Abstract
AIM: To evaluate the survival benefits of different treatment strategies for hepatocellular carcinoma (HCC) patients with portal vein tumor thrombus (PVTT) and to determine the prognosis factors.
METHODS: Between 2007 and 2009, 338 HCC patients treated for PVTT were retrospectively studied. The patients were divided into 4 groups that underwent different treatments: the conservative treatment group (n = 75), the transarterial chemoembolization (TACE) group (n = 86), the hepatic resection group (n = 90), and the hepatic resection associated with postoperative TACE group (n = 87). Survival rates were determined using the Kaplan-Meier method and differences between the groups were identified through log-rank analysis. Cox’s proportional hazard model was used to identify the risk factors for survival.
RESULTS: The mean survival periods for patients in the conservative treatment, TACE, hepatic resection and hepatic resection associated with postoperative TACE groups were 3.8, 7, 8.2 and 15.1 mo, respectively. Significant differences were observed in the survival rates. For the surgical resection associated with postoperative TACE group, the survival rates after 1, 2 and 3 years were 49%, 37% and 19%, respectively. These results were significantly higher than those of the other groups (P < 0.05). Meanwhile, the 1, 2 and 3 year survival rates for the surgical resection group were 28%, 20% and 15%, whereas those for the TACE group were 17.5%, 0% and 0%, respectively. These values significantly increased after hepatic resection compared with those after TACE (P < 0.05).
CONCLUSION: Surgical resection is the most effective therapeutic strategy for HCC patients with PVTT and results in high hepatic functional reserve. For patients who can tolerate the procedure, postoperative TACE is necessary to prevent recurrence and prolong the survival period.
Keywords: Hepatocellular carcinoma, Portal vein tumor thrombosis, Conservative treatment, Transarterial chemoembolization, Surgical resection, Postoperative transarterial chemoembolization
Core tip: Hepatocellular carcinoma (HCC) with portal vein tumor thrombosis (PVTT) is generally considered to be related to or an absolute contraindication for hepatic resection or adjuvant chemotherapeutic methods such as transarterial chemoembolization (TACE). Only conservative and palliative treatments are available. However, many experts have indicated that surgery or TACE prolongs survival and lowers recurrence compared to palliative treatments. Thus, treatments of HCC with PVTT remain controversial. Our study explored appropriate treatment strategies and identified prognostic factors by comparing the survival periods and rates for HCC-PVTT patients with 4 kinds of treatments.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most frequent malignant tumor in the world and leads to 500000 deaths globally every year[1]. Approximately 30% of HCC patients have concurrent portal vein tumor thrombus (PVTT)[2], which appears to be terminal stage HCC. PVTT is closely related to intrahepatic metastasis and recurrence after surgical resection and/or transarterial chemoembolization (TACE) and results in poor prognosis. The mean survival period for patients with untreated HCC is 2.4-4 mo compared with 24.4 mo for patients with untreated HCC who do not have PVTT[3-5]. PVTT is considered to be related to or is an absolute contraindication for hepatic resection or adjuvant chemotherapeutic methods such as TACE. Only conservative and palliative treatments are currently available. However, the development of surgical techniques and adjuvant chemotherapy, hepatic resection, TACE and hepatic resection associated with postoperative TACE have been reported to prolong survival periods compared to conservative treatments[6-8]. Thus, the treatment and management of HCC with PVTT remain complicated and controversial. Our study compared the survival periods and rates of HCC-PVTT patients after different treatment strategies and identified prognostic factors.
MATERIALS AND METHODS
Patients
The inclusion criteria were as follows: (1) HCC with tumor thrombus in the first branch and/or main trunk of the portal vein, as confirmed by preoperative imaging or intraoperative exploration; (2) solitary tumor or multiple nodules that are mainly located in 1 or 2 adherent hepatic lobes and PVTT that can be removed with the tumors during preoperative assessment; (3) no distant metastasis; (4) no preoperative TACE/radiofrequency ablation; (5) candidates for hepatic resection and postoperative TACE with moderate hepatic function (Child-Pugh A or B) and sufficient functional hepatic reserve; (6) candidates for TACE with moderate hepatic function (Child-Pugh A or B) but insufficient hepatic functional reserve; (7) conservative therapy; and (8) tumor size ≤ 10 cm.
