Abstract
AIM: To compare transcatheter arterial chemoembolization (TACE) and 3D conformal radiotherapy (3D-CRT) with TACE monotherapy in hepatocellular carcinoma (HCC).
METHODS: We searched all the eligible studies from the Cochrane Library, PubMed, Medline, Embase, and CNKI. The meta-analysis was performed to assess the survival benefit, tumor response, and the decline in α-fetoprotein (AFP) level. According to the heterogeneity of the studies, pooled OR with 95%CI were calculated using the fixed-effects or random-effects model. An observed OR > 1 indicated that the addition of 3D-CRT to TACE offered survival benefits to patients that could be considered statistically significant. Statistical analyses were performed using Review Manager Software.
RESULTS: Ten studies met the criteria to perform a meta-analysis including 908 HCC participants, with 400 patients in the TACE/3D-CRT combination group and 508 in the TACE alone group. TACE combined with 3D-CRT significantly improved 1-, 2- and 3-year overall survival compared with TACE monotherapy (OR = 1.87, 95%CI: 1.37-2.55, P < 0.0001), (OR = 2.38, 95%CI: 1.78-3.17, P < 0.00001) and (OR = 2.97, 95%CI: 2.10-4.21, P < 0.00001). In addition, TACE plus 3D-CRT was associated with a higher tumor response (complete remission and partial remission) (OR = 3.81; 95%CI: 2.70-5.37; P < 0.00001), and decline rates of AFP level (OR = 3.24, 95%CI: 2.09-5.02, P < 0.00001).
CONCLUSION: This meta-analysis demonstrated that TACE combined with 3D-CRT was better than TACE monotherapy for patients with HCC, which needs to be confirmed by large multicenter trials.
Keywords: Hepatocellular carcinoma, Chemoembolization, Three-dimensional conformal radiotherapy, Meta-analysis
Core tip: Transcatheter arterial chemoembolization (TACE) is the most commonly used palliative therapy for patients with unresectable hepatocellular carcinoma (HCC), which can prolong survival with unsatisfactory long-term effects. 3D conformal radiotherapy (3D-CRT) has been utilized for HCC in a series of trials, with promising results. This meta-analysis demonstrated that TACE combined with 3D-CRT was better than TACE monotherapy for the treatment of HCC, which still needs to be confirmed by large prospectively randomized, controlled, multicenter trials.
INTRODUCTION
Liver cancer is the fifth most frequently diagnosed cancer worldwide and the second most frequent cause of cancer death[1]. The highest liver cancer rates are found in East and Southeast Asia, especially in China. It is recognized that only a small proportion of patients with early-stage hepatocellular carcinoma (HCC) may benefit from surgical resection. Transcatheter arterial chemoembolization (TACE) is the most commonly used palliative therapy for patients with unresectable HCC, which can prolong survival[2-5]. However, the long-term curative effect is unsatisfactory for TACE alone, because of tumor relapse from intracapsular or extracapsular invasion by HCC and remaining tumor cells after the treatment[6]. In addition, severe side effects are observed with the use of repeated rigorous TACE, including liver and renal failure, bone marrow depression, postembolization syndrome, and liver abscess[7].
Traditionally, radiotherapy has played a minor role in the treatment of hepatic cancers because of the low tolerance of the whole organ to irradiation, with a limit of 30-35 Gy[8]. The advent of new elegant 3D conformal radiotherapy (3D-CRT) has allowed the tumor to receive a higher dose and the surrounding normal liver tissue to receive a lower dose[9,10]. 3D-CRT has been utilized for HCC in a series of trials, with promising results in Asian countries, including improvements in response rate, disease control and overall survival.
Therefore, we performed this meta-analysis to evaluate whether the addition of 3D-CRT to TACE could offer any survival benefit to Asian patients with advanced HCC.
MATERIALS AND METHODS
Literature search
PubMed, Medline, Embase, Chinese BioMedical Literature Database (CBM), and the Cochrane Library were searched for studies published from October 2000 to October 2013. The following medical subject heading (MeSH) terms were used: (“hepatocellular carcinoma” or “HCC”) AND (“transcatheter arterial chemoembolization” or “TACE”) AND (“three-dimensional conformal radiotherapy” or “3D-CRT”).
