Abstract
In an effort to improve the therapeutic index of cancer chemotherapy, we developed an advanced nanopreparation based on the combination of landscape phage display to obtain new targeting ligands with micellar nanoparticles for tumor targeting of water insoluble neoplastic agents. With paclitaxel as a drug, this self-assembled nanopreparation composed of MCF-7-specific phage protein and polyethylene glycol phosphatidyl ethanolamine (PEG- PE) micelles showed selective toxicity to target cancer cells rather than non-target, non- cancer cells in vitro. In vivo, the targeted phage-micelles triggered a dramatic tumor reduction and extensive necrosis as a result of improved tumor delivery of paclitaxel. The enhanced anticancer effect was also verified by an enhanced apoptosis and reduced tumor cell proliferation following the treatment with the targeted micellar paclitaxel both in vitro and in vivo. The absence of hepatotoxicity and pathological changes in tissue sections of vital organs, together with maintenance of overall health of mice following the treatment, further support its translational potential as an effective and safe chemotherapy for improved breast cancer treatment.
Keywords: Micelle, Phage display, Drug delivery, Cancer targeting
1. Introduction
Major problems associated with the use of conventional chemotherapy in cancer treatment include insufficient therapeutic outcomes and dose-limiting toxicity. Upon intravenous administration, these small molecule agents typically distribute nonspecifically among tumors and normal tissues, and only a small fraction of the injected doses deposits at tumor sites (1). Thus, conventional chemotherapeutics destroy not only actively dividing tumor cells but also other tissue cells with high proliferation rates, such as those in hair follicles, the GI tract and bone marrow. Among FDA approved chemotherapies for breast cancer treatment, paclitaxel (PCT) is one of the most active agents used for the treatment of metastatic breast cancer (2). Despite its potency, PCT shows some undesirable properties. Its poor solubility leads to limited bioavailability, and the clinical use of PCT has produced dose-limiting toxicities, including hematologic ones (3), necessitating development of delivery systems to enhance its solubility and in vivo efficacy (4). Early on, Bristol-Myers Squibb developed a commercial formulation, in which PCT is prepared in cosolvents containing Cremophor EL and ethanol. However, this formulation has been associated with severe hypersensitivity reactions induced by Cremophor EL (4).
The utility of polymeric micelles offers an efficient solution for the solubilization and tumor-targeted delivery of a variety of sparingly soluble therapeutic agents (5, 6). Polymeric micelle formulations currently under clinical investigation include doxorubicin - encapsulated Pluronic micelles (SP1049C) (7), micelles formed by a copolymer of PEG and DOX-conjugated poly (aspartic acid) (NK911) (8), and PEG-PLA micelles loaded with paclitaxel (Genexol-PM) (9). Clinical data have shown that these micellar formulations have improved half-life, increased bioavailability and reduced toxicity (6-9). Additional improvement of the tumor-targeted efficiency of micellar drugs can be achieved by the surface modification of a micellar formulation with tumor-specific ligands, which selectively recognize tumor cell-associated antigens or receptors (10, 11).
While phage display technology is emerging as a powerful platform for the discovery of new tumor-targeted ligands (12), current use of such peptides for the design of cancer targeted nanomedicines often necessities a multistep scheme involving the chemical synthesis of the identified peptides followed by additionally chemical conjugation of the synthetic peptides to the nanocarriers (13). As an alternative, we explored the extension of the phage display technology to the concept of landscape phages (14-16). In this revised scenario, the so-called landscape phage fusion protein (in this case, a tumor specific peptide fused to the N-terminus of the major coat protein pVIII of filamentous phages) is used directly for the development of tumor-targeted nanomedicines (14).
Recently, we screened such a landscape phage fusion protein specific for breast cancer MCF-7 cells (17-20). Its amphiphilicity allows its application for micellar targeting of water-insoluble drugs to pathogenic sites. Its self-assembly with PEG2000-PE conjugates produces micellar nanoparticles capable for the delivery of hydrophobic PCT to specific tumor cells (11). Our initial in vitro results supported the concept that MCF-7-targeted PCT phage-micelles demonstrated enhanced the tumor cell binding and cytotoxicity against MCF-7 breast cancer cells (11). Herein, we sought to assess the effect of the targeted therapy on cellular apoptosis, necrosis and proliferation as well as its in vivo antitumor efficacy and potential side-effects.
2. Materials and Methods
2.1 Propagation of phage and purification of phage fusion protein
Phages were propagated and isolated as described previously (17, 21). Briefly, phages were subjected to a series of processes including endotoxin removal, PEG-precipitation and structure disassembly by the incubation with sodium cholate. Isolation of the phage major coat protein was carried out using sepharose 6-based size exclusion chromatography. Protein concentrations were determined by absorbance at 280 nm. Amino acid sequence of phage fusion protein was identified as ADMPGTVLPDPAKAAFDSLQASATEYIGYAWAMVVVIVGATIGIKLFKKFTSKAS
2.2. Preparation of paclitaxel-loaded micelles modified with MCF-7-specific phage fusion proteins (termed MCF-7-targeted PCT phage-micelles)
Twenty mM of PEG2000-PE in chloroform was mixed with paclitaxel in chloroform at a drug-to-lipid weight ratio of 1.5:100. After an evaporation step, the resultant lipid film was rehydrated with MCF-7-specific phage protein in 10 mM sodium cholate at a protein-to polymer weight ratio 1: 200, followed by vortexing and overnight dialysis against PBS, (pH 7.4) to remove sodium cholate. Non-incorporated PCT was removed by filtration of the crude micellar suspension through a 0.2 μm polycarbonate membrane. As controls, non-targeted PCT micelles were prepared using a similar procedure.
