Abstract
Phosphatidylserine (PS) is the major anionic phospholipid class particularly enriched in the inner leaflet of the plasma membrane in neural tissues. PS is synthesized from phosphatidylcholine or phosphatidylethanolamine by exchanging the base head group with serine in reactions are catalyzed by phosphatidylserine synthase 1 and phosphatidylserine synthase 2 located in the endoplasmic reticulum. Activation of Akt, Raf-1 and protein kinase C signaling, which supports neuronal survival and differentiation, requires interaction of these proteins with PS localized in the cytoplasmic leaflet of the plasma membrane. Furthermore, neurotransmitter release by exocytosis and a number of synaptic receptors and proteins are modulated by PS present in the neuronal membranes. Brain is highly enriched with docosahexaenoic acid (DHA), and brain PS has a high DHA content. By promoting PS synthesis, DHA can uniquely expand the PS pool in neuronal membranes and thereby influence PS-dependent signaling and protein function. Ethanol decreases DHA-promoted PS synthesis and accumulation in neurons, which may contribute to the deleterious effects of ethanol intake. Improvement of some memory functions has been observed in cognitively impaired subjects as a result of PS supplementation, but the mechanism is unclear.
Keywords: Docosahexaenoic acid, serine, signal transduction, membranes, neuron, cognition
1. Introduction
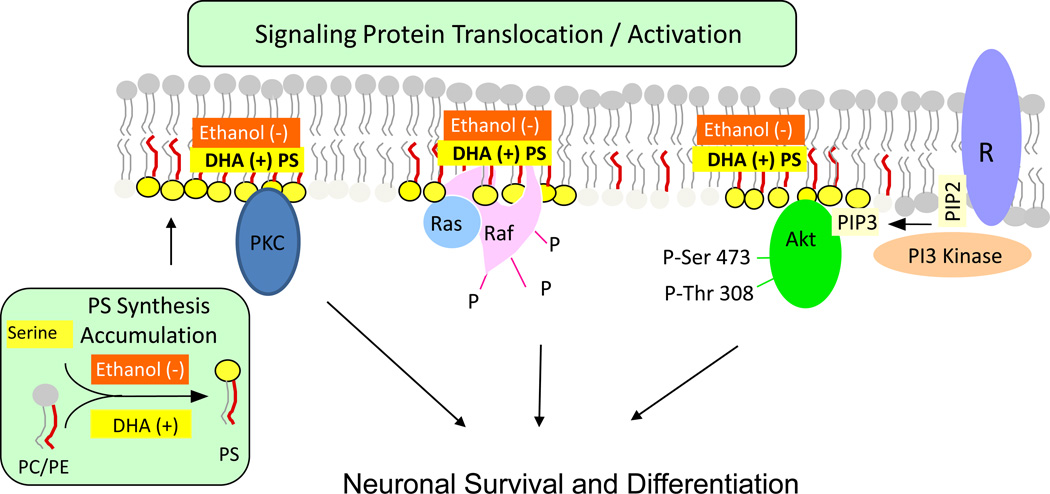
Phosphatidylserine (PS) is the major acidic phospholipid class that accounts for 13–15 % of the phospholipids in the human cerebral cortex [1]. In the plasma membrane, PS is localized exclusively in the cytoplasmic leaflet where it forms part of protein docking sites necessary for the activation of several key signaling pathways. These include the Akt, protein kinase C (PKC) and Raf-1 signaling that is known to stimulate neuronal survival, neurite growth and synaptogenesis [2–7]. Modulation of the PS level in the plasma membrane of neurons has significant impact on these signaling processes. The mechanism of PS-mediated activation of these neuronal signaling pathways is illustrated in Fig. 1.
Fig. 1.
Activation of neuronal signaling pathways facilitated by PS. Activation of Akt, protein kinase C and Raf-1 requires translocation from the cytosol to the cytoplasmic surface of the plasma membrane. Translocation is initiated by specific stimuli, for example, growth factor-dependent PIP3 generation from PIP2 by PI3 kinase in the case of Akt. Binding to the membrane occurs in part through an interaction of these proteins with PS present in anionic domains of the lipid bilayer, activating the signaling pathways leading to neuronal differentiation and survival. DHA facilitates this mechanism by increasing PS production in neurons, while ethanol has the opposite effect because it inhibits the DHA-induced increase in PS production. R: receptor
In the synapses, PS plays an important role in exocytosis by influencing Ca2+-dependent membrane fusion between synaptic vesicles and the target plasma membrane, which is mediated by synaptotagmin and soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex [8–11]. PS also modulates the AMPA glutamate receptor [12], interacts with synapsin I [13], and alters the conformation of the microtubule associated protein tau [14]. Furthermore, abnormal PS asymmetry in the synaptosomal membrane has been observed in mild cognitive impairment and Alzheimer’s disease [15]. The recent discovery of the critical role of PS in activating important signal transduction pathways and modulating neurotransmitter release and receptor function as well as implications in neuropathophysiology have renewed interest in PS in relation to brain function.
This review focuses on the metabolism and function of PS in the nervous system. Further details can be obtained from previous reviews dealing with PS function in the mammalian brain [16], cell and molecular biology involved in PS metabolism [17], the synthesis and intracellular transport of PS [18,19], the effects of docosahexaenoic acid (DHA) on neuronal PS function [5], the interrelationship between phosphatidylethanolamine (PE) and PS metabolism [20–22], and the effects of PS on membrane properties [23].
2. Phosphatidylserine synthesis in the brain
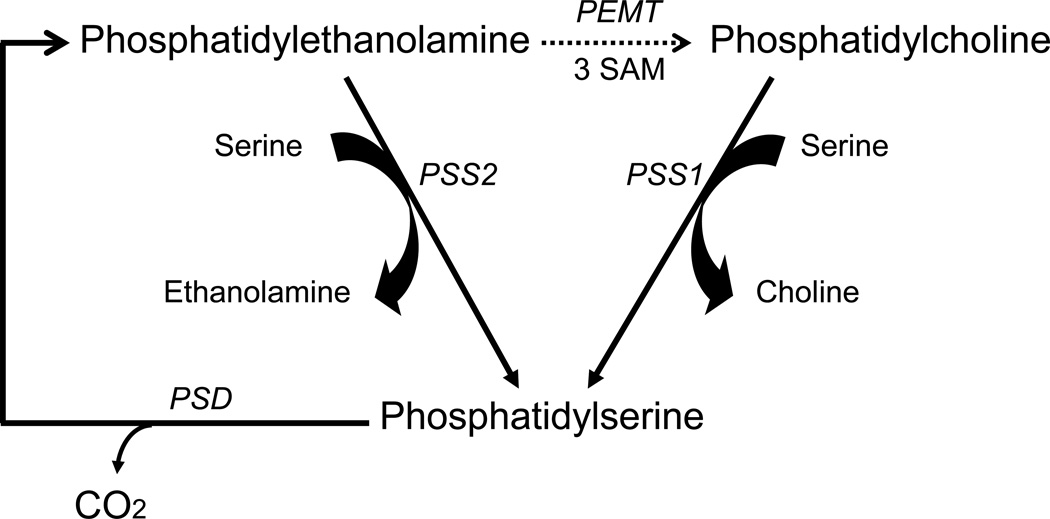
In mammalian tissues, PS is synthesized from either phosphatidylcholine (PC) or PE exclusively by Ca2+-dependent reactions where the head group of the substrate phospholipids is replaced by serine [20], as illustrated in Fig. 2. These base-exchange reactions are catalyzed by phosphatidylserine synthases (PSS) and so far two isoforms, PSS1 and PSS2 encoded by two separate genes, Pss1 and Pss2, respectively, have been identified. PSS1 utilizes PC as its substrate, and PSS2 utilizes PE. These enzymes are localized in the endoplasmic reticulum, particularly enriched in the mitochondria associated membrane regions of the endoplasmic reticulum [24].
Fig. 2.
PS synthesis and metabolism in the brain. PS is synthesized by replacement of the choline group of PC by serine in a reaction catalyzed by PSS1, and also by replacement of the ethanolamine group of PE by serine in a reaction catalyzed by PSS2. These synthetic reactions occur in the endoplasmic reticulum. PS is decarboxylated to PE in the mitochondria by PS decarboxylase (PSD). The phosphatidylethanolamine methyltransferase (PEMT) reaction that utilizes S-adenosylmethionine (SAM) to convert PE to PC is indicated as a dashed arrow because more recent findings have demonstrated that previously reported methylation activity in the brain [32,33] is quantitatively insignificant [35,36].
Together with testis and kidney, brain is one of the tissues that have high capacity to synthesize PS [25]. Also, the expression of PSS in the brain is among the highest. The serine base exchange enzymatic activities of rat cerebellar homogenates, cerebral cortical homogenates and cerebral cortical membranes were shown to be recovered in the insoluble floating fraction of TritonX-100 extracts, suggesting the localized presence of PS synthases in membrane lipid rafts [26,27]. Although intriguing, the possible contribution of microsomal contamination cannot be excluded. PS production is increased in cells of neuronal origin by compounds that trigger Ca2+ release, a finding consistent with the fact that PS synthesis is a Ca2+-dependent process [28].
The PC and PE substrates utilized for PS production can be de novo synthesized in microsomes by transfer of either phosphocholine or phosphoethanolamine from the respective cytidine diphosphate derivatives to 1,2-diacylglycerol [29]. PC synthesis is upregulated during neuronal differentiation. For example, substantial increases of PC were observed along with activation of two cytidine diphosphate-choline pathway enzymes, choline cytidylyltransferase and choline kinase when Neuro-2A or PC12 cells undergo differentiation and form neurites [30,31]. PC also can be synthesized by the phosphatidylethanolamine N-methyl transferase (PEMT) reaction through three sequential methylations of the ethanolamine head group of PE. Polyunsaturated PC species are synthesized largely by the PEMT pathway [32], suggesting that the PEMT reaction might be particularly important in regulating phospholipid molecular species composition where polyunsaturated species are abundant. Although PEMT was reported to be present in rat brain and bovine caudate nucleus, the activities detected were low [33,34]. Subsequent studies indicated that the PEMT pathway is negligible in neurons [35], and the only tissue where it is quantitatively significant is liver [36]. Nevertheless, the PEMT pathway appears to be important for normal hippocampal development, as indicated by the finding that Pemt knockout mice show more neuronal apoptosis and less hippocampal expression of calretinin, a marker of neuronal differentiation [37]. Considering the high level of polyunsaturated PS species in the brain, it is possible that the PC ultimately derived from the hepatic PEMT reaction may be an important substrate for neural PS synthesis catalyzed by PSS1.
2.1. Phosphatidyserine synthase 1 function
Although PSS1 expression is ubiquitous, studies with [3H]serine indicate that the brain has the highest specific activity for choline exchange which represents PSS1 activity. Purified human PSS1 can convert either PC or PE to PS in enzymatic assays in vitro, but PSS1 utilizes only PC in intact cells. An explanation for this difference may be selective phospholipid substrate availability in the membrane microdomains where PSS1 is localized. PC and PSS1 can provide sufficient PS to support neuronal differentiation, for the axon extension in cultured sympathetic neurons is not impaired by a PSS2 deficiency [38]. Furthermore, transactivation of the Pss1 promoter by the Sp and N-Myc transcription factors is high in neonatal brain, leading to higher PSS1 expression and activity as compared with other neonatal mouse tissues [39]. These findings indicate that PSS1 has an important role in PS synthesis in the developing brain. Primary cultures of cortical astrocytes have higher PSS1 activity than primary cortical neuron cultures, suggesting that astrocytes may be a major site of PS synthesis from PC in some brain regions [39].
The 1-stearoyl-2-docosahexanoyl (18:0, 22:6) PC molecular species is the preferred substrate for PS synthesis in cerebral cortical microsomes, and 18:0,22:6 is the most abundant PS species in the brain even though it is not an abundant brain PC species [5,6,40]. Although present in larger amounts, 1-palmitoyl-2-docosahexanoyl (16:0, 22:6) PC is not utilized efficiently by PSS1, and 16:0, 22:6 is a minor PS species in the brain. These results suggest that 18:0, 22:6-PC is particularly favored for the conversion to PS in the brain and provide additional evidence that PC is an important substrate for brain PS synthesis in vivo. Furthermore, MALDI-imaging mass spectrometry studies of mouse brain indicate that 18:0, 22:6-PC is selectively enriched in Purkinje neurons [41], suggesting that this substrate also may be an important source of PS in the cerebellum.
2.2. PSS2 function
Neurons obtained from neonatal mice contain relatively high levels of PSS2, indicating that PE also is an important source of neuronal PS during development. As opposed to PSS1, PSS2 utilizes only PE for PS synthesis under all conditions [42,43]. The tissue expression profile of PSS2 is different from that of PSS1. While PSS1 expression is ubiquitous, PSS2 is expressed highly in testis, brain and kidney [44], suggesting that PSS2 may have specialized roles in these tissues.
Recent studies with purified flag-tagged PSS2 demonstrate that the enzyme utilizes PE substrates containing either palmitate or stearate in the sn-1 position equally well. However, PSS2 prefers PE with DHA as opposed to either arachidonic acid or oleic acid (18:1n-9) in the sn-2 position. PSS2 isolated from a variety of cultured cell lines, as well as from microsomes of a Chinese hamster ovary cell mutant that lacks PSS1, also preferentially utilizes PE containing DHA in the sn-2 position. These findings, together with the higher expression and activity of PSS2 in the brain, suggest that PSS2 plays a key role in producing the high level of DHA-containing PS in the brain [44].
The serine base exchange activity has been observed in the Triton insoluble floating fractions from both rat cerebrocortex and cerebrocortical plasma membrane preparations [27]. The Triton insoluble fractions mainly converted PE to PS, indicating dominant presence of PSS2 activity in these membrane preparations. PKC also was present in the plasma membrane enriched fraction. Although this membrane fraction was enriched in Na/K-ATPase, it also contained 10% as much NADPH cytochrome c reductase activity as in the microsomes. Therefore, it is possible that the PSS activity detected in this cerebrocortical plasma membrane fraction was due to microsomal contamination rather than the enzyme actually being present in the plasma membrane. If the PSS2 activity is indeed enriched in the plasma membrane preparation, local PS synthesis at the cerebrocortical plasma membrane may have a significant role in modulating PKC signaling where PS binding is required.
2.3. Deletion of phosphatidylserine synthase genes
Deletion of both Pss1 and Pss2 causes embryonic lethality in mice, indicating that PS synthesis is an essential metabolic function [45]. When only one of these genes is deleted, PS synthesis is reduced, but sufficient quantities still are produced for normal development and most physiological functions. Deletion of Pss1 does not produce a phenotype, and the mice are viable, fertile and have a normal life span. In these Pss1-deleted mice, the serine base exchange activity is decreased by 67% and 85% in liver homogenate and microsomes, respectively, and the liver PS content is reduced. However, the PS content in the brain is not altered and axonal extension is normal [45]. The Pss2 deletion does not cause a reduction in the PS content in liver, testis and brain, or a decrease in neuronal axon extension [38]. The total serine head group exchange activity is reduced over 90% in the testis, liver and brain [44], but unchanged in hepatocytes [38]. Elevation of PSS1 activity without any change in PSS1 mRNA expression has been observed in some studies of Pss2-deficient mice [38]. The fact that deletion of either Pss1 or Pss2 genes does not affect the PS level in the brain suggests possible compensatory mechanisms at the biochemical level; less PS metabolism through decarboxylation or phospholipase reactions, and/or less regulation of PS synthesis by the remaining PSS in these mutants. The only abnormality resulting from deletion of Pss2 is infertility in some of the male mice [46].
3. Composition of brain phosphatidylserine
The PS content in human brain is maintained at the 13–14% level throughout the life [1]. As shown in Table 1 the synaptic plasma membrane, olfactory bulb and hippocampus of rats and mice contain markedly higher PS as compared to non-neuronal tissues such as liver and adrenal [38,47–50].
Table 1.
Phospholipid composition in rodent tissues
| Mol % | |||
|---|---|---|---|
| PS | PC | PE | |
| Rat brain synaptic plasma membrane | |||
| 15.2a | 43.5 | 36.2 | |
| 13.2b | 41.6 | 34.2 | |
| Rat hippocampus | 16.9 ± 0.9c | 37.2 ± 0.5 | 45.9 ± 1.1 |
| Rat olfactory bulb | 17.4 ± 0.9d | 49.4 ± 1.3 | 33.2 ± 1.8 |
| Rat liver | 3.1 ± 0.2d | 69.7 ± 5.6 | 27.2 ± 2.3 |
| Rat adrenal | 2.5 ± 0.2d | 62.6 ± 2.2 | 34.9 ± 2.2 |
| Mouse brain | 14.4 ± 0.8e | 32.7 ± 0.8 | 49.3 ± 0.6 |
| Mouse liver | 2.7 ± 0.2e | 55.3 ± 1.6 | 38.7 ± 1.5 |
The fatty acid composition of PS, and for comparison that of PE and PC, contained in the gray and white matter of human brain, is shown in Table 2 [1, 51]. There are substantial differences in the fatty acid composition in gray and white matter PS. Gray matter PS contains considerably more DHA and less 18:1n-9 than white matter. Appreciable differences also occur in the PE fatty acid composition in gray and white matter, whereas comparatively small differences occur in the PC composition. DHA accounts for more than one-third of the total fatty acid and 80% of the polyunsaturated fatty acid in gray matter PS. A substantial amount of DHA is present in gray matter PE, but only a relatively small amount is present in PC. Gray matter PS and PE contain considerably more 18:0 and much less 16:0 than PC. Arachidonic acid is present primarily in PE, and there is little arachidonic acid in PS. According to one study [51], PE also contains the largest amount of docosapentaenoic acid (22:5n- 6), an arachidonic acid-derived product. Only trace amounts of linoleic acid (18:2n-6) are present in brain PS, PE and PC.
Table 2.