Between January 2007 and December 2009, 338 HCC-PVTT patients who met the inclusion criteria were treated at the Hepatobiliary Surgery Department or the Interventional Therapy Department of Guangxi Medical University, China. The mean age of the patients (309 men and 29 women) was 46.1 ± 10.1 years (range: 31-76 years). A total of 152 patients (44.9%) had alpha-fetoprotein (AFP) levels > 400 ng/mL. Serum test results for 66 patients (19.5%) were positive for hepatitis B surface antigen, most of whom had underlying cirrhosis (75.1%). According to the Child-Pugh classification, 315 patients (93.2%) were classified as Child-Pugh A and 23 patients (6.8%) as Child-Pugh B. Mean tumor diameter was 6.5 cm (range: 3.6-9.6 cm). Tumor thrombi were found in the first branch in 261 patients (77.2%) and extended to the main trunk of the portal vein in 77 patients (22.8%).
Study design
A total of 338 patients were divided into the following 4 groups and underwent different treatments: (1) conservative treatment group, which consisted of 75 patients treated with immunotherapy combined with nutritional therapy; (2) the chemotherapy group, which consisted of 86 patients treated with TACE; (3) the hepatic resection group, which consisted of 90 patients in whom tumors and PVTT were resected en bloc or thrombi were removed from the portal vein branch and/or trunk; and (4) the hepatic resection combined with postoperative TACE group, which consisted of 87 patients who underwent postoperative TACE after hepatectomy and thrombectomy.
Liver resection
Left hemihepatectomy was performed in 22 patients, right hemihepatectomy in 19 patients, left partial hepatectomy in 23 patients, right partial hepatectomy in 23 patients, partial median hepatectomy in 36 patients, and complete caudate lobe resection and extended left lateral segmentectomy in 3 patients. PVTT was removed in all patients who underwent hepatectomy. Hepatic vein tumor thrombus was simultaneously removed in 6 patients (3 with inferior vena cava thrombus and 3 with superior vena cava tumor thrombus), whereas extrahepatic bile conduct tumor thrombi were removed from 10 patients.
TACE
The indications for adjuvant or postoperative TACE in patients were based on tumor location, tumor number, absence of ascites and total bilirubin level < 3 mg/dL. TACE was performed after diagnostic hepatic angiography. Contrast medium was injected into the arteries via a 4.1-French RC1 catheter, which was introduced into the abdominal aorta via the right superficial femoral artery using the Seldinger technique. Afterwards, the number, locations, size and arterial branches supplying the tumors were identified. Iodized oil (10-20 mL), gelfoam particles with doxorubicin (30-50 mg) and cisplatinum (50-100 mg) were injected into the arterial branches. After 1 mo, follow-up computed tomography (CT) was performed to determine the effects of TACE. Based on liver function and tumor shrinkage, TACE was repeated in the TACE group as well as in the surgical resection combined with postoperative adjuvant chemotherapy group at 1 mo intervals. The number of TACE cycles varied from 1 to 7.
Follow-up
Before conservative therapy, hepatectomy or TACE, all patients were evaluated in terms of baseline history and physical examination, serum laboratory tests, imaging studies [such as ultrasound, CT, magnetic resonance imaging (MRI) and angiography] and pathological diagnosis.
After initial hepatectomy or TACE, serum AFP level was routinely measured and B-scan ultrasonography, dynamic CT or MRI was performed at the end of the first month and then every 3 mo. When intrahepatic recurrence was suspected, dynamic CT, MRI, angiography or pathological investigation via fine-needle aspiration cytology under guided imaging was performed to confirm a patient’s condition. All patients were followed up by the Hepatobiliary Surgery Department or the Interventional Therapy Department until June 30, 2012, or until death.
Statistical analysis
Statistical data analyses were performed using SPSS 18.0 statistical software. Comparisons of the 2 groups’ baseline characteristics were performed using the χ2 test. Survival time was defined as the period between the initial conservative therapy, hepatic resection or TACE and the date of death or the end of the study for surviving patients. Survival rates were determined using the Kaplan-Meier method and differences between groups were identified using log-rank analysis. Cox’s proportional hazards model was used to identify the risk factors for survival. P < 0.05 was considered statistically significant.