Loading of PCT into micelles was determined using a reversed phase HPLC system (Hitachi, Japan) with mobile phase composed of distilled water and acetonitrile at a volume ratio of 40:60, and flow rate of 1.0 ml/min. PCT was detected at 227nm. A colorimetric assay (22) was used to determine final PEG2000-PE concentration of the micellar formulation. Final protein content was detected using micro BCA™ protein assay kit (Pierce, Rockford, IL).
2.3. Size distribution, morphology and stability of micellar preparations
Micellar size was detected using the dynamic light scattering with a Beckman Coulter N4 Plus Particle Analyzer (Beckman Coulter, Fullerton, CA) in triplicate. The morphology of MCF-7 targeted phage-micelles was examined by transmission electronic microscopy according to the protocol described previously (11). For colloidal stability assessment, micellar sizes at 4°C were determined at predetermined times.
2.4. Zeta potential of micellar preparations
Zeta-potential was analyzed with a ZetaPLUS apparatus (Brookhaven, Holtsville, NY) in triplicate.
2.5. Cell lines
MCF-7 cells (ATCC® Number: HTB-22™) or C166 cells (ATCC® Number: CRL-2581™) were purchased from ATCC (Manassas, VA). No authentication was done by the authors.
2.6. Cytotoxicity
Target MCF-7 cells or non-target C166 cells were seeded into 96-well microplates at a density of 5×103 cells/well or 3.5×103cells/well and cultured for overnight. Cells were treated with varying concentrations of free PCT in DMSO, PCT-loaded non targeted micelles and PCT-loaded micelles modified with MCF-7-targeted phage protein (PCT-loaded MCF-7-targeted phage-micelles) and drug-free MCF-7-targeted phage-micelles for 48h, followed by the CellTiter-Blue® assay. The fluorescence intensity was measured using a multi-detection microplate reader (Bio-Tek, Winooski, VT) with 525/590 nm excitation/emission wavelengths.
2.7. In vitro assay for apoptosis and necrosis
Annexin V conjugates and 7-aminoactinomycin D (7-ADD) were used to determine the level of apoptosis and necrosis induced by non-targeted PCT micelles and MCF-7-targeted PCT phage micelles according to the manufacturer's protocol (Annexin V Conjugates for Apoptosis Detection kits, Invitrogen). Following the treatment with micellar PCT at a concentration of 500ng/ml for 48h or 72h, cells were harvested and diluted in Annexin-binding buffer to ∼1 × 106 cells/mL. A cell suspension of 100 μL was stained with 8 μL of the Annexin V conjugate and 10 μL of 7-ADD for 15min at room temperature followed by flow cytometric analysis.
2.8. In vitro assay for cell proliferation
Using immunofluorescent staining of the incorporated bromodeoxyuridine (BrdU) followed by flow cytometric analysis, the effect of micellar PCT on the proliferation of targeted MCF-7 cells was determined using the manufacture's protocol (BD Pharmingen™BrdU Flow Kits, BD Biosciences). After 24h treatment with 500ng/ml non-targeted- or targeted PCT micelles, MCF-7 cells were labeled with 10 μM BrdU for 30min, followed by immunofluorescent staining of incorporated BrdU coupled with 7-ADD staining for total DNA content. Both cells with active DNA synthesis (BrdU incorporation) and their cell cycle position (7-AAD staining intensities) were determined using two-color flow cytometry.
2.9. MCF-7 tumor xenograft model
Female, 6-8weeks nu/nu (athymic) mice (Charles River Laboratories, Wilmington, MA) were housed and kept on a 12:12 light: dark cycle in sterilized cages with ad libitum access to sterile food and water. All animal treatments were carried out in accordance with the guidelines of Northeastern University's IACUC.
To establish and maintain the estrogen-responsive MCF-7 tumor in vivo, estradiol-containing silastic implants were house-made as described previously (23) and inserted subcutaneously over the dorsal thorax through a 5.0 mm incision in mice anesthetized with an i.p. injection of ketamine plus xylazine. The incision was closed with 3-0 non-absorbable surgical sutures. Animals were allowed to recover for at least 48 h before tumor cell implantation.
For the development of subcutaneous MCF-7 tumor xenografts, 2 × 106 MCF-7 cells were re-suspended in 100 μL of 10% serum-supplemented MEM mixed with 100 μL of Matrigel HC (BD Biosciences, San Jose, CA) and injected subcutaneously into the left flank of the lightly anesthetized mice (isoflurane) with a 23 gauge needle.
2.10. Treatment
Groups of MCF-7 tumor-bearing mice (n=5) were treated when tumors reached an estimated mean volume of 250 mm3. Mice were randomly assigned to experimental groups. Tail vein injections with drug formulations at a total PCT dosage of 40 mg/kg were divided into eight doses given on day 0, +2, +4, +6, +8, +10, +12 and +14.
2.11. Monitoring and evaluation
In addition to daily monitoring for the signs of morbidity and mortality, tumor volumes and body weights were determined three times per week. The tumor volume for each mouse was estimated with calipers and calculated using the formula: tumor volume by caliper (mm3) = [length × (width) 2]/2. Antitumor activity was assessed with parameters including the tumor volume from day 0 of treatment (%) and tumor growth inhibition (%). These parameters are defined as below.