Fatty acid composition of human brain glycerophosphatides
| Fatty acid | Fatty Acyl Composition (% of total fatty acid in each phospholipid class) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylserine | Phosphatidylethanolamine | Phosphatidylcholine | ||||||||||
| Gray matter | White matter | Gray matter | White matter | Gray matter | White matter | |||||||
| Aa | Bb | A | B | A | B | A | B | A | B | A | B | |
| 16:0 | 2.3 | 4.2 | 1.7 | 1.9 | 6.7 | 5.7 | 6.7 | 3.4 | 45.0 | 45.9 | 34.3 | 30.2 |
| 16:1 | 0.3 | 0.3 | 0.4 | 0.3 | 0.4 | 0.4 | 1.4 | 0.5 | 3.1 | 2.4 | 1.0 | 2.3 |
| 18:0 | 25.4 | 45.3 | 35.8 | 44.1 | 26.0 | 28.4 | 9.0 | 9.3 | 9.3 | 11.2 | 13.4 | 12.9 |
| 18:1 | 21.5 | 14.0 | 39.7 | 41.4 | 11.9 | 10.3 | 42.4 | 38.9 | 31.4 | 30.3 | 45.2 | 47.1 |
| 20:4 | 1.6 | 3.0 | 2.0 | 1.2 | 13.8 | 11.2 | 6.4 | 16.5 | 4.1 | 3.6 | 1.3 | 0.8 |
| 22:5n-6 | 5.0 | 0.7 | 4.8 | 0.1 | 14.3 | 1.2 | 13.7 | 0.7 | ndc | trd | nd | 0.2 |
| 22:5n-3 | 3.3 | 0.5 | 0.9 | 0.2 | tr | 1.1 | 0.5 | 0.9 | nd | tr | 0.3 | 0.3 |
| 22:6 | 36.6 | 23.2 | 5.6 | 1.3 | 24.3 | 30.5 | 3.4 | 8.6 | 3.1 | 2.5 | 0.1 | 0.2 |
According to the positional distribution of the main fatty acids in bovine brain gray matter PS, 18:0 and DHA represent the most abundant fatty acids at the sn-1 and sn-2 positions, respectively [52]. Likewise, 18:0 and DHA are highly enriched at the sn-1 and sn-2 positions of PE, respectively. In contrast, the sn-2 position of PC contains only minor level of DHA, and 16:0 is more abundant than 18:0 at the sn-1 position. The molecular species analysis in the mouse brain by mass spectrometry also confirms the relatively high concentration of 18:0 and DHA in PS as shown in Table 3. The most abundant PS molecular species is 18:0,22:6-PS, which varies from 38% in the cerebellum to 59% in the cortex. There is little 16:0,22:6-PS except in the olfactory bulb where it accounts for 22% of the PS. Three species that contain DHA are di-polyunsaturated, and together, they comprise 2 to 4% of the PS in these regions of the mouse brain.
Table 3.
PS molecular species distribution in mouse brain regionsa.
| % of the total PS molecular species in each of the brain regions | |||||||
|---|---|---|---|---|---|---|---|
| Cortex | Nucleus Accumbens |
Hippocampus (CA1) |
Hippocampus (CA3) |
Hypothalamus | Cerebellum | Olfactory Bulb |
|
| 16:0, 18:1 | 3.3 ± 0.3b | 3.4 ± 0.3 | 3.6 ± 0.5 | 3.7 ± 0.8 | 3.9 ± 0.7 | 5.0 ± 0.2 | 7.2 ± 1.2 |
| 16:0, 22:5 | 2.0 ± 0.4 | 1.3 ± 0.2 | 1.6 ± 1.0 | 1.7 ± 0.7 | 2.1 ± 0.9 | 2.5 ± 0.7 | 3.3 ± 1.2 |
| 16:0, 22:6 | 1.0 ± 2.1 | 0.0 ± 0.0 | 1.4 ± 1.6 | 0.4 ± 0.8 | 0.9 ± 1.9 | 1.1 ± 2.1 | 22.3 ± 3.2 |
| 18:0, 18:1 | 11.6 ± 3.2 | 21.0 ± 4.5 | 18.4 ± 4.2 | 23.8 ± 2.3 | 20.6 ± 4.9 | 25.5 ± 3.7 | 9.7 ± 1.5 |
| 18:0, 20:4 | 4.5 ± 0.8 | 5.4 ± 0.6 | 5.6 ± 0.7 | 7.1 ± 0.9 | 5.7 ± 0.8 | 5.3 ± 0.5 | 3.2 ± 0.8 |
| 18:0, 22:4 | 4.2 ± 1.0 | 6.4 ± 0.7 | 4.8 ± 1.8 | 5.4 ± 0.6 | 7.6 ± 1.7 | 2.5 ± 0.5 | 2.7 ± 0.3 |
| 18:0, 22:5 | 2.2 ± 0.4 | 3.0 ± 0.6 | 2.7 ± 0.9 | 2.7 ± 0.6 | 2.6 ± 0.7 | 1.7 ± 1.7 | 1.9 ± 0.2 |
| 18:0, 22:6 | 59.3 ± 5.8 | 50.1 ± 5.9 | 53.2 ± 6.1 | 43.6 ± 2.8 | 44.6 ± 5.9 | 38.3 ± 1.3 | 40.8 ± 2.3 |
| 18:1, 18:1 | 4.3 ± 0.7 | 4.0 ± 0.5 | 4.2 ± 1.0 | 6.4 ± 1.0 | 7.3 ± 1.6 | 11.6 ± 0.8 | 3.6 ± 0.5 |
| 18:1, 22:6 | 4.4 ± 2.1 | 3.1 ± 0.7 | 2.8 ± 0.9 | 2.8 ± 0.7 | 2.3 ± 0.1 | 2.0 ± 0.9 | 3.3 ± 1.1 |
| 22:6, 22:4 | 1.8 ± 0.9 | 1.9 ± 1.1 | 1.0 ± 0.6 | 1.6 ± 0.5 | 2.0 ± 1.0 | 2.3 ± 0.4 | 1.1 ± 0.3 |
| 22:6, 22:5 | 0.3 ± 0.2 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.5 ± 0.4 | 0.1 ± 0.2 |
| 22:6, 22:6 | 1.2 ± 0.8 | 0.5 ± 0.2 | 0.8 ± 1.1 | 0.7 ± 0.4 | 0.4 ± 0.2 | 2.0 ± 1.1 | 0.7 ± 0.2 |
K. Hamazaki and H.Y. Kim, unpublished data.
% of total PS.
The greater similarity in fatty acid composition and positional distribution between PS and PE than PS and PC in gray matter might indicate that PE is the more important glycerophosphatide substrate for PS synthesis in neurons. However, the metabolism of neuronal PS, PE and PC is interrelated as shown in Fig. 2, complicating any interpretations based on fatty acid compositional data. For example, the mitochondrial phosphatidylserine decarboxylase (PSD) reaction converts PS to PE [20], and a substantial amount of brain PE is synthesized by this reaction [53,54]. Therefore, one cannot discern whether the similarities in PS and PE fatty acid compositions are due primarily to PSS2 mediated conversion of PE to PS, or conversely, PSD mediated conversion of PS to PE. The fatty acyl composition in these phospholipids is further tailored by deacylation and reacylation reactions [5]. Therefore, conclusions regarding PS biosynthesis based on fatty acid compositional similarities are highly tenuous.
3.1. Phosphatidylserine alkyl ethers and plasmalogens
Alkyl ether phosphoglycerides contain a 1-O-alkyl hydrocarbon chain, and plasmalogens are alkenyl ether phosphoglycerides that contain a 1-O-alkenyl hydrocarbon chain. [U-14C]serine is incorporated into 1-O- alkyl, 2-acyl PS in cultured cerebral hemisphere cells obtained from 16 day rat embryos, demonstrating that the developing brain can synthesize PS alkyl ethers [53]. In addition, small amounts of serine plasmalogens were detected in myelin from monkey, ox, mouse and human brain [51,55]. Previously, it has been reported that PS contained 13% 1-O-alkenyl hydrocarbon chains in the white matter whereas gray matter PS contained only 0.3% 1-O-alkenyl chains. By contrast, the PE in these fractions contained 47% and 21% 1-O-alkenyl hydrocarbon chains. In terms of the 1-O-alkenyl hydrocarbon chain composition, 18:0 accounts for more than half in gray matter, while18:1 was the most abundant 1-O-alkenyl hydrocarbon chain in white matter [51]. However, modern analytical techniques such as mass spectrometry detect 1-O-alkeny, 2-acyl species mostly in PE but rarely in PS from mammalian tissues.
PS alkyl ethers also have been detected in the lens of human eyes by mass spectrometry with collision-induced dissociation and ozone-induced dissociation [56]. These alkyl ethers contain saturated (16:0 and 18:0) and monounsaturated (18:1) hydrocarbon chains. Studies using liquid chromatography combined with tandem mass spectrometry also demonstrate the presence of serine plasmalogens in postmortem samples of human retina and optic nerve obtained from males and females between the ages of 72 and 94 years [57]. Polyunsaturated molecular species were present in the retinal serine plasmalogens. The function of the retinal PS alkyl ethers and serine plasmalogens is unknown, but the more highly unsaturated molecular species are thought to be involved in retinal signaling [57].
4. Sources of serine for the brain
Serine is required by the brain for the synthesis of proteins and three classes of lipids, PS, sphingolipids, and N-acylserines. As illustrated in Fig. 3, serine is obtained either by uptake from the cerebral circulation or by synthesis from glucose. The serine concentration in normal human plasma is 11.2 mg/L (107 µM), which accounts for 3% of the total plasma free amino acid content [58]. Serine is transported across the blood brain barrier by three Na+-dependent neutral amino acid transporters present on the abluminal surface of the capillary endothelium [59]. The concentration of free amino acids in the brain extracellular fluid is estimated to be 10 % of the amount present in the plasma [59], so the serine concentration to which the neural cells are directly exposed is estimated to be about 10 µM.
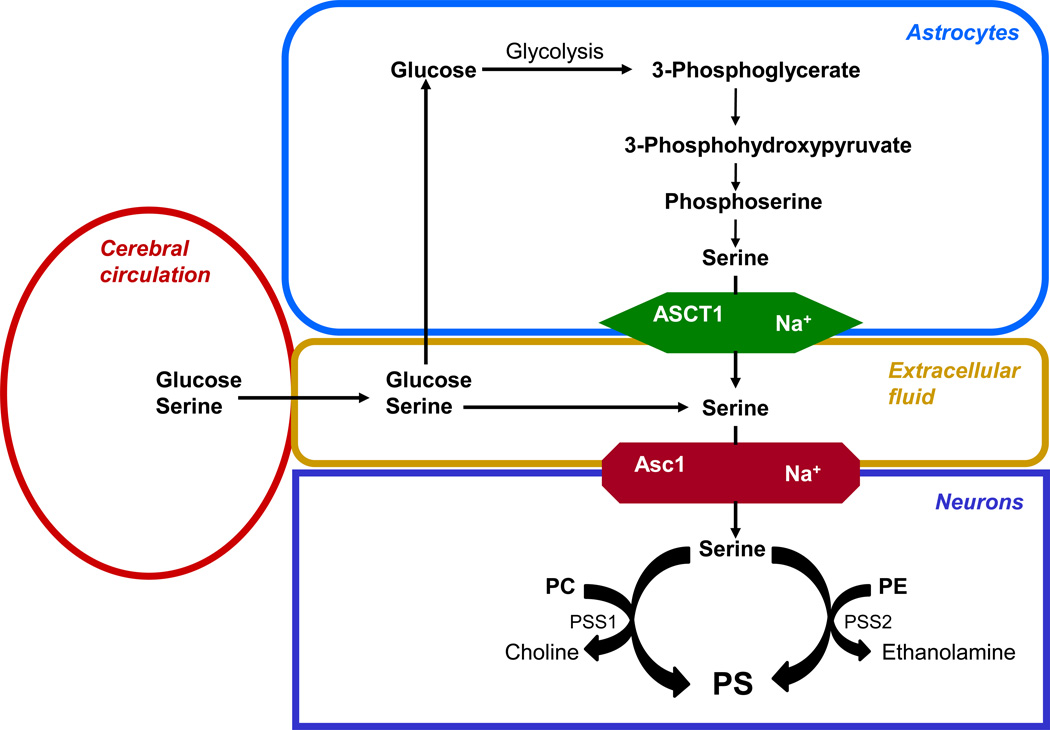
Fig. 3.
Pathways for providing serine to the brain. Serine can be taken up from the plasma or synthesized from glucose. The synthesis from glucose occurs in astrocytes where part of the 3-phosphoglycerate produced by glycolysis is converted to serine. The serine is transported out of the astrocytes by the Na+-dependent ASCT1 transporter. Serine derived from the astrocytes or plasma is taken up by the neurons through the Na+-dependent Asc1 transporter. The neurons utilize part of the incorporated serine for PS production catalyzed by either the PSS1 or PSS2 reactions.
Serine also is synthesized in astrocytes from glucose taken up by the brain. As opposed to most other cells, astrocytes do not convert all of the 3-phosphoglycerate intermediate formed by glycolysis to pyruvate. Instead, as shown in Fig. 3, these cells convert some 3-phosphoglycerate to serine in a pathway that requires three enzymatic steps; 3-phosphoglycerate dehydrogenase, 3-phosphohydroxypyruvate aminotransferase and 3-phosphoserine phosphatase [60]. The serine that is synthesized from glucose in astrocytes is released into the extracellular fluid by the astrocyte Na+-dependent ASCT1 transporter, and serine is one of the neuronal trophic factors contained in astrocyte-conditioned medium.
Neurons cannot convert glucose to serine because they do not express the rate-limiting enzyme, 3-phosphoglycerate dehydrogenase. Therefore, they require a preformed source of serine, as indicated by studies showing that serine is an essential nutrient for survival and neuritogenesis of hippocampal and Purkinje neurons [60]. The Na+-dependent Asc1 transporter expressed in neurons facilitates the uptake of serine from the extracellular fluid, which is provided by either the astrocytes or the amino acid pool of the cerebral circulation [60].
A small amount of PS is present in plasma lipoproteins [61], and the blood brain barrier contains low density lipoprotein receptors and binding sites for high density lipoproteins [62–64]. It is possible that PS contained in lipoproteins might enter the brain and serve as an additional source of serine for the brain, although extensive studies with cholesterol indicated that plasma lipoproteins themselves are not transported into the brain [65].
4.1. Serinc proteins
Serinc proteins are contained in the endoplasmic reticulum and are reported to deliver serine to the enzymes that synthesize serine-containing lipids. The current evidence indicates that they directly interact with PS synthases and sphingosine synthase, and thereby facilitate the synthesis of PS and sphingolipids [66]. Serinc 1 and serinc 2 mRNAs are expressed in rat brain hippocampal neurons, and serinc 5 mRNA is expressed in myelin throughout the brain. During kainate-induced seizures, serinc 1 and serinc 2 mRNAs are up-regulated while serinc 5 mRNA is down-regulated, suggesting that the biosynthesis of serine-containing lipids may be linked to activity-dependent neural plasticity.
4.2. D-serine
L-serine, the enantiomer contained in the plasma and synthesized from glucose by astrocytes, is utilized for protein synthesis and the synthesis of lipids that contain serine, including PS, sphingolipids and the N-acylserines. In addition to L-serine, a small amount of the D-enantiomer is present in the brain. D-serine is synthesized from L-serine by serine racemase, an enzyme expressed constitutively in astrocytes. Serine racemase is posttranslationally regulated by PKC and the scaffold protein PICK1 which binds serine racemase and increases D-serine formation. Conversely, the interaction of activated PKC with the scaffold protein causes the phosphorylation of serine racemase, and this inhibits D-serine production [67]. The regulation of D-serine production is one factor that controls the amount of D-serine available to the neurons. The other factors that control the availability of D-serine are synaptic uptake by the neuronal Asc1 transporter and degradation by D-amino acid oxidase [68,69].
Phosphatidyl-D-serine accounts for 0.9 % of the total PS in the rat cerebral cortex. It is synthesized in a crude homogenate of rat cerebrum in a Ca2+-dependent process, but the enzyme was not identified and the rate of D-serine incorporation was only 1/10th the rate of PS synthesis from L-serine. Phosphatidyl-D-serine also is contained in heart, spleen, lung, testis, liver or kidney, but in much smaller amounts, and it has not been detected in the cerebellum [70,71].
D-serine is an obligatory co-agonist of the NMDA glutamate receptor involved in synaptic plasticity and memory. D-serine binds to the glycine modulatory site of the NR1 subunit of the NMDA glutamate receptor. This augments the affinity of the receptor for glutamate and thereby increases synaptic plasticity in the hippocampus [72]. Furthermore, glutamatergic synapse formation in cerebrocortical neurons induced by astrocytes through the transforming growth factor-β cascade is dependent on D-serine formation [73].
Studies with transgenic mice that express the superoxide dismutase 1 G93A mutant indicate that D-serine may be involved in the pathogenesis and progression of the neurodegenerative disease amyotrophic lateral sclerosis. A two-fold increase in D-serine occurs in the spinal cord of the superoxide dismutase 1 mutant mice. This suggests a mechanism in which a D-serine-mediated increase in glutamate binding to the NMDA glutamate receptor elicits excitotoxicity that leads to motor neuron degeneration [74].