RESULTS
The clinical characteristics of the 338 HCC-PVTT patients are summarized in Table 1. No statistical differences were found in the clinical and pathological variables, including sex, age, AFP, hepatitis B surface antigen, Child-Pugh classification, tumor location, tumor size and tumor thrombus locations in the 4 groups (P > 0.05).
Table 1.
Clinical and pathological characteristic of 338 patients n (%)
| Conservative treatment (n = 75) | TACE (n = 86) | Hepatic resection (n = 90) | Hepatic resection with TACE (n = 87) | P value | |
| Gender male | 69 (92) | 80 (93) | 81 (90) | 79 (90) | 0.381 |
| female | 6 (8) | 6 (6.9) | 9 (10.4) | 8 (10) | |
| Age (yr) | 49.5 ± 8.7 | 45.6 ± 10.2 | 49.3 ± 10.7 | 44.2 ± 11.1 | 0.714 |
| Child Pugh classification A | 69 | 78 | 84 | 84 | 0.601 |
| B | 6 | 8 | 6 | 3 | |
| AFP (ng/mL) ≥ 400 | 27 | 38 | 48 | 39 | 0.704 |
| < 400 | 48 | 48 | 42 | 48 | |
| HBsAg (+) | 21 | 18 | 12 | 15 | 0.702 |
| (-) | 54 | 68 | 78 | 72 | |
| Tumor number = 1 | 48 | 32 | 51 | 51 | 0.124 |
| > 1 | 27 | 54 | 39 | 36 | |
| Tumor size (cm) | 7.5 ± 2.1 | 6.5 ± 2.7 | 6.9 ± 1.6 | 6.2 ± 2.5 | 0.168 |
| Tumor location | 0.319 | ||||
| Left lobe | 21 | 24 | 27 | 18 | |
| Right lobe | 36 | 42 | 39 | 54 | |
| Left and right lobe | 15 | 18 | 21 | 15 | |
| Caudate lobe | 3 | 2 | 3 | 0 | |
| Tumor thrombus location | 0.528 | ||||
| Left branch | 24 | 34 | 30 | 33 | |
| Left branch extending to main trunk | 6 | 6 | 6 | 3 | |
| Right branch | 33 | 32 | 36 | 39 | |
| Right branch extending to main trunk | 6 | 4 | 9 | 3 | |
| Left and right branch extending to main trunk | 6 | 10 | 9 | 9 | |
| Portal vein diameter (cm) | 0.161 | ||||
| < 1.3 | 30 | 48 | 48 | 48 | |
| ≥ 1.3 | 45 | 38 | 42 | 41 |
TACE: Transarterial chemoembolization.
Survival periods
There were significant differences in the survival rates between the 4 groups (Table 2). For the surgical resection associated with postoperative TACE group, the survival rates after 1, 2 and 3 years were 49%, 37% and 19%, respectively, and the mean survival period was 15.1 mo, significantly higher than those of the other 3 groups (P < 0.05). For the surgical resection group, the 1, 2 and 3 year survival rates were 28%, 20% and 15%, whereas those for the TACE group were 17.5%, 0% and 0%, respectively. The values for these groups significantly increased after surgical resection compared with those after TACE (P < 0.05) and the mean survival periods were 7 and 8.2 mo, respectively. Meanwhile, the 1, 2 and 3 year survival rates for the conservative treatment group were 0% and the mean survival period was only 3.8 mo, the lowest of the 4 groups (P < 0.05).
Table 2.
Mean survival periods and survival rates of different groups
| Groups | Mean survival periods (mo) | 1 yr survival rate | 2 yr survival rate | 3 yr survival rate |
| Conservative treatment | 3.8 | 0% | 0% | 0% |
| TACE | 7.0 | 17.5% | 0% | 0% |
| Hepatic resection | 8.2 | 28% | 20% | 15% |
| Hepatic resection with TACE | 15.1 | 49% | 37% | 19% |
TACE: Transarterial chemoembolization.