-
Tumor volume from day 0 of treatment (%)
= (Tumor volume at day after treatment / Tumor volume at treatment day 0) ×100.
-
Tumor growth inhibition (%)
= [(Tumor volume in untreated control group - Tumor volume in treated group) at Endpoint/ (Tumor volume in untreated control group) at Endpoint] ×100.
2.12. Tumor volume estimate by MRI
At the end of experiments, MCF-7 xenografts were anesthetized using 2% isoflurane. The animals were scanned on a 7 T preclinical MRI system (BioSpec 70/20 USR, Bruker BioSpin Corp, Billerica, MA). After a pilot scan to define the region of interest, a multislice T1 RARE spin-echo sequence was used with repetition time (TR) of 1738 ms, echo time (TE) of 10ms, and slice thickness of 1mm. Tumor volume was estimated using ITK-SNAP (version 2.4.0).
2.13. Harvest of blood and tissues
At the end of experiments, blood was collected from anesthetized mice via the retro-orbital plexus into a heparinized (1 U) microcentrifuge tube. Plasma was stored at -80 °C until use. Tumors and vital organs including heart, liver, kidney, and spleen were harvested, weighted and formalin-fixed.
2.14. Hematoxylin & eosin (H&E) staining
H&E staining was carried out for the identification of tumor necrosis and for the examination of histological changes in vital organs following the treatment. Briefly, formalin-fixed paraffin embedded tissue- or tumor samples (5μm) were stained with hematoxylin solution followed by rinsing in running tap water and then re-stained with eosin Y, dehydrated, cleared and slide-mounted. The slides were visualized by light microscopy at either a 20 × (tumor), 100× (tumor and spleen) or 200 × magnifications (liver, kidney and heart).
2.15. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining
A TUNEL assay to assess apoptosis was carried out according to manufacturer's protocol (FragELTM DNA Fragmentation Detection Kits, Calbiochem, Billerica, MA). Before staining, paraffin-embedding tumor sections (5μm) mounted on glass slides were deparaffined, rehydrated, permeated with 20μg/ml of proteinase K solution and treated with 0.3% H2O2 for inactivation of endogenous peroxidase. The tumor sections were subjected to treatment with terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling reaction mixture at 37 °C for 1.5h. After the reaction termination with a stop solution, the detection was performed using diaminobenzidine (DAB) solution after incubation with blocking buffer and horseradish peroxidase (HRP) conjugate. The slides were then counterstained with methyl green and visualized by microscopy at a 400× magnification. Cells with nuclear labeling with brown-color were considered TUNEL- positive. The apoptotic index was defined as the percentage of positive nuclei of a total of 2000 ± 100 tumor cells by manual counting.
2.16. Immunohistochemical staining for proliferation marker Ki-67 antigens
Before staining, paraffin-embedding tumor sections (5μm) were mounted on glass slides. They were subjected to a series of treatment, including depraffination, rehydration, heat-induced antigen retrieval, incubation in a blocking buffer containing 10% FBS and 1% BSA and inhibition of endogenous peroxidase activity with 0.3% H2O2. Sections were incubated overnight with rabbit anti-Ki-67 antibody (1:100) (Enzo Life Sciences, Inc, Farmingdale, NY) at 4 °C in a humidified chamber, followed by incubation with an anti-rabbit secondary antibody (1:500) (Cell Signaling, Inc, MA, USA) at room temperature for 30 min. Sections were then stained with DAB for 15min, rinsed with tap water, counterstained with hematoxylin, and dehydrated with ethanol and xylene. The slides were visualized by microscopy (Nikon, Japan) at a 400× magnification. Cells with nuclear staining with brown color were considered Ki67-positive. The proliferation index was defined as the percentage of positive nuclei of a total of 2000 ± 100 tumor cells by manual counting.
2.17. Quantitative analysis of tumor-associated PCT
Micellar paclitaxel were intravenously injected into MCF-7 tumor-bearing mice (n=3) at a single dose of 7.5mg/kg PCT. After mice were euthanized at 6h post-injection, tumors and tissues, including livers and spleens, were harvested and homogenized in PBS. The resultant homogenates were deproteinized and liquid–liquid extracted with t- butylmethylether. The dried tumor- and tissue extracts were reconstituted and analyzed for PCT by a reversed phase HPLC using the method described in section 2.2. The selective delivery of PCT was evaluated using PCT Tumor-to-RES ratio, which was defined as tumor-associated PCT divided by the sum of PCT associated with both liver and spleen.
2.18. Quantitation of plasma alanine transaminase (ALT) and aspartate aminotransferase (AST) activity
Plasma ALT and AST activity were determined using assay kits according to the manufacturer's protocol (Alanine Transaminase Activity Assay Kit and Aspartate Aminotransaminase Activity Assay Kit from the Biomedical Research Center, State of University of New York at Buffalo).
2.19. Statistical analysis
Results were expressed as mean ± SEM or mean ± SD. The statistical significance of results was analyzed using SPSS (version 18). Differences among the means of experimental treatment groups were compared using one-way ANOVA followed by a LSD post hoc test. A p-value of < 0.05 was considered statistically significant.
