Abstract
Colonoscopy is usually perceived as an invasive and potentially painful procedure, being also affected by a small, but definite, risk of major complications (cardiopulmonary complications, perforation, hemorrhage) and even mortality. To improve both acceptability and safety, PillCam Colon Capsule Endoscopy (CCE) (Given Imaging Ltd, Yoqneam, Israel) has been developed. CCE represents a non-invasive technique that is able to explore the colon without sedation and air insufflation. The Second Generation of Colon Capsule Endoscopy (PillCam Colon 2) (CCE-2) was proven to be an accurate tool to detect colonic neoplastic lesions when used in average risk individuals. To date, the evidence supports the use of CCE-2 in case of colonoscopy failure, in patients unwilling to perform colonoscopy and when colonoscopy is contraindicated. Other potential applications, such as colorectal cancer screening or diagnostic surveillance of inflammatory bowel disease need to be clarified. In this paper, the current “state of the art”, potential application of CCE and future needs are evaluated.
Keywords: Colon capsule endoscopy, Incomplete colonoscopy, Regimen of preparation, Accuracy, Fields of application
Core tip: Colon capsule endoscopy (CCE) allows a minimally invasive, painless colonic investigation without requiring intubation, insufflation or sedation. Indications for CCE are recommended by the European Society of Gastrointestinal Endoscopy guidelines that suggested CCE-2 can be used in average risk patients, in patients with a previous incomplete colonoscopy, in patients unwilling to perform a conventional colonoscopy or in those for whom colonoscopy is not possible or contraindicated. There are issues that still need to be clarified. In the present paper a revision of the literature is provided. Also potential applications and future needs will be discussed.
INTRODUCTION
Colorectal cancer (CRC) is the second most common cause of cancer-related death in developed countries, with 500000 deaths per year worldwide[1]. The procedure of choice for CRC prevention is colonoscopy, which allows the identification and removal of the pre-malignant adenomatous polyps[2-4]. Although the risk to have colonoscopy-related severe complications is small, even considering post-procedural complaints, conventional colonoscopy is perceived as an invasive, and potentially painful procedure, which requires conscious or deep sedation and takes place in unpleasant setting. The anxiety and the psychological inhibition related to all these well-known aspects are the determiner for the low compliance of healthy individuals to undergo colonoscopy for colon cancer prevention, which still remains, therefore, a challenge[5,6]. Another drawback of colonoscopy is the rate of incomplete procedures. Some factors such as a redundant or tortuous colon, inadequate bowel preparation, presence of acute angulations or lumen obstructions, can lead to failed caecal intubation. Literature reports variable results in terms of completeness rate. A large scale screening colonoscopy study recently reported a completion rate of 91.1%[7].
Colon capsule endoscopy (CCE) was initially released in 2006 by Given Imaging (Yoqneam, Israel.)[8]. More recently the technology has been implemented and a second generation of colon capsule endoscopy is now available[9]. It allows a minimally invasive, painless colonic investigation without requiring intubation, insufflation or sedation, allowing to pursue normal daily activities during the procedure.
Indications for CCE recommended by the European Society of Gastrointestinal Endoscopy (ESGE) guidelines suggested that CCE-2 can be used in average risk patients, in patients with a previous incomplete colonoscopy, in patients unwilling to perform a conventional colonoscopy or in those for whom colonoscopy is not possible or contraindicated[10].
In the present paper a revision of the literature is provided. Also potential applications and future needs will be discussed.
WHAT WE KNOW
Characteristics of the device and CCE procedure
The Given® Diagnostic System is comprised of three main subsystems: ingestible capsule endoscope (second-generation colon capsule), Data Recorder and a RAPID workstation. The second-generation CCE (CCE-2) is 11.6 mm × 31.5 mm in size[9,11,12]. It has been endowed with a battery lasting about 10 hours and has 2 cameras, one at each end, with an angle of view of 172° degrees for each camera, allowing a near full visual coverage of the colon. In order to enhance colon visualization and to save battery energy and video reading, the capsule is equipped with an adaptive frame rate (AFR), which alternates from 35 images per second while in motion to 4 images per second when virtually stationary. At the moment of the ingestion, the capsule works using AFR allowing proper visualization of the esophagus also, then it slows down to 14 images per minute. When small bowel images are detected, the system switches on the capsule into the AFR mode. This advanced system[9,10,12] for the control of capsule image rate is the result of a bidirectional communication between the capsule and the Data Recorder, which constantly analyzes and recognizes the transmitted images and adapts in a split second the frame rate. Data Recorder also drives patients by means of visual and audio signals through the procedure activities. It buzzes and vibrates and shows instructions on its liquid crystal display to instruct the patient during the day of the procedure (i.e., to ingest the booster after the capsule has left the stomach end entered the small bowel). Upon completion of the examination, data from the Recorder are downloaded to the Workstation, that is provided with a dedicated software (Rapid Software) for video viewing and processing.