Selection criteria
Studies were considered eligible if they met the following inclusion criteria: (1) prospective cohort or case-control studies; (2) patients in the treatment group received combination therapy consisting of 3D-CRT and TACE with TACE alone in the control group; (3) participants had unresectable HCC; and (4) data were reported on outcomes of overall survival (OS). Studies were excluded if: (1) they were reviews, commentaries, editorials, case reports, and letters; (2) patients underwent surgery; (3) there was a lack of key information for calculation with methods developed by Parmar et al[11], Williamson et al[12], and Tierney et al[13]; and (4) they were duplicated or redundant publications.
Data extraction
Each study was evaluated and classified by two independent investigators (Liqun Zou and Binglan Zhang). Disagreements were resolved in consultation with a third investigator. The extracted items comprised: first author, publication year, country, study design, number of patients, number of combination group and sole TACE group, tumor size and stage, survival rates.
Statistical analysis
Statistical analyses were performed using Review Manager Software (RevMan 5.2; Cochrane Collaboration, Oxford, United Kingdom). OR with 95%CI were calculated for the quantitative aggregation of survival results. An observed OR > 1 indicated that the addition of 3D-CRT to TACE could offer survival benefits to patients and would be considered statistically significant. Forrest plots were used to estimate the therapeutic effect on survival. Heterogeneity was defined as P < 0.10 or I2 > 50%[14]. Data that were not significantly heterogeneous (P ≥ 0.10, I2 ≤ 50%) were calculated using a fixed-effects model. If not, a random-effects model was used. Begg’s test was used to assess potential publication bias, P > 0.05 demonstrated that there was no potential publication bias[15].
RESULTS
Selection of studies
This meta-analysis yielded a total of 653 trials. After reviewing the titles and abstracts, 598 studies were excluded and 55 left for further evaluation. Of the 55 trials, 22 were excluded for inadequate control groups, 15 for being reviews or case reports, five for surgical intervention, and three for interventions other than TACE alone in the control group. Finally, there were 10 studies[16-25] fulfilling the inclusion criteria that were sent for review in our meta-analysis (Figure 1).
Figure 1.
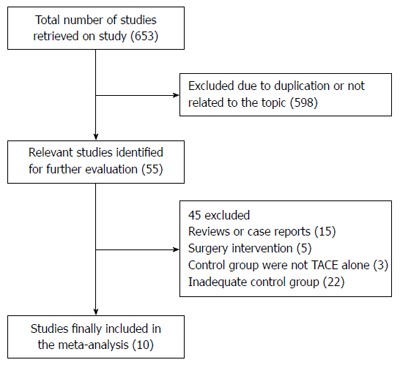
Route and results of including trials in the meta-analysis.
The main characteristics of included studies are listed in Table 1. Among the included studies published between 2001 and 2010, two were randomized clinical trials (RCTs) and eight were nonrandomized observational studies. All the trials originated from Asia because of the high incidence of hepatitis B infection here. In addition to two articles that did not report liver function, all the other eight studies included patients with liver function of Child-Pugh class A or B. The Karnofsky score of most patients was ≥ 70. There were 400 patients in the TACE and 3D-CRT combination group, and 508 patients in the TACE alone group. Among the 10 studies, there were eight, nine and eight that reported comparative data for overall survival rate at 1, 2 and 3 years, respectively (Table 2); seven and four studies that reported comparative data for tumor response and decline in α-fetoprotein (AFP) level, respectively.
Table 1.