5. Intracellular phosphatidylserine transport
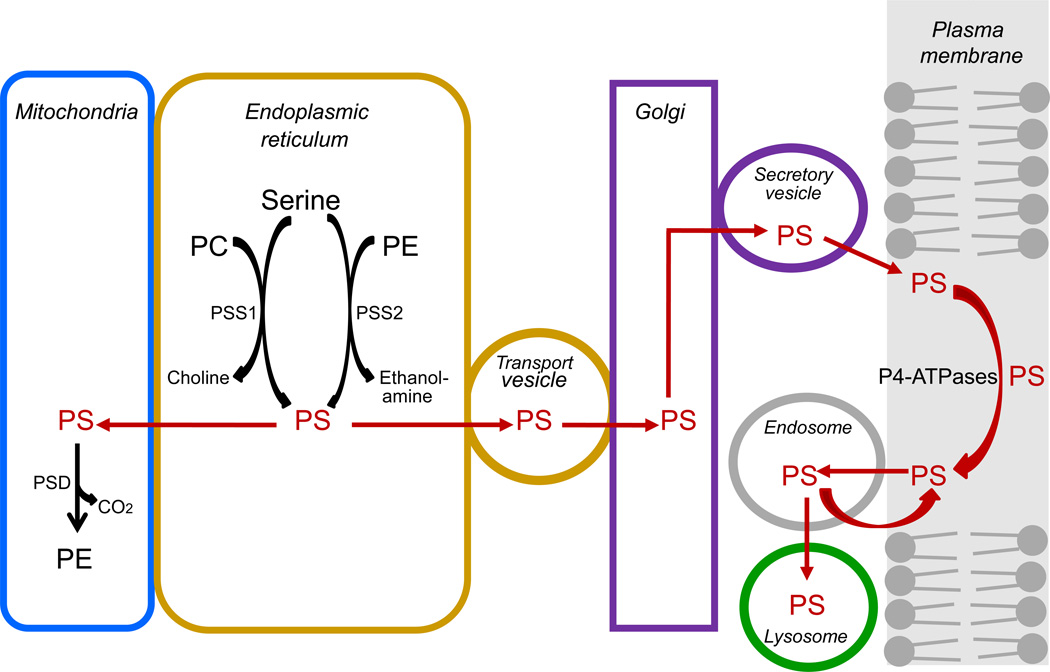
Fig. 4 illustrates intracellular transport of PS after its synthesis in the endoplasmic reticulum. Some of the newly synthesized PS is transferred from the endoplasmic reticulum to the mitochondria [24,75], and the remainder is transported by vesicles to the Golgi. Subsequently, PS is transported by secretory vesicles from the Golgi to the plasma membrane, where it is localized entirely in the cytoplasmic leaflet of the bilayer. PS is essential for retrograde membrane trafficking through endosomes, and the largest amount of PS in intracellular organelles is contained in recycling endosomes [23]. Retrograde trafficking requires binding of the pleckstrin homology (PH) domain of evectin to PS present in recycling endosomes [76]. During endocytosis, plasma membrane PS enters the sorting endosomes and is either recycled to the plasma membrane or delivered to lysosomes where it is degraded by phospholipases [23]. Staurosporine disrupts the endosomal recycling of PS, causing PS to redistribute from the plasma membranes to endosomal membranes. The mechanism of this effect has not been determined, but the existing evidence indicates that it is not due to staurosporine-mediated inhibition of PKC or activation of caspase-3 [77]. Recent evidence indicates that non-vesicular transfer of PS from the endoplasmic reticulum to the plasma membrane also may occur through the action of a subclass of oxysterol binding proteins, including human oxysterol binding proteins ORP5 and ORP10 [78].
Fig. 4.
Intracellular transport of PS. PS synthesized in the endoplasmic reticulum is either transferred to the mitochondria or to transport vesicles for delivery to the Golgi. The PS is then transported from the Golgi to the plasma membrane where it localizes exclusively in the cytoplasmic leaflet of the lipid bilayer and is maintained there by the action of P4-ATPases, a group of ATP-dependent aminophospholipid transferases. Excess PS is removed from the plasma membrane by endocytosis, and it is either recycled to the membrane or delivered to lysosome where it is degraded.
Under physiological conditions, PS in the plasma membrane is exclusively localized in the cytosolic leaflet by an energy dependent mechanism. The membrane asymmetry is maintained by transporting PS from the exoplasmic to the cytoplasmic leaflet by P4-APTases, a group of aminophospholipid translocases [79,80]. Externalization of PS occurs during apoptosis or platelet activation, which serves as a signal for phagocytosis of apoptotic cells [81], or blood coagulation [82]. While exposure of PS on a neural cell surface ordinarily produces deleterious effects in the nervous system, it may enhance neural stem cell transplantation in neurodegenerative diseases. This is suggested by recent studies with a neural stem cell line derived from mouse embryonic stem cells. The neural stem cells spontaneously fuse in vitro with co-cultured rat or mouse cortical neurons and microglia. Fusion occurs through an interaction between PS exposed on the neural stem cell surface with the CD36 receptor of the microglia. The fused cells can differentiate to neurons and astrocytes, thereby facilitating the restoration of function in the diseased brain [83].
5.1. Detection of intracellular phosphatidylserine localization and movement
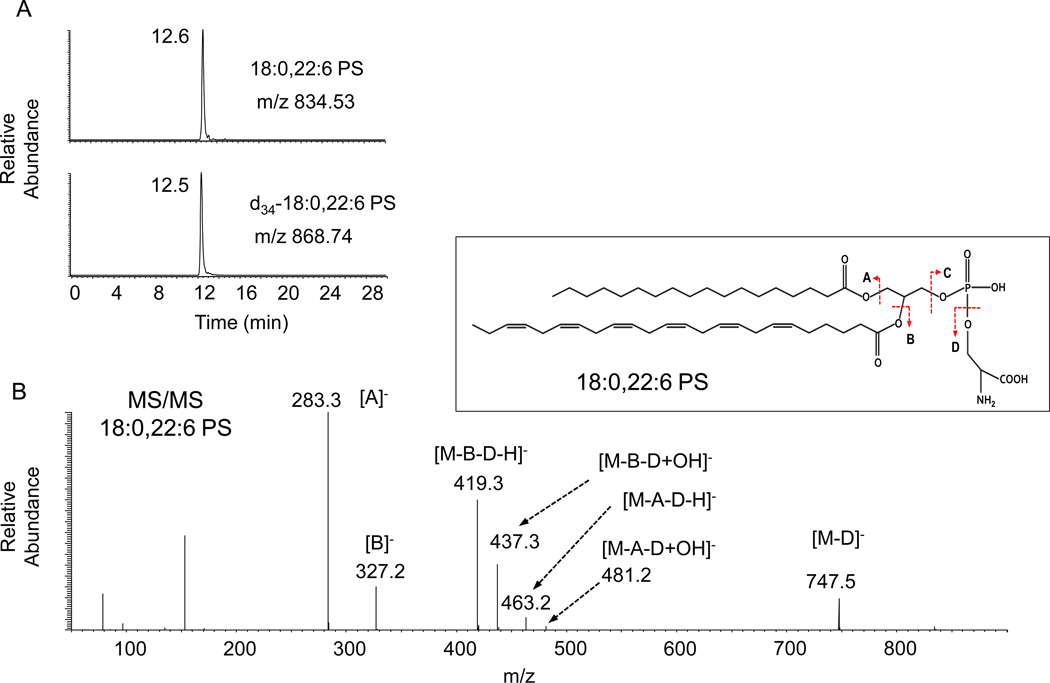
Separation of organelles by differential centrifugation followed by analysis of lipid content has been widely used to obtain information regarding the cellular distribution of PS. The analysis of PS also can be achieved by mass spectroscopic approaches after chromatographic separation from other lipid classes. Of note, isotope dilution mass spectrometry coupled with high performance liquid chromatography has been a powerful tool to accurately quantify not only the total amount of PS but also the PS molecular species (Fig. 5), a feat that cannot be accomplished by any other techniques. Using this method, 18:0, 22:6-PS was shown to account for the increase of total PS level in the plasma membrane of neuronal cells that were supplemented with DHA [3], which underscores the unique role of DHA in neuronal signaling [5]. The major limitations of this in vitro approach include the inevitable cross-contamination between subcellular fractions and the loss of information regarding PS asymmetry in the membrane bilayer.
Fig. 5.
Detection of 18:0, 22:6-PS by isotope dilution mass spectrometry coupled with high performance liquid chromatography. Ion chromatograms reconstructed for [M-H]− (A) and tandem MS spectrum (B) demonstrates detection of 18:0, 22:6-PS in the plasma membrane. Quantitation is based on d35-18:0, 22:6-PS spiked into the biological samples as an internal standard. Extracted ion chromatograms for m/z 868.74 and 834.53 represents 18:0, 22:6- and d35-18:0, 22:6-PS, respectively. The fragments detected in the MS/MS spectrum indicate that the PS species contains 18:0 and 22:6 fatty acyl chains. Inset: the structure of 18:0, 22:6-PS and fragmentation in MS/MS.
A fluorescence microscopic method was developed to detect the asymmetric localization and intracellular movement of PS in intact cells [84]. This in situ method is based on the expression of green fluorescent protein-labeled annexin V which binds to PS with high affinity in a calcium-dependent manner. This approach showed that PS is localized to the cytoplasmic side of the plasma membrane in the cell body and dendrites of the neurons [84]. Treatment of the cells with ionomycin, a Ca2+ ionophore, resulted in the strong localization of the annexin V to the plasma membrane, recycling endosomes and the nuclear envelope, indicating the presence of PS in these subcellular compartments. Considering the low proportion of PS reported in the nucleus [16] and substantially delayed localization of annexin V signal in the nuclear envelop, it is possible that PS migrates to the nucleus after stimulation with ionomycin. This suggests that PS may have a role in the nucleus when neuronal cells are activated by Ca2+-dependent signaling pathways. However, these putative nuclear actions of PS have not been explored.
A concern using this approach is the manipulation of Ca2+ at a non-physiological concentration by ionophores, which may hamper its application to other biological systems. To assess the intracellular distribution of PS in intact cells under a more physiological condition, a calcium-independent probe utilizing the stereo-specific PS-binding domain of green fluorescent protein (GFP)-tagged lactadherin was used [85]. This study demonstrated that the majority of the PS is located in the cytosolic leaflet of the plasma membrane, endosomes and lysosomes. However, both GFP-annexin V and GFP-lactadherin probes failed to detect significant amount of PS in the cytosolic leaflet of the Golgi complex or the endoplasmic reticulum where PS is synthesized, indicating the luminal localization of most PS in these organelles. Development of more sensitive and specific probes to capture the dynamic intracellular transport of PS in living cells may yield better understanding of the molecular mechanism underlying the function of this lipid.
5.2. Aminophospholipid translocases
Abnormalities occur in the nervous system function if PS does not remain localized to the cytoplasmic leaflet of the plasma membrane. This asymmetry normally is maintained by the action of an aminophospholipid flippase, a translocase that transports PS unidirectionally from the external to the cytoplasmic leaflet of the membrane lipid bilayer [80,86]. Flippases utilize Mg2+ and ATP as a source of energy, are stimulated by PS, and are inhibited by Ca2+ and vanadate [87]. Bovine brain contains four isoforms of the flippase ATPase II, a P4-type ATPase that utilizes ATP and Mg2+ and is PS-dependent. PS is essential for dephosphorylation of the phosphoenzyme intermediate of ATPase II that is formed during the catalytic cycle [88].
Angelman syndrome, an inherited neurobehavioral disease manifested by severe mental retardation, ataxia and epilepsy, is associated with a deficiency of ATP10C, a P4-type ATPase that maintains the asymmetric distribution of membrane phospholipids [89]. A deficiency in Atp8a1, a gene encoding a P-type ATPase that is expressed in hippocampal neurons of the dentate gyrus, CA1 and CA3, is associated with externalization of PS in hippocampal cells. This causes increased activity and delayed hippocampus-dependent learning in C57BL/6 mice [90]. Mutations in the Atp8a2 gene, which is expressed in the brain, spinal cord and retina, inactivates the P-type ATPase ATP8A2. Mutations in Atp8a2 in the mouse cause axonal degeneration, decreased photoreceptor response and viability, and degeneration of spiral ganglion cells [91,92].
6. Phosphatidylserine metabolism
Besides base exchange, PS can undergo three types of metabolic reactions. The serine moiety of PS can be decarboxylated by PSD, converting the PS to PE as shown in Fig. 2. In addition, phospholipases can hydrolyze one of the fatty acyl groups from PS, converting the PS to lysophosphatidylserine. An acyltransferase also can attach a fatty acid to the serine residue of PS via amide linkage, forming N-acylphosphatidylserine, the likely intermediate in the synthesis of the N-acylserine class of lipid signaling molecules.
6.1. Phosphatidylserine decarboxylase
The PSD enzyme is localized at the inner mitochondrial membrane and produces PE as an alternative to the de novo PE synthesis via cytidine diphosphate-ethanolamine pathway [53,93,94]. A role of the PSD reaction in neurite development also is suggested by the finding that nerve growth factor increases the decarboxylation of PS while stimulating neurite outgrowth in PC12 cells [95]. Another function of the PSD reaction is to supply PE in coordination with cardiolipin for mitochondrial biogenesis and protein import into the inner mitochondrial membrane [96].
The mechanism of PSD biogenesis has been demonstrated in yeast. Two proteases, MPP and Oct1, process the PSD precursor protein. Autocatalytic cleavage subsequently occurs at a conserved LGST motif, producing PSDα and PSDβ. The β-subunit forms an anchor in the inner mitochondrial membrane, and the α-subunit binds to it. PSD requires integration into the inner mitochondrial membrane for full enzymatic activity [97].
Studies with rat brain cortical mitochondria indicate that the most effective PS substrate for the PSD reaction is the 18:0,22:6 molecular species [98]. Therefore, the PE formed by PS decarboxylation is expected to contain an abundance of DHA in the sn-2 position. However, the mitochondrial PE molecular species profile does not reflect this substrate preference, presumably due to the contribution of other mechanisms such as the cytidine diphosphate-ethanolamine pathway which preferentially produces mono- and di-unsaturated PE molecular species [99]. PE synthesized from PS by the PSD reaction accumulates in the inner mitochondrial membrane, whereas PE produced by the mitochondrial cytidine diphosphate-ethanolamine pathway is mainly localized to the outer membrane [100].
The PSD reaction has been reported to account for 7% of the total PE in rat brain [54]. This probably is a minimum value, for turnover studies in 16-day rat embryos indicate that 20% of the PE in the cerebral hemispheres is produced by PS decarboxylation [53]. However, there is some uncertainty regarding these quantitative results because of questions about the sizes and specific radioactivity of the precursor pools. Substantial conversion of PS to PE was also observed in cultured cells. For example, in BHK-21 cells, 40 – 50% of the PS labeled with [3H]serine was converted to PE in 7.5 hours [101], and PS decarboxylation was the major pathway for PE synthesis in BHK-21 and Chinese hamster ovary cells [18,93,102–104]. These results differ from those obtained in liver where PE synthesis occurs primarily by the cytidine diphosphate-ethanolamine pathway [105].
Deletion of Pisd, the gene that encodes PSD, causes embryonic lethality even if the cytidine diphosphate-ethanolamine pathway for PE synthesis is functioning normally [21]. Likewise, deletion of the cytidine diphosphate-ethanolamine pathway causes embryonic lethality even if PSD function is normal. Therefore, both sources of PE are essential for normal development [20].
6.2. Lysophosphatidylserine
PS is not hydrolyzed by the Ca2+-dependent synaptosomal phospholipase activated by K+ depolarization that hydrolyzes PC [106],but it is converted to lysophosphatidylserine by a Ca2+- independent phospholipase in brain [107]. This is quantitatively a minor pathway, but it may have functional importance. Lysophosphatidylserine is a poor substrate for lysophospholipid acyltransferases present in human brain and myelin including LPEAT2, even with DHA-CoA as the substrate [108–112]. ABHD12, a membrane bound serine hydrolase that contains an α/β hydrolase domain, is a major lysophosphatidylserine lipase in mammalian brain. Homozygous mutations in human ABHD12 cause PHARC, a rare autosomal recessive neurodegenerative disease. Demyelination, retinal dystrophy and cerebellar atrophy occur in PHARC disease, producing polyneuropathy, hearing loss, ataxia, retinitis pigmentosa and cataracts. ABHD12−/− mice have a neuroinflammatory response associated with accumulation of lysophosphatidylserine containing very long chain fatty acids in the brain. This occurs early in life and produces microglial activation, leading to auditory and motor defects and neurobehavioral abnormalities. The pathological and symptomatic similarities between the ABHD12−/− mouse and PHARC indicate that lysophosphatidylserine accumulation probably is the cause of the neurodegenerative abnormalities that occur in PHARC disease [113].
Other functional effects of lysophosphatidylserine have been reported in neuronal systems. Lysophosphatidylserine has been observed to enhance differentiation induced by nerve growth factor in PC12 cells and to stimulate migration of U87 glioma cells [114]. Lysophosphatidylserine generated in activated neutrophils acts as a pro-resolving lipid mediator, promoting phagocytosis of apoptotic neutrophils through activation of the macrophage G-protein coupled receptor G2A. This in turn leads to increases in cAMP and prostaglandin E2 and a protein kinase A-dependent augmentation of Rac1 activity [115]. Whether this process plays a role in the resolution of inflammation in the brain remains to be determined.
6.3. N-Acylphosphatidylserine
By attaching a fatty acyl residue to the serine moiety via amide linkage, PS can be converted to N-acylphosphatidylserine [116]. N-Acylphosphatidylserine is present in mouse brain, and it comprises about 0.1% of the lipid in porcine brain. N-palmitoylphosphatidylserine, the most abundant species, accounts for 40% of the N-acylphosphatidylserine in the brain. The remainder consists of N-linked saturated and unsaturated fatty acids containing 14–30 carbons. N-Acylphosphatidylserine is structurally similar to N-acylphosphatidylethanolamine (NAPE), and as in the case of NAPE, arachidonic acid is one of the N-linked fatty acids present in N-acylphosphatidylserine. NAPE was shown to be synthesized by a Ca2+-dependent transacylation reaction in which the sn-1 fatty acyl group of PC is transferred to the ethanolamine moiety of PE [117,118]. Based on the structural similarity between N-acylphosphatidylserine and NAPE, it is conceivable that an analogous PC-dependent transacylation mechanism provides the N-linked fatty acid for N-acylphosphatidylserine synthesis [116].