Bowel preparation
Differently from conventional colonoscopy, with CCE it is not possible to clean the colon during the procedure. Therefore colonic preparation is crucial, since even small amount of debris could interfere with colon capsule capability to identify colonic polyps and ultimately with the outcome of the procedure. Colonic preparation for CCE is not limited to achieve an adequate cleansing level, but it is also aimed to distend the colonic wall filling the lumen of clean liquids and promote the capsule propulsion and excretion, ensuring that the journey is completed within the battery life-time[13,14]. Since preliminary studies using the same preparation as colonoscopy (PEG solution) showed low ingestion rates (20%), a protocol combining PEG (4l) and boosts with sodium phosphate (75 mL) was adopted and was demonstrated to allow a complete colon examination in most of the cases[15-17]. Subsequent studies proposed modifications in the timing and doses of the components, the inclusion of diet recommendations (low-residue diet, liquid diet the day before) and suppository (in case of delayed capsule excretion), prokinetics (for delayed stomach emptying) and different kind of boosters. In particular, because of the known concerns related to the administration of sodium phosphate, other boosters, have been investigated. Unfortunately, these studies resulted in unsatisfactory outcomes in terms of significant reduction of capsule excretion and completion rate. When considering that cecal intubation rates of higher than 90% and 95% are respectively recommended for routine and screening colonoscopies[18], CCE could not be considered an efficient option if only 75% of patients achieve a complete examination, as observed when administering a PEG instead of a NaP booster[13]. Moreover an incomplete capsule examination, in contrast to an incomplete colonoscopy, leaves uninvestigated the site more commonly harboring colonic polyps, that is the left colon.
All these studies resulted in 2 important information: (1) split regimens of PEG (2L + 2L) are recommended in order to improve the cleansing level; and (2) sodium phosphate boosters should be recommended in order to achieve a reasonable capsule excretion (i.e., complete colonoscopy). As booster, low dose of sodium phosphate (45-55 mL) was shown to achieve an adequate capsule excretion rate with the significant advantage to decrease the risk of sodium phosphate toxicity (acute nephropathy, electrolyte imbalance, kidney failure)[9,12,14] .
For this reasons, ESGE guidelines recommends the inclusion of a split dose of PEG and one or two low doses of sodium phosphate boosters in the protocol of preparation for CCE, as detailed in Table 1.
Table 1.
| Schedule | Intake | |
| Day -2 | Bedtime | Senna, 4 tb (48 mg) |
| Day -1 | All Day | Clear Liquid Diet |
| Evening (7-9 pm) | 2 L PEG | |
| Exam Day | 7-9 am | 2 L PEG |
| 10:00 AM | Capsule Ingestion1 | |
| (about 1 h after last intake of PEG) | ||
| after small bowel detection | 1st Boost | |
| 40 mL NaP + 1 L water with Gastrografin3 | ||
| (50 mL) | ||
| 3 h after 1st Boost | 2nd Boost | |
| 20 mL NaP + 0.5 L water with Gastrografin3 (25 mL)2 | ||
| 2 h after 2nd Boost | Suppository | |
| 10 mg Bisacodyl2 | ||
Ten mg Metoclopramide tablet if capsule delayed in stomach > 1 h;
Only if capsule not excreted yet;
Sodium-amidotrizoate and meglumine-amidotrizoate.
Recently, Rex et al[19] reported the results of a United States trial where sodium phosphate was replaced by Suprep (sodium sulfate, potassium sulfate and magnesium sulfate) (Braintree Lab Inc, United States), maintaining the split dose of PEG. Results of different regimens of preparation are listed in Table 2.
Table 2.
Accuracy of colon capsule endoscopy 2 for polyp detection
| Ref. | Pts | Adequate cleansing | Excretion rate |
Polyp ≥ 6 mm |
Polyp ≥ 10 mm |
||
| Sensitivity (95%CI) | Specificity (95%CI) | Sensitivity (95%CI) | Specificity (95%CI) | ||||
| Eliakim et al[9] | 104 | 78% | 81%1 | 89% | 76% | 88% | 89% |
| (68%-86%) | (70%-97%) | (72%-78%) | (56%-98%) | (86%-90%) | |||
| Spada et al[12] | 117 | 85% | 81%1 | 84% | 64% | 88% | 95% |
| (73%-88%) | (74%-95%) | (52%-76%) | (76%-99%) | (90%-100%) | |||
| Rex et al[19] | 884 | 80% | 91%2 | 88% | 82% | 92% | 95% |
| (76%-83%) | (82%-93%) | (80%-83%) | (82%-97%) | (94%-95%) | |||
Within 8 h;
10 h post ingestion.
