and aggression disorders (Hughes et al., 2007; Nevels, Dehon, Alexander, & Gontkovsky, 2010).
Medication Assessment and Intervention Priorities for Adolescents with Co-Occurring Disorders
The adage that for teenagers with ADHD, comorbidity is the rule rather than the exception has been widely supported in the literature, with co-occurrence rates as high as 85% (Larson, Russ, Kahn, & Halfon, 2011; Pliszka et al., 2007). Mood, anxiety, substance use, disruptive behavior, and learning disorders all commonly co-exist with ADHD. Untreated, each class of co-occurring disorder may adversely affect several aspects of ADHD treatment, from client engagement to medication compliance and outcomes. Thus, it is vital that prescribers and behavior therapists alike pay careful attention to additional behavioral health conditions affecting teens with ADHD.
Co-occurring behavioral disorders often share symptoms with ADHD that interact in ways that complicate differential assessment. Therefore, all teenagers with ADHD should undergo a comprehensive mental health evaluation that focuses on areas of symptomatic overlap: anxiety (including excessive worry, repetitive behaviors, trauma history), mood (lability, excessive irritability, dysphoria, grandiosity, impulsivity), learning problems (academic performance, standardized test scores for basic literacy, history of academic supports and special education), drug and alcohol involvement (substances used, frequency, setting, consequences), and disruptive behavior (symptoms of oppositional defiant and conduct disorders).
It is clear that addressing co-occurring conditions with medication and/or behavioral treatments, as appropriate, is crucial for treatment success. However, as with assessment, treatment of ADHD and its comorbid symptoms can be complicated by the overall clinical picture. For example, stimulant medications can adversely affect mood in bipolar individuals, worsen anxiety and repetitive behavior among anxious youth, and be diverted or misused by teens with substance use and disruptive behavior disorders. A number of strategies for treating ADHD in clients with comorbid disorders advocate for using non-stimulant medications in these circumstances. One strategy, the Texas Children’s Medication Algorithm for Pharmacotherapy of ADHD, proposes distinct treatment approaches for ADHD with comorbid anxiety, depression, aggression, tics, and substance use disorders (Pliszka et al., 2006). All strategies stress the concomitant treatment of both conditions while critically evaluating the efficacy of medications in addressing target symptoms, to avoid problems associated with polypharmacy.
Providing ADHD Medication Interventions to Adolescent Substance Users
Behavior therapists who routinely treat adolescent substance use (ASU) need to adopt reliable strategies for assessing and treating ADHD. As indicated above, ADHD is highly prevalent among adolescents with substance use problems, with comorbidity estimates ranging from 25% to 60% of ASU clients (see Tims et al., 2002). Moreover, co-occurring ADHD presents a major barrier to successful ASU treatment (Mariani & Levin, 2007). Compared to drug-using teens without ADHD, those with ADHD tend to transition more quickly from substance use to dependence (Tapert, Baratta, Abrantes, & Brown, 2002), drop out of treatment earlier (Wise, Cuffe, & Fischer, 2001), demonstrate a worse symptom course and worse treatment outcomes (Biederman et al., 1998), have worse mental health prognoses (Bukstein et al., 2005), and return to using substances in greater numbers and more rapidly after ASU treatment (Tomlinson, Brown, & Abrantes, 2004; Whitmore & Riggs, 2006). These data underscore the importance of integrating evidence-based interventions for ADHD into substance use treatment for teenagers with comorbid diagnoses (Whitmore & Riggs, 2006).
Are ADHD medications a viable option for adolescents with substance use problems? One common concern among clinicians and families alike is that prescribed ADHD medications will exacerbate substance problems and/or create risk for misuse or diversion by substance-involved teens (Kollins, 2007). However, the best available evidence strongly suggests that ADHD medication does not present additional risk for substance misuse. Several literature reviews have concluded that ADHD medication during childhood does not exacerbate risk for ASU (e.g., Fischer & Barkley, 2003; Wilens, Faraone, Biederman, & Gunawardene, 2003). A recent meta-analysis of longitudinal studies (Humphreys, Eng, & Lee, 2013) found that initiating stimulant medication in children with ADHD neither protects them from developing a substance use disorder nor creates greater risk for one. Similarly, in the most complete study to date Molina and colleagues (2013) reported that lifetime ADHD medication had a neutral impact on ASU, neither increasing nor decreasing ASU risk. To our knowledge this is the only study to measure the effects of medication use across the developmental span—not just initial medication acceptance in childhood—on ASU outcomes. More research on adherence to specific medication regimens (Sanchez et al., 2005) and medication use across multiple years (Langberg & Becker, 2012) in both child and adolescent samples is needed to fully address questions about the longitudinal impact of medication on ASU problems.
