Abstract
Autoimmune pancreatitis (AIP) is characterized by obstructive jaundice, a dramatic clinical response to steroids and pathologically by a lymphoplasmacytic infiltrate, with or without a pancreatic mass. Type 1 AIP is the pancreatic manifestation of an IgG4-related systemic disease and is characterized by elevated IgG4 serum levels, infiltration of IgG4-positive plasma cells and extrapancreatic lesions. Type 2 AIP usually has none or very few IgG4-positive plasma cells, no serum IgG4 elevation and appears to be a pancreas-specific disorder without extrapancreatic involvement. AIP is diagnosed in approximately 2%-6% of patients that undergo pancreatic resection for suspected pancreatic cancer. There are three patterns of autoimmune pancreatitis: diffuse disease is the most common type, with a diffuse, “sausage-like” pancreatic enlargement with sharp margins and loss of the lobular contours; focal disease is less common and manifests as a focal mass, often within the pancreatic head, mimicking a pancreatic malignancy. Multifocal involvement can also occur. In this paper we describe the features of AIP at ultrasonography, computed tomography, magnetic resonance and positron emission tomography/computed tomography imaging, focusing on diagnosis and differential diagnosis with pancreatic ductal adenocarcinoma. It is of utmost importance to make an early correct differential diagnosis between these two diseases in order to identify the optimal therapeutic strategy and to avoid unnecessary laparotomy or pancreatic resection in AIP patients. Non-invasive imaging plays also an important role in therapy monitoring, in follow-up and in early identification of disease recurrence.
Keywords: Autoimmune pancreatitis, Pancreatic imaging, Ultrasonography, Computed tomography, Magnetic resonance
Core tip: In this paper we describe the features of autoimmune pancreatitis (AIP) at ultrasonography, computed tomography, magnetic resonance and positron emission tomography/computed tomography imaging, focusing on diagnosis and differential diagnosis with pancreatic ductal adenocarcinoma, which has a similar imaging appearance but a completely different therapeutic management. It is of utmost importance to make an early correct differential diagnosis between these two diseases in order to identify the optimal therapeutic strategy and to avoid unnecessary laparotomy or pancreatic resection in AIP patients. Non-invasive imaging plays also an important role in therapy monitoring, in follow-up and in early identification of disease recurrence.
INTRODUCTION
Autoimmune pancreatitis (AIP) is a distinct form of pancreatitis frequently characterized by obstructive jaundice and by a dramatic clinical response to steroids; pathologically, it is characterized by a lymphoplasmacytic infiltrate, with or without a pancreatic mass. The term AIP was first used in 1995 by Yoshida et al[1] to describe a type of chronic pancreatitis associated with a Sjogren-like syndrome. Recently AIP was divided into type 1 and type 2 which have distinct histopathology, clinical features and different diagnostic criteria[2-4].
Type 1 AIP is also called lymphoplasmacytic sclerosing pancreatitis (LPSP) or AIP without granulocyte epithelial lesions (GEL) and pathology of the pancreas shows four characteristic features[3-7]: (1) Dense periductal infiltration of plasma cells and lymphocytes; (2) Peculiar storiform fibrosis; (3) Venulitis with lymphocytes and plasma cells often leading to obliteration of the affected veins; and (4) Abundant IgG4-positive plasma cells.
Type 1 AIP seems to be the pancreatic manifestation of an IgG4-related systemic disease, characterized by elevated IgG4 serum levels, infiltration of IgG4-positive plasma cells and extrapancreatic lesions (e.g., sclerosing cholangitis, sclerosing sialoadenitis and retroperitoneal fibrosis). This form of AIP presents predominantly with obstructive jaundice in elderly male subjects; both pancreatic and extrapancreatic manifestations respond to steroid therapy. The clinical diagnosis of LPSP can be made without need for a histology sample[3-7].
Type 2 AIP is also defined idiopathic duct-centric pancreatitis (IDCP) or AIP with GEL[3-10]. It shares with LPSP some histopathological features, such as periductal lymphoplasmocytic infiltrates and storiform fibrosis. A characteristic feature of IDCP are GELs: intraluminal and intraepithelial neutrophils, leading to destruction and obliteration of pancreatic duct lumen. IDCP usually has none or very few IgG4-positive plasma cells, no serum IgG4 elevation and appears to be a pancreas-specific disorder without extrapancreatic involvement. Approximately 30% of reported cases of IDCP are associated with inflammatory bowel disease, frequently ulcerative colitis. Patients with IDCP are, on average, a decade younger than LPSP patients and the disease does not show a sex preference. Because IDCP patients are seronegative and lack other organ involvement, definitive diagnosis requires pancreatic histology[3-7,11].
DIAGNOSTIC CRITERIA
In 2011, the International Consensus Diagnostic Criteria (ICDC)[3] were developed by the International Association of Pancreatology after a review of existing criteria, including Japanese Pancreas Society criteria (JPS 2002, 2006)[12], HISORt criteria of the Mayo Clinic (2006, 2009)[13,14], Korean criteria (2007)[15], Asian criteria (2008)[16] and Mannheim criteria (2009)[17]. ICDC are composed of five cardinal features such as imaging of the pancreatic parenchyma on computed tomography (CT) and magnetic resonance (MR) and duct on endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), serology, other organ involvement, histology and response to steroid therapy[3]. ICDC can be used to diagnose type 1 and type 2 AIP independently[3].
EPIDEMIOLOGY
The true incidence of AIP is unknown. AIP was diagnosed in approximately 2%-6% of patients that underwent pancreatic resection for suspected pancreatic cancer[18,19]. In Japan the incidence of AIP was reported to be 0.82 per 100000 population[20].
PATHOPHISIOLOGY
The precise pathogenesis of AIP has not been elucidated. It is still unclear if IgG4 plays a direct pathogenic role in developing AIP or if their presence is an epiphenomenon[21,22]. Molecular mimicry by a microbial pathogen, which leads to a cross reaction with endogenous antigens, has been postulated as a cause of many autoimmune conditions including AIP[23,24].