Clinical characteristics of 10 included trials
| Ref. | Year | Study design | n (CMT/TACE) | Tumor stage | KPS | Child-Pugh Class (A/B/C) | Anticancer drug of TACE |
| Chia-Hsien Cheng et al[25] | 2001 | Nonrandomized | 33 (17/16) | II, IIIA, IVA | ≥ 70 | 33/0/0 | doxorubicin, cisplatin, MMC |
| Lan et al[24] | 2005 | Nonrandomized | 102 (42/60) | II, III | 10-Hydroxycamptothecine, DDP, 5-FU | ||
| Li et al[23] | 2003 | Nonrandomized | 82 (41/41) | 50/32/0 | 10-Hydroxycamptothecine, 5-FU, MMC or ADM | ||
| Liao et al[22] | 2010 | Randomized | 48 (24/24) | III, IV | 34/14/0 | 5-FU, DDP, ADM | |
| Liu et al[21] | 2005 | Nonrandomized | 114 (54/60) | ≥ 70 | 83/31/0 | MMC, ADM, CBP | |
| Shang et al[20] | 2007 | Nonrandomized | 76 (40/36) | T1-2N0M0 | ≥ 70 | 5-FU, DDP, ADM or MMC | |
| Shim et al[19] | 2005 | Nonrandomized | 73 (38/35) | III, IVa | 65/8/0 | doxorubicin | |
| Wu et al[18] | 2004 | Nonrandomized | 81 (41/40) | I, II | ≥ 70 | 55/26/0 | MMC, ADM, CBP or DDP |
| Zeng et al[17] | 2004 | Nonrandomized | 203 (54/149) | 158/45/0 | 5-FU, DDP, MMC | ||
| Zhao et al[16] | 2006 | Randomized | 96 (49/47) | T1N0M0, T2N0M0 | ≥ 70 | 96/0/0 | 10-Hydroxycamptothecine, DDP, 5-FU |
5-FU: 5-fluorouracil; ADM: Doxorubicin; CBP: Carboplatin; CMT: Combination therapy; KPS: Karnofsky score; MMC: Mitomycin C.
Table 2.
Outcomes of the combination therapy and transcatheter arterial chemoembolization alone groups
| Ref. | Year |
1-yr survival |
2-yr survival |
3-yr survival |
|||
| TACE + 3D-CRT | TACE alone | TACE + 3D-CRT | TACE alone | TACE + 3D-CRT | TACE alone | ||
| Chia-Hsien Cheng et al[25] | 2001 | 58.0% | 56.0% | ||||
| Lan et al[24] | 2005 | 57.1% | 61.7% | 40.5% | 30.0% | 26.2% | 16.7% |
| Li et al[23] | 2003 | 73.2% | 54.8% | 58.7% | 27.3% | 41.9% | 12.8% |
| Liao et al[22] | 2010 | 74.0% | 50.0% | 30.0% | 14.0% | ||
| Liu et al[21] | 2005 | 66.5% | 53.9% | 48.4% | 37.2% | 37.4% | 17.8% |
| Shang et al[20] | 2007 | 78.0% | 50.0% | 60.0% | 32.0% | 34.0% | 18.0% |
| Shim et al[19] | 2005 | 36.8% | 14.3% | ||||
| Wu et al[18] | 2004 | 90.2% | 89.7% | 75.6% | 58.7% | 44.6% | 24.0% |
| Zeng et al[17] | 2004 | 71.5% | 59.6% | 42.3% | 26.5% | 24.0% | 11.1% |
| Zhao et al[16] | 2006 | 82.0% | 55.0% | 63.0% | 28.0% | 43.0% | 15.0% |
TACE: Transcatheter arterial chemoembolization; 3D-CRT: Three-dimensional conformal radiotherapy.
Survival rates
One-year survival: Eight trials (802 patients) were identified with the outcome measurements of 1-year survival rates. Meta-analysis showed a significant improvement in the 1-year survival favoring combination therapy (OR = 1.87, 95%CI: 1.37-2.55, P < 0.0001) (Figure 2). There was no heterogeneity among the trials included, using fixed-effects model (heterogeneity χ2 = 9.02, P = 0.25; I2 = 22%).
Figure 2.