Moreover, there is emerging evidence that ADHD medication can have additive benefits when combined with behavioral treatment for adolescents with comorbid ADHD and ASU (Mariani & Levin, 2007). The largest study to date, a multisite trial of stimulant medication versus placebo combined with cognitive-behavioral therapy for adolescents with comorbid ADHD and ASU (Riggs et al., 2011), found that ADHD medications were well-tolerated by ASU clients, rarely misused or diverted, and produced benefits in multiple areas (e.g., parent reports of ADHD symptoms, urine assays for substance use). Altogether, it seems prudent to include ADHD medication in routine treatment planning for most teenagers with co-occurring ASU, with perhaps an additional degree of monitoring for medication compliance and diversion.
Feasibility of Medication Integration in Routine Behavioral Practice
MIP strategies contain several features intended to heighten their compatibility and sustainability in routine working conditions of behavioral care. First, they are designed to increase the efficiency of behavioral services for adolescent ADHD by fully integrating medication interventions with behavioral interventions; this also reduces patient burden associated with separate involvement in, and competing demands from, psychiatric versus behavioral services. Second, MIP strategies can be implemented in any treatment setting —specialty behavioral health clinics, school-based clinics, family service agencies—in which behavioral therapists practice. Third, MIP can function as a stand-alone protocol, or instead, be combined with another manualized treatment to build a multicomponent treatment package for comorbid cases (e.g., Riggs et al., 2011). Fourth, MIP strategies are anchored by family therapy techniques that have been widely validated on diverse samples of high-risk families, including minority and inner city adolescents (Henggeler & Sheidow, 2012; Tanner-Smith et al., 2012), and as such hold great promise for applicability and adaptability to heterogeneous adolescent populations. Finally, MIP strategies can be delivered in conjunction with either family-based treatments or individual-based treatments that can flexibly include caregivers in multiple treatment sessions. Thus they can be adopted by clinicians with allegiance to a wide range of treatment approaches, and they need not preempt or conflict with preferred intervention routines.
Acknowledgments
The authors would like to thank Chris A. Ziegler Dendy and the dedicated staff of the Roberto Clemente Center for their contributions in developing the Medication Integration Protocol.
Funding
Preparation of this article was supported by the National Institute on Drug Abuse (K02DA026538 and R21DA031305).
Contributor Information
Aaron Hogue, The National Center on Addiction and Substance Abuse at Columbia University, New York, New York, USA.
Molly Bobek, The National Center on Addiction and Substance Abuse at Columbia University, New York, New York, USA.
Gregory Z. Tau, Division of Child and Adolescent Psychiatry at Columbia University, New York, New York, USA and New York State Psychiatric Institute.