3. Results
3.1. Characterization of PCT micelles
Given its amphiphilic property, it was hypothesized that the MCF-7-specific landscape phage fusion protein would self-assemble with PEG2000-PE conjugate to produce micellar nanoparticles. Dynamic light scatter analysis showed a narrow size distribution of this nanopreparation with a size range from 8nm to 20nm (Figure 1A), comparable to the size distribution of non-targeted PCT micelles (Figure 1B). TEM image revealed the spherical morphology of the targeted micellar nanoparticles with a uniform size distribution (Figure 1C). The final micellar formulation contained 15.5 ± 0.3 mM PEG2000- PE and 190 ± 17.2 μg/ml phage fusion protein. The encapsulation efficiency of PCT was 66.7 ± 18.1% for non-targeted PCT micelles and 71.8 ± 15.4 % for targeted PCT phage- micelles (mean ± SD, n=3). Both MCF-7-targeted phage-micelles and non-targeted micelles had a negative charge (Figure 1D). The phage-micelle formulations also showed colloidal stability (Figure 1E).
Figure 1. Characterization of micellar paclitaxel (PCT).
Dynamic light scattering analysis of size distribution of MCF-7-targeted PCT phage micelles (A) and non-targeted PCT micelles (B); (C) TEM image of the targeted phage micelles; (D) Zeta-potential; (E) Storage stability at 4°C.
3.2. In vitro evaluation of antitumor activity
3.2.1. Cytotoxicity
Cytotoxicity study for 48h showed a significantly higher potency of MCF-7-targeted PCT phage-micelles in induction of MCF-7 tumor cell death compared to controls, including free PCT formulation and non-targeted PCT micelles at the concentration tested (Figure 2A), but this effect was absent with non-target, non-cancer C166 cells (Figure 2B). While the control free PCT in DMSO and non-targeted PCT micelles caused ∼15% MCF-7 cell killing, the MCF-7 targeted PCT phage-micelles significantly increased MCF-7 cell death by ∼36% at the same PCT concentration of 2.9 nM (Figure 2A). In the case of control non-target C166 cells, no significantly different cytotoxicity was observed between free PCT, non-targeted PCT micelles and MCF-7-targeted phage-micelles at the same concentration of PCT tested (Figure 2B). At the concentration tested, the drug-free targeted phage micelles had ignorable toxicity to MCF-7 cells (Figure 2A) and less than 10% C166 cell killing (Figure2 B). These results were in consistent with cytotoxicity for 72h treatment (11), suggesting the selective cytotoxicity of MCF targeted phage-micelle towards target cells rather than non-target, non-cancer cells.
Figure 2. In vitro antitumor activity of MCF-7 targeted PCT phage micelles.
Following the treatment with targeted and non-targeted micellar PCT, (A-B) cytotoxicity of MCF-7 cells and of C166 cells; (C) FACS analysis of Annexin V conjugates and 7-ADD co-staining MCF-7 cells; (D) FACS analysis of immunofluorescent staining of MCF-7 cells with BrdU incorporation coupled with 7-ADD staining for the determination of cells with active DNA synthesis and cell cycle position. Mean ± SEM, n= 3, p<0.05.
3. 2. 2. Enhanced in vitro apoptosis with MCF-7-targeted PCT phage-micelles
Flow cytometric analysis of Annexin V conjugates and 7-ADD co-staining of MCF-7 cells allowed us to differentiate and quantify the early and the late apoptotic cell populations following the treatment of non-targeted PCT micelles and MCF-7-targeted PCT phage-micelles. The scatter plots (Figure 2C) were divided into four regions to differentiate cell populations, including the viable cells which appeared in a lower left region with a lower fluorescence intensity staining by both Annexin V conjugates and 7- ADD; the early apoptosis cells presented in a lower right region with high fluorescence intensity staining of Annexin V conjugates and low fluorescence intensity of 7-ADD staining, and the late apoptosis cells located in upper right region with high fluorescence intensity staining of both Annexin V conjugates and 7-ADD. While the majority of untreated cells remained viable, both micellar PCT preparations triggered a reduction in the number of viable cells concomitant with an increased number of apoptotic cells after 48h treatment. Compared to untreated cells, the late apoptotic cells were increased by 51- fold when the targeted formulation was used versus 35-fold in case of the non-targeted one, and the early apoptotic cells were increased by 12.4-fold by the targeted formulation versus 8.4-fold by the non-targeted one. The total percent of apoptotic cells (including both the early and the late apoptosis) was 56 % with the targeted formulation versus 38.5% with the non-targeted one (Figure 2C). The enhanced in vitro apoptosis by MCF-7-targeted PCT phage-micelles was observed for 72h treatment as well.
3.2.3. Enhanced in vitro anti-proliferation with MCF-7-targeted PCT phage-micelles
Flow cytometric analysis of the MCF-7 cell population profiles for DNA content and BrdU labeling following exposure to micellar PCT clearly showed that a dramatic decrease in the number of MCF-7 cells actively synthesizing DNA (S phase) was accompanying by a significant increase in the percentage of cells in the G2/M cell cycle. Compared to untreated MCF-7 cells, treatment with MCF-7-targeted PCT phage-micelles inhibited the incorporation of BrdU by over 4-fold versus 3-fold with the non-targeted formulation, which is indicative of enhanced anti-proliferation induced by the MCF-7-targeted PCT phage-micelles (Figure 2D).