Predictors of postoperative survival
Multivariate analysis revealed that the location of the tumor thrombus and the number of TACE cycles are independent predictors of postoperative survival (Table 3, Figure 1). The mean postoperative survival period for patients with HCC and PVTT in the first branch or higher order was significantly longer than those with PVTT extending to the main trunk of the portal vein (14.5 ± 3.6 mo vs 8.3 ± 2.1 mo, P = 0.012). The survival period for patients who received ≥ 3 postoperative TACE cycles (21.6 ± 4.1 mo) was significantly longer than those for patients who did not receive postoperative TACE (15.1 ± 2.4 mo) or who received only 1-2 cycles of postoperative TACE (8.2 ± 1.3 mo) (P < 0.0001). The tumor number, location and size and other clinicopathological characteristics were not related to postoperative survival.
Table 3.
Factors affecting the survival status of hepatocellular carcinoma patients with portal vein tumor thrombus
| Mean survival periods (mo) | P value | |
| Tumor thrombus location | 0.012 | |
| First branch | 8.3 ± 2.1 | |
| Branch extending to main trunk | 14.5 ± 3.6 | |
| Number of postoperative TACE cycles | < 0.0001 | |
| 0 | 8.2 ± 1.3 | |
| 1-2 | 15.1 ± 2.4 | |
| ≥ 3 | 21.6 ± 4.1 |
TACE: Transarterial chemoembolization.
Figure 1.
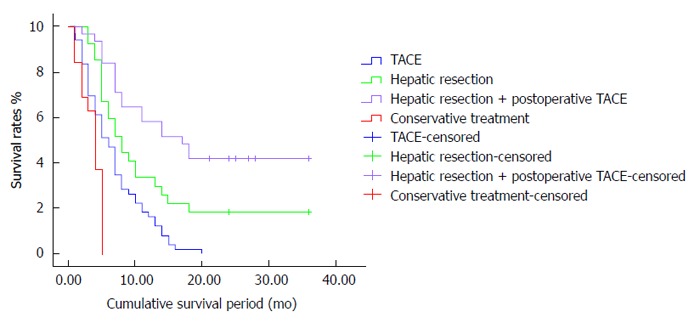
Survival curves of hepatocellular carcinoma patients with portal vein tumor thrombus in different treatment groups. TACE: Transarterial chemoembolization.
Factors affecting HCC-PVTT patients’ survival
Cox’s proportional hazard model was used to analyze the pretreatment and treatment variables of all 338 patients. Multivariate analysis revealed that the strategy of treatment (TACE) is an independent prognostic factor for HCC-PVTT patients (Table 3). Compared with TACE, hepatic resection significantly reduced the recurrence rate and prolonged the survival period (mean survival period: 7 mo vs 8.2 mo, P < 0.05).
DISCUSSION
Significance of hepatic resection
PVTT is generally considered to be related to or an absolute contraindication for hepatic resection. Only conservative or palliative treatments are currently available. Of the surgical techniques developed, hepatic resection along the portal tributary is effective in eradicating the main solitary tumor, as well as the tumor’s surgical margins, possible satellites, nodules and PVTT, and reducing the high risk microportal invasion area and intrahepatic metastasis of HCC[9]. Based on the theory proposed by Fan et al[10], hepatectomy plus thrombectomy can reduce portal vein pressure and thus prevent the occurrence of intractable ascites and bleeding of esophageal varices. The method also allows recovery of blood flow in the portal vein, improves liver function, reduces tumor burden and increases the efficacy of postoperative multimodality treatments, such as TACE, hepatic artery infusion, portal vein infusion and biotherapy. Fan et al[10] also reported that the mean survival periods of HCC-PVTT patients in the conservative therapy group, chemotherapy group, hepatic resection group and hepatic resection plus adjunctive chemotherapy group were 3.6, 7.3, 10.1 and 15.1 mo, respectively. The overall 0.5, 1, 2 and 3 year survival rates of patients who underwent surgical resection combined with adjunctive chemotherapy were 55.8%, 39.3%, 30.4% and 15.6%, respectively, the highest of the 4 groups (P < 0.0001). The survival period of patients who underwent surgical resection was significantly longer than that of patients who underwent conservative therapy (P = 0.001). Meanwhile, the survival rates of patients who underwent surgical resection were significantly higher than those of patients who underwent chemotherapy (P = 0.019). These findings are similar to the results of the current study. In our study, the 1, 2 and 3 year survival rates for the surgical resection associated with postoperative TACE group were 49%, 37% and 19%, respectively, and the mean survival period was 15.1 mo, significantly higher than those of the other 3 groups (P < 0.05). The 1, 2 and 3 year survival rates for the surgical resection group were 28%, 20% and 15%, respectively, whereas those for the TACE group were 17.5%, 0% and 0%. The values for these 2 groups increased more significantly after surgical resection than for those after TACE (P < 0.05). The mean survival periods were 7 and 8.2 mo in the surgical resection and TACE groups, respectively. Meanwhile, the 1, 2 and 3 year survival rates for the conservative treatment group were all 0% and the mean survival period was 3.8 mo, the lowest of the 4 groups (P < 0.05). Hepatic resection can reportedly improve patients’ quality of life and survival rates[11-13]. Thus, hepatic resection is an effective radical therapy for HCC-PVTT patients and results in sufficient hepatic functional reserve.