Frances R. Levin, Division on Substance Abuse at Columbia University, New York, New York, USA and New York State Psychiatric Institute.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: APA; 2000. text revision. [Google Scholar]
- Barkley RA. Associated cognitive, developmental, and health problems. In: Barkley RA, editor. Attention Deficit Hyperactivity Disorder: A handbook for diagnosis and treatment. 3. New York: Guilford; 2006. pp. 122–183. [Google Scholar]
- Biederman J, Wilens T, Mick E, Faraone SV, Spencer T. Does Attention-Deficit/Hyperactivity Disorder impact the developmental course of drug and alcohol abuse and dependence? Biological Psychiatry. 1998;44:269–273. doi: 10.1016/s0006-3223(97)00406-x. [DOI] [PubMed] [Google Scholar]
- Bukstein OG, Cornelius J. Psychopharmacology of adolescents with substance use disorders: Using diagnostic-specific treatment. In: Liddle HA, Rowe CL, editors. Adolescent substance abuse: Research and clinical advances. Cambridge University Press; 2006. pp. 241–263. [Google Scholar]
- Bukstein OG, Cornelius J, Trunzo AC, Kelly TM, Wood DS. Clinical predictors of treatment in a population of adolescents with alcohol use disorders. Addictive Behaviors. 2005;30:1663–1673. doi: 10.1016/j.addbeh.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Bussing R, Porter P, Zima BT, Mason D, Garvan C, Reid R. Academic outcome trajectories of students with ADHD: Does exceptional education status matter? Journal of Emotional and Behavioral Disorders. 2012;20:131–143. [Google Scholar]
- Chan Y, Dennis ML, Funk RR. Prevalence and comorbidity of major internalizing and externalizing problems among adolescents and adults presenting to substance abuse treatment. Journal of Substance Abuse Treatment. 2008;34:14–24. doi: 10.1016/j.jsat.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpita BF, Daleiden EL, Weisz JR. Modularity in the design and application of therapeutic interventions. Applied & Preventive Psychology. 2005;11:141–56. [Google Scholar]
- Coolidge FL, Thede LL, Young SE. Heritability and the comorbidity of attention deficit hyperactivity disorder with behavioral disorders and executive function deficits: A preliminary investigation. Developmental Neuropsychology. 2000;17:273–287. doi: 10.1207/S15326942DN1703_1. [DOI] [PubMed] [Google Scholar]
- Cummings CM, Fristad MA. Medications prescribed for children with mood disorders: Effects of a family-based psychoeducation program. Experimental and Clinical Psychopharmacology. 2007;15:555–562. doi: 10.1037/1064-1297.15.6.555. [DOI] [PubMed] [Google Scholar]
- Cutting L, Denckla M. Attention: Relationships between Attention-Deficit/Hyperactivity Disorder and learning disabilities. In: Swanson H, Harris K, Graham S, editors. Handbook of learning disabilities. New York: Guilford; 2003. pp. 125–139. [Google Scholar]
- Dendy CAZ. Teaching Teens with ADD and ADHD: A Quick Reference Guide for Teachers and Parents. Bethesda, MD: Woodbine House; 2000. [Google Scholar]
- Dendy CAZ. Teenagers with ADD and ADHD: A Guide for Parents and Professionals. 2. Bethesda, MD: Woodbine House; 2006. [Google Scholar]
- Diamond GM, Liddle HA, Hogue A, Dakof GA. Alliance-building interventions with adolescents in family therapy: A process study. Psychotherapy: Theory, Research, Practice, & Training. 1999;36:355–368. [Google Scholar]
- DuPaul G, Evans SW. School-based interventions for adolescents with Attention-Deficit/Hyperactivity Disorder. Adolescent Medicine: State of Art Reviews. 2008;19:300–312. [PubMed] [Google Scholar]
- Evans SW, Owens JS, Bunford N. Evidence-based psychosocial treatments for children and adolescents with Attention-Deficit/Hyperactivity Disorder. Journal of Clinical Child and Adolescent Psychology. doi: 10.1080/15374416.2013.850700. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE, Coles EK, Gnagy EM, Chronis-Tuscano A, O’Connor BC. A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder. Clinical Psychology Review. 2009;29:129–140. doi: 10.1016/j.cpr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. European Child & Adolescent Psychiatry. 2010;19:353–364. doi: 10.1007/s00787-009-0054-3. [DOI] [PubMed] [Google Scholar]