3.3. In vivo evaluation of antitumor efficacy
To assess the in vivo antitumor efficacy of micellar PCT formulations, subcutaneous MCF-7 tumor xenografts were developed followed by the initiation of treatment at a mean tumor volume of 250 mm3. While untreated mice continued tumor growth within the 35-day observation period, tumor-bearing mice had the strongest treatment response with MCF-7-targeted PCT phage-micelles. A faster and greater tumor remission was seen as early as 3-5 days after the treatment's initiation. This MCF-7- targeted therapy triggered a mean tumor volume decrease of ∼20% between day 3 and 5 and ∼ 34% between days 9 and 12 (p<0.005, compared to untreated mice; p<0.05, compared to non-targeted formulation). Even within the period of time after therapy's discontinuation between day 14 and 35, the antitumor activity remained. The tumor volume reduction remained at ∼ 40% between days 19 and 24 and up to 37% between days 28 and 35 (p<0.005, compared to untreated mice) (Figure 3A). Control, non-targeted PCT micelles led to a slight tumor reduction with a tumor volume decrease of 5 to 10% between days 3 and 12 (p<0.05, compared to targeted PCT phage-micelles) (Figure 3A). Discontinuation of the non-targeted therapy was accompanied by an increase of tumor volume to its initial pretreatment tumor volume. Five-weeks after initiation of treatment, average tumor weight in the MCF-7-targeted phage-Doxil group was 55 ± 2 mg versus 111 ± 18mg in the untreated group (p<0.005), and 87 ± 8 mg in non-targeted PCT group (p<0.05) (Figure 3B).
Figure 3. In vivo antitumor activity following the treatment with micellar PCT in MCF-7 tumor-bearing nude mice.
(A) Estimation of tumor volume using a caliper. Tumor volume from day 0 of treatment (%) = [(Tumor volume at days after treatment) / (Tumor volume at treatment day 0)] ×100; (B) Final tumor weight; (C) A representative set of regular MR images of tumors untreated and treated with non-targeted PCT micelles and MCF-7 targeted PCT phage-micelles at the endpoint of treatment; (D) Estimation of tumor volume using MR images; (E) Tumor growth inhibition (%) estimated by MRI, defined as the difference between the tumor volume of the untreated group and the tumor volume of the treated group divided by the tumor volume in untreated group ×100. Mean ± SEM, n= 5 * p<0.05 or p<0.005.
We also used magnetic resonance imaging (MRI) to independently assess tumor volume. A representative set of mouse MR images and 3D reconstitution of tumors in each group are shown (Figure 3C). In comparison with the groups of untreated mice and mice treated with non-targeted PCT micelles, the treatment with MCF-7-targeted phage-micelles was associated with the smallest tumor sizes and a corresponding increased tumor growth inhibition (Figure 3 D-E). The targeted PCT phage-micelle treatment effect was clearly greater than with the PCT-loaded micelles in the non-targeted formulation (p<0.05).
3.4. Ex vivo evaluation of antitumor activity
3.4.1. Enhanced necrosis by MCF-7-targeted PCT phage-micelle
The in vivo antitumor activity was further verified with the use of H&E staining of tumor sections. Notably, the tumor sections treated with MCF-7-targeted PCT phage-micelles had rare live tumor cells surrounding an extensive necrotic center (Figure 4A). Conversely, the majority of cells in untreated tumors as well as those treated with the non-targeted formulation remained viable. The high magnification images revealed only a limited increase in the necrotic area by non-targeted PCT micelles when compared to untreated tumors.
Figure 4. Ex vivo antitumor activity following the treatment with micellar PCT in MCF-7 tumor-bearing nude mice.
(A) Representative images of H & E staining of tumor sections. Necrotic cells showing eosinophilic cytosol (pink) accompanied by the absence of hemotoxylin-stained nuclei (blue); viable cells showing eosinophilic cytosol (pink) accompanied by hemotoxylin-stained nuclei (blue); (B) Representative images of tumor sections with TUNEL staining, showing enhanced in vivo apoptosis by the treatment with MCF-7-targeted PCT phage-micelles; (C) Apoptosis index (%), defined as the percentage of TUNEL- positive nuclei of a total of 2000 ± 100 tumor cells by manual counting; (D) Representative images of tumor sections with immunohistochemical staining for the Ki-67 proliferation marker antigens, showing enhanced anti-proliferation by the treatment with MCF-7-targeted PCT- phage-micelles; (E) Proliferation Index (%) defined as the percentage of Ki67-positive nuclei of a total of 2000 ±100 tumor cells by manual counting. Mean ± SEM, n= 5, p<0.05 or p<0.001.
3.4.2. Enhanced apoptosis by MCF-7-targeted PCT phage-micelle
The in vivo antitumor activity was also confirmed by a TUNEL assay to identify apoptosis in tumor sections. Although spontaneously-occurring apoptotic cells were rarely seen in the untreated tumors, apoptosis became extensive in drug-treated groups (Figure 4B). Notably, treatment with MCF-7-targeted PCT phage-micelles produced highest level of apoptotic cells. The mean apoptosis index was 6.3 ± 1.5 with targeted therapy versus 2.5 ±1.3 with non-targeted PCT micelles and 0.9 ± 0.5 in untreated tumors (Figure 4C).
3.4. 3. Reduced proliferation by the treatment with MCF-7-targeted PCT phage-micelles
Using antibody against the Ki67 tumor proliferation biomarker, the presence of proliferating tumor cells was indicated by brown-color staining nuclei in tumor sections (Figure 4D). Untreated tumors had quantitatively the highest proliferation index. MCF-7 targeted phage-micelles inhibited the expression of Ki67 proliferation-associated antigen in tumor tissues (Figure 4E).