CLINICAL ISSUES
The clinical presentation of AIP can be divided into acute and subacute phase. In the acute phase, the classic presentation of AIP is that of obstructive jaundice with abdominal imaging showing pancreatic enlargement[2-5,13]. Thus it is imperative to differentiate AIP from pancreatic cancer, especially in localized forms. Less commonly AIP presents with mild abdominal pain and elevated pancreatic enzymes, which may also be consistent with acute pancreatitis. In the subacute phase, after initial treatment, AIP can present with pancreatic atrophy and steatorrhea resembling chronic pancreatitis. Severe unremitting abdominal pain requiring narcotic pain medication is hardly ever present[3]. The presence of such severe pain should prompt a re-evaluation of the diagnosis. Diabetes mellitus (DM) is seen in up to 50% of patients with AIP and resolves in a proportion of patients with corticosteroid therapy[20,25].
OTHER ORGAN INVOLVEMENT
As previously stated, type 1 AIP is the pancreatic manifestation of a systemic disease. The involvment of other organs can lead to characteristic symptoms, such as xeroftalmia and xerostomia (Sjogren-like syndrome), jaundice (bile ducts involvement), and swelling in the groin (regional lymphoadenopathy). Other organ involvement that can be seen on abdominal imaging includes retroperitoneal fibrosis and renal involvement (interstitial nephritis). When present, these signs strengthen the diagnosis of AIP, and also prompt the histologic confirmation of AIP itself[5,26-28]. Less commonly, gallbladder and gastric involvement have also been described[29]. Symptoms related to other organ involvement often improve with treatment and can be useful for the assessment of treatment response[4].
IMAGING
There are three recognized patterns of AIP: diffuse, focal and multifocal. Diffuse disease is the most common type, with a diffuse, “sausage-like” pancreatic enlargement with sharp margins, loss of the lobular contours, and absence of pancreatic clefts (Figure 1)[30,31]. Focal disease is less common than diffuse disease and manifests as a focal mass, often within the pancreatic head, an appearance that may mimic that of a pancreatic malignancy (Figure 2). Focal disease tends to be relatively well demarcated and, when present, upstream dilation of the main pancreatic duct is typically milder than what is observed in patients with pancreatic carcinoma. In some patients with focal AIP, only the dorsal pancreas or the pancreatic tail is involved[32]. Multifocal involvement can also be evident.
Figure 1.
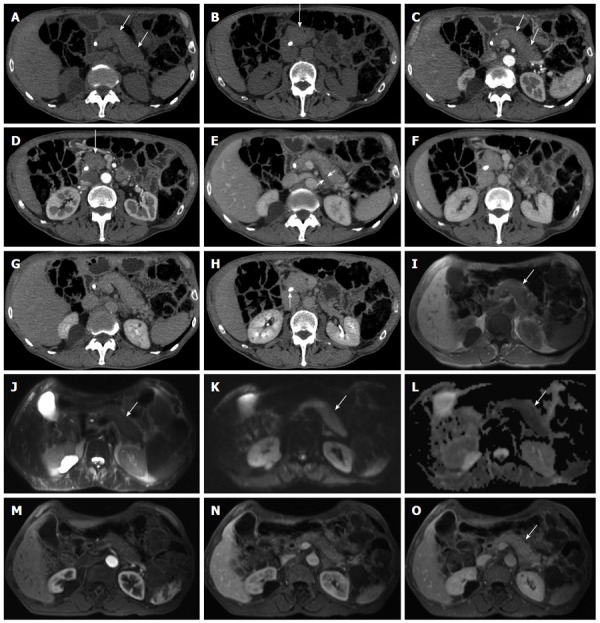
Diffuse-type autoimmune pancreatitis. A-H: Computed tomography: the pancreas appears diffusely enlarged (arrows in A-D) with a hypodense peripancreatic rim, better visible in the venous phase (arrow in E). The lesion shows fair enhancement resulting almost isodense in the delayed phase (G-H). A plastic biliary endoprothesis is visible in the common bile duct (arrow in H); I-O: Magnetic resonance: the entire organ is slightly hypointense on T1-weighted images (arrow in I) and slightly hyperintense on T2-weighted images (arrow in J), with diffusion coefficient restriction (arrows in K and L) with intermediate-high b values. At dynamic examination the pancreatic lesion presents fair enhancement resulting almost isodense in the delayed phase (arrow in O).
Figure 2.
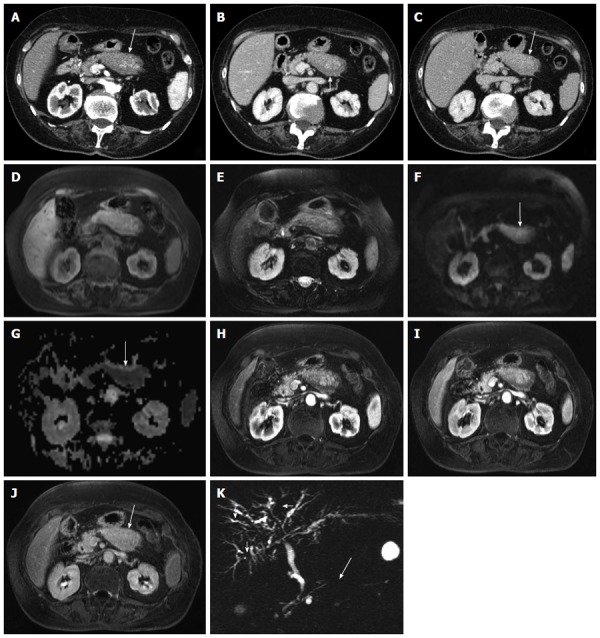
Focal-type autoimmune pancreatitis. A-C: Computed tomography: the body of the pancreas appears focally enlarged (arrow in A) with a hypodense peripancreatic rim, better visible in the venous phase (arrow in B). The lesion shows fair enhancement resulting almost isodense in the delayed phase (arrow in C); D-K: Magnetic resonance: the affected portion of the pancreas is slightly hypointense on T1-weighted fat-saturated (arrow in D) images and slightly hyperintense on T2-weighted fat-saturated images (E), with diffusion coefficient restriction (arrows in F-G) with intermediate-high b values. At dynamic examination the pancreatic lesion shows fair enhancement resulting almost isodense in the delayed phase (arrow in J). At magnetic resonance cholangiopancr-eatography the main pancreatic duct shows a focal stenosis (long arrow in K) without upstream dilation. The intrahepatic bile ducts present irregular slightly stenotic portions (short arrows in K), due to involvement in the autoimmune process.