6.3.1. N-Acylserines
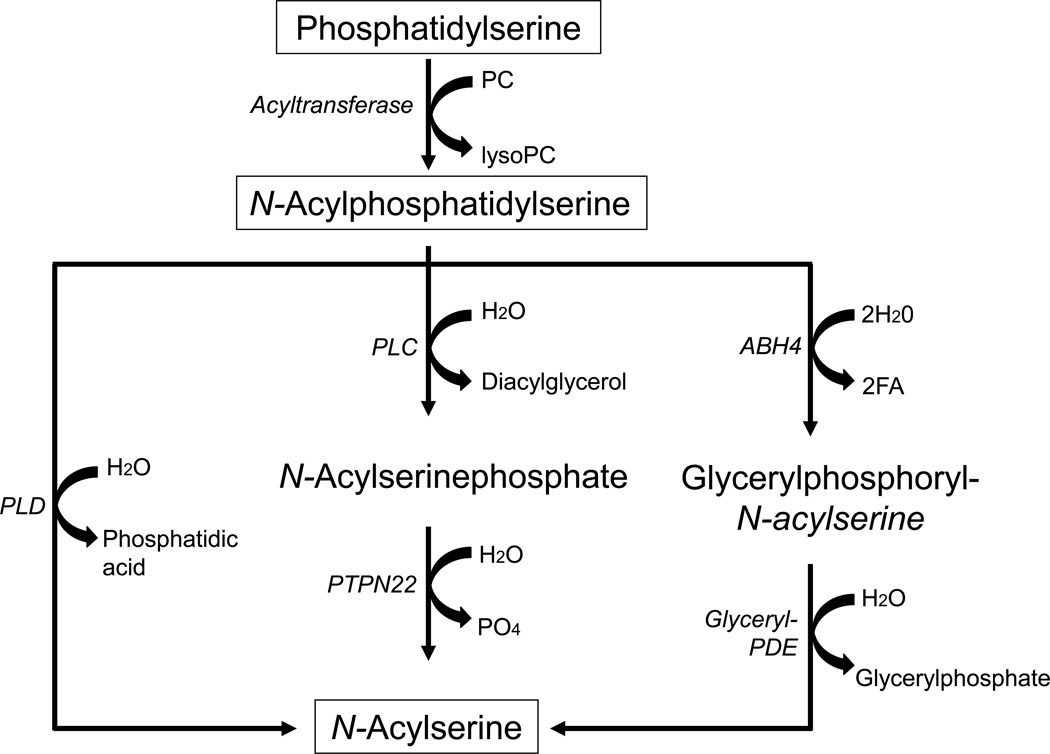
N-acylserines contain a fatty acyl group attached to serine via amide linkage. They are structural analogues of the N-acylethanolamines, and N-arachidonoylserine is a structural analogue of N-arachidonoylethanolamine (anandamide), an endogenous cannabinoid [119,120]. N-acylethanolamines are produced from NAPE through several different enzymatic pathways, including a phospholipase D-mediated hydrolysis reaction [121,122]. Based on the analogy between NAPE and N-acylphosphatidylserine, it has been suggested that N-acylserine also is produced by a phospholipase D-mediated hydrolysis of NAPS [116]. However, it is possible that N-acylserine production from N-acylphosphatidylserine may occur through several other enzymatic reactions that have been reported for N-acylethanolamine production from NAPE, including sequential hydrolysis by phospholipase C and the phosphatase PTPN22 [123], or double O-deacylation followed by glycerolphosphodiesterase 1-mediated hydrolysis [124]. Fig. 6 shows the hypothetical pathway for the formation of N-acylphosphatidylserine from PS and potential reactions for the release of N-acylserine from N-acylphosphatidylserine.
Fig. 6.
Hypothetical biosynthetic pathways involved in the formation of N-acylserine. This figure shows the likely mechanism for the synthesis of N-acylphosphatidylserine from PS which involves the transfer of the sn-1 fatty acyl residue from PC to the serine residue of PS mediated by a N-acyltransferase [116], and three potential pathways for the production of N-acylserine from N-acylphosphatidylserine; hydrolysis by phospholipase D (PLD) [116], hydrolysis by phospholipase C (PLC) followed by removal of the phosphate residue of N-acylserine phosphate by the PTPN22 phosphatase [123], or a double O-dacylation of N-acylphosphatidylserine by α,β-hydrolase ABH4 followed by glycerol-phosphodiesterase (PDE)-1-mediated hydrolysis [124].
N-palmitoylserine and N-stearoylserine are the two most abundant N-acylserines present in bovine brain [125]. While the function of these N-acylserines is unknown, functional effects have been reported for N-arachidonoylserine, which also is present in the brain. N-Arachidonoylserine activates N-type-Ca2+ channels (Cav2.2) in rat sympathetic neurons, suggesting that it might provide Ca2+ for synaptic transmission [126]. N-Arachidonoylserine also produces vasorelaxation and thereby may increase cerebral blood flow. Two mechanisms appear to contribute to this effect, phosphorylation of Akt and ERK1/2 in the endothelium [127] and direct activation of large conductance Ca2+-activated K+ channels in the vascular smooth muscle [128], In addition, N-arachidonoylserine has proangiogenic effects on microvascular endothelial cells through a signal transduction mechanism activated by the G-protein-coupled receptor 55 [129]. N-oleoylserine is another functionally active N-acylserine. It stimulates osteoblast proliferation through a Gi protein coupled receptor that activates ERK1/2 [130]. Although N-oleoylserine has not been reported in the brain, its putative precursor N-oleoylphosphatidylserine is present in mouse brain [116].
7. Phosphatidylserine in neuronal signal transduction
PS participates in key signaling pathways in the neuronal system. Unlike other membrane phospholipids, PS does not produce its signaling effects through a phospholipase-mediated hydrolysis that leads to the formation of bioactive products. Instead, PS functions as a constitutive component of membrane anionic domains that bind and thereby activate cytosolic proteins involved in neuronal signaling [2–4,6,131]. The high concentration of negatively charged PS in the inner leaflet of the plasma membrane facilitates the binding of these signaling proteins through electrostatic interactions or Ca2+ bridges. At least three major pathways including phosphatidylinositol 3-kinase (PI3K)/Akt, Raf/Ras and protein kinase C are shown to be PS-dependent. Activation of Akt, Raf-1 and protein kinase Cα requires the translocation from cytosol to the plasma membrane for which interaction with PS is critically important.
7.1. Molecular basis of the interaction between phosphatidylserine and proteins
Ca2+-dependent C2 domains, named after the second homology region in conventional protein kinase C, are the most well-known PS binding domains. The protein kinase Cα-C2 domain (~130 residues) consists of a characteristic eight-stranded antiparallel β-sandwich connected by three interstrand loops where two or three calcium ions cooperatively bind to several conserved Asp residues (D187, D193, D246 and D254) [132]. The binding of calcium ions changes the electrostatic potential of the loops, enabling a positively charged canonical membrane binding site to interact with the negatively charged membrane [133]. R249 and R252 in the calcium binding loops also contribute to the non-specific electrostatic attraction, and N189 plays a critical role in the selectivity for PS by interacting specifically with the serine head group [134]. In addition, bound calcium ions form a bridge between the protein kinase Cα-C2 domain and PS [135]. Other notable Ca2+-dependent C2 domain-containing proteins include protein kinase Cβ, protein kinase Cγ, synaptotagmin III [129], and phospholipase Cδ [136].
Besides the C2-containing proteins, the binding of annexins to PS is also Ca2+-dependent. In this case, the PS-binding domains are composed of 4 annexin repeats, each of which contains 5 α-helices connected by surface loops where calcium ions bind [137,138]. This binding plays an essential “bridging” role by connecting carbonyl and carboxyl groups in the loops of annexin to the phosphoglycerol backbone and serine headgroup of PS in the membrane [137,139].
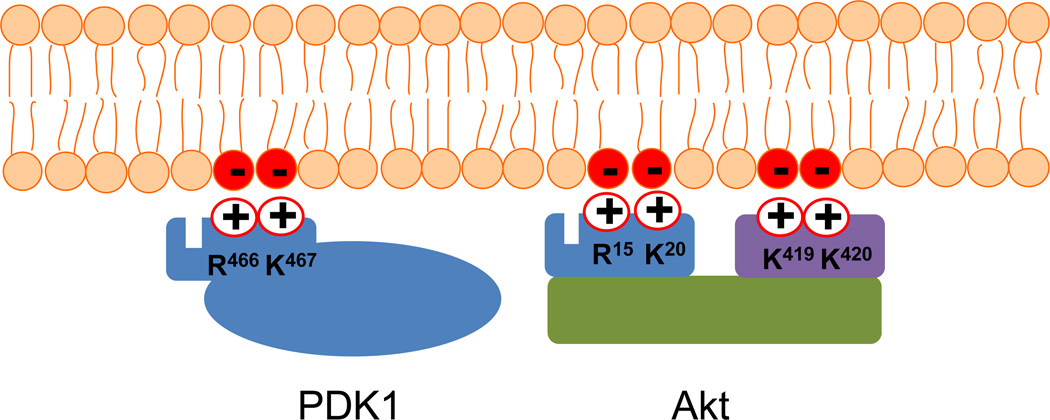
Specific electrostatic interaction between PS and signaling proteins including Akt and phosphoinositide-dependent kinase (PDK) 1 has recently been identified [3,140]. The positively charged residues including R15 and K20 located outside the phosphatidylinositol-3,4,5-trisphosphate (PIP3) binding pocket in the PH domain, along with K419 and K420 in the regulatory domain of Akt1, are identified as PS-specific binding sites. These binding sites are critical for facilitating and securing Akt membrane translocation triggered by the interaction of PIP3 with the PH domain. Similarly, cationic grooves composed of R466 and K467 in the PH domain of PDK1 selectively bind PS. Furthermore, a cluster of basic residues including R143, K144 and K148 present in the anterior half of the cysteine-rich domain of Raf-1 interacts with PS [141], although it is not clear whether these residues also interact with other acidic membrane phospholipids such as phosphatidylinositol. In addition, electrostatic interactions generally facilitate the binding between the anionic PS and positively charged proteins [85].
Ca2+-independent stereo-specific binding to PS has been observed for proteins that contain discoidin C2 domains (e.g. lactadherin), which are structurally unrelated to the C2 domains of protein kinase C [142,143]. These domains contain a β-barrel core with hydrophobic residues that interact with PS in a stereo-specific manner. In addition, the C1 domain of conventional protein kinase C stereo-specifically interacts with PS [144].
7.1.1. PI3K/Akt signaling
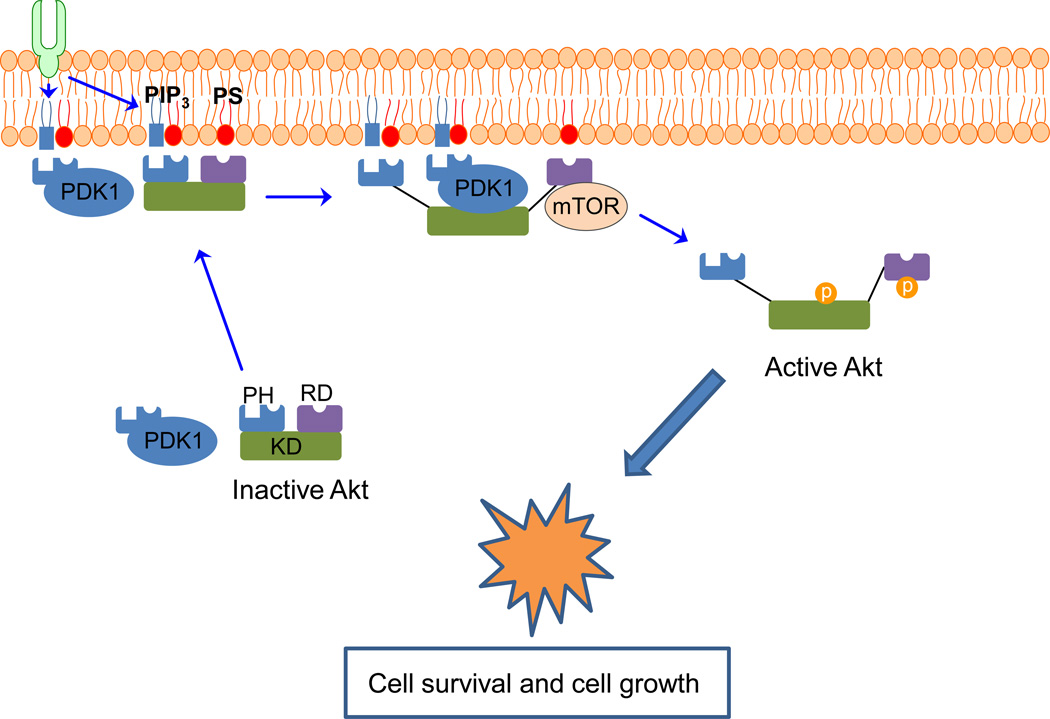
Akt is a critical protein for cell survival and proliferation [145]. Inactive Akt resides in a folded structure in the cytosol, with its PH and regulatory domains blocking access of the upstream kinases to the T308 and S473 phosphorylation sites in the kinase domain. Receptor-activated PIP3 formation triggers Akt translocation to the plasma membrane. The PH domain of Akt contains the PIP3 binding pocket. It also contains basic residues R15 and K20 that bind to PS in anionic domains of the plasma membrane cytoplasmic leaflet. These interactions are required for Akt binding to the plasma membrane as indicated by the finding that the mutation of R15 and K20 diminishes the membrane binding and disables the translocation [3]. Membrane binding of the PH domain causes the inter-domain conformational changes to allow PDK1 to phosphorylate T308 in the kinase domain for Akt activation. Furthermore, binding of basic residues K419 and K420 in the regulatory domain of Akt to PS leads to conformational changes that allow phosphorylation of S473 by mTORC2, which is known to potentiate Akt activity [146].
Activation of Akt and subsequent downstream phosphorylation of BAD (Bcl-2 antagonist of cell death) suppress caspase-3 activity and promote cell survival. The importance of neuronal enrichment of PS is underscored by the findings that PS concentration-dependently influences endogenous Akt signaling and neuronal survival [3]. Increasing the membrane PS by DHA supplementation [2,4], or expressing the PSS1 (R95K) mutant lacking regulatory capability [147] significantly reduces cell death caused by serum starvation in neuronal cells. In contrast, decreasing the PS levels in a PSS1-deficient mutant Chinese hamster ovary cells [104], produces the opposite effect [3]. The role of PS in promoting Akt signaling is particularly important for sustaining neuronal survival under conditions where the production of PIP3 is limited.
PDK1 is a ubiquitously expressed serine/threonine kinase that acts downstream of PI3K and activates Akt [148,149]. It is present in the cytosol and is constitutively active due to auto-phosphorylation. However, association of PDK1 with the plasma membrane is necessary for its signaling function. After binding to the membrane, PDK1 phosphorylates T308 in the kinase domain. The PH domain of PDK1 also specifically interacts with PS [140]. Disruption of the PS-PDK1 interaction by mutation abolishes membrane localization and function of PDK1 leading to diminished phosphorylation of Akt T308. Collectively, it seems that the influence of PS on PI3K/PDK1/Akt signaling pathway is more profound than previously thought. The basic residues of Akt and PDK1 specifically interacting with membrane PS as well as crucial requirement of PS in PI3K/Akt signaling are illustrated in Figs. 7 and 8.
Fig. 7.
Schematic presentation of the specific interaction between anionic PS and basic residues in Akt and PDK1. The basic residues R466/K467 and R15/K20 that bind to PS (red circles) are located near the PIP3 binding pocket in PDK1 and Akt, respectively. K419 and K420 are located in the regulatory domain of Akt.
Fig. 8.
Molecular mechanism of PS involvement in Akt activation. Akt is recruited to the plasma membrane initially by the specific binding of the pleckstrin homology domain (PH) to PIP3 which is generated by growth factor receptor stimulation. The membrane translocation is secured by the electrostatic interaction of membrane PS with specific PS-binding residues in the Akt pleckstrin homology and regulatory (RD) domains. The Akt interaction with PIP3 and PS causes Akt interdomain conformational changes that expose T308 and S473 for phosphorylation and activation by PDK1 and mTORC2 kinases, respectively. PDK1 co-localizes with Akt at the plasma membrane through the interaction with not only PIP3 but also PS. After phosphorylation, active Akt is released from the membrane to perform its downstream functions such as regulating cell survival and cell growth. KD: kinase domain.
7.1.2. Raf/Ras signaling
Raf-1 is a serine-threonine kinase that plays a key role in cell growth, survival and differentiation through the mitogen-activated protein kinase kinase (MEK)/ERK signal transduction pathway [150]. Raf-1 functions by directly phosphorylating MEK, thereby activating the mitogen-activated protein kinase/ERK pathway. Like Akt, Raf-1 is present in the cytosol as an inactive form and is activated by extracellular signals that cause it to associate with acidic plasma membrane domains. Association of Raf 1 with membranes is prerequisite for its activation. This critically depends on the electrostatic interaction of Raf-1 with membrane PS, as evidenced by the fact that disruption of Raf-PS interaction by eliminating the positive charges on residues R143, K144 and K148 abolishes the translocation of Raf-1 to the membrane [141]. Consistently, increasing the PS level enhances the binding affinity of Raf-1 for the membrane and promotes the membrane translocation and subsequent activation of Raf-1 induced by growth factor stimulation [4,141]. As a result, ERK phosphorylation is enhanced, caspase-3 is inhibited, and apoptotic neuronal cell death is suppressed. Furthermore, Ras GTPase, particularly K-Ras that contains a polybasic sequence, nanoclusters on the plasma membrane most likely due to the electrostatic interaction with the acidic plasma membrane surface. Staurosporine can redistribute the plasma membrane PS to endomembranes and acutely decreases plasma membrane PS. Under such condition, K-Ras is mislocalized to the endosomes and degraded, and K-Ras-dependent proliferation is abrogated, indicating the importance of PS interaction in K-Ras signaling [77].
