Abstract
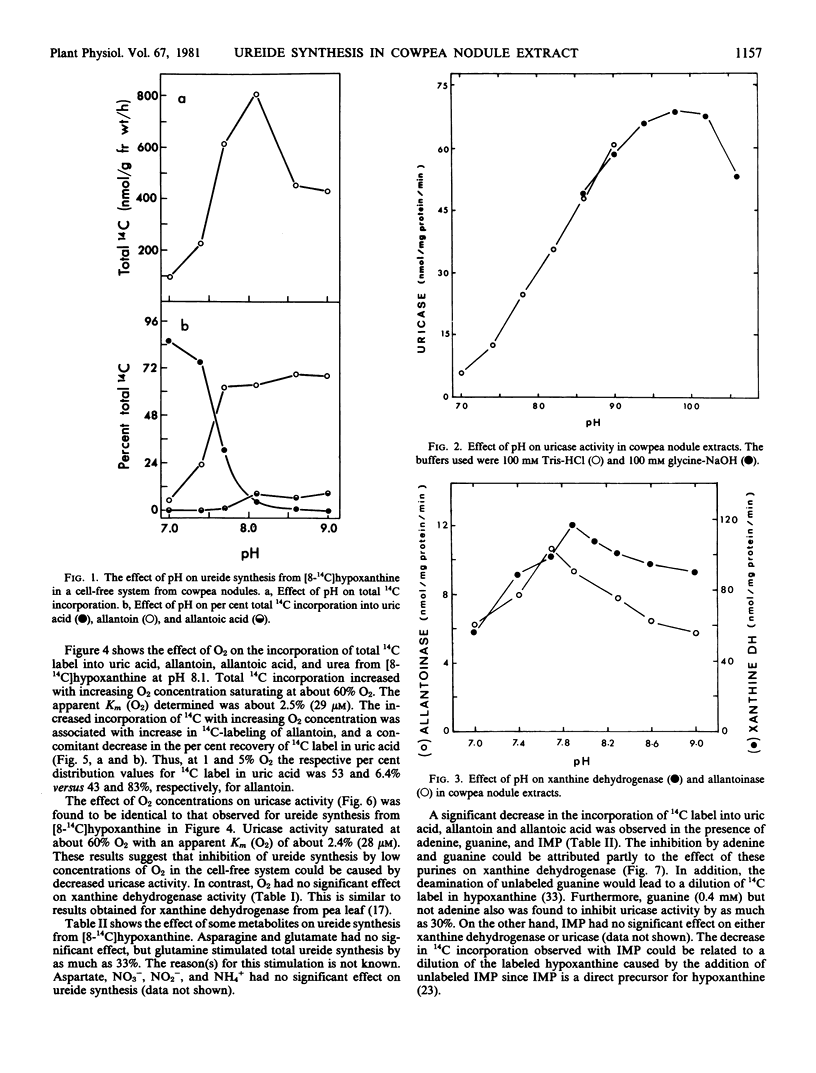
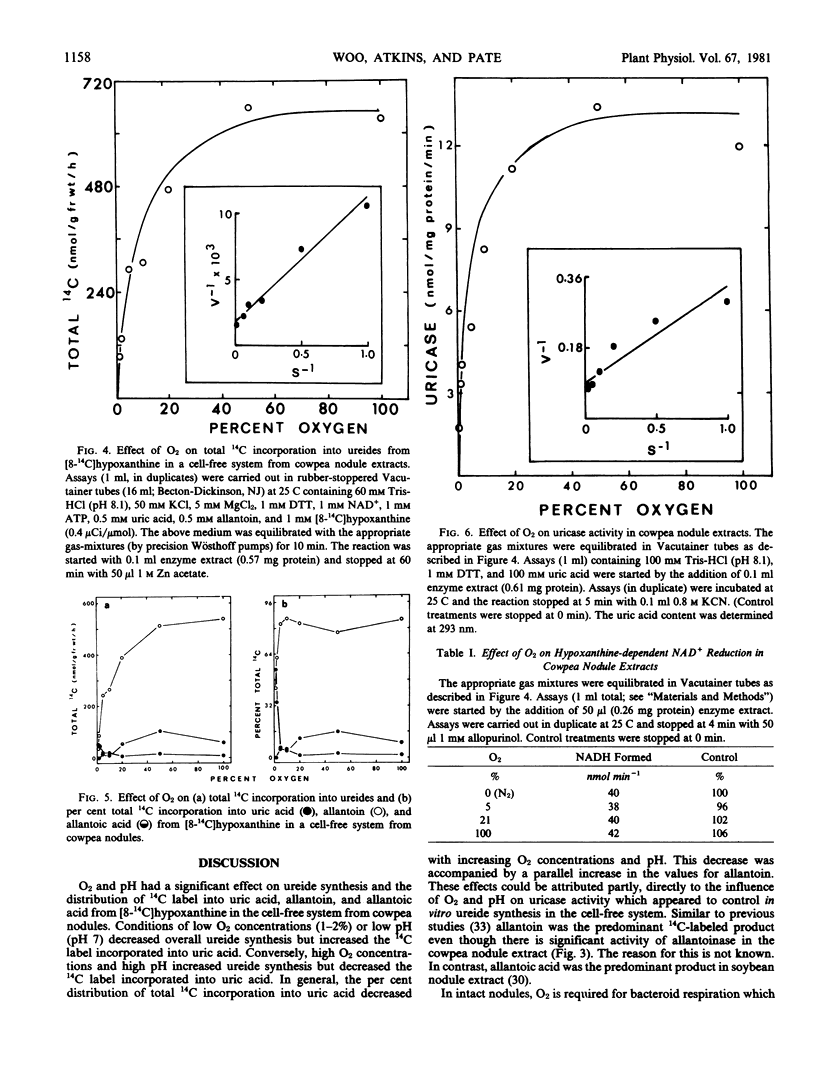
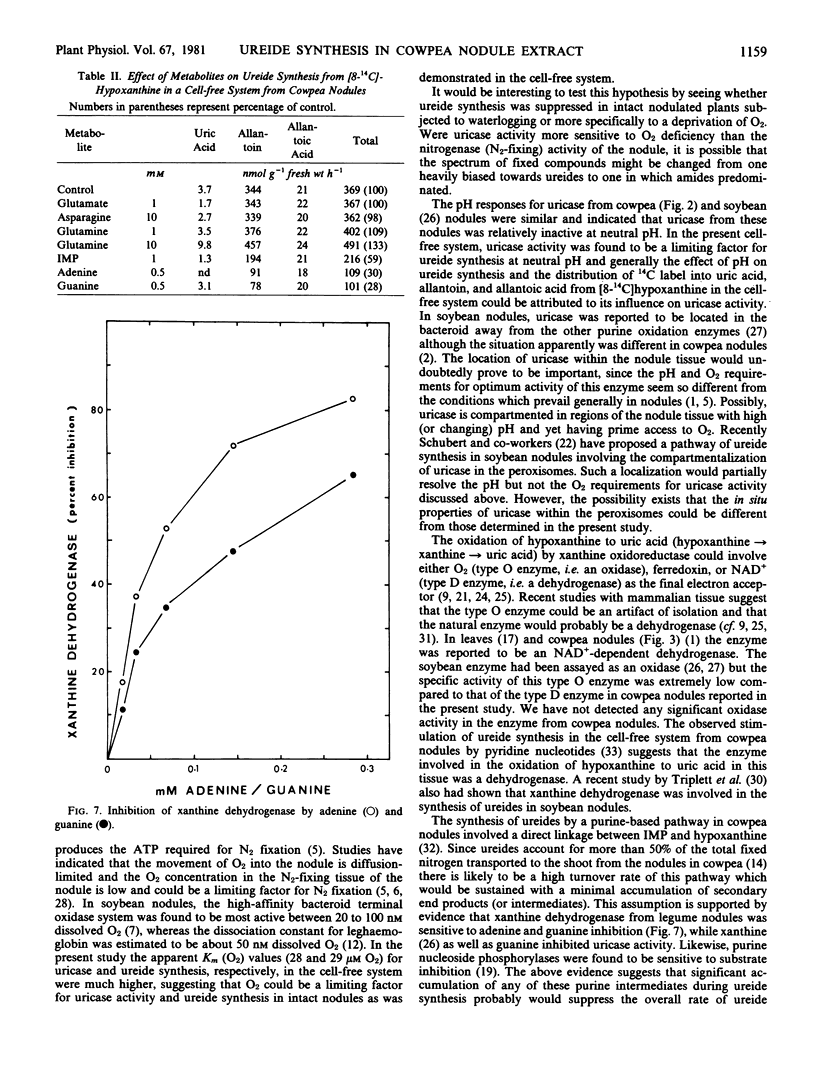
The effect of O2 and pH on the in vitro synthesis of 14C-labeled ureides from [8-14C]hypoxanthine in a cell-free system from cowpea nodules was investigated. Under conditions which suppressed uricase (EC 1.7.3.3) activity, namely low O2 concentrations and low pH, ureide synthesis was inhibited and the 14C label incorporated into uric acid was increased. Conversely, conditions which increased uricase activity, namely high O2 concentrations and high pH, also stimulated ureide synthesis, and the 14C label was incorporated principally into allantoin. The overall response of the system to O2 concentration and pH indicated that the per cent distribution of total 14C label incorporated into uric acid was inversely related to that into allantoin. In the present study there was evidence that uricase (EC 1.7.3.3) controlled the in vitro rate of ureide synthesis in the cell-free system. Adenine and guanine inhibited xanthine dehydrogenase (EC 1.2.1.37) and as a consequence ureide synthesis from [8-14C]hypoxanthine was also inhibited.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A. Properties of leghaemoglobin in vivo, and its isolation as ferrous oxyleghaemoglobin. Biochim Biophys Acta. 1969;188(2):222–229. doi: 10.1016/0005-2795(69)90069-5. [DOI] [PubMed] [Google Scholar]