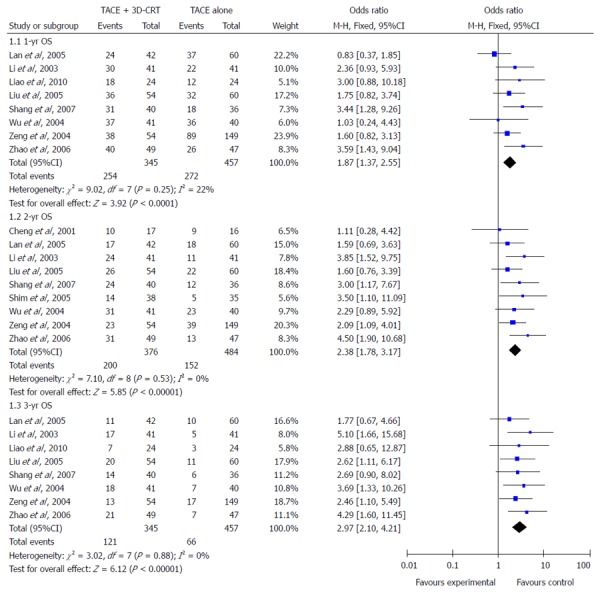
Outcomes of overall survival of combination therapy compared with sole transcatheter arterial chemoembolization therapy alone.
Two-year survival: Data for 2-year survival rate were reported in nine studies (860 patients) and there was also no heterogeneity among these studies (heterogeneity χ2 = 7.10, P = 0.53; I2 = 0%), thus the fixed-effects model was used to pool the results. Meta-analysis showed that combination of 3D-CRT and TACE was associated with a higher 2-year survival rate compared with TACE alone (OR = 2.38, 95%CI: 1.78-3.17, P < 0.00001) (Figure 2).
Three-year survival: Eight trials (802 patients) were identified with the outcome measurements of 3-year survival rates. Analysis of the 3-year survival (425 participants) also showed a significant benefit with the combination therapy method (OR = 2.97, 95%CI: 2.10-4.21, P < 0.00001) (Figure 2). In the χ2 and I2 tests, there were no heterogeneous findings (heterogeneity χ2 = 3.02, P = 0.88; I2 = 0%).
Tumor response
Seven trials (698 patients) were identified with outcome measurements of complete response (CR) and partial response (PR). There was no heterogeneity among the trials included, using a fixed-effects model (heterogeneity χ2 = 5.90, P = 0.43; I2 = 0%). The pooled analysis showed that compared with TACE alone, the combination method significantly improved CR + PR (OR = 3.81; 95%CI: 2.70-5.37; P < 0.00001) (Figure 3).
Figure 3.
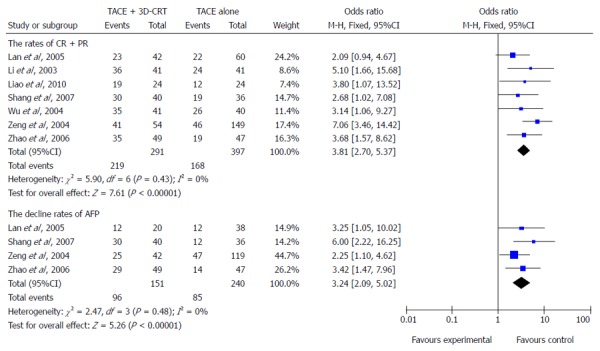
Better tumor response and higher decline in α fetal protein level were shown in patients treated with combination therapy.
Decline in AFP level
Four trials (391 participants) were identified with the outcome measurements of decline in AFP level. There was no evidence of heterogeneity among the trials included, using a fixed-effects model (heterogeneity χ2 = 2.47, P = 0.48; I2 = 0%). Meta-analysis showed that combination of 3D-CRT and TACE was associated with a greater decrease in AFP level compared with TACE monotherapy (OR = 3.24, 95%CI: 2.09-5.02, P < 0.00001) (Figure 3).
Publication bias
Begg’s funnel plot was performed to assess the publication bias in all the included studies for evaluation of survival rates, tumor response and decline in AFP level. Begg’s funnel plot did not reveal any evidence of significant asymmetry in the 1-year OS (P = 0.621), 2-year OS (P = 0.835), and 3-year OS (P = 0.138) (Figure 4). There was also no indication of publication bias in Begg’s test of tumor response (P = 0.652) and the decline in AFP level (P = 0.497) (Figure 5).