7.1.3. Protein kinase C signaling
Protein kinase C signaling regulates a broad spectrum of physiological processes such as differentiation, proliferation, gene expression, and apoptosis. Among 10 isoforms of protein kinase C, conventional isoforms including α, βI, βII and γ interact with PS in a Ca2+ dependent manner [131]. Protein kinase Cα contains two functional domains, an amino terminal regulatory domain and a carboxyl terminal catalytic domain. The regulatory domain is composed of an auto-inhibitory pseudo-substrate sequence followed by two tandem C1 domains and a C2 domain. In the inactive state, Protein kinase Cα resides in the cytosol with the pseudo-substrate sequence occupying the substrate binding pocket in the catalytic domain. Activation of protein kinase Cα is triggered by receptor-mediated hydrolysis of PIP2 that generates two second messengers, diacylglycerol and inositol trisphosphate, the latter increasing the intracellular Ca2+ concentration. The C1 domain binds to diacylglycerol, which is promoted by the stereo-specific binding of the C1 domain to PS. On the other hand, Ca2+ binds to the C2 domain, which prompts the interaction of C2 domain with membrane PS and PIP2 [131,132]. These membrane interactions result in a conformational change of protein kinase Cα which causes the release of the pseudo-substrate sequence from the catalytic domain. This opens the activation loop for substrate phosphorylation and downstream signaling [131]. Because membrane interaction is the prerequisite event for the allosteric regulation, protein kinase Cα activity is membrane PS-dependent. In neuronal cells, increases of cellular PS levels by DHA supplementation promote the membrane translocation, activation and substrate phosphorylation capability of protein kinase C [6].
7.2. Receptors
PS modulates the properties and function of several membrane-bound receptors that play a key role in neuronal function. The binding affinity of the AMPA glutamate receptor is increased by PS in rat telencephalic membranes [12]. PS has a similar effect on the central type benzodiazepine receptor in cerebral cortical membranes. Interaction with the anionic domain of the membrane increases the affinity of this receptor for flunitrazepam without affecting the maximal binding capacity [151]. The cannabinoid type 2 receptor, which is present in brain microglia and implicated in neuroinflammatory processes, also is modulated by PS. In a liposome-reconstituted system, G protein activation by agonist-bound recombinant cannabinoid type 2 receptor increased with increasing PS content up to 50 mol% in the liposome. Facilitated receptor activation due to the increased negative electric surface potential of the proteoliposomes appeared to be responsible for the activation rather than efficient G protein coupling to the receptor [152].
7.3. Exocytosis
Synaptic neurotransmission is achieved by the Ca2+-regulated exocytic release of neurotransmitters. The SNARE-assisted membrane fusion between neurotransmitter-containing synaptic vesicles and synaptic plasma membrane is necessary for exocytosis. The fusing membranes are forced to come close together by the SNARE complex while the Munc-18 protein is proposed to enable lipid mixing of the fusing membranes [153]. Synaptotagmin, an integral membrane protein localized on the synaptic vesicle, is the Ca2+ sensor that is essential for fusion. Ca2+ enters into the nerve terminal through the voltage gated Ca2+ channel and binds to the C2 domain of synaptotagmin, triggering the fusion pore formation which allows neurotransmitter release within a few hundred microseconds. PS is required for the Ca2+ binding to synaptotagmin and this binding is dramatically enhanced by increasing PS concentration in the membrane [8,10], indicating a crucial role of PS in the fusion process and exocytosis [154,155]. In addition to the synaptic plasma membrane [48], synaptic vesicles also contain PS [156]. Although a significant fraction of PS is localized in the cytoplasmic face of the synaptic vesicles[157], only the target plasma membrane PS is critically important for fusion [158].
7.4. Other phosphatidylserine-interacting proteins
The proteins shown to interact with PS are listed in Table 4. PS affects the function of synapsin and tau, two proteins that play a critical role in neuronal function. Interaction with PS results in a conformational change in synapsin I, allowing insertion of the amino-terminal segment containing hydrophobic residues into the synaptic membrane [13]. In addition, PS alters the conformation and antigenic properties of tau. As a result, the ability of mitogen-activated protein kinase to phosphorylate tau is impaired and tau association with microtubules is decreased [14].
Table 4.
Proteins and enzymes modulated by PS
| Protein | Role of PS | Functional effect | Reference |
|---|---|---|---|
| Akt | Membrane binding of pleckstrin homology and regulatory domains, producing interdomain conformational change | Enables phosphorylation by PDK1 and mTORC2, activating Akt signaling pathway | [3] |
| BASP1 | Localizing protein to inner surface of presynaptic membrane | Forms cation-selective ion channel across membrane | [160] |
| Diacylglycerol kinase-θ | Binding to membrane activates interfacial activity | Stimulates substrate binding and catalytic activity | [165] |
| Evectin-2 | Binding to PH domain | Enables membrane trafficking from recycling endosomes to Golgi | [76] |
| Na, K-ATPase | Stabilizing interaction of FXYD protein with enzyme α-subunit | Stabilizes and modulates kinetic properties of the enzyme | [168] |
| nNOS | Electrostatic interaction with hydrophobic/basic amino acid cluster in residues 732–754 | Produces conformational change for calmodulin binding | [159] |
| PDK1 | Recruitment to plasma membrane via PH domain binding | Activates PDK1 | [140] |
| PKCα | Binding to N189 in C2 domain | Activates PKCα signal transduction | [134] |
| iPLA2 | Membrane recruitment and oligomerization | Modulates activity and increases specific activity | [164] |
| PLCδ1 | Electrostatic interaction with Ca2+ and N647 of C2 domain | Membrane targeting | [136] |
| Rasal | Membrane recruitment by interaction between C2A domain and Ca2+ | Induces membrane curvature and activates Ras GTPase activating protein activity | [166] |
| Sphingosine kinase 1 | Binding through T54 and N89 targets enzyme to membrane | Activates the enzyme, regulating cell growth and prevents apoptosis | [163] |
| Synapsin I | Association with synaptic membrane | Maintains pool of neurotransmitter-loaded vesicles for neuronal activity | [13] |
| tau | Binding to membrane PS, altering conformation and inhibiting mitogen-activated protein kinase phosphorylation of epitopes | Prevents association with microtubules and increases calpain-mediated proteolysis | [14] |
| Lactadherin | Binding to C2 domain binds through residues K45, R70, R146 | Inhibits targeting of nascent protein chains to translocon and translocation across endoplasmic reticulum membrane | [161,162] |
| GRP1 | Transient membrane association through pleckstrin homology domain binding | Facilitates two-dimensional search for PIP3 target | [167] |
PS also influences the properties of neuronal nitric oxide synthase, a key enzyme in oxidative functions in the brain. The calmodulin-binding domain of neuronal nitric oxide synthase interacts through a cluster of basic amino acid residues with acidic membrane domains that contain either PS or phosphatidic acid [159].
BASP1 is a myristoylated brain protein present in the inner surface of the presynaptic plasma membrane. It forms a pore-like oligomeric structure that induces cation selective single channel currents across negatively charged planar lipid bilayers that contain either PS or phosphatidylinositol [160]. Lactadherin, which inhibits the translocation of nascent polypeptide chains across the endoplasmic reticulum, binds tightly to PS through its C2 domain [161,162].
A number of other enzymes and proteins interact with PS. These include sphingosine kinase 1, cytosolic phospholipase Cδ1, and Na,K-ATPase. Sphingosine kinase 1 regulates cell growth and prevents apoptosis. It is present in an inactive form in the cytosol and is activated in response to extracellular signals by binding to PS in the plasma membrane. Studies with HEK293 cells and HeLa cells demonstrated that when S225 is phosphorylated, sphingosine kinase 1 undergoes a conformational change that allows T48 and N150 to interact with PS in the cytoplasmic leaflet of the plasma membrane [163]. Phospholipase Cδ1 also is present in an inactive form in the cytosol and translocates to the plasma membrane when the cell is activated. Membrane targeting is mediated by interactions between the C2 domain of the enzyme, Ca2+ and PS. The localization of phospholipase Cδ1 to specific anionic domains of the plasma membrane is governed by N647 binding to PS [136]. These findings were obtained in enzymatic assays or from cultured cell lines of non-neural origin. However, it seems reasonable to suggest that similar effects of PS on these enzymes might occur in neural cells.
Liposomes containing acidic phospholipids, including PS, bind to the calcium-independent cytosolic phospholipase A2, and this binding increased the specific activity of the enzyme [164]. Liposomes containing 20 mol% PS also increased the turnover rate of diacylglycerol kinase-θ, an important modulator of diacylglycerol signaling. The acidic phospholipids function synergistically with polybasic proteins to increase the activity of diacylglycerol kinase-θ by 10- to 30-fold [165].
Rasal, a member of the Gap1 gene family, increases Ras GTPase activity when it co-localizes with Ras at the plasma membrane. Rasal is recruited from the cytoplasm to the membrane as a result of a receptor-mediated increase in intracellular Ca2+. Binding to the membrane occurs through a Ca2+- dependent interaction of the C2 domains of Rasal with acidic lipids. The C2A domain binds to PS and the C2B domain binds to inositol phospholipids [166]. GRP1, a general receptor for phosphoinositides, transiently associates with the membrane through the binding between the PH domain and PS, which enables a two-dimensional search for its PIP3 target [167].
Structural studies indicate that PS also is involved in modulating the activity of Na,K-ATPase. PS stabilizes the interaction of purified Na,K-ATPase with a small regulatory protein, FXYD, that modulates the kinetic properties of the enzyme. PS binds to transmembrane segment 9 of the Na,K-ATPase α-subunit adjacent to FXYD, and this stabilizes the complex against inactivation by heat or detergents [168]. A mutation study indicates the presence of a specific PS binding pocket in the transmembrane segments 8–10 of Na,K-ATPase [169].
Evectin-2, a protein expressed in a broad range of tissues that targets recycling endosomes to the Golgi, binds to PS through a PH domain in COS-1 cells. A similar post-Golgi protein, evectin-1, is expressed specifically in the nervous system [76] but it is not known whether it also binds through a PS-dependent mechanism.
7.5. Neurotransmitters
Molecular dynamic simulations indicate that the neurotransmitter dopamine and its metabolic precursor L-dihydroxyphenylalanine associate with the head groups of lipid bilayers and localize at the lipid-water interface of the membrane. This occurs predominantly through hydrogen bonding. In a lipid bilayer consisting of PC, PE and PS, a composition similar to the membrane cytoplasmic leaflet and presynaptic vesicles, the presence of 22 mol % PS increased the stability of dopamine and L-dihydroxyphenylalanine binding due to formation of a hydrogen bond network with a long lifetime. Although dopamine formed hydrogen bonds with each of the phospholipids, its affinity for PS was greater than for either PC or PE. The localization of intracellular dopamine and L-dihydroxyphenylalanine to the membrane cytoplasmic surface suggests these compounds might be metabolized effectively by enzymes that also bind to the membrane [170].
Equilibrium dialysis studies with glycine or γ-aminobutyric acid and dimyristoylphosphatidylcholine bilayers indicate that these zwitterionic neurotransmitters also accumulate at the membrane interface. Glycine and γ-aminobutyric acid binding increased when 10 % anionic lipids, either dimyristoylphosphatidylserine or dimyristoylphosphatidylglycerol, were added to the dimyristoylphosphatidylcholine. The concentration of these neurotransmitters at the membrane interface was 5- to 10-times greater than in the aqueous phase. Molecular simulations indicated that the attraction was due mainly to electrostatic interactions between the amino group of the neurotransmitters and the lipid phosphate groups. The fact that similar results were obtained with dimyristoylphosphatidylserine and dimyristoylphosphatidylglycerol indicates that the increase in binding due to surface charge rather than to a specific role of PS. However, the cytoplasmic leaflet of neuronal membranes, including synaptic membranes, contains 20 to 30 % PS and little or no phosphatidylglycerol. Therefore, it is most likely that PS is primarily responsible for this effect under physiological conditions [171].
8. Interactions between phosphatidylserine and docosahexaenoic acid
Brain is enriched with DHA, and the PS content in the grey matter of human brain is particularly high as shown in Table 2. PC and PE containing DHA are the best substrate for PS biosynthesis [40,44]. As a consequence, the PS level is high in the brain where DHA is abundant. In contrast, DHA-depletion from the brain lowers the PS level [50]. For example, dietary depletion of n-3 fatty acids can reduce DHA in the brain and increase the n-6 counterpart docosapentaenoic acid (22:5n-6, DPAn-6). Since phospholipids containing DPAn-6 are not as good substrates for PS synthesis as DHA-containing phospholipid species, the brain PS level is reduced [40,49,50]. It is important to note that modern western diets are excessively abundant in n-6 fatty acids compared to n-3 fatty acids, and therefore, DHA enrichment in the brain is compromised as indicated by the considerable accumulation of DPAn-6 observed in the postmortem human hippocampus [172]. Such a fatty acid profile is comparable to that observed in rodent brains depleted in DHA at least to a moderate extent, and therefore, the PS level is likely not at a maximum in modern human brains although systematic analysis has not yet been performed.
In agreement with the preference for DHA-containing substrates in PS biosynthesis, the level of a single molecular species 18:0, 22:6-PS is remarkably high in most brain regions (Table 3). LPAAT4, a lysophosphatidic acid acyltransferase that is highly expressed in the brain and has a high specificity for DHA-CoA, is involved in 18:0,22:6-PC formation [173]. This PC species may be utilized by PSS1 to form a portion of the 18:0,22:6-PS that accumulates in the brain. In the olfactory bulb, 16:0, 22:6-PS is particularly high (Table 3), suggesting that PSS2, which does not discriminate the sn-1 fatty acid composition [44], contributes substantially to the PS biosynthesis in this region of the brain.
Neuronal cell culture studies indicate that the DHA-mediated PS increase may function to enhance neuronal development and prevent apoptosis. Enhanced neuronal development is indicated by the finding that DHA supplementation increases neurite length and branching in cultured hippocampal neurons [174,175], and it promotes neurogenic differentiation of neural stem cells [176]. Furthermore, addition of DHA to DHA-deficient embryonic hippocampal neurons in culture can restore neurite length [174]. Recent studies indicate that endogenous metabolism of DHA to N-docosahexaenoylethanolamine (synaptamide) is primarily responsible for the observed DHA effects on neurite growth, synaptogenesis and neurogenesis [177,178]. Nevertheless, the DHA-induced PS increase and facilitation of PS-dependent signaling pathways leading to neuronal differentiation may also be a contributing mechanism for the observed DHA effects [5].
Evidence obtained with neuronal cell cultures also suggests that PS and DHA prevent apoptosis through a common mechanism. Supplementation of Neuro 2A cells with DHA for 24 hours prevented apoptotic cell death induced by serum starvation [2,4]. This protective effect was associated with an increase in the membrane PS content and facilitated translocation of Akt and Raf-1 to the cell membrane. While 22:5n-6, the omega-6 fatty acid analogue of DHA, also increased the PS content of Neuro 2A cells, the increase was smaller and the protection was less against cell death compared to DHA [2,179]. Consistently, hippocampal neurons from DHA-depleted animals showed reduced amounts of PS and were more susceptible to cell death. As opposed to the findings with Neuro 2A cells, DHA supplementation did not increase the PS content in Chinese hamster ovary-K1 cells, NIH3T3 cells or HEK cells, suggesting that the PS increase induced by DHA occurs primarily in neuronal cells [180]. The protective action of DHA did not occur when Neuro 2A cells were incubated in a serine-free medium, suggesting that this effect is PS-dependent [2,4]. Increasing PS by DHA supplementation promotes the membrane interaction of Akt via PS binding residues and facilitates membrane-induced conformational changes required for activation of Akt [3]. This PS-derived mechanism is particularly important for neuronal survival under adverse conditions where the generation of PIP3, a primary survival signaling molecule, is limited. In summary, the neuroprotective effect of DHA is linked to an increase of the PS content in neuronal cells, which promotes the activation of the Akt and Raf-1 signaling pathways and thereby enhances neuronal survival as illustrated in Fig. 1.
Related effects of DHA status on PS metabolism have been observed in experimental animals. Dietary depletion of DHA in rat pups reduced [3H]serine incorporation into PS [181], and decreased the content of PS in the olfactory bulb, brain cortex and hippocampus [49,50]. Therefore, the metabolic linkage between DHA and PS observed in neuronal cultures seems to occur also in vivo [4].
8.1 Ethanol-induced effects
Findings in cultured neuronal cells and animal models indicate that ethanol can disrupt the beneficial interaction between DHA and PS. Exposure of Neuro 2A cells to 25 mM ethanol decreased the DHA-mediated accumulation of PS, Akt phosphorylation and cell survival as indicated in Fig. 1. Administration of ethanol to pregnant rats also decreased the DHA and PS content of the fetal hippocampus and increased the number of apoptotic hippocampal cells [182]. Likewise, administration of ethanol to rats during the prenatal and developmental period decreased microsomal PS biosynthetic activity without altering PSS expression, and thus the amount of PS, particularly the 18:0, 22:6 species in the cerebral cortex [183]. The ethanol-mediated decrease in the 18:0, 22:6-PS molecular species is also consistent with the finding that incubation of brain microsomes with high concentrations of ethanol increased oleoyl-CoA incorporation into PS and diverted polyunsaturated fatty acids into triglycerides [184]. Considering the significant roles played by PS in neuronal survival and function discussed avove, a reduction in DHA-stimulated synthesis of PS may be one factor that produces the deleterious effect of ethanol on the central nervous system.
9. Phospahtidylserine and cognitive function
A decrease of the DHA content in PS has been reported in cognitive impairment. A small reduction in the DHA content of hippocampal PS was observed in 12 month-old senescence-accelerated prone mice that have a shorter life span, learning and memory deficit, and an increase in hippocampal Aβ-peptide content [185]. The decrease in DHA was associated with a corresponding increase in the AA content of hippocampal PS. Likewise, the DHA content of PS in the superior temporal and mid-frontal cortex was reduced by 12 and 14 %, respectively, in brain tissues obtained from patients with Alzheimer’s disease [186]. However, there is no information as to how a decrease in the DHA content of PS might contribute to the pathogenesis of cognitive impairment, and a decrease in DHA content is not a uniform finding in animal models of cognitive impairment. For example, substantial fatty acyl compositional changes, including reductions in AA, have been observed in brain PS of aged Wistar rats with cognitive deficits, but there is no difference in the DHA content of the PS [187]. Therefore, the putative linkage between DHA reductions in PS and cognitive impairment remains open to question.