Accuracy and clinical indications
Colon Capsule Endoscopy (CCE-2) was demonstrated to be a feasible and reliable tool to detect colonic lesions, such as polyps and tumors (Figure 1). Results of published studies are shown in Table 2. To date, 3 studies[9,12,19] (involving more than 1000 patients) evaluated the performance of CCE-2, compared to colonoscopy. The relative low number of patients studied is a clear limitation and further data are needed. However, it should be emphasized that these studies show comparable results in terms of accuracy, cleanliness, excretion rate and safety, suggesting that they represent the actual CCE-2 performance. The low specificity observed in trials was mainly related to a consistent number of false positive cases generated by size mismatching between standard colonoscopy and CCE. Only a minority of false positives was related to findings visualized by CCE and not confirmed by colonoscopy, being not possible to exclude the risk of missed polyps by colonoscopy (i.e., false negative at colonoscopy). Regarding the accuracy of CCE in detecting colo-rectal cancers, to date 10 cancers have been detected by conventional colonoscopy in comparative trials: CCE-2 identified cancers in all the cases, suggesting a potential 100% sensitivity[9,12,20].
Figure 1.
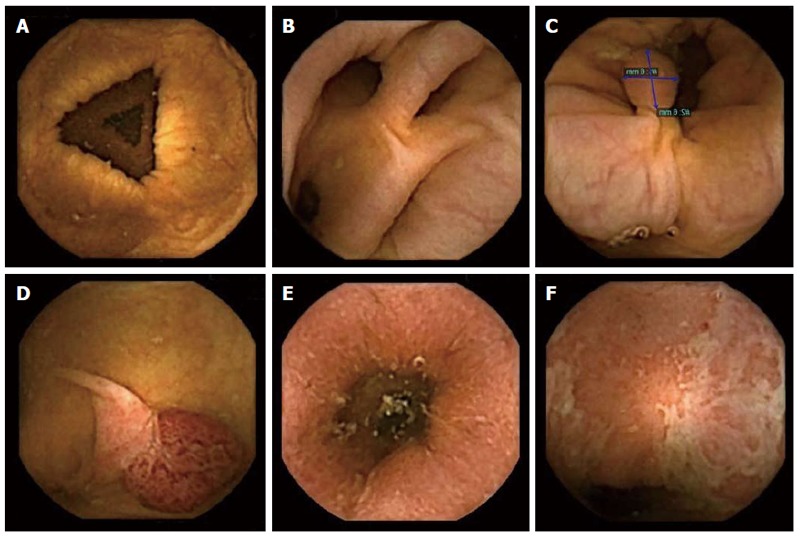
Normal colon and findings visualized at colon capsule endoscopy. A: Normal colon; B: Diverticula; C, D: Polyps; E, F: Ulcerative colitis.
Based on the available evidence, CCE-2 is not alternative to conventional colonoscopy but it should be considered a complementary test in specific settings. According to the ESGE guidelines CCE[10] can be used in average risk subjects (i.e., patients with non-alarm symptoms) who do not appear to be at increased risk of colorectal neoplasia. In this setting, a non-invasive tests may be proposed and, among non-invasive tests, CCE might be preferred over non-imaging tests (i.e., fecal tests), because of its ability to detect non-neoplastic conditions that may be regarded as clinically useful (e.g., vascular malformations). On the other hand, patients with alarm symptoms (because of symptoms or signs, a family or a personal history of CRC) are at increased (5-10 fold increased risk of malignancy) risk of colorectal neoplasia. These patients should be referred to colonoscopy. However, in patients not compliant to colonoscopy, the use of CCE should be considered and discussed with the patient.
To date, most of the evidence for CCE refers to patients with a previous incomplete colonoscopy. The most frequent causes of incomplete colonoscopies include left-sided angulations caused by diverticular disease or post-surgical adhesions, extensive looping or stenosing colorectal cancer[21]. Because of the risk of missed neoplasia in the non-visualized colon, further tests may be advisable depending on patients’ risk factors (i.e., left-sided polyps, family history, clinical indication). Such tests usually consist of radiological imaging (CT colonography or barium enema), colonoscopy using different endoscopes (pediatric or variable stiffness colonoscopes, balloon-assisted enteroscopes) or with anaesthetist-assistance[22]. In several studies[23-29], CCE was proven to be able to complement a previous incomplete colonoscopy, being able to visualize the colonic segments not visualized by previous incomplete conventional colonoscopy and to detect additional findings that would have been missed since they were localized in unseen segments (Table 3). Moreover, in this setting, when compared to CT-colonography, (i.e., the first choice imaging technique in case of incomplete colonoscopy), CCE was demonstrated to have a higher diagnostic yield for significant polyps (i.e., polyps ≥ 6 mm)[29], and it is better tolerated[30]. Interestingly, lesions missed by CTC were mainly flat and/or sessile lesions, lesser than 10 mm, located in the right side of the colon. Although this might suggest the capability of CCE to detect flat and/or sessile lesions, further studies confirming these results are awaited.