Figure 4.
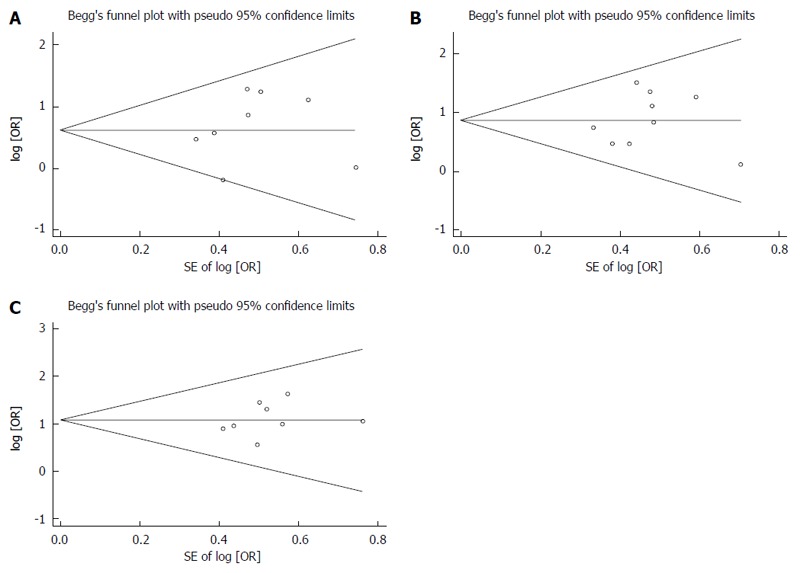
No significant asymmetry was revealed in 1-year (A), 2-year (B) and 3-year (C) overall survival.
Figure 5.
No publication bias was found in tumor response (A) and reduction in α fetal protein level (B).
DISCUSSION
The hepatic artery provides at least 80% of the blood supply to HCC, therefore, TACE has been suggested as a standard therapeutic method for patients who are unsuitable for surgical management[2,26]. However, TACE alone frequently results in incomplete tumor necrosis[27]. Previous meta-analysis has shown that the efficacy of TACE combined with radiofrequency ablation (RFA), high-intensity focused ultrasound (HIFU), or percutaneous ethanol injection (PEI) was significantly better than that of TACE alone in patients with HCC[3,28-35]. PEI has been widely used in treating HCC, but the effectiveness is limited to the diameter of HCC lesions[36]. RFA monotherapy was found to be associated with a higher tumor progression rate, and the main cause is the residual tumor tissue after RFA. Additionally, RFA cannot be a suitable treatment for tumors with multiple nodules. Consequently, combination of TACE and other local therapies may have several theoretical advantages. So far, there is no meta-analysis performed to assess the efficacy of the combination of TACE and 3D-CRT compared with TACE alone for treatment of HCC, and we performed this meta-analysis in which 10 studies were included eventually. However, incidence of complication in CRT with TACE might be higher than TACE alone.
Our meta-analysis demonstrated that the combination of 3D-CRT and TACE was associated with higher survival rates (1-year OR = 1.87, 95%CI: 1.37-2.55, P < 0.0001; 2-year OR = 2.38, 95%CI: 1.78-3.17, P < 0.00001; 3-year OR = 2.97, 95%CI: 2.10-4.21, P < 0.00001). In addition, the combination of TACE and 3D-CRT had a significantly better tumor response (OR = 3.81; 95%CI: 2.70-5.37; P < 0.00001) and greater decline in AFP level (OR = 3.24, 95%CI: 2.09-5.02, P < 0.00001). Thus, the method with TACE plus 3D-CRT was a better choice than the method with only TACE for treatment of patients with HCC.