Transabdominal ultrasonography
Conventional ultrasonography (US) is often the first imaging exam performed in presence of any abdominal symptom since it is noninvasive, inexpensive, easy to perform and widely available. US of diffuse form of AIP shows a diffusely enlarged and hypoechoic pancreatic parenchyma. In the focal and multifocal forms of AIP only the affected regions of the pancreas appear hypoechoic. This appearance, however, is not specific and includes many features commonly seen in other types of acute and chronic pancreatitis.
At color-Doppler, the enlarged pancreas can show hypervascularity[33]. Conventional US is often not able to show the irregular focal or diffuse narrowing of the main pancreatic duct or of the intrahepatic bile duct, which represents one of the main diagnostic criteria[3]. Contrast-enhanced US can successfully visualize fine vessels in pancreatic lesions and may play a pivotal role in the depiction and differential diagnosis of pancreatic tumors[34].
Computed tomography
Cross sectional pancreatic imaging is the cornerstone to the diagnosis of AIP. Quadriphasic abdominal CT and MR examinations are the imaging modalities of choice to diagnose AIP. CT scan is of utmost importance in diagnosing AIP and in confirming or ruling out pancreatic cancer. Classic features of diffuse AIP at CT are a diffusely enlarged hypodense sausage-shaped pancreas with sharp and smooth borders; decreased enhancement of the pancreatic gland in the early phase and moderate and persisting delayed enhancement in the late phase are found in 90% of the cases, a finding due to fibrosis[3,14,35,36]. Supplementary findings include a hypodense capsule-like peripheral rim with subtle delayed enhancement[35] sorrounding the pancreas (12%-40% of cases), which is believed to represent fluid, flegmon or fibrous tissue due to inflammatory changes of the peripancreatic tissues[30,31,35,36].
When AIP presents as a focal enlargement of the pancreas, it is more often located in the pancreatic head[37]. A segmental enlargement of the pancreas is seen in 30%-40% of the patients with AIP. The enlarged segment of the pancreas is typically isoattenuating or hypoattenuating to the spared, non-enlarged portion of parenchyma and may be indistinguishable from pancreatic cancer[30,36,38,39].
Unlike from many other causes of pancreatitis, peri-pancreatic stranding is usually minimal in AIP but can occur[40]. Involution of the pancreatic tail and regional lymphoadenopathy may also be seen[37]. Segmental or diffuse narrowing of the main pancreatic duct, involvement of the distal common bile duct, and multiple cholangitis-like bile duct strictures have been described but are better depicted on MR or MRCP or by means of ERCP than at CT[41,42].
Atrophic pancreatic parenchyma represents a late burnt-out phase of the disease[30,36]. This appearance may also persist after steroid therapy.
Magnetic resonance
At MR, AIP shows a similar appearance to CT: the pancreas is diffusely, focally or multifocally enlarged, and the involved portion is hypointense on T1-weighted images, slightly hyperintense on T2-weighted images, and has heterogeneously diminished enhancement in the early phase and delayed enhancement in the late phase of contrast enhancement[30,35,43,44]. The capsule-like rim described at CT is usually hypointense on both T1 and T2-weighted images, and has delayed moderate enhancement on contrast-enhanced MR[35,44].
Other imaging hallmarks of AIP include multiple narrowings of the main pancreatic duct or an irregularly narrowed main pancreatic duct in the affected segment[12,30]. Narrowing of the main pancreatic duct in AIP is usually longer than 3 cm in the diffuse form of AIP[45]. MRCP is a less invasive and more easily performed technique than ERCP but Kamisawa et al[45] stated that it cannot completely replace ERCP for diagnosing AIP, since narrowing of the main pancreatic duct in AIP cannot be always visualized on MRCP as clearly as on ERCP and in some studies[46] the narrowed main pancreatic duct could not be seen at MRCP at all. However, MRCP findings of a segmental or skipped non-visualized main pancreatic duct accompanied by less upstream main pancreatic duct dilatation than what is usually seen with adenocarcinoma may suggest the presence of focal AIP[45,47,48]. The irregular narrowing of the main pancreatic duct, which is usually longer than the stenosis caused by pancreatic adenocarcinoma, is one of the useful findings to differentiate focal AIP from pancreatic adenocarcinoma[49,50] together with the absence of upstream duct dilation, since ductal stenosis is not as strict as the one of adenocarcinoma[43,51]. A study by Muhi et al[39] revealed that 4 mm is the optimal cutoff value of ductal dilation to differentiate between focal AIP and pancreatic cancer[39]. Moreover, according to some studies, secretin stimulation during MRCP is of key importance to differentiate focal AIP and pancreatic adenocarcinoma, since the main pancreatic duct in focal AIP is not completely obstructed and tends to penetrate the mass after secretin administration, with the so-called “penetrating duct sign”, which has been described to be highly specific for benign strictures[52,53]. Another useful finding among AIP ductal abnormalities, not frequently seen in pancreatic cancer, is the presence of secondary pancreatic ducts deriving from the narrowed portion of the main pancreatic duct in AIP patients.
Bile duct abnormalities can be also recognized. These include smooth narrowing of the intrapancreatic portion of the common bile duct[40,43], or irregularity and stricturing of the intra- and extra-hepatic bile ducts with features similar to those seen in primary sclerosing cholangitis. Enhancing duct wall thickening is also a recognized feature and, less commonly, intra-hepatic bile duct dilation may also be observed[40,43].