Table 3.
Results of colon capsule endoscopy in incomplete conventional colonoscopy
| Ref. | No. | Completeness (%) | CCE complementary findings (%) |
| Pioche et al[24] | 107 | 83 | 34 |
| Alarcón-Fernández et al[25] | 34 | 85 | 23.5 |
| Triantafyllou et al[26] | 75 | 90.7 | 44 |
| Nogales et al[27] | 96 | 93 | 4512 |
| Baltes et al[28] | 74 | 95 | 49/2812 |
| Spada et al[29] | 100 | 98 | 241 |
Significant polyps;
Cancers. CCE: Colon capsule endoscopy.
To date, there is insufficient data to support the use of CCE in the diagnostic work-up or in the surveillance of patients with suspected or known inflammatory bowel disease (IBD) (Figure 1). Mucosal healing (MH) is now adopted as a principal endpoint for medical treatment of ulcerative colitis in clinical trials and may be used in clinical practice because it may alter the course of the disease and reduce the need for hospitalization or surgery[31-33]. MH is usually assessed by colonoscopy and has been defined as the Mayo Clinic endoscopy subscore of 0 or 1 (normal mucosa or erythema without mucosal friability, erosions or ulceration). As diagnosis of ulcerative colitis requires biopsy and histological confirmation, CCE cannot be recommended for initial diagnosis. However, it is potentially a useful tool to guide therapy, especially for checking mucosal healing when considering discontinuation of medication. Sung et al[34] reported the sensitivity of first-generation CCE for detecting active colonic inflammation to be 89% and specificity to be 75% in UC. Consensus guidelines issued by the European Society of Gastrointestinal Endoscopy (ESGE) on CCE have established that CCE-2 may be useful to monitor inflammation in ulcerative colitis, which may help to guide therapy[10]. To date, there have been only a few studies on this topic, with results showing that CCE is a safe procedure to monitor mucosal status and healing in ulcerative colitis, but that it cannot replace conventional colonoscopy. These studies have all been conducted in adults, and only one of the studies used CCE-2[34-37]. Recently, the potential role of CCE (i.e., second generation CCE) in IBD was evaluated in 30 consecutive pediatric patients with ulcerative colitis. The sensitivity of CCE for disease activity was 96 % and specificity was 100 %. The positive and negative predictive values of CCE-2 were 100 % and 85 %, respectively. In the same trial, CCE had a higher overall tolerability than colonoscopy and interobserver agreement was excellent in all cases (≥ 0.86)[38]. Results of these studies are listed in Table 4.
Table 4.
Colon capsule endoscopy accuracy for ulcerative colitis
| Ref. | n | Type of CCE | Sensitivity | Specificity | PPV | NPV |
| Sung et al[34] | 100 | CCE1 | 89 (80-95) | 75 (51-90) | 93 (84-97) | 65 (43-83) |
| Oliva et al[38] | 29 | CCE2 | 96 (79-99) | 100 (61-100) | 100 (85-100) | 85 (49-97) |
CCE: Colon capsule endoscopy.
Finally, to date, the potential role of CCE in colorectal cancer screening programs is unknown since specific trials in this setting are missing. There are several screening models available. The most common strategy in Europe adopts a two-stage population-based approach. In this model, individuals who are identified as at risk, by either gFOBT or fecal immunological test (FIT) occult blood tests, are referred for colonoscopy. However, while these tests select out a population at risk for colonic cancers and adenomas, the majority of individuals who undergo colonoscopy do not have neoplasia. Studies have demonstrated the usefulness of occult blood tests in screening, but the relatively high rate of false-positive tests is a concern. From the available data, at least 40 % of people undergoing a screening colonoscopy following a positive FOBT will not have neoplasia detected, and therefore the procedure was unnecessary. This represents a major burden on screening resources and a substantial risk for screening participants, due to the invasive nature of colonoscopy and the potential for significant, albeit infrequent, adverse events. In a recent trial, Holleran et al[20], aiming to reduce the number of negative standard colonoscopies, evaluated if CCE could provide a screening filter test for people who have positive FIT results. In a total of 62 FIT+/participants optical colonoscopy detected at least one polyp in 36 participants (58 %), significant lesions in 18 (29 %), and cancer in 1 (2 %). The Authors demonstrated that there was good correlation between CCE and optical colonoscopy for any lesion and for significant lesions (r = 0.62 and 0.84, respectively). The negative predictive value of CCE was high both for any polyp (90 %) and for significant lesions (96 %). Results suggest that CCE is an effective means of detecting cancer and polyps in a positive FIT screening cohort. It would be a useful “filter test” in this situation, reducing the number of colonoscopies performed by 71 %. A cost-effective analysis using a Markov model showed that FIT repeated every year is the most cost-effective strategy and although CCE every 5 years is as effective as FIT 1-year, it is not a cost-effective alternative[39]. Although CCE is not a cost-effective alternative when assuming an equal adherence, it may become an efficient option when assuming that adherence to CCE was higher compared to colonoscopy for CRC screening, a feature which has not been demonstrated yet.