In the past, there was a concern that the normal liver tissue was sensitive and had poor tolerance to radiation, so radiotherapy was limited in the treatment of HCC. Fortunately, with the advent of 3D planning systems, 3D-CRT can minimize the irradiation of normal tissue and improve the distribution of target irradiation dose to tumors. Numerous clinical studies of TACE in combination with 3D-CRT for patients with HCC have emerged in recent years. In our meta-analysis, all the included trials adopted the 3D-CRT technique. About 20% of the blood supply for HCC comes from the portal vein, resulting in a small number of tumor cells remaining viable, and the tumor may recur after TACE. 3D-CRT can be a consolidation planned procedure to target residual hepatic tumor. Furthermore, Seong et al[37] have reported that the anticancer drugs applied during TACE are retained in the tumor and may have a radiosensitizing effect.
In addition, we evaluated the adverse effects of TACE and 3D-CRT in the included studies. Five trials reported the development of radiation-induced liver disease, but no differences in liver function tests were found between the combination and control groups. Other common adverse effects were the postembolization syndrome including leukocyte count decline, fever, mild nausea, abdominal pain, and elevation of serum aminotransferase level or total bilirubin, which were transient and the patients often recovered in a short time.
Although there was no heterogeneity and publication bias in our meta-analysis, this study had several possible limitations. First, the number of RCTs was very limited, and only two RCTs were included. Second, the basic characteristics of included cases are not all the same, including clinical stage, microvascular infiltration, tumor number and size, and different stage of liver function. In addition, the interventional measures used (anticancer drugs of TACE, TACE course, radiation dose and methods) were different. Future studies need better design and more strict management of conduction. More patient information should be collected in the trials, including status of infection by hepatitis virus and degree of tumor cell differentiation. Lastly, a major limitation was that the studies were all from Asian countries, owing to no articles being published comparing the efficacy of TACE plus 3D-CRT vs TACE alone in non-Asian areas.
In conclusion, this meta-analysis based on 10 included studies indicates that TACE combined with 3D-CRT is a promising treatment for HCC. Importantly, these results need to be validated in further prospectively randomized, controlled multicenter clinical trials.
COMMENTS
Background
Transcatheter arterial chemoembolization (TACE) has been recognized as a standard treatment for unresectable hepatocellular carcinoma (HCC), but TACE alone has achieved limited success. With the advent of 3D conformal radiotherapy (3D-CRT), it has been utilized for HCC in a series of trials, with promising results. However, the role of TACE combined with 3D-CRT remains unclear.
Research frontiers
3D-CRT allows higher radiotherapy doses for HCC and minimizes liver injury, which can result in promising outcomes, including increases in response rate, tumor control and overall survival. In the current study, the authors performed a meta-analysis to assess the efficacy of 3D-CRT plus TACE compared with TACE alone for HCC, and this is believed to be the first meta-analysis to do this.
Innovations and breakthroughs
Previous studies assessing the effectiveness of 3D-CRT plus TACE compared with TACE alone have reported conflicting results. Consequently, we performed a meta-analysis to evaluate whether the addition of 3D-CRT to TACE could offer any survival benefits for HCC. The results indicated that 1-, 2-, and 3-year overall survival, tumor response, and decline in AFP level treated with 3D-CRT plus TACE were significantly higher than those with TACE alone.
Applications
This current limited evidence demonstrated that TACE plus 3D-CRT was better than TACE monotherapy for treatment of HCC, which can improve the overall survival rate and provides better prognosis for patients with HCC.
Terminology
TACE is performed with the infusion of a mixture of chemotherapy drugs and has been widely used to treat HCC. 3D-CRT is operated by a 3D treatment planning system and can minimize liver injury and increase irradiation dose to HCC.
Peer review
This manuscript presents a meta-analysis of 10 studies (two of them were randomized trials) comparing TACE alone to its combination with 3D-CRT in the treatment of HCC. Although, as stated by the authors at the end of the discussion, there is a variety of drugs and techniques used and more studies will be required, this paper provides an important overview on this subject.