Diffusion-weighted magnetic resonance imaging (DWI) has been increasingly used to evaluate diseases involving abdominal organs. Quantitative measurement of the diffusivity of water molecules in various tissues are described by the apparent diffusion coefficient (ADC) value. ADC is correlated to blood microcirculation, as well as molecular diffusion of water, frequently altered in various disease processes due to changes in physiological and morphological characteristics, such as cell density and tissue viability. Decreased ADC values correlate with increased lesion cellularity and total nuclear area, both restricting water diffusion. In general, malignant tumors have higher cellularity than benign lesions[54]. At DWI, AIP and pancreatic cancer are both detected as high signal intensity areas at high b-values images; however, pancreatic cancer usually present as a solitary area, while diffuse or multiple high-intensity areas are suggestive for AIP[55,56]. A longitudinal high intensity area also suggests AIP more than pancreatic cancer[55]. It has been found that mean ADC values are significantly lower in AIP than in pancreatic cancer, which has ADC values lower than normal pancreatic parenchyma[57,58]. Muhi et al[39] found that the optimal ADC cutoff value (100% sensitivity and 89% specificity) for differentiating mass-forming AIP from pancreatic carcinoma would be 0.88 × 10-3 mm2/s. Similarly Kamisawa et al[55] found ADC values to be significantly lower in AIP patients (1.012 × 10-3 ± 0.112 × 10-3 mm2/s) than in pancreatic cancer patients (1.249 × 10-3 ± 0.113 × 10-3 mm2/s). The reason of these findings resides in the anatomo-pathological features of these lesions: although cancer cell infiltration with desmoplastic stroma is the typical histopathological feature of pancreatic cancer, the cellularity of the dense lymphoplasmocytic infiltrate in AIP is greater than that of pancreatic cancer, therefore increased cellularity in AIP induce lower ADC values in AIP than in pancreatic cancer[12,22,28].
18F-fluorodeoxyglucose positron emission tomography/CT
Many patients with AIP are likely to be among those who receive fluorodeoxyglucose positron emission tomography (FDG-PET) because of suspected pancreatic cancer. However, even FDG-PET cannot always differentiate between these two lesions because inflammatory foci in the pancreas also accumulate FDG with the same avidity as a pancreatic neoplasm[59,60]. AIP causes intense FDG uptake by the pancreas[61,62]. Ozaki et al[63] showed FDG uptake in all AIP patients of their series and in 73.1% of pancreatic cancer patients. In contrast, previous studies had found that the sensitivity of FDG uptake to be higher (96%) in patients with pancreatic cancer[60], and lower (83%) in those with AIP[62]. Typical FDG-PET findings for AIP[63,64] are heterogeneous longitudinal accumulation and multiple localizations, whereas those for pancreatic cancer are nodular homogeneous accumulation, and solitary localization. When FDG accumulation in AIP is focal, differentiation from pancreatic cancer can be difficult. The longitudinal FDG uptake found in AIP is due to diffuse distribution of the inflammatory process, and FDG uptake by inflammatory cells possibly results in heterogeneous accumulation because of the scattered distribution of inflammatory cells. However, diffuse-type pancreatic cancer may also show a similar longitudinal shape, although such cases are rare. FDG uptake by extrapancreatic organs may assist in differentiating the two conditions.
DIFFERENTIAL DIAGNOSIS
The most common presentation of AIP is with obstructive jaundice and pancreatic enlargement that mimics the presentation of pancreatic cancer[14], and 5%-21% of patients undergoing resection for suspected pancreatic cancer have a final diagnosis of benign disease, including AIP[65,66]. As mentioned above, pancreatic enlargement can be focal or diffuse: when AIP presents as focal pancreatic enlargement with mass effect differentiating AIP from pancreatic cancer at imaging can be challenging. Since AIP responds extremely well to steroid therapy, it is of utmost importance to differentiate it from pancreatic cancer to avoid unnecessary laparotomy or pancreatic resection.
Obstructive jaundice caused by pancreatic cancer typically progresses steadily, whereas AIP jaundice sometimes fluctuates or, in rare cases, improves spontaneously[4,55,67].
Although false positive elevation of IgG, IgG4 and other antinuclear antibodies can be seen in pancreatic cancer[3], a marked elevation of serum IgG4 (> 2 times the upper limit of normal) is strongly suggestive of AIP in the setting of obstructive jaundice/pancreatic mass[3].
At CT the “sausage-like” appearance of the pancreas is the typical finding in AIP and is rarely seen in pancreatic cancer[56]. Enhancement of an enlarged pancreas on the delayed phase of CT and MR is characteristic of AIP[56]. As fibroinflammatory changes involve the peripancreatic adipose tissue, a capsule-like rim surrounding the pancreas is specifically detected in some AIP patients[30,32,44].
Some studies[52,68] state that MRCP findings such as skipped strictures of the main pancreatic duct without significant upstream dilation and the “penetrating duct sign” are most frequently seen in AIP patients.
As mentioned above, both AIP and pancreatic cancer are detected as high signal intensity areas on DWI images[55,56]. However, these areas are differently shaped, being diffuse, solitary or multiple in AIP, whereas all patients with pancreatic cancer have solitary areas[55,56]. In addition ADC values have been demostrated to be significantly lower in AIP than in pancreatic cancer[55,56].
Morover, while clarifying the differential diagnosis between AIP and pancreatic cancer, it has to be clear that the presence of other organ involvement and responsiveness to steroids are both highly suggestive of AIP.
The differential diagnosis between diffuse AIP and lymphoma may be difficult, since both entities determine enlargement of the pancreatic parenchyma and appear hypoattenuating in the pancreatic phase. Therefore, the differential diagnosis is based on ancillary findings, such as retroperitoneal and pelvic enlarged lymphnodes, splenic lesions, or both; when necessary fine needle aspiration or core biopsy are performed[69].
TREATMENT
Both subtypes of AIP are exquisitely sensitive to steroid therapy. The response to corticosteroid therapy can be both diagnostic and therapeutic. When typical imaging features and collateral evidence for AIP are absent and pancreatic cancer has been reliably ruled out, a steroid trial of oral prednisone for 2 wk can be started. Response to steroids is based on objective data such as radiologic evidence a dramatic decrease in the pancreatic mass or other organ involvement, resolution of the obstructive jaundice without biliary stenting, and normalization of liver function tests. If there is no such improvement or if the cancer antigen 19.9 level is rising, then the diagnosis of AIP should be reconsidered.