What we would like to know
Colon Capsule Endoscopy (CCE) was widely demonstrated to be a feasible and reliable instrument to detect bowel lesions, such as polyps and tumors. When considering polyps ≥ 6 mm and ≥ 10 mm, sensitivity (84%-89% and 88%-89% respectively) and specificity (64%-76% and 60%-89%) are definitely appropriate for its purpose[9,12]. Starting from this point, it was organized the first International Colon Capsule International Workshop in Tarquinia (Italy) in 2012. The potential indication and perspectives of CCE were discussed. During that meeting there was a general agreement that the first indication that needed to be explored was incomplete conventional colonoscopy. During the last 2 years, the role of CCE in case of a previous incomplete colonoscopy was explored. To date, there is a good evidence that CCE is a highly technically feasible examination for patients with previously incomplete colonoscopy, being able to complete the vast majority of the previous incomplete colonoscopy and to detect significant findings not visualized by incomplete colonoscopy (Table 3). Nevertheless, there are some issues that still need to be clarified. These mainly relate to the timing of capsule after incomplete colonoscopy and how to proceed with the preparation if CCE is performed immediately after colonoscopy. It would be important to know if CCE is feasible and can be performed immediately after an incomplete colonoscopy. This would be crucial since patients would not be asked to perform an additional preparation and it would allow endoscopists to complete colonoscopy the same day without referring the patient to other physicians and/or sessions. It is basically unknown how to proceed with the preparation if CCE is feasible immediately after incomplete colonoscopy. In particular, it is unknown if in such cases the regimen of preparation for CCE may be limited to the administration of boosters or if additional doses of lavage solutions are required.
The possibility to perform a colonoscopy immediately after CCE is extremely appealing since it would offer the advantage to perform conventional colonoscopy using the same regimen of preparation recommended for CCE. This scenario was never explored and it may be feasible only if certain circumstances occur. First, the video of CCE should be evaluated in a relatively short time, without any risk to decrease the overall accuracy of CCE. In this sense, the QuickView (i.e., a tool in the Rapid Software to decrease to reading time) my offer the chance to review the colonic video within few minutes. However, the accuracy of QuickView for significant findings was never evaluated. Second, the regimen of preparation should provide a CCE colon transit time sufficiently short to meet logistic constraints. Bowel preparation is no longer a problem in terms of cleansing level: the latest preparation regimen allows to obtain an adequate cleansing level in > 80% of patients. Nevertheless, colonic transit time is still an enigma when a CCE is performed, having patients with a very short transit time and others without capsule excreted at the end of the battery life. A regimen of preparation that meets the need of an adequate cleansing level with a homogeneous and relatively short CCE colonic transit time is highly desirable.
Although, CCE is specifically designed to explore the colon, it offers excellent images also in the small bowel. If the capsule is activated before the ingestion (i.e., CCE works for the entire gut using the ADR), it is possible to explore the esophagus (using a specific ingestion protocol), small bowel and colon, leaving only the stomach poorly explored. The indications for a pan-endoscopy, however, need to be clarified and the procedure should be validated.
CCE might also play a role in increasing the compliance to colonoscopy. Conventional colonoscopy is perceived as an invasive and painful procedure, which requires conscious or deep sedation and takes place in unpleasant setting. No colorectal imaging test may be performed on an out-of-clinic basis. This represents a major drawback compared with fecal tests. Because CCE automatically detects small bowel mucosa, it has the potential to become the first colorectal imaging test to be performed out-of-clinic. The out-of-clinic procedure (i.e., “home procedure”), in combination with the non invasiveness of CCE might be two of the most attractive and relevant features that might increase the compliance to colonoscopy. Adler et al[40], evaluated the feasibility and efficiency of CCE when offered as an out-of-clinic procedure. They showed that as an out-of-clinic procedure, CCE is feasible and easily performed. However, we still do not know if a home-based procedure may be associated with better acceptability and compliance to colonoscopy. This is a relevant issue since, if such hypothesis will be confirmed, CCE potentially has the features to play a relevant role in order to increase adherence to colorectal cancer screening.