Efficacy of postoperative TACE
Cancer recurrence in the remnant liver is the major contributor to postoperative death in HCC patients[8,14], possibly because of preoperative, invisible intrahepatic metastasis in the residual liver, tumor cell dissemination during hepatic resection manipulation, or the multicentric origin of the tumor[15]. Invisible intrahepatic metastasis via the portal venous system is the primary mechanism for intrahepatic recurrence[16-18]. Lipiodol selectively accumulates in the tumors when delivered intra-arterially and acts as a carrier for anticancer drugs. Postoperative TACE can effectively block the tumor’s nutrient vessels, thus allowing large doses of sustainable chemotherapeutic drugs to kill the residual microscopic HCC cells in the remnant liver and circulation without damaging normal liver cells[19-21]. Fan et al[10] showed that the overall 0.5, 1, 2 and 3 year survival rates of patients who underwent surgical resection combined with adjunctive chemotherapy were 55.8%, 39.3%, 30.4% and 15.6%, respectively, and the mean survival period was 15.1 mo. However, the overall 0.5, 1, 2 and 3 year survival rates of patients who underwent surgical resection only were 46.8%, 22.7%, 9.8% and 0%, respectively, and the mean survival period was 10.1 mo. These results show a significant difference between the 2 groups (P < 0.001). We found that for the surgical resection associated with postoperative TACE group, the survival rates after 1, 2 and 3 years were 49%, 37% and 19%, respectively, and the mean survival was 15.1 mo. Meanwhile, the 1, 2 and 3 year survival rates for the surgical resection group were 28%, 20% and 15%, respectively, and the mean survival was 8.2 mo. These results show a significant difference between these 2 groups (P < 0.05). Our results and those of Jia indicate that postoperative TACE can reduce the recurrence rate and prolong the survival period. Therefore, postoperative TACE should be recommended for HCC-PVTT patients with residual liver function that can tolerate TACE to eliminate micrometastases that could not be removed by hepatic resection or malignant cell shedding during the surgical procedure.
Efficacy of TACE for unresectable HCC
The natural history of unresectable HCC with PVTT is poor. The mean survival period for these patients reportedly ranges from only 2.4 to 4 mo[3,4]. As previously stated, PVTT is also generally considered to be related to or an absolute contraindication for TACE. However, TACE has been shown to yield promising results[7,8]. The benefits of TACE are as follows: (1) blockage of the main nutrient vessels of the tumor and prevention of compensatory circulation growth; and (2) reduction in portal vein pressure and prevention of intractable ascites and bleeding of esophageal varices[22]. Luo et al[7] reported that mean survival periods for the TACE and conservative groups were 7.1 and 4.1 mo, respectively. The 3, 6, 12 and 24 mo overall survival rates for the TACE and conservative groups were 85.6%, 56.4%, 30.9% and 9.2%, and 63.6%, 28%, 3.8% and 0%, respectively. The TACE group had a significantly better overall survival than the conservative group (P < 0.001). Our findings reveal that the mean survival periods for the TACE and conservative groups were 7.8 and 3.0 mo, respectively. The 1, 2 and 3 year overall survival rates for the TACE and conservative groups were 7.5%, 0% and 0%, and 0%, 0% and 0%, respectively. TACE resulted in a significantly better overall survival than conservative treatment (P < 0.05). However, Huang et al[23] showed that although TACE had been confirmed as an effective and safe therapeutic strategy for terminal stage HCC patients, the number of TACE cycles should not exceed 3 because repeated chemotherapy or chemoembolization can damage the remnant liver parenchyma, particularly in cirrhotic patients, and lead to liver function impairment or deterioration[24,25]. Consequently, the number of postoperative TACE cycles should be decided based on the patient’s liver function and response to TACE. However, TACE should be recommended as an effective and safe treatment for patients with unresectable HCC and PVTT because it leads to significantly better survival than conservative treatments.