3.5. Improved drug tumor delivery by MCF-7-targeted PCT phage-micelles
To investigate whether or not the enhanced antitumor activity was attributable to improved tumor delivery of PCT, we analyzed for tumor-associated paclitaxel at 6h post-injection. The quantitative results showed that the targeted phage micelles delivered 1.8- fold more paclitaxel to tumors than non-targeted formulations (p<0.05) (Figure 5A). Also, the tumor-selective delivery of PCT was improved by the targeted therapy with a Tumor-to-RES organ ratio of 0.42 compared to a ratio of 0.30 for non-targeted PCT (p<0.05) (Figure 5B).
Figure 5. Enhanced tumor delivery of PCT by MCF-7-targeted PCT phage-micelles.
(A) Quantification of tumor-associated PCT expressed as ng PCT per g of tumor tissue; (B) PCT Tumor-to-RES ratio. A one-way ANOVA was followed by LSD post hoc tests to analyze the statistic. Mean ± SEM, n= 3, p<0.05.
3.6. Assessment of potential toxicity of MCF-7-targeted PCT phage-micelles
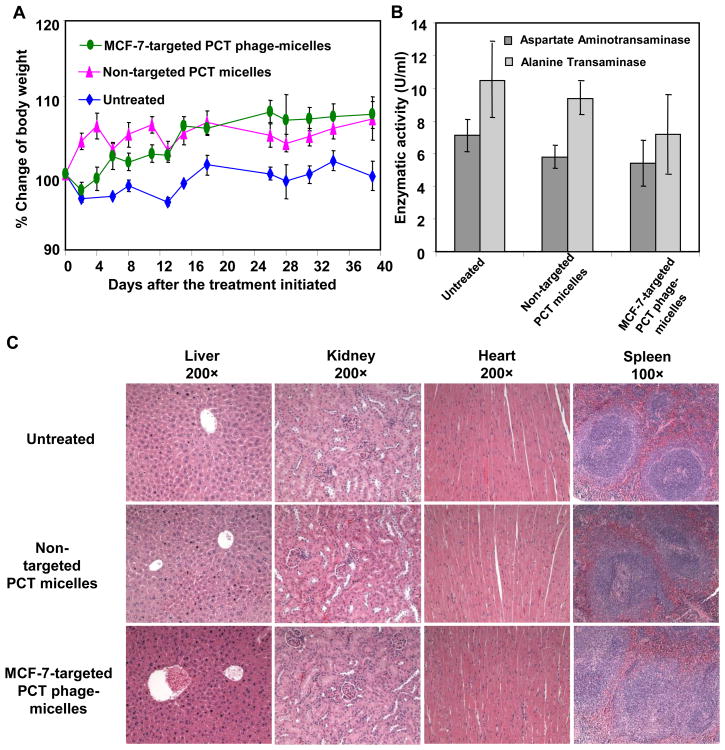
The potential side-effects associated with the treatment with MCF-7-targeted phage micelles, was investigated. Neither untreated nor drug-treated mice had abnormalities in body weight (Figure 6A), appetite or behavior. The treatment also produced no apparent hepatotoxicity based on the activity of plasma ALT and AST (Figure 6B). No pathological changes based on the tissue sections of liver, heart, kidney, lung and spleen were detected as well (Figure 6C).
Figure 6. Evaluation of potential side-effects of micellar PCT.
(A) Mouse body weight following treatment; (B) The effect of treatment on liver enzyme activity of mice; (C) Histological examination of tissue sections of vital organs following the treatment. Mean ± SEM, n=3.
4. Discussion
To meet the need for tumor-targeted delivery of hydrophobic neoplastic agents, we have combined a polymeric micelle-based drug delivery system and a phage-derived fusion protein specific for MCF-7 breast cancer cells as a targeting ligand. Polymeric micelles represent an efficient system for the delivery of a broad variety of hydrophobic drugs (5, 6, 23, 24). Loading such drugs into the hydrophobic micellar core dramatically increases a drug's solubility and bioavailability (5, 6), protects them from various destructive factors after parenteral administration, and improves their pharmacokinetics and biodistribution. Their nanometer-size (typically, between 5 and 50 nm) allow micellar drugs to passively target tumor sites (5) via the enhanced permeability and retention (EPR) effect (25). The attachment of targeting moieties onto the micelle surface can potentate the efficacy of micelle-loaded antitumor agents (26). Indeed, the MCF-7-targeted PCT-loaded phage-micelles preferentially bind to target cells compared to non-target cells (11, 27) and accordingly induce a higher cytotoxicity, apoptosis and anti-proliferative activity of MCF-7 cells than non-targeted formulations in vitro. Animal studies further validated the enhanced system's antitumor activity without noticeable toxicity induced by the targeted formulations.
One of mechanisms of paclitaxel induction of tumor cells death is the activation of an apoptotic cascade (28, 29). PCT loaded in the micellar nanocarriers maintains its pharmacologic activity and promotes MCF-7 breast cancer cell apoptosis. The treatment with micellar paclitaxel had a significant increase in the number of Annexin V- positive staining cells in vitro and of TUNEL-positive staining cells in vivo. Notably, the apoptotic effect was more pronounced when PCT was loaded into micelles modified with MCF-7-specific phage protein. In addition to apoptosis, necrosis may also be an important cause leading to cancer cell death in the presence of PCT. The remarkable necrosis occurring in tumors treated with MCF-7-targeted phage micelles further demonstrates its antitumor activity.