Once the diagnosis of AIP has been established, the best initial treatment is oral prednisone for 4 wk. Beginning at week 4, with continued objective response to therapy, the dose should be tapered.
Up to 40% of patients (mostly with type 1 AIP) will have disease relapse after the first course of corticosteroid therapy[70,71]. Proximal bile duct involvement can be a predictor of disease relapse.
The most severe cases of AIP are not responsive to pharmacologic treatment and requires surgical intervention. In cases with focal involvement of the pancreatic head region, pancreatico-duodenectomy is most frequently performed. Focal forms of AIP with body-tail involvement are treated with distal spleno-pancreatectomy. Diffuse forms of AIP, not responsive to corticosteroid therapy can require total pancreatectomy[72].
FOLLOW-UP
Laboratory findings and clinical evaluation are of great importance in the follow-up of patients with AIP, but imaging, mainly performed with CT and MR, plays a pivotal role.
Corticosteroid therapy induces the resolution of pancreatic changes. The gland swelling decreases, the physiological lobularity of the pancreatic contour is again visible and the other pancreatic (parenchymal eterogeneity and tail retraction) and peripancreatic (peripancreatic fat stranding and hypodense halo) changes improve. This improvement can be partial or complete and sometimes the pancreas can become slightly atrophic[51,73]. In patients with partial response retraction of the pancreatic tail can persist or a focal mass-like swelling can still be visible after therapy.
Manfredi et al[69] reported that the enhancement pattern returned to its normal appearance in the majority of patients, with the previously affected parenchyma resulting isoattenuating to the spleen or the unaffected adjacent parenchyma in the pancreatic phase.
At MR, steroid treatment resulted in significant changes in signal intensity on both T1- and T2-weighted images as compared to the pre-treatment images: the previously affected pancreatic parenchyma regains its physiological signal intensity in the majority of treated patients[46]. In more than 65% of the cases the affected parenchyma presents a post-therapy physiological contrastographic behaviour, resulting isointense to the non-affected parenchyma in every dynamic phase[46]. After steroid therapy, the main pancreatic duct has normal caliber, persisting narrowed only in a small percentage of patients, infrequently with a slight upstream dilation[46,69]. Therapy induces also the regularization of the common bile duct[46,69].
MR is also useful in the post-therapy follow up with DWI sequences: after steroid therapy, high intensity areas on DWI disappear or are markedly decreased in the same way as the pancreatic enlargement. The reduced ADC values of the inflammatory lesions usually increase to nearly those of normal pancreas. Remaining or recurring areas of low ADC indicate disease recurrence[55,74].
Disease recurrence occurs more frequently in young patients with focal forms of AIP. It tends to be morphologically similar to the previous presentation of the disease and with the same imaging features. Rarely AIP recurrence presents as diffuse form of the disease[69,75].
CONCLUSION
In conclusion, in the light of the recent literature and the latest published guidelines, it is clear that noninvasive imaging modalities play a progressively more important role in the diagnosis of AIP. Imaging is also of utmost importance for differential diagnosis, therapy monitoring, follow-up and early identification of disease recurrence.
Footnotes
P- Reviewer: Gao BL, Petersen LJ, Tsushima Y, Yazdi HR S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
References
- 1.Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 2.Sugumar A, Klöppel G, Chari ST. Autoimmune pancreatitis: pathologic subtypes and their implications for its diagnosis. Am J Gastroenterol. 2009;104:2308–2310; quiz 2311. doi: 10.1038/ajg.2009.336. [DOI] [PubMed] [Google Scholar]
- 3.Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–358. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 4.Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas. 2010;39:549–554. doi: 10.1097/MPA.0b013e3181e4d9e5. [DOI] [PubMed] [Google Scholar]
- 5.Sugumar A. Diagnosis and management of autoimmune pancreatitis. Gastroenterol Clin North Am. 2012;41:9–22. doi: 10.1016/j.gtc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Frulloni L, Amodio A, Katsotourchi AM, Vantini I. A practical approach to the diagnosis of autoimmune pancreatitis. World J Gastroenterol. 2011;17:2076–2079. doi: 10.3748/wjg.v17.i16.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugumar A, Chari ST. Autoimmune pancreatitis. J Gastroenterol Hepatol. 2011;26:1368–1373. doi: 10.1111/j.1440-1746.2011.06843.x. [DOI] [PubMed] [Google Scholar]
- 8.Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27:1119–1127. doi: 10.1097/00000478-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, Leins A, Longnecker D, Klöppel G. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552–563. doi: 10.1007/s00428-004-1140-z. [DOI] [PubMed] [Google Scholar]
- 10.Klöppel G, Detlefsen S, Chari ST, Longnecker DS, Zamboni G. Autoimmune pancreatitis: the clinicopathological characteristics of the subtype with granulocytic epithelial lesions. J Gastroenterol. 2010;45:787–793. doi: 10.1007/s00535-010-0265-x. [DOI] [PubMed] [Google Scholar]