The accuracy of CCE in the detection of flat/sessile polyps is basically unknown. These lesions in some circumstances are difficult to detect and may be easily missed at optical colonoscopy also a preliminary retrospective evaluation of patients enrolled in prospective comparative trials that used the colonoscopy as gold standard reported that CCE can detect flat lesions with high diagnostic yield[41]. Authors adopted Paris classification to classify lesions both at colonoscopy and CCE. Interestingly lesions that were classified as non-polypoid at colonoscopy, looked like as protruding lesions by CCE. The main reason for this seems to be related to the fact that during CCE colon is naturally distended by water and, therefore, lesions are not stretched into the colonic wall by air, as it happens during colonoscopy. Flat lesions have gained special attention because they were noted to have a higher risk of being cancerous than polypoid lesions. Studies performed in Western populations indicate a prevalence ranging between 5% and 25%. Therefore, prospective trial confirming these preliminary results and evaluating the accuracy of CCE in the detection of flat and sessile lesions are strongly needed.
Although appealing, the role of CCE in CRC screening programs is basically unknown. To date, the only information available in literature comes from a single center, Irish trial where it was demonstrated that when used in a cohort of FIT+ screening patients, CCE is an effective means of detecting cancer and polyps and it can be a useful “filter test” to select those patients who deserve a conventional colonoscopy for polypectomy. Starting from this, we should ask ourselves if and how CCE can take part in a CRC screening program. Two big trials are undergoing in Europe to evaluate the potential role of CCE in CRC screening programs. One Italian trial (CCANDY trial) that will enroll about 400 FIT+ patients is aimed to assess sensitivity, specificity, positive and negative predictive values in detecting CRC and advanced adenomas. The second study that is running in the Netherlands (ORCA trial) will enrol up to 1000 patients with the aim to determine the uptake and diagnostic yield of primary population screening for CRC by means of CCE. These 2 trials are very much awaited since they will clarify (1) if CCE accuracy for target lesions in CRC screening programs is sufficiently high to be included in the “Olympus” of the screening tests; and (2) if the uptake is good enough to ensure an equal or even higher participations to CRC screening programs.
On the other hand, it would be highly desirable to know if CCE is able to retake those FIT+ patients who are unwilling to perform a conventional colonoscopy. In such cases, two scenarios might appear: (1) CCE detects significant finding. This might convince and drive the patient to a therapeutic colonoscopy; and (2) CCE will not detect any finding. Patients will be referred for a following screening colonoscopy after 3 years[10].
CONCLUSION
An Editorial a few years ago was titled “The future is wireless”[42]. In the setting of colonoscopy, we do not know if such prediction will be realized. Conventional colonoscopy is the gold standard and probably will continue to be the gold standard for the next years. CCE is, and probably will continue to be, a complementary test in case of average risk patients unwilling to perform a colonoscopy, in case of incomplete colonoscopy or in case of patients unable to perform colonoscopy. For such indications, CCE is and it should be considered one of the options to explore the colon that offers some advantages when compared to the other alternatives since it is a non-invasive endoscopic test that directly visualize the colonic mucosa with high accuracy for significant lesions. Second generation of colon capsule was demonstrated to be consistently more accurate than the previous CCE generation and that it is a no sense to continuously refer to the first generation of CCE that showed disappointing results. That first generation is part of the past and it is not available anymore on the market. However, the evidence for the second generation of CCE is limited and there are a lot of topics that “we would like to know”. Trials are strongly needed in order to understand the potential role of CCE in the field of colonoscopy. CCE it is meant to be one of the most promising tool in the endoscopic field and, considering the fast developing technologies, what we really would like to know about CCE is where the future will lead us.
Footnotes