PCT has been shown to stabilize microtubules against depolymerization (29, 30) and arrest cell within the mitotic phase of the cell cycle, leading to inhibition of functional cell division and of cell proliferation activity (31). When BrdU and 7-ADD were used to co-stain the MCF-7 cells treated with micellar PCT, the phenomena of mitotic arrest were clearly seen concurrently, reducing the number of cells actively synthesizing DNA (S phase) in vitro. The enhanced anti-proliferative activity by the targeted therapy was also verified in an in vivo setting: a decrease in the tumor Ki67 labeling index generally reflects a reduced growth fraction of the tumor cells (29, 32). Furthermore, these measures of proliferation may also serve as indicators for survival prediction. Their predictive value on survival is considered better than other established prognostic factors such as tumor size, histological grade and number of involved lymph nodes (29).
The net balance of enhanced apoptosis together with the reduced proliferation and the extensive tumor necrosis consequently produced a significant cytotoxicity in vitro and tumor reduction in vivo, which is consistent with previous reports (33-36). This enhanced antitumor activity was correlated with improved tumor drug delivery. In fact, this phage-targeted vehicle has demonstrated enhanced binding affinity and specificity toward targeted MCF-7 cells compared to normal cells in vitro (11, 27) and has shown improved tumor selective delivery in vivo.
In addition to effectiveness as an anticancer agent, the absence of generalized toxicity is indispensable for a viable drug delivery system in translational applications. The use of MCF-7-targeted PCT phage-micelles was unaccompanied by signs of toxicity based on the absence of detectable hepatotoxicity and pathological change associated with vital organs, together with maintenance of apparent overall health and a lack of behavioral deficits in the mice during the treatment period. Further assessment of the potential immunogenicity would be our ongoing effort.
In sum, to generate this targeted therapy we exploited the amphiphilic character of the MCF-7 cancer cell-specific phage protein to prepare targeted phage-micelles for the delivery of water-insoluble antineoplastic drugs to tumors. The targeted phage-micelles produced a dramatic tumor reduction as well as extensive apoptosis and necrosis as a result of the improved tumor delivery of paclitaxel. These results are indicative of its translational potential with an efficient and safe nanomedicine for improved breast cancer treatment.
Acknowledgments
Financial Information: This work was supported by NIH grant # R01 CA125063-01 and the Animal Health and Disease Research grant 2006-9, College of Veterinary Medicine, Auburn University to V.A. Petrenko and by NIH grant #1U54CA151881 to V. P. Torchilin.
An Abbreviations List
- PCT
paclitaxel
- 7-ADD
7-aminoactinomycin D
- BrdU
bromodeoxyuridine
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
- DAB
diaminobenzidine
- HRP
horseradish peroxidase
- ALT
alanine transaminase
- AST
aspartate aminotransferase
- H&E
hematoxylin & eosin
References
- 1.Lammers T, Hennink WE, Storm G. Tumour-targeted nanomedicines: principles and practice. Br J Cancer. 2008;99:392–7. doi: 10.1038/sj.bjc.6604483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hennenfent KL, Govindan R. Novel formulations of taxanes: a review. Old wine in a new bottle? Ann Oncol. 2006;17:735–49. doi: 10.1093/annonc/mdj100. [DOI] [PubMed] [Google Scholar]
- 3.Shade RJ, Pisters KM, Huber MH, Fossella F, Perez-Soler R, Shin DM, et al. Phase I study of paclitaxel administered by ten-day continuous infusion. Invest New Drugs. 1998;16:237–43. doi: 10.1023/a:1006157226693. [DOI] [PubMed] [Google Scholar]
- 4.Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235:179–92. doi: 10.1016/s0378-5173(01)00986-3. [DOI] [PubMed] [Google Scholar]
- 5.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 6.Sutton D, Nasongkla N, Blanco E, Gao J. Functionalized micellar systems for cancer targeted drug delivery. Pharm Res. 2007;24:1029–46. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- 7.Danson S, Ferry D, Alakhov V, Margison J, Kerr D, Jowle D, et al. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br J Cancer. 2004;90:2085–91. doi: 10.1038/sj.bjc.6601856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumura Y, Hamaguchi T, Ura T, Muro K, Yamada Y, Shimada Y, et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br J Cancer. 2004;91:1775–81. doi: 10.1038/sj.bjc.6602204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004;10:3708–16. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 10.Torchilin VP. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. Aaps J. 2007;9:E128–47. doi: 10.1208/aapsj0902015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T, Petrenko VA, Torchilin VP. Paclitaxel-loaded polymeric micelles modified with MCF-7 cell-specific phage protein: enhanced binding to target cancer cells and increased cytotoxicity. Mol Pharm. 2010;7:1007–14. doi: 10.1021/mp1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krumpe LR, Mori T. The Use of Phage-Displayed Peptide Libraries to Develop Tumor-Targeting Drugs. Int J Pept Res Ther. 2006;12:79–91. doi: 10.1007/s10989-005-9002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holig P, Bach M, Volkel T, Nahde T, Hoffmann S, Muller R, et al. Novel RGD lipopeptides for the targeting of liposomes to integrin-expressing endothelial and melanoma cells. Protein Eng Des Sel. 2004;17:433–41. doi: 10.1093/protein/gzh055. [DOI] [PubMed] [Google Scholar]