- Fischer M, Barkley RA. Childhood stimulant treatment and risk for later substance abuse. Journal of Clinical Psychiatry. 2003;64(Suppl 11):19–23. [PubMed] [Google Scholar]
- Fletcher JM, Lyon G, Fuchs L, Barnes M. Learning disabilities: From identification to intervention. New York: Guilford; 2007. [Google Scholar]
- Fristad MA. Psychoeducational treatment for school-aged children with bipolar disorder. Development and Psychopathology. 2006;18:1289–1306. doi: 10.1017/S0954579406060627. [DOI] [PubMed] [Google Scholar]
- Ge’ist R, Heinmaa M, Stephens D, Davis R, Katzman DK. Comparison of family therapy and family group psychoeducation in adolescents with anorexia nervosa. Canadian Journal of Psychiatry. 2000;45:173–178. doi: 10.1177/070674370004500208. [DOI] [PubMed] [Google Scholar]
- Henggeler SW, Sheidow AJ. Empirically supported family-based treatments for conduct disorder and delinquency in adolescents. Journal of Marital and Family Therapy. 2012;38:30–58. doi: 10.1111/j.1752-0606.2011.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogue A, Bobek M. Medication Integration Protocol (MIP): Provider Manual. The National Center on Addiction and Substance Abuse at Columbia University; New York: 2013. Available from the first author upon request. [Google Scholar]
- Hogue A, Liddle HA. Family-based treatment for adolescent substance abuse: Controlled trials and new horizons in services research. Journal of Family Therapy. 2009;31:126–154. doi: 10.1111/j.1467-6427.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking JD, Cisler RA, Couper DJ, Gastfriend DR, Kivlahan DR, Anton RF. Design and analysis of trials of combination therapies. Journal of Studies on Alcohol, Supplement. 2005;15:34–42. doi: 10.15288/jsas.2005.s15.34. [DOI] [PubMed] [Google Scholar]
- Hughes CW, Emslie GJ, Crismon M, Posner K, Birmaher B, Ryan N, Trivedi MH. Texas children’s medication algorithm project: Update from Texas consensus conference panel on medication treatment of childhood major depressive disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:667–686. doi: 10.1097/chi.0b013e31804a859b. [DOI] [PubMed] [Google Scholar]
- Humphreys KL, Eng T, Lee SS. Stimulant medication and substance use outcomes: A meta-analysis. JAMA Psychiatry. 2013;70:740–749. doi: 10.1001/jamapsychiatry.2013.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Improving the quality of healthcare for mental and substance use conditions. Washington, DC: National Academies Press; 2006. [Google Scholar]
- Jensen PS, Hinshaw SP, Swanson JM, Greenhill LL, Conners CK, Arnold LE, Wigal T. Findings from the NIMH Multimodal Treatment Study of ADHD (MTA): Implications and applications for primary care providers. Developmental and Behavioral Pediatrics. 2001;22:60–73. doi: 10.1097/00004703-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Kollins SH. Abuse liability of medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) The American Journal on Addictions. 2007;16:35–44. doi: 10.1080/10550490601082775. [DOI] [PubMed] [Google Scholar]
- Langberg JM, Becker SP. Does long-term medication use improve the academic outcomes of youth with Attention-Deficit/Hyperactivity Disorder? Clinical Child and Family Psychology Review. 2012:1–19. doi: 10.1007/s10567-012-0117-8. [DOI] [PubMed] [Google Scholar]
- Larson K, Russ SA, Kahn RS, Halfon N. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127:462–470. doi: 10.1542/peds.2010-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle HA. Conceptual and clinical dimensions of a multidimensional, multisystems engagement strategy in family-based adolescent treatment. Psychotherapy: Theory, Research, Practice, & Training. 1995;32:39–58. [Google Scholar]