P- Reviewer: Antunes H, Carter D, Di Nardo G, Kotwal VS S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 3.Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812–815. doi: 10.1136/gut.48.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 7.Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 8.Eliakim R, Fireman Z, Gralnek IM, Yassin K, Waterman M, Kopelman Y, Lachter J, Koslowsky B, Adler SN. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38:963–970. doi: 10.1055/s-2006-944832. [DOI] [PubMed] [Google Scholar]
- 9.Eliakim R, Yassin K, Niv Y, Metzger Y, Lachter J, Gal E, Sapoznikov B, Konikoff F, Leichtmann G, Fireman Z, et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41:1026–1031. doi: 10.1055/s-0029-1215360. [DOI] [PubMed] [Google Scholar]
- 10.Spada C, Hassan C, Galmiche JP, Neuhaus H, Dumonceau JM, Adler S, Epstein O, Gay G, Pennazio M, Rex DK, et al. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2012;44:527–536. doi: 10.1055/s-0031-1291717. [DOI] [PubMed] [Google Scholar]
- 11.Spada C, Hassan C, Riccioni ME, Costamagna G. False positive at colon capsule endoscopy or false negative at conventional colonoscopy? Endoscopy. 2010;42:427–428; author reply 428. doi: 10.1055/s-0029-1244126. [DOI] [PubMed] [Google Scholar]
- 12.Spada C, Hassan C, Munoz-Navas M, Neuhaus H, Deviere J, Fockens P, Coron E, Gay G, Toth E, Riccioni ME, et al. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc. 2011;74:581–589.e1. doi: 10.1016/j.gie.2011.03.1125. [DOI] [PubMed] [Google Scholar]
- 13.Spada C, Riccioni ME, Hassan C, Petruzziello L, Cesaro P, Costamagna G. PillCam colon capsule endoscopy: a prospective, randomized trial comparing two regimens of preparation. J Clin Gastroenterol. 2011;45:119–124. doi: 10.1097/MCG.0b013e3181dac04b. [DOI] [PubMed] [Google Scholar]
- 14.Spada C, Hassan C, Ingrosso M, Repici A, Riccioni ME, Pennazio M, Pirozzi GA, Pagano N, Cesaro P, Spera G, et al. A new regimen of bowel preparation for PillCam colon capsule endoscopy: a pilot study. Dig Liver Dis. 2011;43:300–304. doi: 10.1016/j.dld.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Van Gossum A, Munoz-Navas M, Fernandez-Urien I, Carretero C, Gay G, Delvaux M, Lapalus MG, Ponchon T, Neuhaus H, Philipper M, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009;361:264–270. doi: 10.1056/NEJMoa0806347. [DOI] [PubMed] [Google Scholar]
- 16.Sieg A, Friedrich K, Sieg U. Is PillCam COLON capsule endoscopy ready for colorectal cancer screening? A prospective feasibility study in a community gastroenterology practice. Am J Gastroenterol. 2009;104:848–854. doi: 10.1038/ajg.2008.163. [DOI] [PubMed] [Google Scholar]
- 17.Sacher-Huvelin S, Coron E, Gaudric M, Planche L, Benamouzig R, Maunoury V, Filoche B, Frédéric M, Saurin JC, Subtil C, et al. Colon capsule endoscopy vs. colonoscopy in patients at average or increased risk of colorectal cancer. Aliment Pharmacol Ther. 2010;32:1145–1153. doi: 10.1111/j.1365-2036.2010.04458.x. [DOI] [PubMed] [Google Scholar]
- 18.Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 19.Rex DK, Adler SN, Aisenberg J, Burch WC, Carretero C, Chowers Y, Fein SA, Fern SE, Fernandez-Urien I, Fich A, et al. Accuracy of PillCam COLON 2 for detecting subjects with adenomas ≥ 6 mm. Gastrointest Endosc. 2013;77:AB29. [Google Scholar]
- 20.Holleran G, Leen R, O’Morain C, McNamara D. Colon capsule endoscopy as possible filter test for colonoscopy selection in a screening population with positive fecal immunology. Endoscopy. 2014;46:473–478. doi: 10.1055/s-0034-1365402. [DOI] [PubMed] [Google Scholar]
- 21.Hanson ME, Pickhardt PJ, Kim DH, Pfau PR. Anatomic factors predictive of incomplete colonoscopy based on findings at CT colonography. AJR Am J Roentgenol. 2007;189:774–779. doi: 10.2214/AJR.07.2048. [DOI] [PubMed] [Google Scholar]
- 22.Rex DK. Achieving cecal intubation in the very difficult colon. Gastrointest Endosc. 2008;67:938–944. doi: 10.1016/j.gie.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Spada C, Riccioni ME, Petruzziello L, Marchese M, Urgesi R, Costamagna G. The new PillCam Colon capsule: difficult colonoscopy? No longer a problem? Gastrointest Endosc. 2008;68:807–808. doi: 10.1016/j.gie.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Pioche M, de Leusse A, Filoche B, Dalbiès PA, Adenis Lamarre P, Jacob P, Gaudin JL, Coulom P, Letard JC, Borotto E, et al. Prospective multicenter evaluation of colon capsule examination indicated by colonoscopy failure or anesthesia contraindication. Endoscopy. 2012;44:911–916. doi: 10.1055/s-0032-1310008. [DOI] [PubMed] [Google Scholar]