- 14.Petrenko V. Evolution of phage display: from bioactive peptides to bioselective nanomaterials. Expert Opin Drug Deliv. 2008;5:825–36. doi: 10.1517/17425247.5.8.825. [DOI] [PubMed] [Google Scholar]
- 15.Kuzmicheva GA, Jayanna PK, Sorokulova IB, Petrenko VA. Diversity and censoring of landscape phage libraries. Protein Eng Des Sel. 2009;22:9–18. doi: 10.1093/protein/gzn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T, Kulkarni N, Bedi D, D'Souza GG, Papahadjopoulos-Sternberg B, Petrenko VA, et al. In vitro optimization of liposomal nanocarriers prepared from breast tumor cell specific phage fusion protein. J Drug Target. 2011;19:597–605. doi: 10.3109/1061186X.2010.550920. [DOI] [PubMed] [Google Scholar]
- 17.Wang T, D'Souza GG, Bedi D, Fagbohun OA, Potturi LP, Papahadjopoulos-Sternberg B, et al. Enhanced binding and killing of target tumor cells by drug-loaded liposomes modified with tumor-specific phage fusion coat protein. Nanomedicine. 2010;5:563–74. doi: 10.2217/nnm.10.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Kulkarni N, D'Souza GG, Petrenko VA, Torchilin VP. On the mechanism of targeting of phage fusion protein-modified nanocarriers: only the binding peptide sequence matters. Mol Pharm. 2011;8:1720–8. doi: 10.1021/mp200080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayanna PK, Bedi D, Gillespie JW, DeInnocentes P, Wang T, Torchilin VP, et al. Landscape phage fusion protein-mediated targeting of nanomedicines enhances their prostate tumor cell association and cytotoxic efficiency. Nanomedicine. 2010;6:538–46. doi: 10.1016/j.nano.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang T, Yang S, Petrenko VA, Torchilin VP. Cytoplasmic delivery of liposomes into MCF-7 breast cancer cells mediated by cell-specific phage fusion coat protein. Mol Pharm. 2010;7:1149–58. doi: 10.1021/mp1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T, Hartner WC, Gillespie JW, Praveen KP, Yang S, Mei LA, et al. Enhanced Tumor Delivery and Antitumor Activity in Vivo of Liposomal Doxorubicin Modified with MCF-7-Specific Phage Fusion Protein. Nanomedicine. 2013 doi: 10.1016/j.nano.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nag A, Mitra G, Ghosh PC. A colorimetric assay for estimation of polyethylene glycol and polyethylene glycolated protein using ammonium ferrothiocyanate. Anal Biochem. 1996;237:224–31. doi: 10.1006/abio.1996.0233. [DOI] [PubMed] [Google Scholar]
- 23.Zhu L, Wang T, Perche F, Taigind A, Torchilin VP. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc Natl Acad Sci U S A. 2013;110:17047–52. doi: 10.1073/pnas.1304987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L, Perche F, Wang T, Torchilin VP. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials. 2014;35:4213–22. doi: 10.1016/j.biomaterials.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63:131–5. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci U S A. 2003;100:6039–44. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T, Petrenko V, Torchilin V. Optimization of Landscape Phage Fusion Protein-Modified Polymeric PEG-PE Micelles for Improved Breast Cancer Cell Targeting. J Nanomedic Nanotechnol. 2012;S4:008. doi: 10.4172/2157-7439.S4-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders DE, Lawrence WD, Christensen C, Wappler NL, Ruan H, Deppe G. Paclitaxel-induced apoptosis in MCF-7 breast-cancer cells. Int J Cancer. 1997;70:214–20. doi: 10.1002/(sici)1097-0215(19970117)70:2<214::aid-ijc13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 29.Cleator S, Parton M, Dowsett M. The biology of neoadjuvant chemotherapy for breast cancer. Endocr Relat Cancer. 2002;9:183–95. doi: 10.1677/erc.0.0090183. [DOI] [PubMed] [Google Scholar]
- 30.Olah E, Csokay B, Prajda N, Kote-Jarai Z, Yeh YA, Weber G. Molecular mechanisms in the antiproliferative action of taxol and tiazofurin. Anticancer Res. 1996;16:2469–77. [PubMed] [Google Scholar]
- 31.Symmans WF, Volm MD, Shapiro RL, Perkins AB, Kim AY, Demaria S, et al. Paclitaxel-induced apoptosis and mitotic arrest assessed by serial fine-needle aspiration: implications for early prediction of breast cancer response to neoadjuvant treatment. Clin Cancer Res. 2000;6:4610–7. [PubMed] [Google Scholar]
- 32.Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–64. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milross CG, Mason KA, Hunter NR, Chung WK, Peters LJ, Milas L. Relationship of mitotic arrest and apoptosis to antitumor effect of paclitaxel. J Natl Cancer Inst. 1996;88:1308–14. doi: 10.1093/jnci/88.18.1308. [DOI] [PubMed] [Google Scholar]
- 34.Archer CD, Parton M, Smith IE, Ellis PA, Salter J, Ashley S, et al. Early changes in apoptosis and proliferation following primary chemotherapy for breast cancer. Br J Cancer. 2003;89:1035–41. doi: 10.1038/sj.bjc.6601173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamm I, Schriever F, Dorken B. Apoptosis: implications of basic research for clinical oncology. Lancet Oncol. 2001;2:33–42. doi: 10.1016/S1470-2045(00)00193-5. [DOI] [PubMed] [Google Scholar]
- 36.Johnston SR, Boeddinghaus IM, Riddler S, Haynes BP, Hardcastle IR, Rowlands M, et al. Idoxifene antagonizes estradiol-dependent MCF-7 breast cancer xenograft growth through sustained induction of apoptosis. Cancer Res. 1999;59:3646–51. [PubMed] [Google Scholar]