Prognostic factors for the survival of HCC-PVTT patients
Our study showed that tumor thrombus location is an independent risk factor for postoperative survival. The mean postoperative survival period for HCC-PVTT patients in the first branch or higher order was 14.5 ± 3.6 mo. By contrast, the mean postoperative survival for HCC-PVTT patients that extends to the main trunk of the portal vein was 8.3 ± 2.1 mo. These values indicate a significant difference (P = 0.012). Cheng et al[26] reported that for HCC patients with PVTT sizes of 2 cm in the portal vein trunk, hepatic resection resulted in better survival than nonsurgical treatments. However, no significant difference in survival was found between hepatic resection and nonsurgical treatments in HCC patients with PVTTs < 2 cm in the portal vein trunk.
Multivariate analysis revealed that the number of TACE cycles (mean survival period: 21.6 ± 4.1 mo for ≥ 3 TACE cycles vs 10.5 ± 2.2 mo for 0-2 TACE cycles, P < 0.05) is another independent survival predictor for HCC-PVTT patients. The results show that the survival period for patients who received ≥ 3 postoperative TACE cycles was significantly longer than that in those who did not receive postoperative TACE or those who received only 1-2 cycles of postoperative TACE (P < 0.0001). Norton[27] showed that the cytotoxic effects of chemotherapy drugs generally follow log-cell kill kinetics, in which cells are killed proportionally. Therefore, tumor cells cannot be eliminated by 1 cycle of chemotherapy. With multiple treatment cycles, the possibility of killing residual tumor cells increases with better prognosis. However, repeat TACE can damage the remnant liver parenchyma, particularly in cirrhotic patients, and result in liver function impairment or deterioration[24,25].
Furthermore, our study showed that the treatment strategy is an independent prognostic factor for survival in HCC-PVTT patients (mean survival period: 7 mo after TACE vs 8.2 mo after hepatic resection, P < 0.05). Approximately 95%-99% of HCC blood is supported by the hepatic artery. Lipiodol selectively accumulates in tumors when delivered intra-arterially and acts as a carrier for anticancer drugs. TACE can effectively provide direct treatment by blocking the nutrient vessels of the tumor and providing large doses of sustainable chemotherapeutic drugs to kill the HCC cells without damaging normal liver cells[19-21]. However, TACE cannot completely block nutrient transport to the tumor because of the small nutrient vessels from the portal vein; therefore, tumor necrosis is not completely achieved[7]. Xia et al[28] showed that the serum levels of angiogenic factors in post-TACE patients were significantly higher than those in postoperative patients, whereas the serum levels of vascular endothelial cells in post-TACE patients were significantly lower than those in postoperative patients. Hepatic resection is more effective in preventing vessel renascence and HCC recurrence. Therefore, TACE remains a conservative or palliative treatment. Hepatic resection significantly reduces the recurrence rate and prolongs survival compared to TACE.
In conclusion, surgical resection is the most effective therapeutic strategy for HCC-PVTT patients and results in high hepatic functional reserve. Postoperative TACE is necessary for preventing disease recurrence and prolonging survival in patients who can tolerate chemoembolization. TACE significantly increases the survival rate of patients with unresectable HCC with PVTT compared with conservative treatment and should therefore be recommended as an effective and safe treatment for this disease.