- Lopez M, Toprac M, Crimson M, Boemer B, Baumgartner J. A psychoeducational program for children with ADHD or depression and their families: Results from the CMAP feasibility study. Community Mental Health Journal. 2005;41:51–66. doi: 10.1007/s10597-005-2599-z. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Levin FR. Treatment strategies for co-occurring ADHD and substance use disorders. The American Journal on Addictions. 2007;16:45–56. doi: 10.1080/10550490601082783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough JJ, McBurnett K, Bukstein O, Wilens TE, Greenhill L, Lerner M, Stein M. Once-daily OROS methylphenidate is safe and well tolerated in adolescents with Attention-Deficit/Hyperactivity Disorder. Journal of Child and Adolescent Psychopharmacology. 2006;16:351–356. doi: 10.1089/cap.2006.16.351. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He J, Burstein M, Swendsen J, Avenevoli S, Case B, Olfson M. Service utilization for lifetime mental disorders in US adolescents: results of the National Comorbidity Survey–Adolescent Supplement (NCS-A) Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:32–45. doi: 10.1016/j.jaac.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GK, Tieman JJ, Shelby-James TM. Multidisciplinary care planning and teamwork in primary care. Medical Journal of Australia. 2008;188:S61–S64. doi: 10.5694/j.1326-5377.2008.tb01747.x. [DOI] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Arnold LE, Swanson JM, Pelham WE, Hechtman L, Marcus S. Adolescent substance use in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder (ADHD)(MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:250–263. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya A, Colom F, Ferrin M. Is psychoeducation for parents and teachers of children and adolescents with ADHD efficacious? A systematic literature review. European Psychiatry. 2011;26:166–175. doi: 10.1016/j.eurpsy.2010.10.005. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Murphy K. Psychosocial treatments for ADHD in teens and adults: A practice-friendly review. Journal of Clinical Psychology. 2005;61:607–619. doi: 10.1002/jclp.20123. [DOI] [PubMed] [Google Scholar]
- Nevels RM, Dehon EE, Alexander K, Gontkovsky ST. Psychopharmacology of aggression in children and adolescents with primary neuropsychiatric disorders: A review of current and potentially promising treatment options. Experimental and Clinical Psychopharmacology. 2010;18:184–201. doi: 10.1037/a0018059. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology. 2008;37:184–214. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EN, Sibley MH, Kipp HL, Smith BH, Evans SE, Bukstein O. Attributions and perception of methylphenidate effects in adolescents with ADHD. Journal of Attention Disorders. 2013 doi: 10.1177/1087054713493320.. [DOI] [PubMed] [Google Scholar]
- Piacentini J, Bennett S, Compton S, Kendall P, Birmaher B, Albano AM, Walkup J. 24- and 36-week outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS) Journal of the American Academy of Child and Adolescent Psychiatry. doi: 10.1016/j.jaac.2013.11.010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka S, Bernet W, Bukstein O, Walter HJ, Arnold V, Beitchman J, Medicus J. American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Crismon M, Hughes CW, Corners CK, Emslie GJ, Jensen PS, Lopez M. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:642–657. doi: 10.1097/01.chi.0000215326.51175.eb. [DOI] [PubMed] [Google Scholar]
- Poulin C. From Attention-Deficit/Hyperactivity Disorder to medical stimulant use to the diversion of prescribed stimulants to non-medical stimulant use: Connecting the dots. Addiction. 2007;102:740–751. doi: 10.1111/j.1360-0443.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- Power TJ, Mautone JA, Soffer SL, Clarke AT, Marshall SA, Sharman J, Jawad AF. A family–school intervention for children with ADHD: Results of a randomized clinical trial. Journal of Consulting and Clinical Psychology. 2012;80:611–623. doi: 10.1037/a0028188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggi VL, Chronis AM. Interventions to address the academic impairment of children and adolescents with ADHD. Clinical Child and Family Psychology Review. 2006;9:85–111. doi: 10.1007/s10567-006-0006-0. [DOI] [PubMed] [Google Scholar]
- Reinecke MA, Curry JF, March JS. Findings from the Treatment for Adolescents with Depression Study (TADS): What have we learned? What do we need to know? Journal of Clinical Child and Adolescent Psychology. 2009;38:761–767. doi: 10.1080/15374410903258991. [DOI] [PubMed] [Google Scholar]