- 11.Novotný I, Díte P, Lata J, Nechutová H, Kianicka B. Autoimmune pancreatitis--recent advances. Dig Dis. 2010;28:334–338. doi: 10.1159/000319410. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, Ohara H, Ito T, Kiriyama S, Inui K, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41:626–631. doi: 10.1007/s00535-006-1868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010–1016; quiz 934. doi: 10.1016/j.cgh.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Chari ST, Takahashi N, Levy MJ, Smyrk TC, Clain JE, Pearson RK, Petersen BT, Topazian MA, Vege SS. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7:1097–1103. doi: 10.1016/j.cgh.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Kwon S, Kim MH, Choi EK. The diagnostic criteria for autoimmune chronic pancreatitis: it is time to make a consensus. Pancreas. 2007;34:279–286. doi: 10.1097/MPA.0b013e31802eff5f. [DOI] [PubMed] [Google Scholar]
- 16.Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, Park SW, Shimosegawa T, Lee K, Ito T, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008;43:403–408. doi: 10.1007/s00535-008-2205-6. [DOI] [PubMed] [Google Scholar]
- 17.Schneider A, Löhr JM. [Autoimmune pancreatitis] Internist (Berl) 2009;50:318–330. doi: 10.1007/s00108-008-2262-1. [DOI] [PubMed] [Google Scholar]
- 18.Yadav D, Notahara K, Smyrk TC, Clain JE, Pearson RK, Farnell MB, Chari ST. Idiopathic tumefactive chronic pancreatitis: clinical profile, histology, and natural history after resection. Clin Gastroenterol Hepatol. 2003;1:129–135. doi: 10.1053/cgh.2003.50016. [DOI] [PubMed] [Google Scholar]
- 19.Smith CD, Behrns KE, van Heerden JA, Sarr MG. Radical pancreatoduodenectomy for misdiagnosed pancreatic mass. Br J Surg. 1994;81:585–589. doi: 10.1002/bjs.1800810435. [DOI] [PubMed] [Google Scholar]
- 20.Nishimori I, Tamakoshi A, Kawa S, Tanaka S, Takeuchi K, Kamisawa T, Saisho H, Hirano K, Okamura K, Yanagawa N, et al. Influence of steroid therapy on the course of diabetes mellitus in patients with autoimmune pancreatitis: findings from a nationwide survey in Japan. Pancreas. 2006;32:244–248. doi: 10.1097/01.mpa.0000202950.02988.07. [DOI] [PubMed] [Google Scholar]
- 21.Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, Pearson RK, Pelaez-Luna M, Petersen BT, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102:1646–1653. doi: 10.1111/j.1572-0241.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 22.Raina A, Yadav D, Krasinskas AM, McGrath KM, Khalid A, Sanders M, Whitcomb DC, Slivka A. Evaluation and management of autoimmune pancreatitis: experience at a large US center. Am J Gastroenterol. 2009;104:2295–2306. doi: 10.1038/ajg.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawa S, Ota M, Yoshizawa K, Horiuchi A, Hamano H, Ochi Y, Nakayama K, Tokutake Y, Katsuyama Y, Saito S, et al. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology. 2002;122:1264–1269. doi: 10.1053/gast.2002.33022. [DOI] [PubMed] [Google Scholar]
- 24.Kountouras J, Zavos C, Chatzopoulos D. A concept on the role of Helicobacter pylori infection in autoimmune pancreatitis. J Cell Mol Med. 2005;9:196–207. doi: 10.1111/j.1582-4934.2005.tb00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, Okumura F, Nishikawa T, Kobayashi K, Ichiya T, et al. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009;58:1504–1507. doi: 10.1136/gut.2008.172908. [DOI] [PubMed] [Google Scholar]
- 26.Sugumar A, Chari S. Autoimmune pancreatitis: an update. Expert Rev Gastroenterol Hepatol. 2009;3:197–204. doi: 10.1586/egh.09.2. [DOI] [PubMed] [Google Scholar]
- 27.Fukukura Y, Fujiyoshi F, Nakamura F, Hamada H, Nakajo M. Autoimmune pancreatitis associated with idiopathic retroperitoneal fibrosis. AJR Am J Roentgenol. 2003;181:993–995. doi: 10.2214/ajr.181.4.1810993. [DOI] [PubMed] [Google Scholar]
- 28.Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol. 2006;41:613–625. doi: 10.1007/s00535-006-1862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leise MD, Smyrk TC, Takahashi N, Sweetser SR, Vege SS, Chari ST. IgG4-associated cholecystitis: another clue in the diagnosis of autoimmune pancreatitis. Dig Dis Sci. 2011;56:1290–1294. doi: 10.1007/s10620-010-1478-9. [DOI] [PubMed] [Google Scholar]
- 30.Sahani DV, Kalva SP, Farrell J, Maher MM, Saini S, Mueller PR, Lauwers GY, Fernandez CD, Warshaw AL, Simeone JF. Autoimmune pancreatitis: imaging features. Radiology. 2004;233:345–352. doi: 10.1148/radiol.2332031436. [DOI] [PubMed] [Google Scholar]
- 31.Yang DH, Kim KW, Kim TK, Park SH, Kim SH, Kim MH, Lee SK, Kim AY, Kim PN, Ha HK, et al. Autoimmune pancreatitis: radiologic findings in 20 patients. Abdom Imaging. 2006;31:94–102. doi: 10.1007/s00261-005-0047-8. [DOI] [PubMed] [Google Scholar]
- 32.Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Kamata N. Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2694–2699. doi: 10.1111/j.1572-0241.2003.08775.x. [DOI] [PubMed] [Google Scholar]
- 33.Susset MA, Kunz A, Sczepanski B, Littmann M, Blank W, Braun B. [Autoimmune pancreatitis (AIMP) - a clinical entity of its own?] Dtsch Med Wochenschr. 2001;126:1294–1298. doi: 10.1055/s-2001-18470. [DOI] [PubMed] [Google Scholar]