9.1. Effects of dietary phosphatidylserine supplements on cognitive function
Dietary PS supplements are reported to improve cognitive function in experimental animals [188,189], and a similar result has been obtained recently with krill PS which has a high content of omega-3 fatty acid. Aged rats given daily doses of krill PS orally for 7 days showed improvement in the Morris water maze test. There was less loss of choline acetyltransferase and acetylcholine esterase transporter mRNA in the hippocampus. The neuroprotective activity of 20 mg/kg krill PS was equivalent to that of 50 mg/kg soy PS in these aged rats [190]. Normal young rats given 100 mg/kg krill PS orally for 30 days also showed improvement in the Morris water maze test [191].
Likewise, cognitive improvement was reported in humans given oral PS supplements [192], and these findings subsequently were confirmed and extended. Human subjects treated for 42 days with 200 mg of soy-based PS showed a more relaxed state before and after mental stress as measured by electro-encephalography [193], and no adverse effects were evident at this dose given three-times a day for 6 to 12 weeks [194]. The ability to recall words increased by 42% in male and female subjects who were older than 60 years and complained of subjective memory loss when they were treated with 300 mg/day of PS containing 37.5 mg of eicosapentaenoic acid and DHA [195]. Improved verbal immediate recall was also observed in a double-blind, placebo controlled clinical trial in a large group of elderly subjects with memory complaints when treated with a daily dose of 300 mg PS containing DHA and eicosapentaenoic acid in a 3:1 ratio. A subset with relatively good cognitive performance at baseline showed the greatest improvement [196]. In a similar double blind study in Japanese subjects between the ages of 50 and 69 years with memory complaints, subjects having low scores at baseline showed greater improvement in delayed verbal recall after treatment for 6 months with soybean PS [197].
Several biochemical responses to PS administration that have been reported in experimental animals could be involved in the mechanism of PS-mediated improvement in cognition. Stimulation of dopamine-dependent adenylate cyclase activity was observed in mouse brain following an intravenous infusion of a sonicated preparation of bovine brain PS [198]. Sonicated suspensions of PS injected intravenously also increased calcium-dependent acetylcholine output from the cerebral cortex in urethane anesthetized rats [199]. Likewise, intravenous injection of purified bovine brain PS for 8 days attenuated the decrease in acetylcholine release from the parietal cortex in aged rats, possibly by providing more choline for acetylcholine synthesis [200]. Furthermore, orally administered krill PS in normal young rats for 30 days produced an increase in neurons positive for brain-derived neurotrophic factor and insulin-like growth factor in the hippocampal CA1 region [191].
A current hypothesis is that these biochemical responses and the resulting cognitive improvements are due to PS mediated effects on neuronal membrane properties [192]. However, experimental evidence indicating that orally or intravenously administered PS actually alters neuronal membrane properties is lacking. How the administered PS is transported in the plasma, how much enters the brain, whether it is taken up intact, and whether it is incorporated into neurons or glia are not known. Dietary phospholipids are hydrolyzed during digestion, so orally administered PS most likely is not absorbed intact. PS preparations are rich in DHA, and DHA supplementation is known to improve hippocampal function [5]. Because the administered PS probably undergoes partial or complete hydrolysis, the beneficial effects of PS on cognition, particularly from krill or bovine sources, possibly are produced by DHA released from the PS rather than the intact PS itself. These issues will have to be investigated in order to obtain some mechanistic insight into how dietary or intravenously administered PS supplements function to produce cognitive improvement.
10. Conclusion
A body of evidence supports the functional significance of PS in the brain. Underlying mechanisms are still unfolding; however, PS facilitates the activation of signaling proteins and receptors that are critical for neuronal survival, differentiation and synaptic neurotransmission. Despite its constitutive nature, membrane PS is often an indispensable participant in signaling events and/or influences the signaling in a concentration-dependent manner. The PS biosynthesis preferentially utilizes DHA-containing phospholipids as substrates. Although membrane phospholipids are under a tight homeostatic regulation, the PS level can be altered according to the DHA status, specifically in the brain. Therefore, diet- or ethanol-induced alteration of the brain DHA level and membrane PS can influence the signaling platform in the membrane and the transmission of the signaling cues. Detailed molecular mechanisms, particularly membrane PS-protein interactions, warrant further investigation in order to obtain more insight into the functional significance of neuronal PS. Such endeavor is likely to generate new targets for controlling physiologic or pathophysiologic processes affecting brain function.
List of Abbreviations
- Akt
protein kinase B
- DHA
docosahexaenoic acid
- ERK
extracellular signal-regulated protein kinase
- NAPE
N-acylphosphatidylethanolamide
- PC
phosphatidylcholine
- PDK1
phosphoinositide-dependent kinase-1
- PE
phosphatidylethanolamine
- PEMT
phosphatidylethanolamine N-methyl transferase
- PH
pleckstrin homology
- PI3K
phosphatidylinositol 3-kinase
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- PKC
protein kinase C
- PS
phosphatidylserine
- PSD
phosphatidylserine decarboxylase
- PSS1
phosphatidylserine synthase 1
- PSS2
phosphatidylserine synthase 2
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Svennerholm L. Distribution and fatty acid composition of normal human brain. J Lipid Res. 1968;9:570–579. [PubMed] [Google Scholar]
- 2.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang BX, Akbar M, Kevala K, Kim HY. Phosphatidylserine is a critical modulator for Akt activation. J Cell Biol. 2011;192:979–992. doi: 10.1083/jcb.201005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HY, Akbar M, Lau A, Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3): Role of phosphatidylserine in antiapoptotic effect. J Biol Chem. 2000;275:35215–35223. doi: 10.1074/jbc.M004446200. [DOI] [PubMed] [Google Scholar]
- 5.Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem. 2007;282:18661–18665. doi: 10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- 6.Kim HY, Akbar M, Kim YS. Phosphatidylserine-dependent neuroprotective signaling promoted by docosahexaenoic acid. Prostagl Leukot Essent Fatty Acids. 2010;82:165–172. doi: 10.1016/j.plefa.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton AC, Keranen LM. Phophatidyl-L-serine is necessary for protein kinase C’s high-affinity interaction with diacylglycerol-containing membranes. Biochemistry. 1994;33:6651–6658. doi: 10.1021/bi00187a035. [DOI] [PubMed] [Google Scholar]
- 8.Brose N, Petrenko AG, Sudhof TC, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface necessary for protein kinase C’s high-affinity interaction with diacylglycerol-containing membranes. Science. 1994;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 9.Augustine GJ. How does calcium trigger neurotransmitter release? Curr. Opin. Neurobiol. 2001;11:320–326. doi: 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- 10.Tucker WC, Weber T, Chapman ER. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science. 2004;304:435–438. doi: 10.1126/science.1097196. [DOI] [PubMed] [Google Scholar]
- 11.Dennison SM, Bowen ME, Brunger AT, Lentz BR. PS alters the interaction of SNARE proteins with membranes so that these proteins promote rather than inhibit fusion. Biophys J. 2006;90:1661–1675. doi: 10.1529/biophysj.105.069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baudry M, Massicotti G, Hauge S. Phosphatidylserine increases the affinity of the AMPA/quasqualate receptor in rat brain membranes. Behav Neural Biol. 1991;55:137–140. doi: 10.1016/0163-1047(91)80134-z. [DOI] [PubMed] [Google Scholar]
- 13.Murray J, Cuccia L, Ianoul A, Cheetham JJ, Johnston LJ. Imaging the selective binding of synapsin to anionic membrane domains. ChemBioChem. 2004;5:1489–1494. doi: 10.1002/cbic.200400097. [DOI] [PubMed] [Google Scholar]
- 14.Shea TB. Phospholipids alter tau conformation, phosphorylation, proteolysis, and association with microtubules: Implication for tau function under normal and degenerative conditions. J Neurosci Res. 1997;50:114–122. doi: 10.1002/(SICI)1097-4547(19971001)50:1<114::AID-JNR12>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 15.Bader Lange ML, Cenini G, Piroddi M, Abdul HM, Sultana R, Galli F, et al. Loss of phospholipid asymmetry and elevated brain apoptotic protein levels in subjects with amnestic mild cognitive impairment and Alzheimer disease. Neurobiol Disease. 2008;29:456–463. doi: 10.1016/j.nbd.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozzi R, Buratta S, Goracci G. Metabolism and function of phosphatidylserine in mammalian brain. Neurochem Res. 2003;28:195–214. doi: 10.1023/a:1022412831330. [DOI] [PubMed] [Google Scholar]
- 17.Vance JE. Molecular and cell biology of phosphatidylserine and phosphatidylethanolamine metabolism. Prog Nucleic Acid Res Mol Biol. 2003;75:69–111. doi: 10.1016/s0079-6603(03)75003-x. [DOI] [PubMed] [Google Scholar]
- 18.Kuge O, Nishijima M. Biosynthetic regulation and intracellular transport of phosphatidylserine in mammalian cells. J Biochem. 2003;133:397–403. doi: 10.1093/jb/mvg052. [DOI] [PubMed] [Google Scholar]
- 19.Vance JE, Vance DE. Phospholipid biosynthesis in mammalian cells. Biochem Cell Biol. 2004;82:113–128. doi: 10.1139/o03-073. [DOI] [PubMed] [Google Scholar]
- 20.Vance JE. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res. 2008;49:1377–1387. doi: 10.1194/jlr.R700020-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Vance DE, Vance JE. Physiological consequences of disruption of mammalian phospholipid biosynthetic genes. J Lipid Res. 2009;50:S132–S137. doi: 10.1194/jlr.R800048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vance JE, Tasseva G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochem Biophy Acta. 2013;18312:543–554. doi: 10.1016/j.bbalip.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 24.Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- 25.Sturbois-Balcerzak B, Stone SJ, Sreenivas A, Vance JE. Structure and expression of the murine phosphatidylserine synthase-1 gene. J Biol Chem. 2001;276:8205–8212. doi: 10.1074/jbc.M009776200. [DOI] [PubMed] [Google Scholar]
- 26.Buratta S, Felicetti M, Mozzi R. Synthesis of phosphatidylserine by base exchange in Triton-insoluble floating fractions from rat cerebellum. J Neurochem. 2007;103:942–951. doi: 10.1111/j.1471-4159.2007.04783.x. [DOI] [PubMed] [Google Scholar]
- 27.Buratta S, Ferrera G, Mozzi R. Presence of phosphatidylserine synthesizing enzymes in the triton insoluble floating fractions from cerebrocortical plasma membranes. Do phosphatidylserine synthesizing enzymes in plasma membrane microdomains play a role in signal transduction? Neurochem Res. 2011;36:774–782. doi: 10.1007/s11064-011-0399-0. [DOI] [PubMed] [Google Scholar]
- 28.Mikhaevitch IS, Singh IN, Sorrentino G, Massarelli R, Kanfer JN. Modulation of phosphatidylserine synthesis by a muscarinic receptor occupancy in human neuroblastoma cell line LA-N-1. Biochem J. 1994;299:375–380. doi: 10.1042/bj2990375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunetti M, Gaiti A, Porcellati G. Synthesis of phosphatidylcholine and phosphatidylethanolamine at different ages in the rat brain in vitro. Lipids. 1979;11:925–931. doi: 10.1007/BF02533507. [DOI] [PubMed] [Google Scholar]
- 30.Carter JM, Waite KA, Campenot RB, Vance JE, Vance DE. Enhanced expression and activation of CTP:phosphocholine cytidylyltransferase β2 during neurite outgrowth. J Biol Chem. 2003;278:44988–44994. doi: 10.1074/jbc.M307336200. [DOI] [PubMed] [Google Scholar]
- 31.Marcucci H, Paoletti L, Jackowski S, Banchio C. Phosphatidylcholine biosynthesis during neuronal differentiation and its role in cell fate determination. J Biol Chem. 2010;285:25382–25393. doi: 10.1074/jbc.M110.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pynn CJ, Henderson NG, Clark H, Kostner G, Bernhard W, Postle AD. Specificity and rate of human and mouse liver and plasma phosphatidylcholine synthesis analyzed in vivo. J Lipid Res. 2011;52:399–407. doi: 10.1194/jlr.D011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mozzi R, Porcellati G. Conversion of phosphatidylethanolamine to phosphatidylcholine in rat brain by the methylation pathway. FEBSLett. 1979;100:363–366. doi: 10.1016/0014-5793(79)80370-1. [DOI] [PubMed] [Google Scholar]
- 34.Blusztajn JK, Zeisel SH, Wurtman RJ. Synthesis of lecithin (phosphatidylcholine) from phosphatidylethanolamine in bovine brain. Brain Res. 1979;179:319–329. doi: 10.1016/0006-8993(79)90447-5. [DOI] [PubMed] [Google Scholar]
- 35.Vance JE, Pau D, Campenot RB, Bussière M, Vance DE. Evidence that the major membrane lipids except cholesterol, are made in axons of cultured rat sympathetic neurons. J Neurochem. 1994;62:329–337. doi: 10.1046/j.1471-4159.1994.62010329.x. [DOI] [PubMed] [Google Scholar]
- 36.Vance DE. Phospholipidmethylation in mammals: from biochemistry to physiological function. Biochim Biophys Acta. 2014;1838:1477–1487. doi: 10.1016/j.bbamem.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 37.daCosta KA, Rai KS, Cracinescu CN, Parikh K, Mehedint MG, Sanders LM, et al. Dietary docosahexaenoic acid supplementation modulates hippocampal development in the Pemt−/− mouse. J Biol Chem. 2010;285:1008–1015. doi: 10.1074/jbc.M109.017137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steenbergen R, Nanowski TS, Nelson R, Young SG, Vance JE. Phospholipid homeostasis in phosphatidylserine synthase-2-deficient mice. Biochim Biophys Acta. 2006;1761:313–323. doi: 10.1016/j.bbalip.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Tasseva G, Cole L, Vance JE. N-Myc and Sp regulate phosphatidylserine synthase-1 expression in brain and glial cells. J Biol Chem. 2011;286:1061–1073. doi: 10.1074/jbc.M110.158709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim HY, Bigelow J, Kevala JH. Substrate preference in phosphatidylserine biosynthesis for docosahexaenoic acid containing species. Biochemistry. 2004;43:1030–1036. doi: 10.1021/bi035197x. [DOI] [PubMed] [Google Scholar]
- 41.Sugiura Y, Konishi Y, Zaima N, Kajihara S, Nakanishi H, Taguchi R, et al. Visualization of the cell-selective distribution of PUFA-containing phosphatidylcholines in mouse brain by imaging mass spectrometry. J Lipid Res. 2009;50:1766–1788. doi: 10.1194/jlr.M900047-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuge O, Hasegawa K, Ohsawa T, Saito K, Nishijima M. Purification and characterization of Chinese hamster phosphatidylserine synthase 2. J Biol Chem. 2003;278:42692–42698. doi: 10.1074/jbc.M307270200. [DOI] [PubMed] [Google Scholar]
- 43.Tomohiro S, Kawaguti A, Kawabe Y, Kitada S, Kuge O. Purification and characterization of human phosphatidylserine synthases 1 and 2. Biochem J. 2009;418:421–429. doi: 10.1042/BJ20081597. [DOI] [PubMed] [Google Scholar]
- 44.Kimura AK, Kim HY. Phosphatidylserine synthase 2: high efficiency for synthesizing phosphatidylserine containing docosahexaenoic acid. J Lipid Res. 2013;54:214–222. doi: 10.1194/jlr.M031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arikketh D, Nelson R, Vance JE. Defining the importance of phosphatidylserine synthase-1 (PSS1): Unexpected viability of PSS1-deficient mice. J Biol Chem. 2008;283:12888–12897. doi: 10.1074/jbc.M800714200. [DOI] [PubMed] [Google Scholar]
- 46.Bergo MO, Gavino BJ, Steenbergen R, Sturbois B, Parlow AF, Sanan DA, et al. Defining the importance of phosphatidylserine synthase 2 in mice. J Biol Chem. 2002;277:47701–47708. doi: 10.1074/jbc.M207734200. [DOI] [PubMed] [Google Scholar]
- 47.Cotman CW, Blank ML, Moehl A, Snyder F. Lipid composition of synaptic plasma membranes isolated from rat brain by zonal centrifugation. Biochemistry. 1969;8:4606–4612. doi: 10.1021/bi00839a056. [DOI] [PubMed] [Google Scholar]
- 48.Breckenridge WC, Gombos G, Morgan IG. The lipid composition of adult rat brain synaptosomal plasma membranes. Biochim Biophys Acta. 1972;266:695–707. doi: 10.1016/0006-3002(72)90012-1. [DOI] [PubMed] [Google Scholar]
- 49.Murthy M, Hamilton J, Greiner RS, Moriguchi T, Salem N, Jr, Kim HY. Differential effects of n-3 fatty acid deficiency on phospholipid molecular species composition in the rat hippocampus. J Lipid Res. 2002;43:611–617. [PubMed] [Google Scholar]
- 50.Hamilton J, Greiner R, Salem N, Jr, Kim HY. N-3 Fatty acid deficiency decreases phosphatidylserine accumulation selectively in neuronal tissues. Lipids. 2000;35:863–869. doi: 10.1007/s11745-000-0595-x. [DOI] [PubMed] [Google Scholar]