- Riggs PD, Winhusen T, Davies RD, Leimberger JD, Mikulich-Gilbertson S, Klein C, Lu D. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with Attention-Deficit/Hyperactivity Disorder and Substance Use Disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:903–914. doi: 10.1016/j.jaac.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb JA, Sibley MH, Pelham WE, Foster E, Molina BSG, Gnagy EM, Kuriyan AB. The estimated annual Cost of ADHD to the US education system. School Mental Health. 2011;3:169–177. doi: 10.1007/s12310-011-9057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins MS, Liddle HA, Turner CW, Dakof GA, Alexander JF, Kogan SM. Adolescent and parent therapeutic alliances as predictors of dropout in multidimensional family therapy. Journal of Family Psychology. 2006;20:108–116. doi: 10.1037/0893-3200.20.1.108. [DOI] [PubMed] [Google Scholar]
- Robin A. Training families with adolescents with ADHD. In: Barkley R, editor. Attention Deficit Hyperactivity Disorder: A handbook for diagnosis and treatment. 3. New York: Guilford; 2006. pp. 499–546. [Google Scholar]
- Sanchez R, Crismon M, Barner J, Bettinger T, Wilson J. Assessment of adherence measures with different stimulants among children and adolescents. Pharmacotherapy. 2005;25:909–917. doi: 10.1592/phco.2005.25.7.909. [DOI] [PubMed] [Google Scholar]
- Sanford M, Boyle M, McCleary L, Miller J, Steele M, Duku E, Offord D. A pilot study of adjunctive family psychoeducation in adolescent major depression: Feasibility and treatment effect. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:386–395. doi: 10.1097/01.chi.0000198595.68820.10. [DOI] [PubMed] [Google Scholar]
- Schultz BK, Storer J, Watabe Y, Sadler J, Evans SW. School-based treatment of attention-deficit/hyperactivity disorder. Psychology in the Schools. 2011;48:254–262. [Google Scholar]
- Schwarz A, Cohen S. More diagnoses of hyperactivity in new C.D.C. data: A rapid rise in rates. The New York Times. 2013 Retrieved from http://www.nytimes.com/2013/04/01/health/more-diagnoses-of-hyperactivity-causing-concern.html.
- Schultz BK, Storer J, Watabe Y, Sadler J, Evans SW. School-based treatment of Attention-Deficit/Hyperactivity Disorder. Psychology in the Schools. 2011;48:254–262. [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, Gnagy EM, Waschbusch DA, Garefino AC, Karch KM. Diagnosing ADHD in adolescence. Journal of Consulting and Clinical Psychology. 2012;80:139–150. doi: 10.1037/a0026577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BH, Barkley RA, Shapiro C. Combined child therapies. In: Barkley RA, editor. Attention Deficit Hyperactivity Disorder: A handbook for diagnosis and treatment. 3. New York: Guilford; 2006. pp. 678–691. [Google Scholar]
- Smith BH, Waschbusch DA, Willoughby MT, Evans S. The efficacy, safety, and practicality of treatments for adolescents with Attention-Deficit/Hyperactivity Disorder (ADHD) Clinical Child and Family Psychology Review. 2000;3:243–267. doi: 10.1023/a:1026477121224. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Carter Sobell LC. Substance use, health, and mental health. Clinical Psychology: Science and Practice. 2007;14:1–5. [Google Scholar]
- Spencer T, Wilens T, Biederman J, Weister R, Read S, Pratt R. Efficacy and safety of mixed amphetamine salts extended release (Adderall XR) in the management of Attention-Deficit/Hyperactivity Disorder in adolescent patients: A 4-week randomized, double-blind, placebo-controlled, parallel-group study. Clinical Therapeutics. 2006;28:266–279. doi: 10.1016/j.clinthera.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The OAS Report: A day in the life of American adolescents: Substance use facts. SAMHSA, Office of Applied Studies; Rockville, MD: 2007. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The NSDUH Report: Adolescent Mental Health: Service Settings and Reasons for Receiving Care. SAMHSA, Office of Applied Studies; Rockville, MD: 2009. [Google Scholar]
- Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 2010;36:207–226. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock R. Language, reading, and motor control problems in ADHD: A potential behavioral phenotype. In: Greenhill L, editor. Learning disabilities: Implications for psychiatric treatment. Washington, DC: American Psychiatric Press; 2000. pp. 129–167. [Google Scholar]