- 34.Kitano M, Kudo M, Maekawa K, Suetomi Y, Sakamoto H, Fukuta N, Nakaoka R, Kawasaki T. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut. 2004;53:854–859. doi: 10.1136/gut.2003.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irie H, Honda H, Baba S, Kuroiwa T, Yoshimitsu K, Tajima T, Jimi M, Sumii T, Masuda K. Autoimmune pancreatitis: CT and MR characteristics. AJR Am J Roentgenol. 1998;170:1323–1327. doi: 10.2214/ajr.170.5.9574610. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi N, Fletcher JG, Fidler JL, Hough DM, Kawashima A, Chari ST. Dual-phase CT of autoimmune pancreatitis: a multireader study. AJR Am J Roentgenol. 2008;190:280–286. doi: 10.2214/AJR.07.2309. [DOI] [PubMed] [Google Scholar]
- 37.Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670–2676. doi: 10.1056/NEJMra061200. [DOI] [PubMed] [Google Scholar]
- 38.Wakabayashi T, Kawaura Y, Satomura Y, Watanabe H, Motoo Y, Okai T, Sawabu N. Clinical and imaging features of autoimmune pancreatitis with focal pancreatic swelling or mass formation: comparison with so-called tumor-forming pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2679–2687. doi: 10.1111/j.1572-0241.2003.08727.x. [DOI] [PubMed] [Google Scholar]
- 39.Muhi A, Ichikawa T, Motosugi U, Sou H, Sano K, Tsukamoto T, Fatima Z, Araki T. Mass-forming autoimmune pancreatitis and pancreatic carcinoma: differential diagnosis on the basis of computed tomography and magnetic resonance cholangiopancreatography, and diffusion-weighted imaging findings. J Magn Reson Imaging. 2012;35:827–836. doi: 10.1002/jmri.22881. [DOI] [PubMed] [Google Scholar]
- 40.Kawamoto S, Siegelman SS, Hruban RH, Fishman EK. Lymphoplasmacytic sclerosing pancreatitis (autoimmune pancreatitis): evaluation with multidetector CT. Radiographics. 2008;28:157–170. doi: 10.1148/rg.281065188. [DOI] [PubMed] [Google Scholar]
- 41.Matos C, Metens T, Devière J, Nicaise N, Braudé P, Van Yperen G, Cremer M, Struyven J. Pancreatic duct: morphologic and functional evaluation with dynamic MR pancreatography after secretin stimulation. Radiology. 1997;203:435–441. doi: 10.1148/radiology.203.2.9114101. [DOI] [PubMed] [Google Scholar]
- 42.Fukukura Y, Fujiyoshi F, Sasaki M, Nakajo M. Pancreatic duct: morphologic evaluation with MR cholangiopancreatography after secretin stimulation. Radiology. 2002;222:674–680. doi: 10.1148/radiol.2223010684. [DOI] [PubMed] [Google Scholar]
- 43.Proctor RD, Rofe CJ, Bryant TJ, Hacking CN, Stedman B. Autoimmune pancreatitis: an illustrated guide to diagnosis. Clin Radiol. 2013;68:422–432. doi: 10.1016/j.crad.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 44.Bodily KD, Takahashi N, Fletcher JG, Fidler JL, Hough DM, Kawashima A, Chari ST. Autoimmune pancreatitis: pancreatic and extrapancreatic imaging findings. AJR Am J Roentgenol. 2009;192:431–437. doi: 10.2214/AJR.07.2956. [DOI] [PubMed] [Google Scholar]
- 45.Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kodama M, Kamata N. Can MRCP replace ERCP for the diagnosis of autoimmune pancreatitis? Abdom Imaging. 2009;34:381–384. doi: 10.1007/s00261-008-9401-y. [DOI] [PubMed] [Google Scholar]
- 46.Manfredi R, Frulloni L, Mantovani W, Bonatti M, Graziani R, Pozzi Mucelli R. Autoimmune pancreatitis: pancreatic and extrapancreatic MR imaging-MR cholangiopancreatography findings at diagnosis, after steroid therapy, and at recurrence. Radiology. 2011;260:428–436. doi: 10.1148/radiol.11101729. [DOI] [PubMed] [Google Scholar]
- 47.Park SH, Kim MH, Kim SY, Kim HJ, Moon SH, Lee SS, Byun JH, Lee SK, Seo DW, Lee MG. Magnetic resonance cholangiopancreatography for the diagnostic evaluation of autoimmune pancreatitis. Pancreas. 2010;39:1191–1198. doi: 10.1097/MPA.0b013e3181dbf469. [DOI] [PubMed] [Google Scholar]
- 48.Vaishali MD, Agarwal AK, Upadhyaya DN, Chauhan VS, Sharma OP, Shukla VK. Magnetic resonance cholangiopancreatography in obstructive jaundice. J Clin Gastroenterol. 2004;38:887–890. doi: 10.1097/00004836-200411000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Kamisawa T, Tu Y, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Involvement of pancreatic and bile ducts in autoimmune pancreatitis. World J Gastroenterol. 2006;12:612–614. doi: 10.3748/wjg.v12.i4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horiuchi A, Kawa S, Hamano H, Hayama M, Ota H, Kiyosawa K. ERCP features in 27 patients with autoimmune pancreatitis. Gastrointest Endosc. 2002;55:494–499. doi: 10.1067/mge.2002.122653. [DOI] [PubMed] [Google Scholar]
- 51.Kamisawa T, Chen PY, Tu Y, Nakajima H, Egawa N, Tsuruta K, Okamoto A, Kamata N. MRCP and MRI findings in 9 patients with autoimmune pancreatitis. World J Gastroenterol. 2006;12:2919–2922. doi: 10.3748/wjg.v12.i18.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carbognin G, Girardi V, Biasiutti C, Camera L, Manfredi R, Frulloni L, Hermans JJ, Mucelli RP. Autoimmune pancreatitis: imaging findings on contrast-enhanced MR, MRCP and dynamic secretin-enhanced MRCP. Radiol Med. 2009;114:1214–1231. doi: 10.1007/s11547-009-0452-0. [DOI] [PubMed] [Google Scholar]
- 53.Ichikawa T, Sou H, Araki T, Arbab AS, Yoshikawa T, Ishigame K, Haradome H, Hachiya J. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology. 2001;221:107–116. doi: 10.1148/radiol.2211001157. [DOI] [PubMed] [Google Scholar]
- 54.Yoshikawa T, Kawamitsu H, Mitchell DG, Ohno Y, Ku Y, Seo Y, Fujii M, Sugimura K. ADC measurement of abdominal organs and lesions using parallel imaging technique. AJR Am J Roentgenol. 2006;187:1521–1530. doi: 10.2214/AJR.05.0778. [DOI] [PubMed] [Google Scholar]