- 25.Alarcón-Fernández O, Ramos L, Adrián-de-Ganzo Z, Gimeno-García AZ, Nicolás-Pérez D, Jiménez A, Quintero E. Effects of colon capsule endoscopy on medical decision making in patients with incomplete colonoscopies. Clin Gastroenterol Hepatol. 2013;11:534–40.e1. doi: 10.1016/j.cgh.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Triantafyllou K, Viazis N, Tsibouris P, Zacharakis G, Kalantzis C, Karamanolis DG, Ladas SD. Colon capsule endoscopy is feasible to perform after incomplete colonoscopy and guides further workup in clinical practice. Gastrointest Endosc. 2014;79:307–316. doi: 10.1016/j.gie.2013.07.061. [DOI] [PubMed] [Google Scholar]
- 27.Nogales O, Lujan M, Fernández-Urien I, González S B, Couto I, Baki W, Olmedo J, Grafía C, González C, Merino B, et al. Utility of Colon Capsule Endoscopy after an incomplete colonoscopy. UEGW 2013. Spanish: Multicentric Spanish Study; 2013. p. 793. [Google Scholar]
- 28.Baltes P, Bota M, Albert JG, Philipper M, Horster HG, Steinbruck I, Hagenmueller F, Bechtler M. PillCam Colon2® After Incomplete Colonoscopy- a Prospective Multi-Center Study. Gastrointest Endosc. 2014;79:AB584. [Google Scholar]
- 29.Spada C, Hassan C, Barbaro B, Iafrate F, Cesaro P, Petruzziello L, Minelli Grazioli L, Senore C, Brizi G, Costamagna I, et al. Colon capsule versus CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut. 2014:Epub ahead of print. doi: 10.1136/gutjnl-2013-306550. [DOI] [PubMed] [Google Scholar]
- 30.Rondonotti E, Borghi C, Mandelli G, Radaelli F, Paggi S, Amato A, Imperiali G, Terreni N, Lenoci N, Terruzzi V, et al. Accuracy of capsule colonoscopy and computed tomographic colonography in individuals with positive results from the fecal occult blood test. Clin Gastroenterol Hepatol. 2014;12:1303–1310. doi: 10.1016/j.cgh.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Kane S. Endoscopic healing should be a goal for everyone with ulcerative colitis. Inflamm Bowel Dis. 2008:Epub ahead of print. doi: 10.1002/ibd.20779. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenstein GR, Rutgeerts P. Importance of mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2010;16:338–346. doi: 10.1002/ibd.20997. [DOI] [PubMed] [Google Scholar]
- 33.Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 34.Sung J, Ho KY, Chiu HM, Ching J, Travis S, Peled R. The use of Pillcam Colon in assessing mucosal inflammation in ulcerative colitis: a multicenter study. Endoscopy. 2012;44:754–758. doi: 10.1055/s-0032-1309819. [DOI] [PubMed] [Google Scholar]
- 35.Ye CA, Gao YJ, Ge ZZ, Dai J, Li XB, Xue HB, Ran ZH, Zhao YJ. PillCam colon capsule endoscopy versus conventional colonoscopy for the detection of severity and extent of ulcerative colitis. J Dig Dis. 2013;14:117–124. doi: 10.1111/1751-2980.12005. [DOI] [PubMed] [Google Scholar]
- 36.Meister T, Heinzow HS, Domagk D, Dortgolz A, Lenze F, Ross M, Domschke W, Lügering A. Colon capsule endoscopy versus standard colonoscopy in assessing disease activity of ulcerative colitis: a prospective trial. Tech Coloproctol. 2013;17:641–646. doi: 10.1007/s10151-012-0965-8. [DOI] [PubMed] [Google Scholar]
- 37.Hosoe N, Matsuoka K, Naganuma M, Ida Y, Ishibashi Y, Kimura K, Yoneno K, Usui S, Kashiwagi K, Hisamatsu T, et al. Applicability of second-generation colon capsule endoscope to ulcerative colitis: a clinical feasibility study. J Gastroenterol Hepatol. 2013;28:1174–1179. doi: 10.1111/jgh.12203. [DOI] [PubMed] [Google Scholar]
- 38.Oliva S, Di Nardo G, Hassan C, Spada C, Aloi M, Ferrari F, Redler A, Costamagna G, Cucchiara S. Second-generation colon capsule endoscopy vs. colonoscopy in pediatric ulcerative colitis: a pilot study. Endoscopy. 2014;46:485–492. doi: 10.1055/s-0034-1365413. [DOI] [PubMed] [Google Scholar]
- 39.Hassan C, Benamouzig R, Spada C, Ponchon T, Zullo A, Saurin JC, Costamagna G. Cost effectiveness and projected national impact of colorectal cancer screening in France. Endoscopy. 2011;43:780–793. doi: 10.1055/s-0030-1256409. [DOI] [PubMed] [Google Scholar]
- 40.Adler SN, Hassan C, Metzger Y, Sompolinsky Y, Spada C. Second-generation colon capsule endoscopy is feasible in the out-of-clinic setting. Surg Endosc. 2014;28:570–575. doi: 10.1007/s00464-013-3206-y. [DOI] [PubMed] [Google Scholar]
- 41.Spada C, Hassan C, Adler SN, Cesaro P, Petruzziello L, Minelli L, Costamagna G. Flat Colorectal Lesions At PillCam Colon Capsule Endoscopy (CCE) Gastrointest Endosc. 2013;77:AB175. [Google Scholar]
- 42.Sharma VK. The future is wireless: advances in wireless diagnostic and therapeutic technologies in gastroenterology. Gastroenterology. 2009;137:434–439. doi: 10.1053/j.gastro.2009.06.029. [DOI] [PubMed] [Google Scholar]