- BAUM H., HUBSCHER G., MAHLER H. R. Studies on uricase. II. The enzyme-substrate complex. Biochim Biophys Acta. 1956 Dec;22(3):514–527. doi: 10.1016/0006-3002(56)90062-2. [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L. Leghaemoglobin and the supply of O2 to nitrogen-fixing root nodule bacteroids: presence of two oxidase systems and ATP production at low free O2 concentration. J Gen Microbiol. 1975 Dec;91(2):345–354. doi: 10.1099/00221287-91-2-345. [DOI] [PubMed] [Google Scholar]
- Davidson J. N. The purification of uricase: Some properties of purified uricase. Biochem J. 1942 Feb;36(1-2):252–258. doi: 10.1042/bj0360252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara S., Yamaguchi M. Effects of Allopurinol [4-Hydroxypyrazolo(3,4-d)Pyrimidine] on the Metabolism of Allantoin in Soybean Plants. Plant Physiol. 1978 Jul;62(1):134–138. doi: 10.1104/pp.62.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge D. F., Atkins C. A., Pate J. S., Rainbird R. M. Allantoin and Allantoic Acid in the Nitrogen Economy of the Cowpea (Vigna unguiculata [L.] Walp.). Plant Physiol. 1978 Oct;62(4):495–498. doi: 10.1104/pp.62.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKI Y. Pathophysiological effects of circulating ferritin. Nature. 1959 Dec 19;184(Suppl 25):1944–1945. doi: 10.1038/1841944b0. [DOI] [PubMed] [Google Scholar]
- Pate J. S., Atkins C. A., White S. T., Rainbird R. M., Woo K. C. Nitrogen Nutrition and Xylem Transport of Nitrogen in Ureide-producing Grain Legumes. Plant Physiol. 1980 May;65(5):961–965. doi: 10.1104/pp.65.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe P. B., Wyngaarden J. B. The mechanism of dietary alterations in rat hepatic xanthine oxidase levels. J Biol Chem. 1966 Dec 10;241(23):5571–5576. [PubMed] [Google Scholar]