COMMENTS
Background
Hepatocellular carcinoma (HCC) with portal vein tumor thrombosis (PVTT) is generally considered to be related or an absolute contraindication for hepatic resection or adjuvant chemotherapeutic methods such as transarterial chemoembolization (TACE). Only conservative and palliative treatments are available. However, many experts have indicated that surgery or TACE prolongs survival and lowers recurrence rates compared to palliative treatments. Thus, treatment of patients with HCC-PVTT remains controversial. This study aimed to explore appropriate treatment strategies for HCC with PVTT and identify the prognostic factors by comparing survival periods and rates for HCC-PVTT patients after 4 kinds of treatments.
Research frontiers
To compare and evaluate the efficacy of different treatments for HCC < 10 cm with PVTT, this study indicates that surgery is the only eradicative treatment. TACE led to better survival than palliative treatments.
Innovations and breakthroughs
This study indicates that surgery remains the only eradicative treatment. TACE led to better survival than palliative treatments.
Applications
This study indicates that surgery or TACE results in better survival than palliative treatments. This result offers treatment choices for clinical surgeons to treat HCC < 10 cm in PVTT patients.
Terminology
Liver resection includes left hemihepatectomy, right hemihepatectomy, left partial hepatectomy, right partial hepatectomy, partial median hepatectomy, complete caudate lobe resection and extended left lateral segmentectomy. PVTT and extrahepatic bile duct tumor thrombi were removed in patients who underwent eradicative hepatectomy. The indications for adjuvant or postoperative TACE in patients were based on tumor location, tumor number, absence of ascites and total bilirubin level < 3 mg/dL. TACE was performed after diagnostic hepatic angiography. Contrast medium was injected into the arteries via a 4.1-French RC1 catheter, which was introduced into the abdominal aorta via the right superficial femoral artery using the Seldinger technique. Afterwards, the number, locations, tumor size and arterial branches supplying the tumors were identified. Iodized oil (10-20 mL), gelfoam particles with doxorubicin (30-50 mg) and cisplatinum (50-100 mg) were injected into the arterial branches. After 1 mo, follow-up computed tomography was performed to determine the effects of TACE. Based on liver function and tumor shrinkage, TACE was repeated in the TACE group as well as in the surgical resection combined with postoperative adjuvant chemotherapy group at 1 mo intervals. The number of TACE cycles varied from 1 to 7.
Peer review
The authors described that appropriate treatment strategies improved survival of hepatocellular carcinoma patients with portal vein tumor thrombus and tumors no larger than 10 cm. The number of patients was 338 with Vp3, enough for statistical analysis.
Footnotes
P- Reviewer: Ohkohchi N S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Wang CH
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Kuo YH, Lu SN, Chen CL, Cheng YF, Lin CY, Hung CH, Chen CH, Changchien CS, Hsu HC, Hu TH, et al. Hepatocellular carcinoma surveillance and appropriate treatment options improve survival for patients with liver cirrhosis. Eur J Cancer. 2010;46:744–751. doi: 10.1016/j.ejca.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Lau WY, Yu SL. Management of portal vein tumor thrombus. In: Lau WY, editor. Hepatocellular carcinoma. Singapore: World scientific publishing. Co Pte Ltd; 2008. pp. 739–760. [Google Scholar]
- 4.Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Guanghui D, Wenming C, Peijun W, Yuxiang Z. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology. 2007;54:499–502. [PubMed] [Google Scholar]
- 5.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 6.Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073–2080. doi: 10.1245/s10434-010-0940-4. [DOI] [PubMed] [Google Scholar]
- 7.Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, Shi M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 8.Minagawa M, Makuuchi M, Takayama T, Ohtomo K. Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg. 2001;233:379–384. doi: 10.1097/00000658-200103000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arii S, Tanaka S, Mitsunori Y, Nakamura N, Kudo A, Noguchi N, Irie T. Surgical strategies for hepatocellular carcinoma with special reference to anatomical hepatic resection and intraoperative contrast-enhanced ultrasonography. Oncology. 2010;78 Suppl 1:125–130. doi: 10.1159/000315240. [DOI] [PubMed] [Google Scholar]