- Tapert SF, Baratta MV, Abrantes AM, Brown SA. Attention dysfunction predicts substance involvement in community youths. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:680–686. doi: 10.1097/00004583-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Taylor E. Developing ADHD. Journal of Child Psychology & Psychiatry. 2009;50:126–132. doi: 10.1111/j.1469-7610.2008.01999.x. [DOI] [PubMed] [Google Scholar]
- Thompson LL, Whitmore EA, Raymond K, Crowley TJ. Measuring impulsivity in adolescents with serious substance and conduct problems. Assessment. 2006;13:3–15. doi: 10.1177/1073191105282247. [DOI] [PubMed] [Google Scholar]
- Tims FM, Dennis ML, Hamilton N, Buchan JB, Diamond G, Funk R, Brantley LB. Characteristics and problems of 600 adolescent cannabis abusers in outpatient treatment. Addiction. 2002;97(s1):46–57. doi: 10.1046/j.1360-0443.97.s01.7.x. [DOI] [PubMed] [Google Scholar]
- Todd RD, Huang H, Henderson CA. Poor utility of the age of onset criterion for DSM-IV attention deficit/hyperactivity disorder: Recommendations for DSM-V and ICD-11. Journal of Child Psychology and Psychiatry. 2008;49:942–949. doi: 10.1111/j.1469-7610.2008.01892.x. [DOI] [PubMed] [Google Scholar]
- Tomlinson KL, Brown SA, Abrantes A. Psychiatric comorbidity and substance use treatment outcomes of adolescents. Psychology of Addictive Behaviors. 2004;18:160–169. doi: 10.1037/0893-164X.18.2.160. [DOI] [PubMed] [Google Scholar]
- Turner WC, Muck RD, Muck RJ, Stephens RL, Sukumar B. Co-occurring disorders in the adolescent mental health and substance abuse treatment systems. Journal of Psychoactive Drugs. 2004;36:455–462. doi: 10.1080/02791072.2004.10524428. [DOI] [PubMed] [Google Scholar]
- Vieta E. Improving treatment adherence in bipolar disorder through psychoeducation. Journal of Clinical Psychiatry. 2005;66(Suppl 1):24–29. [PubMed] [Google Scholar]
- Visser S, Bitsko R, Danielson M, Perou R, Blumberg S. Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children—United States, 2003 and 2007. Morbidity and Mortality Weekly Report. 2010;59:1439–1443. [PubMed] [Google Scholar]
- Whitmore EA, Riggs PD. Developmentally informed diagnostic and treatment considerations in comorbid conditions. In: Liddle HA, Rowe CL, editors. Adolescent Substance Abuse: Research and Clinical Advances. Cambridge University Press; 2006. pp. 264–283. [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Wilens TE, McBurnett K, Bukstein O, McGough J, Greenhill L, Lerner M, Lynch JM. Multisite controlled study of OROS methylphenidate in the treatment of adolescents with attention-deficit/hyperactivity disorder. Archives of Pediatrics & Adolescent Medicine. 2006;160:82–90. doi: 10.1001/archpedi.160.1.82. [DOI] [PubMed] [Google Scholar]
- Wilson HK, Cox DJ, Merkel RL, Moore M, Coghill D. Effect of extended release stimulant-based medications on neuropsychological functioning among adolescents with Attention-Deficit/Hyperactivity Disorder. Archives of Clinical Neuropsychology. 2006;21:797–807. doi: 10.1016/j.acn.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Wise BK, Cuffe SP, Fischer T. Dual diagnosis and successful participation of adolescents in substance abuse treatment. Journal of Substance Abuse Treatment. 2001;21:161–165. doi: 10.1016/s0740-5472(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Wolraich ML, Wibbelsman CJ, Brown TE, Evans SW, Gotlieb EM, Knight JR, Wilens T. Attention-Deficit/Hyperactivity Disorder among adolescents: A review of the diagnosis, treatment, and clinical implications. Pediatrics. 2005;115:1734–1746. doi: 10.1542/peds.2004-1959. [DOI] [PubMed] [Google Scholar]
- Wu LT, Gersing K, Burchett B, Woody GE, Blazer DG. Substance use disorders and comorbid Axis I and II psychiatric disorders among young psychiatric patients: Findings from a large electronic health records database. Journal of Psychiatric Research. 2011;45:1453–1462. doi: 10.1016/j.jpsychires.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuvekas SH, Vitiello B. Stimulant medication use among US children: a twelve-year perspective. American Journal of Psychiatry. 2012;169:160–166. doi: 10.1176/appi.ajp.2011.11030387. [DOI] [PMC free article] [PubMed] [Google Scholar]