- Smith S. T., Rajagopalan K. V., Handler P. Purification and properties of xanthine dehydroganase from Micrococcus lactilyticus. J Biol Chem. 1967 Sep 25;242(18):4108–4117. [PubMed] [Google Scholar]
- Stirpe F., Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem. 1969 Jul 25;244(14):3855–3863. [PubMed] [Google Scholar]
- Trijbels F., Vogels G. D. Degradation of allantoin by Pseudomonas acidovorans. Biochim Biophys Acta. 1966 Feb 14;113(2):292–301. doi: 10.1016/s0926-6593(66)80068-1. [DOI] [PubMed] [Google Scholar]
- Triplett E. W., Blevins D. G., Randall D. D. Allantoic Acid Synthesis in Soybean Root Nodule Cytosol via Xanthine Dehydrogenase. Plant Physiol. 1980 Jun;65(6):1203–1206. doi: 10.1104/pp.65.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waud W. R., Rajagopalan K. V. Purification and properties of the NAD+-dependent (type D) and O2-dependent (type O) forms of rat liver xanthine dehydrogenase. Arch Biochem Biophys. 1976 Feb;172(2):354–364. doi: 10.1016/0003-9861(76)90087-4. [DOI] [PubMed] [Google Scholar]
- Woo K. C., Atkins C. A., Pate J. S. Biosynthesis of Ureides from Purines in a Cell-free System from Nodule Extracts of Cowpea [Vigna unguiculata (L) Walp.]. Plant Physiol. 1980 Oct;66(4):735–739. doi: 10.1104/pp.66.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]