- 51.O’Brien JS, Fillerup DL, Mead JF. Quantification and fatty acid and fatty aldehyde composition of the ethanolamine, choline, and serine glycerophosphatides in human cerebral grey and white matter. J lipid Res. 1964;5:329–338. [PubMed] [Google Scholar]
- 52.Yabuuchi H, O’Brien JS. Positional distribution of fatty acids in glycerophosphatides of bovine gray matter. J Lipid Res. 1968;9:65–67. [PubMed] [Google Scholar]
- 53.Yavin E, Zeigler BP. Regulation of phospholipid metabolism in differentiating cells from rat brain cerebral hemispheres in culture. Serine incorporation into serine phosphoglycerides: base exchange and decarboxylation patterns. J Biol Chem. 1977;252:260–267. [PubMed] [Google Scholar]
- 54.Butler M, Morell P. The role of phosphatidylserine decarboxylase in brain phospholipid metabolism. J Neurochem. 1983;41:1445–1454. doi: 10.1111/j.1471-4159.1983.tb00844.x. [DOI] [PubMed] [Google Scholar]
- 55.Horrocks LA. The alk-1-enyl group content of mammalian myelin phosphoglycerides by quantitative two-dimensional thin-layer chromatography. J Lipid Res. 1968;9:469–472. [PubMed] [Google Scholar]
- 56.Deeley JM, Thomas MC, Truscott RJW, Mitchell TW, Blanksby SJ. Identification of abundant alkyl ether glycerophospholipids in the human lens by tandem mass spectrometry techniques. Anal Chem. 2009;81:1920–1930. doi: 10.1021/ac802395d. [DOI] [PubMed] [Google Scholar]
- 57.Nagy K, Brahmbhatt VV, Berdeaux O, Bretillon L, Destaillats F, Acar N. Comparative study of serine plasmalogens in human retina and optic nerve: identification of atypical species with odd carbon chains. J Lipid Res. 2012;53:776–783. doi: 10.1194/jlr.D022962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stein WH, Moore S. The free amino acids of human blood plasma. J Biol Chem. 1954;211:915–926. [PubMed] [Google Scholar]
- 59.O’Kane RL, Viña JR, Simpson I, Hawkins RA. Na+-dependent neutral amino acid transporters A, ASC, and N of the blood-brain barrier: mechanisms for neutral amino acid removal. Am J Physiol. 2004;287:E622–E629. doi: 10.1152/ajpendo.00187.2004. [DOI] [PubMed] [Google Scholar]
- 60.Hirabayashi Y, Furuya S. Roles of L-serine and sphingolipid synthesis in brain development and neuronal survival. Prog Lipid Res. 2008;47:188–203. doi: 10.1016/j.plipres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Phillips GB, Dodge GT. Composition of phospholipids and of phospholipid fatty acids of human plasma. J Lipid Res. 1967;8:676–681. [PubMed] [Google Scholar]
- 62.Méresse S, Delbart C, Fruchart JC, Cecchelli R. Low-density lipoprotein receptor on endothelium of brain capillaries. J Neurochem. 1989;53:340–345. doi: 10.1111/j.1471-4159.1989.tb07340.x. [DOI] [PubMed] [Google Scholar]
- 63.De Vries HE, Breedveld B, Kuiper J, De Boer AG, Van Berkel TJC, Breimer DD. High-density lipoprotein and cerebral endothelial cellsin vitro: Interactions and transport. Biochem Pharmacol. 1995;50:271–273. doi: 10.1016/0006-2952(95)00127-l. [DOI] [PubMed] [Google Scholar]
- 64.Dehouck B, Fenart L, Dehouck MP, Pierce A, Torpier G, Cecchelli R. A new function for the LDL receptor: Transcytosis of LDL across the blood–brain barrier. J Cell Biol. 1997;138:877–889. doi: 10.1083/jcb.138.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dietschy JM, Turley SD. Cholesterol metabolism in the central nervous system during esarly development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 66.Inuzuka M, Hayakawa M, Ingi T. Serinc, an activity-regulated protein family, incorporates serine into membrane lipid synthesis. J Biol Chem. 2005;280:35776–35783. doi: 10.1074/jbc.M505712200. [DOI] [PubMed] [Google Scholar]
- 67.Vargas-Lopes C, Madeira C, Kahn SA, Albino do Couto I, Bado P, Houzel JC, et al. Protein kinase C activity regulates D-serine availability in the brain. J Neurochem. 2011;116:281–290. doi: 10.1111/j.1471-4159.2010.07102.x. [DOI] [PubMed] [Google Scholar]
- 68.Rutter AR, Fradley RL, Garrett EM, Chapman KL, Lawrence JM, Rosahl TW, et al. Evidence from gene knockout studies implicates Asc-1 as the primary transporter mediating D-serine uptake in the mouse CNS. Eur J Neurosci. 2007;25:1757–1766. doi: 10.1111/j.1460-9568.2007.05446.x. [DOI] [PubMed] [Google Scholar]
- 69.Pollegioni L, Sacchi S. Metabolism of the neuromodulator D-serine. Cell Mol Life Sci. 2010;67:2387–2314. doi: 10.1007/s00018-010-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Omori T, Mihara H, Kurihara T, Esaki N. Occurrence of phosphatidyl-d-serine in the rat cerebrum. Biochem Biophys Res Commun. 2009;382:415–418. doi: 10.1016/j.bbrc.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 71.Omori T, Mihara H, Kurihara T, Esaki N. The distribution of phosphatidyl-D-serine in the rat. Biosci Biotechno Biochem. 2010;74:1953–1955. doi: 10.1271/bbb.100271. [DOI] [PubMed] [Google Scholar]
- 72.Yang JH, Wada A, Yoshida K, Miyoshi Y, Sayano T, Esaki K, et al. Brain-specific Phgdh deletion reveals a pivotal role for L-serine biosynthesis in controlling the level of D-serine, an N-methyl-D-aspartate receptor co-agonist, in adult brain. J Biol Chem. 2010;285:41380–41390. doi: 10.1074/jbc.M110.187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diniz LP, Almeida JC, Tortelli V, Lopes CV, Setti-Perdigão P, Stipurski J, et al. Astrocyte-induced synaptogenesis is mediated by transforming growth factor β signaling through modulation of D-serine levels in cerebral cortex neurons. J Biol Chem. 2012;287:41432–41445. doi: 10.1074/jbc.M112.380824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thompson M, Marecki JC, Marinesco S, Labrie V, Roder JC, Barger SW, et al. Paradoxical roles of serine racemase and D-serine in the G93A mSOD1mouse model of amyotrophic lateral sclerosis. J Neurochem. 2012;120:598–610. doi: 10.1111/j.1471-4159.2011.07601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voelker DR. Reconstitution of phosphatidylserine import into rat liver mitochondria. J Biol Chem. 1989;264:8019–8025. [PubMed] [Google Scholar]
- 76.Uchida Y, Hasegawa J, Chinnapen D, Inoue T, Okazaki S, Kato R, et al. Intracellular phosphatidylserine is essential for retrograde membrane traffic through endosomes. Proc Natl Acad Sci USA. 2011;108:15846–15851. doi: 10.1073/pnas.1109101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho KJ, Park JH, Piggott AM, Salim AA, Gorfe AA, Parton RG, et al. Staurosporines disrupt phosphatidylserine trafficking and mislocalize Ras proteins. J Biol Chem. 2012;287:43573–43584. doi: 10.1074/jbc.M112.424457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maeda K, Anand K, Chiapparino A, Kumar A, Poletto M, Kaksonen M, et al. Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature. 2013;501:257–261. doi: 10.1038/nature12430. [DOI] [PubMed] [Google Scholar]
- 79.Coleman JA, Kwok MCM, Molday RS. Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J Biol Chem. 2009;284:32670–32679. doi: 10.1074/jbc.M109.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baldridge RD, Graham TR. Identification of residues defining phospholipid flippase substrate specificity of type IV ATPqases. Proc Nat Acad Sci USA. 2012;109:E290–E298. doi: 10.1073/pnas.1115725109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Brantton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 82.Bevers EM, Comfurius P, van Rijn JL, Hemker HC, Zwaal RF. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur J Biochem. 1982;122:429–436. doi: 10.1111/j.1432-1033.1982.tb05898.x. [DOI] [PubMed] [Google Scholar]
- 83.Cusulin C, Monni E, Ahlenius H, Wood J, Brune JC, Lindvall O, et al. Embryonic stem cell-derived neural stem cells fuse with microglia and mature neurons. Stem Cells. 2012;30:2657–2671. doi: 10.1002/stem.1227. [DOI] [PubMed] [Google Scholar]
- 84.Calderon F, Kim HY. Detection of intracellular phosphatidylserine in living cells. J Neurochem. 2008;104:1271–1279. doi: 10.1111/j.1471-4159.2007.05079.x. [DOI] [PubMed] [Google Scholar]
- 85.Yeung T, Gilbert GE, Shi J, Kapus A, Grinstein S. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- 86.Daleke DL. Phospholipid flippases. J Biol Chem. 2007;282:821–825. doi: 10.1074/jbc.R600035200. [DOI] [PubMed] [Google Scholar]
- 87.Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–242. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- 88.Ding J, Wu Z, Crider BP, Ma Y, Li X, Slaughter C, Gong L, Xie XS. Identification and functional expression of four isoforms of ATPase II, the putative aminophospholipid translocase: Effect of isoform variation of the ATPase activity and phospholipid selectivity. J Biol Chem. 2000;275:23378–23386. doi: 10.1074/jbc.M910319199. [DOI] [PubMed] [Google Scholar]
- 89.Meguro M, Kashigawi A, Mitsuya K, Nakao M, Kondo I, Saitoh S, et al. A novel maternally expressed gene, ATP10C, encodes a putative aminophospholipid translocase associated with Angelman syndrome. Nature Genetics. 2001;28:19–20. doi: 10.1038/ng0501-19. [DOI] [PubMed] [Google Scholar]
- 90.Levano K, Punia V, Raghunath M, Debata PR, Curcio GM, Mogha A, et al. Atp8a1 deficiency is associated with phosphatidylserine externalization in the hippocampus and delayed hippocampus-dependent learning. J Neurochem. 2012;120:302–313. doi: 10.1111/j.1471-4159.2011.07543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu X, Libby RT, de Vries WN, Smith RS, Wright DL, Bronson RT, et al. Mutations of a P-type ATPase gene cause axonal degeneration. PLoS Genet. 2012;8(8):e1002853. doi: 10.1371/journal.pgen.1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coleman JA, Zhu X, Djajadi HR, Molday LL, Smith RS, Libby RT, et al. Phospholipid flippase ATP8A2 is required for normal visual and auditory function and photoreceptor and spiral ganglion cell survival. J Cell Sci. 2014;127:1138–1149. doi: 10.1242/jcs.145052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Voelker DR, Frazier JL. Isolation and characterization of a Chinese hamster ovary cell line requiring. J Biol Chem ethanolamine or phosphatidylserine for growth and exhibiting defective phosphatidylserine synthase activity. 1986;261:1002–1008. [PubMed] [Google Scholar]
- 94.Borkenhagen LF, Kennedy EP, Fielding L. Enzymatic formation and decarboxylation of phosphatidylserine. J Biol Chem. 1961;236:PC28–PC30. [Google Scholar]
- 95.Ikemoto A, Kobayashi T, Emoto K, Umeda M, Watanabe S, Okuyama H. Effects of docosahexaenoic and arachidonic acids on the synthesis and distribution of aminophospholipids during neuronal differentiation of PC12 cells. Arch Biochem Biophys. 1999;364:67–74. doi: 10.1006/abbi.1999.1110. [DOI] [PubMed] [Google Scholar]
- 96.Gohil VM, Greenberg ML. Mitochondrial membrane biogenesis: phospholipids and proteins go hand in hand. J Cell Biol. 2009;184:469–472. doi: 10.1083/jcb.200901127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Horvath SE, Böttinger L, Vögtle FN, Wiedemann N, Meisinger C, Becker T, et al. Processing and topology of the yeast mitochondrial phosphatidylserine decarboxylase 1. J Biol Chem. 2012;287:36744–36755. doi: 10.1074/jbc.M112.398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kevala JH, Kim HY. Determination of substrate preference in phosphatidylserine decarboxylation by liquid chromatography–electrospray ionization mass spectrometry. Anal Biochem. 2001;292:130–138. doi: 10.1006/abio.2001.5076. [DOI] [PubMed] [Google Scholar]
- 99.Bleijerveld OB, Brouwers JFHM, Vaandrager AB, Helms JB, Houweling M. The CDP-ethanolamine pathway and phosphatidylserine decarboxylation generate different phosphatidylethanolamine molecular species. J Biol Chem. 2007;282:28362–28372. doi: 10.1074/jbc.M703786200. [DOI] [PubMed] [Google Scholar]
- 100.Camici O, Corazzi L. Import of phosphatidylethanolamine for the assembly of rat brain mitochondrial membranes. J Membrane Biol. 1995;148:169–176. doi: 10.1007/BF00207272. [DOI] [PubMed] [Google Scholar]
- 101.Voelker DR. Disruption of phosphatidylserine translocation to the mitochondria in baby hamster kidney cells. J Biol Chem. 1985;260:14671–14676. [PubMed] [Google Scholar]
- 102.Voelker DR. Phosphatidylserine functions as the major precursor of phosphatidylethanolamine in cultured BHK-21 cells. Proc Natl Acad Sci USA. 1984;81:2669–2673. doi: 10.1073/pnas.81.9.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuge O, Nishijima M, Akamatsu Y. Phosphatidylserine-dependent neuroprotective signaling promoted by docosahexaenoic acid. Proc Natl Acad Sci USA. 1985;82:1926–1930. doi: 10.1073/pnas.82.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuge O, Nishijima M, Akamatsu Y. Phosphatidylserine biosynthesis in cultured Chinese hamster ovary cells. II. Isolation and characterization of phosphatidylserine auxotrophs. J Biol Chem. 1986;261:5790–5794. [PubMed] [Google Scholar]
- 105.Leonardi R, Frank MW, Jackson PD, Rock CO, Jackowski S. Elimination of the CDP-ethanolamine pathway disrupts hepatic lipid homeostasis. J Biol Chem. 2009;284:27077–27089. doi: 10.1074/jbc.M109.031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bradford PG, Marinetti GV, Abood LG. Stimulation of phospholipase A2 and secretion of catecholamines from brain synaptosomes by potassium and A23187. J Neurochem. 1983;41:1684–1693. doi: 10.1111/j.1471-4159.1983.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 107.Kelleher JA, Sun GY. Enzymic hydrolysis of arachidonoyl-phospholipids by rat brain synaptosomes. Neurochem Int. 1985;7:825–831. doi: 10.1016/0197-0186(85)90038-5. [DOI] [PubMed] [Google Scholar]
- 108.Cao J, Shan D, Revett T, Li D, Wu L, Liu W, et al. Molecular identification of a novel mammalian brain isoform of acyl-CoA:lysophospholipid acyltransferase with prominent ethanolamine lysophospholipid acylating activity, LPEAT. J Biol Chem. 2008;283:19049–19057. doi: 10.1074/jbc.M800364200. [DOI] [PubMed] [Google Scholar]
- 109.Ross BM, Kish SJ. Characterization of lysophospholipid metabolizing enzymes in human brain. J Neurochem. 1994;63:1839–1848. doi: 10.1046/j.1471-4159.1994.63051839.x. [DOI] [PubMed] [Google Scholar]
- 110.James OA, MacDonald G, Thompson W. Acylation of lysophosphatidylserine by rat brain microsomes. J Neurochem. 1979;33:1061–1066. doi: 10.1111/j.1471-4159.1979.tb05242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rhodes PG, Hu ZY, Sun GY. Lysophosphatidylserine enhances the transfer of 22:6n3 to lysophosphatidic acid in rat brain microsomes. Life Sci. 1991;49:225–232. doi: 10.1016/0024-3205(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 112.Vaswani KK, Ledeen RW. Purified rat brain myelin contains measurable acyl-CoA:lysophospholipid acyltransferase(s) but little, if any, glycerol-3-phosphate acyltransferase. J Neurochem. 1989;52:69–74. doi: 10.1111/j.1471-4159.1989.tb10899.x. [DOI] [PubMed] [Google Scholar]
- 113.Blankman JL, Long JZ, Trauger SA, Siuzdak G, Cravatt BF. ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc Natl Acad Sci USA. 2013;110:1500–1505. doi: 10.1073/pnas.1217121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Makide K, Kitamura H, Sato Y, Okutani M, Aoki J. Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostagl other Lipid Mediat. 2009;89:135–139. doi: 10.1016/j.prostaglandins.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 115.Frasch SC, Bratton DL. Emerging role for lysophosphatidylserine in resolution of inflammation. Prog Lipid Res. 2012;51:199–207. doi: 10.1016/j.plipres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guan Z, Li S, Smith DC, Shaw WA, Raetz CRH. Identification of N-acylphosphatidylserine molecules in eukaryotic cells. Biochemistry. 2007;46:14500–14513. doi: 10.1021/bi701907g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schmid HHO, Schmid PC, Natarajan V. N-acylated glycerophospholipids and their derivatives. Prog Lipid Res. 1990;29:1–43. doi: 10.1016/0163-7827(90)90004-5. [DOI] [PubMed] [Google Scholar]
- 118.Cadas H, di Tomaso E, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci. 1997;17:1226–1242. doi: 10.1523/JNEUROSCI.17-04-01226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guiffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 120.Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.DeMarzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 122.Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- 123.Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, et al. A biosynthetic pathway for anandamide. Proc Natl Acad Sci USA. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Simon GM, Cravatt BF. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1and detection of the glycerophospho N-acyl ethanolamine precursors in mouse brain. J Biol Chem. 2008;283:9341–9349. doi: 10.1074/jbc.M707807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tan B, O’Dell DK, Yu YW, Monn MF, Hughes HV, Burstein S, et al. Identification of endogenous acyl amino acids based on a targeted lipidomics approach. J Lipid Res. 2010;51:112–119. doi: 10.1194/jlr.M900198-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo J, Williams DJ, Ikeda SR. N-Arachidonoyl L-serine, a putative endocannabinoid, alters the activation of N-type Ca2+ channels in sympathetic neurons. J Neurophysiol. 2008;100:1147–1151. doi: 10.1152/jn.01204.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Batkai S, et al. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci USA. 2006;103:2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Godlewski G, Offertáler L, Osei-Hyiaman D, Mo FM, Harvey-White J, Davis MI, et al. The endogenous brain constituent N-arachidonoyl L-serine Is an activator of large conductance Ca2+-ctivated K+ channels. J Pharmacol Exp Therap. 2009;328:351–361. doi: 10.1124/jpet.108.144717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang X, Maor Y, Wang JF, Kunos G, Groopman JE. Endocannabinoid-like N-arachidonoyl serine is a novel pro-angiogenic mediator. Br J Pharmacol. 2010;160:1583–1594. doi: 10.1111/j.1476-5381.2010.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smoum R, Bar A, Tan B, Milman G, Attar-Namdar M, Ofek O, et al. Oleoyl serine, an endogenous N-acyl amide, modulates bone remodeling. Proc Natl Acad Sci, USA. 2010;107:17710–17715. doi: 10.1073/pnas.0912479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Medkova M, Cho W. Mutagenesis of the C2 domain of protein kinase C-α. J Biol Chem. 1998;273:17544–17552. doi: 10.1074/jbc.273.28.17544. [DOI] [PubMed] [Google Scholar]