- 10.Fan J, Zhou J, Wu ZQ, Qiu SJ, Wang XY, Shi YH, Tang ZY. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2005;11:1215–1219. doi: 10.3748/wjg.v11.i8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tazawa J, Maeda M, Sakai Y, Yamane M, Ohbayashi H, Kakinuma S, Miyasaka Y, Nagayama K, Enomoto N, Sato C. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol. 2001;16:660–665. doi: 10.1046/j.1440-1746.2001.02496.x. [DOI] [PubMed] [Google Scholar]
- 12.Ando E, Tanaka M, Yamashita F, Fukumori K, Sumie S, Yano Y, Sata M. Chemotherapy for hepatocellular carcinoma with portal hypertension due to tumor thrombus. J Clin Gastroenterol. 2000;31:247–249. doi: 10.1097/00004836-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Inoue K, Nakamura T, Kinoshita T, Konishi M, Nakagohri T, Oda T, Takahashi S, Gotohda N, Hayashi T, Nawano S. Volume reduction surgery for advanced hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:362–366. doi: 10.1007/s00432-004-0566-7. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda S, Okuda K, Imamura M, Imamura I, Eriguchi N, Aoyagi S. Surgical resection combined with chemotherapy for advanced hepatocellular carcinoma with tumor thrombus: report of 19 cases. Surgery. 2002;131:300–310. doi: 10.1067/msy.2002.120668. [DOI] [PubMed] [Google Scholar]
- 15.Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagasue N, Ono T, Yamanoi A, Kohno H, El-Assal ON, Taniura H, Uchida M. Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis. Br J Surg. 2001;88:515–522. doi: 10.1046/j.1365-2168.2001.01732.x. [DOI] [PubMed] [Google Scholar]
- 17.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197:753–758. doi: 10.1016/j.jamcollsurg.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, Miller CM, Schwartz ME. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533–539. doi: 10.1097/00000658-200204000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueno K, Miyazono N, Inoue H, Nishida H, Kanetsuki I, Nakajo M. Transcatheter arterial chemoembolization therapy using iodized oil for patients with unresectable hepatocellular carcinoma: Evaluation pf three kinds of regimens and analysis of prognosis factors. Cancer. 2000;88:1574–1581. [PubMed] [Google Scholar]
- 21.Huang YH, Wu JC, Lui WY, Chau GY, Tsay SH, Chiang JH, King KL, Huo TI, Chang FY, Lee SD. Prospective case-controlled trial of adjuvant chemotherapy after resection of hepatocellular carcinoma. World J Surg. 2000;24:551–555. doi: 10.1007/s002689910090. [DOI] [PubMed] [Google Scholar]
- 22.Bruix J, Llovet JM. Two decades of advances in hepatocellular carcinoma research. Semin Liver Dis. 2010;30:1–2. doi: 10.1055/s-0030-1247219. [DOI] [PubMed] [Google Scholar]
- 23.Huang JF, Li SQ, Liang LJ. Efficacy of transarterial chemoembolization for advanced hepatocellular carcinoma. Zhonghua Gandan Waike Zazhi. 2010;1:3–6. [Google Scholar]
- 24.Ono T, Yamanoi A, Nazmy El Assal O, Kohno H, Nagasue N. Adjuvant chemotherapy after resection of hepatocellular carcinoma causes deterioration of long-term prognosis in cirrhotic patients: metaanalysis of three randomized controlled trials. Cancer. 2001;91:2378–2385. [PubMed] [Google Scholar]
- 25.Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747–1752. doi: 10.1002/cncr.10407. [DOI] [PubMed] [Google Scholar]
- 26.Cheng SQ, Wu MC, Chen H, Shen F, Yang JH, Cong WM, Wang PJ, Zhao YX. [Significance of typing of tumor thrombi in determination of treatment and assessment of prognosis of hepatocellular carcinoma with tumor thrombi in the portal vein] Zhonghua Yixue Zazhi. 2004;84:3–5. [PubMed] [Google Scholar]
- 27.Norton L. Adjuvant breast cancer therapy: current status and future strategies--growth kinetics and the improved drug therapy of breast cancer. Semin Oncol. 1999;26:1–4. [PubMed] [Google Scholar]
- 28.Xia HT, Guo GH, Huang XQ, Wang J. Variations in serum level of cytokines associated with vascular endothelial cells before and after treatment with either TACE and liver resection in patients with hepatocellular carcinoma. Zhonghua Gandan Waike Zazhi. 2012;17:23–26. [Google Scholar]