- 55.Kamisawa T, Takuma K, Anjiki H, Egawa N, Hata T, Kurata M, Honda G, Tsuruta K, Suzuki M, Kamata N, et al. Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. Am J Gastroenterol. 2010;105:1870–1875. doi: 10.1038/ajg.2010.87. [DOI] [PubMed] [Google Scholar]
- 56.Takuma K, Kamisawa T, Gopalakrishna R, Hara S, Tabata T, Inaba Y, Egawa N, Igarashi Y. Strategy to differentiate autoimmune pancreatitis from pancreas cancer. World J Gastroenterol. 2012;18:1015–1020. doi: 10.3748/wjg.v18.i10.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muraoka N, Uematsu H, Kimura H, Imamura Y, Fujiwara Y, Murakami M, Yamaguchi A, Itoh H. Apparent diffusion coefficient in pancreatic cancer: characterization and histopathological correlations. J Magn Reson Imaging. 2008;27:1302–1308. doi: 10.1002/jmri.21340. [DOI] [PubMed] [Google Scholar]
- 58.Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, Araki T, Fujii H. High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: preliminary results. AJR Am J Roentgenol. 2007;188:409–414. doi: 10.2214/AJR.05.1918. [DOI] [PubMed] [Google Scholar]
- 59.Shreve PD. Focal fluorine-18 fluorodeoxyglucose accumulation in inflammatory pancreatic disease. Eur J Nucl Med. 1998;25:259–264. doi: 10.1007/s002590050226. [DOI] [PubMed] [Google Scholar]
- 60.Sperti C, Pasquali C, Decet G, Chierichetti F, Liessi G, Pedrazzoli S. F-18-fluorodeoxyglucose positron emission tomography in differentiating malignant from benign pancreatic cysts: a prospective study. J Gastrointest Surg. 2005;9:22–28; discussion 28-29. doi: 10.1016/j.gassur.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Nakamoto Y, Sakahara H, Higashi T, Saga T, Sato N, Okazaki K, Imamura M, Konishi J. Autoimmune pancreatitis with F-18 fluoro-2-deoxy-D-glucose PET findings. Clin Nucl Med. 1999;24:778–780. doi: 10.1097/00003072-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Nakamoto Y, Saga T, Ishimori T, Higashi T, Mamede M, Okazaki K, Imamura M, Sakahara H, Konishi J. FDG-PET of autoimmune-related pancreatitis: preliminary results. Eur J Nucl Med. 2000;27:1835–1838. doi: 10.1007/s002590000370. [DOI] [PubMed] [Google Scholar]
- 63.Ozaki Y, Oguchi K, Hamano H, Arakura N, Muraki T, Kiyosawa K, Momose M, Kadoya M, Miyata K, Aizawa T, et al. Differentiation of autoimmune pancreatitis from suspected pancreatic cancer by fluorine-18 fluorodeoxyglucose positron emission tomography. J Gastroenterol. 2008;43:144–151. doi: 10.1007/s00535-007-2132-y. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J, Shao C, Wang J, Cheng C, Zuo C, Sun G, Cui B, Dong A, Liu Q, Kong L. Autoimmune pancreatitis: whole-body 18F-FDG PET/CT findings. Abdom Imaging. 2013;38:543–549. doi: 10.1007/s00261-012-9966-3. [DOI] [PubMed] [Google Scholar]
- 65.Weber SM, Cubukcu-Dimopulo O, Palesty JA, Suriawinata A, Klimstra D, Brennan MF, Conlon K. Lymphoplasmacytic sclerosing pancreatitis: inflammatory mimic of pancreatic carcinoma. J Gastrointest Surg. 2003;7:129–137; discussion 137-139. doi: 10.1016/s1091-255x(02)00148-8. [DOI] [PubMed] [Google Scholar]
- 66.Kennedy T, Preczewski L, Stocker SJ, Rao SM, Parsons WG, Wayne JD, Bell RH, Talamonti MS. Incidence of benign inflammatory disease in patients undergoing Whipple procedure for clinically suspected carcinoma: a single-institution experience. Am J Surg. 2006;191:437–441. doi: 10.1016/j.amjsurg.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 67.Law R, Bronner M, Vogt D, Stevens T. Autoimmune pancreatitis: a mimic of pancreatic cancer. Cleve Clin J Med. 2009;76:607–615. doi: 10.3949/ccjm.76a.09039. [DOI] [PubMed] [Google Scholar]
- 68.Hur BY, Lee JM, Lee JE, Park JY, Kim SJ, Joo I, Shin CI, Baek JH, Kim JH, Han JK, et al. Magnetic resonance imaging findings of the mass-forming type of autoimmune pancreatitis: comparison with pancreatic adenocarcinoma. J Magn Reson Imaging. 2012;36:188–197. doi: 10.1002/jmri.23609. [DOI] [PubMed] [Google Scholar]
- 69.Manfredi R, Graziani R, Cicero C, Frulloni L, Carbognin G, Mantovani W, Mucelli RP. Autoimmune pancreatitis: CT patterns and their changes after steroid treatment. Radiology. 2008;247:435–443. doi: 10.1148/radiol.2472070598. [DOI] [PubMed] [Google Scholar]
- 70.Chari ST, Murray JA. Autoimmune pancreatitis, Part II: the relapse. Gastroenterology. 2008;134:625–628. doi: 10.1053/j.gastro.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 71.Gardner TB, Chari ST. Autoimmune pancreatitis. Gastroenterol Clin North Am. 2008;37:439–460, vii. doi: 10.1016/j.gtc.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 72.Kamisawa T, Satake K. Clinical management of autoimmune pancreatitis. Adv Med Sci. 2007;52:61–65. [PubMed] [Google Scholar]
- 73.Sahani DV, Sainani NI, Deshpande V, Shaikh MS, Frinkelberg DL, Fernandez-del Castillo C. Autoimmune pancreatitis: disease evolution, staging, response assessment, and CT features that predict response to corticosteroid therapy. Radiology. 2009;250:118–129. doi: 10.1148/radiol.2493080279. [DOI] [PubMed] [Google Scholar]
- 74.Taniguchi T, Kobayashi H, Nishikawa K, Iida E, Michigami Y, Morimoto E, Yamashita R, Miyagi K, Okamoto M. Diffusion-weighted magnetic resonance imaging in autoimmune pancreatitis. Jpn J Radiol. 2009;27:138–142. doi: 10.1007/s11604-008-0311-2. [DOI] [PubMed] [Google Scholar]
- 75.Frulloni L, Lunardi C. Serum IgG4 in autoimmune pancreatitis: a marker of disease severity and recurrence? Dig Liver Dis. 2011;43:674–675. doi: 10.1016/j.dld.2011.06.010. [DOI] [PubMed] [Google Scholar]