- 133.Murray D, Honig B. Electrostatic control of the membrane targeting of C2 domains. Mol. Cell. 2002;9:145–154. doi: 10.1016/s1097-2765(01)00426-9. [DOI] [PubMed] [Google Scholar]
- 134.Stahelin RV, Rafter JD, Das S, Cho W. The molecular basis of differential subcellular localization of C2 domains of protein kinase C-α and group IVa cytosolic phospholipase A2. J Biol. Chem. 2003;278:12452–12460. doi: 10.1074/jbc.M212864200. [DOI] [PubMed] [Google Scholar]
- 135.Verdaguer N, Corbalan-Garcia S, Ochoa WF, Fita I, Gomez-Fernandez JC. Ca2+ bridges the C2 membrane-binding domain of protein kinase C directly to phosphatidylserine. EMBO J. 1999;18:6329–6338. doi: 10.1093/emboj/18.22.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ananthanarayanan B, Das S, Rhee SG, Murray D, Cho W. Membrane targeting of C2 domains of phospholipase C-δ isoforms. J Biol Chem. 2002;277:3568–3575. doi: 10.1074/jbc.M109705200. [DOI] [PubMed] [Google Scholar]
- 137.Swairjo MA, Concha NO, Kaetzel MA, Dedman JR, Seaton BA. Ca2+-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nature Struct Biol. 1995;2:968–974. doi: 10.1038/nsb1195-968. [DOI] [PubMed] [Google Scholar]
- 138.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signaling to membrane dynamics. Mol Cell.Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 139.Huber R, Romisch J, Paques E-P. The crystal and molecular structure of human annexin V, an anticoagulant protein that binds to calcium and membranes. EMBO J. 1990;9:3867–3874. doi: 10.1002/j.1460-2075.1990.tb07605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lucas N, Cho W. Phosphatidylserine binding is essential for plasma membrane recruitment and signaling function of 3-phosphoinositide-dependent kinase 1. J Biol Chem. 2011;286:41265–41272. doi: 10.1074/jbc.M111.300806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Improta-Brears T, Ghosh S, Bell RM. Mutational analysis of Raf-1 cysteine rich domain: requirement for a cluster of basic aminoacids for interaction with phosphatidylserine. Mol Cell Biochem. 1999;198:171–178. doi: 10.1023/a:1006981411691. [DOI] [PubMed] [Google Scholar]
- 142.Macedo-Ribeiro S, Bode W, Huber R, Quinn-Allen MA, Kim SW, Ortel TL, et al. Crystal structures of the membrane-binding C2 domain of human coagulation factor V. Nature. 1999;402:434–439. doi: 10.1038/46594. [DOI] [PubMed] [Google Scholar]
- 143.Shi J, Heegaard CW, Rasmussen JT, Gilbert GE. Lactadherin binds selectively to membranes containing phosphatidyl-L-serine and increased curvature. Biochim. Biophys. Acta. 2004;1667:80–90. doi: 10.1016/j.bbamem.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 144.Johnson JE, Giorgione J, Newton AC. The C1 and C2 domains of protein kinase C are independent membrane targeting modules, with specificity for phosphatidylserine conferred by the C1 domain. Biochemistry. 2000;39:11360–11369. doi: 10.1021/bi000902c. [DOI] [PubMed] [Google Scholar]
- 145.Brazil DP, Hemmings BA. Ten Years of protein kinase B signaling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 146.Andjelkovic M, Alessi DR, Meier R, Fermandez A, Lamb NJC, Frech M, et al. Role of Translocation in the Activation and Function of Protein Kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 147.Kuge O, Hasegawa K, Saito K, Nishijima M. Control of phosphatidylserine biosynthesis through phosphatidylserine-mediated inhibition of phosphatidylserine synthase I in Chinese hamster ovary cells. Proc Natl Acad Sci USA. 1998;95:4199–4203. doi: 10.1073/pnas.95.8.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PRJ, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 149.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Holmes AB, Gaffney PRJ, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 150.Leevers SJ, Paterson HF, Marshall CJ. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 151.Levi de Stein M, Medina JH, De Robertis E. In vivo and in vitro modulation of central type benzodiazepine receptors by phosphatidylserine. Molec Brain Res. 1989;5:9–15. doi: 10.1016/0169-328x(89)90012-0. [DOI] [PubMed] [Google Scholar]
- 152.Kimura T, Yeliseev AA, Vukoti K, Rhodes SD, Cheng K, Rice KC, et al. Recombinant cannabinoid type 2 receptor in liposome model activates G protein in response to anionic lipid constituents. J Biol Chem. 2012;287:4076–4087. doi: 10.1074/jbc.M111.268425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Südhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bhalla A, Tucker WC, Chapman ER. Synaptogamin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentrations to SNARE-mediated membrane fusions. Mol Biol Cell. 2005;16:4755–4764. doi: 10.1091/mbc.E05-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hui E, Johnson CP, Yao J, Dunning FM, Chapman ER. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell. 2009;138:709–721. doi: 10.1016/j.cell.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 157.Deutsch JW, Kelly RB. Lipids of synaptic vesicles: relevance to the mechanism of membrane fusion. Biochemistry. 1981;20:378–385. doi: 10.1021/bi00505a024. [DOI] [PubMed] [Google Scholar]
- 158.Wang Z, Liu H, Gu Y, Chapman ER. Reconstituted synaptotagmin I mediates vesicle docking, priming, and fusion. J Cell Biol. 2011;195:1159–1170. doi: 10.1083/jcb.201104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Watanabe Y, Nishio M, Hamaji S, Hagashi Y, Hu Y, Hidaka H. Neuronal nitric oxide synthase-membrane phospholipid interactions. Arch Biochem Biophys. 1998;358:68–73. doi: 10.1006/abbi.1998.0820. [DOI] [PubMed] [Google Scholar]
- 160.Ostroumova OS, Schagima LV, Mosevitsky MI, Zakharov VV. Ion channel activity of brain abundant protein BASP1 in planar lipid bilayers. FEBS J. 2011;278:461–469. doi: 10.1111/j.1742-4658.2010.07967.x. [DOI] [PubMed] [Google Scholar]
- 161.Yamamoto H, Kida Y, Sakaguchi M. Phosphatidylserine-binding protein lactadherin inhibits protein translocation across ER membrane. Biochem Biophys Res Commun. 2013;434:620–626. doi: 10.1016/j.bbrc.2013.03.131. [DOI] [PubMed] [Google Scholar]
- 162.Baihong HY, Subramanian V, Choi BH, Liang Y, Hanikishore A, Chakraboty G, et al. NMR structure of C2 domain of MFG-E8 and insights into its molecular recognition with phosphatidylserine. Biochim Biophys Acta. 2013;1828:1083–1093. doi: 10.1016/j.bbamem.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 163.Stahelin RV, Hwang JH, Kim JH, Park ZY, Johnson KR, Obeid LM, et al. The mechanism of membrane targeting of human sphingosine kinase 1. J Biol Chem. 2005;280:43030–43038. doi: 10.1074/jbc.M507574200. [DOI] [PubMed] [Google Scholar]
- 164.Morrison K, Witte K, Mayers JB, Schuh AL, Audhya A. Roles of acidic phospholipids and nucleotides in regulating membrane binding and activity of a calcium-independent phospholipase A2 isoform. J Biol Chem. 2012;287:38824–38834. doi: 10.1074/jbc.M112.391508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tu-sekine B, Rabin DM. Dual regulation of diacylglycerol kinase (DGK)-θ. Polybasic protein promote activation by phospholipids and increase substrate affinity. J Biol Chem. 2012;287:41619–41627. doi: 10.1074/jbc.M112.404855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sot B, Behrmann E, Raunser S, Wittinghofer A. Ras GTPase activating (GAP) activity of the dual specificity GAP protein Rasal requires colocalization and C2 domain binding to lipid membranes. Proc Natl Acad Sci USA. 2013;110:111–116. doi: 10.1073/pnas.1201658110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lai CL, Srivastava A, Pilling C, Chase AR, Falke JJ, Voth GA. Molecular mechanism of membrane searching of the GRP1 PH domain. J Mol Biol. 2013;425:3073–3090. doi: 10.1016/j.jmb.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mishra NK, Peleg Y, Cirri E, Belogus T, Lifshitz Y, Voelker DR, et al. FXYD proteins stabilize Na,K-ATPase: Amplification of specific phosphatidylserine-protein interactions. J Biol Chem. 2011;286:9699–9712. doi: 10.1074/jbc.M110.184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kapri-Pardes E, Katz A, Havlv H, Mahmmoud Y, Ilan M, Khalfin-Penigel I, et al. Stabilization of the a2 isoform of Na,K-ATPase by mutations in a phospholipid binding pocket. J Biol Chem. 2011;286:42888–42899. doi: 10.1074/jbc.M111.293852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Orlowski A, Gryzbck M, Bunker A, Pasenliewicz-Gierula M, Vattulainen I, Mannisto PT, et al. Strong preference for dopamine and L-dopa towards lipid head group: importance for lipid composition and implications for neurotransmitter metabolism. J Neurochem. 2012;122:681–690. doi: 10.1111/j.1471-4159.2012.07813.x. [DOI] [PubMed] [Google Scholar]
- 171.Wang C, Ye F, Velardez GF, Peters GH, Westh P. Affinity of four polar neurotransmitters for lipid bilayer membranes. J Phys Chem B. 2011;115:196–203. doi: 10.1021/jp108368w. [DOI] [PubMed] [Google Scholar]
- 172.Hamazaki K, Choi KH, Kim HY. Phospholipid profile in the postmortem hippocampus of patients with schizophrenia and bipolar disorder: No changes in docosahexaenoic acid species. J Psych Res. 2010;44:688–693. doi: 10.1016/j.jpsychires.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Eto M, Shindou H, Shimizu T. A novel lysophosphatidic acid acyltransferase enzyme (LPAAT4) with a possible role for incorporating docosahexaenoic acid into brain glycerophospholipids. Biochem Biophys Res Commun. 2014;443:718–724. doi: 10.1016/j.bbrc.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 174.Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 175.Cao D, Xue R, Xu J, Liu Z. Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J Nutr Biochem. 2004;16:538–546. doi: 10.1016/j.jnutbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 176.Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139:991–997. doi: 10.1016/j.neuroscience.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 177.Kim HY, Moon HS, Cao D, Lee J, Kevala K, Jun SB, et al. N-docosylhexaenoylethanolamide promotes development of hippocampal neurons. Biochem J. 2011;435:327–336. doi: 10.1042/BJ20102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rashid MA, Katakura M, Khavebava G, Kim HY. N- docosylhexaenoylethanolamine is a potent neurogenic factor for neural stem cell differentiation. J. Neurochem. 2013;125:869–884. doi: 10.1111/jnc.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim HY, Akbar M, Lau A. Effects of docosapentaenoic acid on neuronal apoptosis. Lipids. 2003;38:453–457. doi: 10.1007/s11745-003-1083-z. [DOI] [PubMed] [Google Scholar]
- 180.Guo M, Stockert L, Akbar M, Kim HY. Neuronal specific increase of phosphatidylserine by docosahexaenoic acid. J Molec Neurosci. 2007;33:67–73. doi: 10.1007/s12031-007-0046-z. [DOI] [PubMed] [Google Scholar]
- 181.Tam O, Innis SM. Dietary polyunsaturated fatty acids in gestation alter fetal cortical phospholipids, Fatty acids and phosphatidylserine synthesis. Develop Neurosci. 2006;28:222–229. doi: 10.1159/000091920. [DOI] [PubMed] [Google Scholar]
- 182.Akbar M, Baick J, Calderon F, Wen Z, Kim HY. Ethanol promotes neuronal apoptosis by inhibiting phosphatidylserine accumulation. J Neurosci Res. 2006;83:432–440. doi: 10.1002/jnr.20744. [DOI] [PubMed] [Google Scholar]
- 183.Wen Z, Kim HY. Inhibition of phosphatidylserine biosynthesis by ethanol in developing rat brain. J Neurosci Res. 2007;85:1568–1578. doi: 10.1002/jnr.21263. [DOI] [PubMed] [Google Scholar]
- 184.Carrasco MP, Jiménez-López JM, Segovia JL, Marco C. Effects of ethanol on the remodeling of neutral lipids and phospholipids in brain mitochondria and microsomes. Neurochem Internat. 2007;50:858–865. doi: 10.1016/j.neuint.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 185.Petursdottir AL, Farr SA, Morley JE, Banks WA, Skuladottir GV. Lipid peroxidation in brain during aging in the senescence-accelerated mouse (SAM) Neurobiol Aging. 2007;28:1170–1178. doi: 10.1016/j.neurobiolaging.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 186.Cunnane SC, Schneider JA, Tangney C, Tremblay-Mercier J, Fortier M, Bennett DA, et al. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J Alzheimer’s Dis. 2012;29:691–697. doi: 10.3233/JAD-2012-110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ullman L, Mimouni V, Roxx S, Porsolt R, Poisson JP. Brain and hippocampus fatty acid composition in the phospholipid classes of age-related cognitive deficit rats. Prostagl Leukot Essent Fatty Acid. 2001;64:189–195. doi: 10.1054/plef.2001.0260. [DOI] [PubMed] [Google Scholar]
- 188.Drago F, Canonico PL, Scapagini U. Behavioral effects of phosphatidylserine in aged rats. Neurobiol Aging. 1981;2:209–213. doi: 10.1016/0197-4580(81)90023-3. [DOI] [PubMed] [Google Scholar]
- 189.Corwin J, Dean RL, III, Bartus RT, Rotrosen J, Watkins DL. Behavioral effects of phosphatidylserine in the aged Fischer 344 rats: Amelioration of passive avoidance deficits without changes in psychomotor task performance. Neurobiol Aging. 1985;6:11–15. doi: 10.1016/0197-4580(85)90065-x. [DOI] [PubMed] [Google Scholar]
- 190.Lee B, Sur BJ, Han JJ, Shim I, Her S, Lee HJ, et al. Krill phosphatidylserine improves learning and memory in Morris water maze in aged rats. Prog Neuropsychopharmacol Biol Psych. 2010;34:1085–1093. doi: 10.1016/j.pnpbp.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 191.Park HJ, Shim HS, Kim KS, Han JJ, Kim JS, Yu AR, et al. Enhanced learning and memory of normal young rats by repeated oral administration of Krill phosphatidylserine. Nutr Neurosci. 2012;16:47–53. doi: 10.1179/1476830512Y.0000000029. [DOI] [PubMed] [Google Scholar]
- 192.Delwaide PJ, Gyselynck-Mambourg AM, Hurlet A, Ylieff M. Double blind randomized controlled study of phosphatidylserine in senile demented patients. Acta Neurol Scand. 1986;73:136–140. doi: 10.1111/j.1600-0404.1986.tb03254.x. [DOI] [PubMed] [Google Scholar]
- 193.Baumeister J, Barthel T, Geiss KR, Weiss M. Influence of phosphatidylserine on cognitive performance and cortical activity after induced stress. Nutr Neurosci. 2008;11:103–110. doi: 10.1179/147683008X301478. [DOI] [PubMed] [Google Scholar]
- 194.Jorissen BL, Brorens F, Van Boxtel MPJ, Riedel WJ. Safety of soy-derived phosphatidylserine in elderly people. Nutr Neurosci. 2002;5:337–343. doi: 10.1080/1028415021000033802. [DOI] [PubMed] [Google Scholar]
- 195.Richter Y, Herzog Y, Cohen T, Steinhart Y. The effect of phosphatidylserine-containin omega-3 fatty acids on memory abilities in subjects with subjective memory complaints; a pilot study. Clin Intervent Aging. 2010;5:313–316. doi: 10.2147/CIA.S13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vakhapova V, Cohen T, Richter Y, Herzog Y, Korczyn AD. Phosphatidylserine containing ω-3 fatty acids may improve memory abilities in non-demented elderly with memory complaints: A double-blind placebo-controlled trial. Dement Geriatr Cogn Disord. 2010;29:467–474. doi: 10.1159/000310330. [DOI] [PubMed] [Google Scholar]
- 197.Kato-Kataoka A, Sakai M, Ebina R, Nonaka C. Soybean derived phosphatidylserine improves memory function of the elderly Japanese subjects with memory complaints. J Clin Biochem Nutr. 2010;47:246–255. doi: 10.3164/jcbn.10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Leon A, Benvegnú D, Toffano G, Orlando P, Massari P. Effect of brain cortex phospholipids on adenylate-cyclase activity in mouse brain. J Neurochem. 1978;30:23–26. doi: 10.1111/j.1471-4159.1978.tb07030.x. [DOI] [PubMed] [Google Scholar]
- 199.Casamenti F, Mantovani P, Amaducci L, Papeu G. Effect of phosphatidylserine on acetylcholine output from the cerebral cortex of rats. J Neurochem. 1979;32:529–533. doi: 10.1111/j.1471-4159.1979.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 200.Casamenti F, Scali C, Papeu G. Phosphatidylserine reverses the age-dependent decrease in cortical acetylcholine release: a microdialysis study. Eur J Pharmacol. 1991;194:11–16. doi: 10.1016/0014-2999(91)90117-9. [DOI] [PubMed] [Google Scholar]