Footnotes
P- Reviewer: Cerwenka HR, Mizuguchi T, Morise Z, Ramia JM, Yan Y, Zhang Q S- Editor: Ma YJ L- Editor: Kerr C E- Editor: Ma S
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Rahbari NN, Mehrabi A, Mollberg NM, Müller SA, Koch M, Büchler MW, Weitz J. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253:453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 3.Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011;(3):CD004787. doi: 10.1002/14651858.CD004787.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers RP. Meta-analysis of transarterial embolization in patients with unresectable hepatocellular carcinoma. Radiology. 2003;227:611–612; author reply 612-613. doi: 10.1148/radiol.2272021187. [DOI] [PubMed] [Google Scholar]
- 5.Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 6.Jansen MC, van Hillegersberg R, Chamuleau RA, van Delden OM, Gouma DJ, van Gulik TM. Outcome of regional and local ablative therapies for hepatocellular carcinoma: a collective review. Eur J Surg Oncol. 2005;31:331–347. doi: 10.1016/j.ejso.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747–1752. doi: 10.1002/cncr.10407. [DOI] [PubMed] [Google Scholar]
- 8.Dhir V, Swaroop VS, Mohandas KM, Dinshaw KA, Desai DC, Nagral A, Sharma V, Jagannath P, Desouza LJ. Combination chemotherapy and radiation for palliation of hepatocellular carcinoma. Am J Clin Oncol. 1992;15:304–307. doi: 10.1097/00000421-199208000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Liu MT, Li SH, Chu TC, Hsieh CY, Wang AY, Chang TH, Pi CP, Huang CC, Lin JP. Three-dimensional conformal radiation therapy for unresectable hepatocellular carcinoma patients who had failed with or were unsuited for transcatheter arterial chemoembolization. Jpn J Clin Oncol. 2004;34:532–539. doi: 10.1093/jjco/hyh089. [DOI] [PubMed] [Google Scholar]
- 10.Kim TH, Kim DY, Park JW, Kim YI, Kim SH, Park HS, Lee WJ, Park SJ, Hong EK, Kim CM. Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am J Clin Oncol. 2006;29:568–575. doi: 10.1097/01.coc.0000239147.60196.11. [DOI] [PubMed] [Google Scholar]
- 11.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. 2002;21:3337–3351. doi: 10.1002/sim.1303. [DOI] [PubMed] [Google Scholar]
- 13.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 16.Zhao MH, Lang FP, Jiang QA, Ma JJ, Song YX. Three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for inoperable primary liver cancer. Zhonghua Fangshe Chongliuxue Zazhi. 2006;15:39–41. [Google Scholar]
- 17.Zeng ZC, Tang ZY, Fan J, Zhou J, Qin LX, Ye SL, Sun HC, Wang BL, Yu Y, Wang JH, et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J. 2004;10:307–316. doi: 10.1097/00130404-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Wu DH, Zhi FC, Chen LH. Evaluating the efficacy of transcatheter arterial chemoembolization combinedwith hypofractionated 3-dimensional conformal radiotherapy for hrpstocellular carcinoma. Zhonghua Xiaohua Zazhi. 2004;24:353–357. [Google Scholar]
- 19.Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005;25:1189–1196. doi: 10.1111/j.1478-3231.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 20.Shang Y, You GX, Xu HY, Chen MC. Prospective randomized clinical study of transcatheter arterial chemoembolization, combined with three-dimensional conformal radiotherapy for primary liver cancer: An analysis of 40 cases. Shijie Huaren Xiaohua Zazhi. 2007;15:3140–3142. [Google Scholar]
- 21.Liu MZ, Wang XS, Cai L, Gu MF, Liu H, Li Q, Cui NJ, Zhang YQ, Li GH, Li JQ. [External radiation and combined transcatheter arterial chemoembolization for unresectable primary liver cancer] Ai Zheng. 2005;24:82–86. [PubMed] [Google Scholar]
- 22.Liao XF, He HJ, Zhou ZS, Hu W, Zhu XP. Three-dimensional conformal radiotherapy combined with interventional therapy in treatment of primary hepatocellular carcinoma. J Prac Oncol. 2010;25:681–683. [Google Scholar]
- 23.Li Y, Yan Y, Zhang HB, Guo ZW, Yan ZC, Li D. Three-dimensional conformal radiation combined with transarterial chemoembolization for unresectable primary liver cancer. Zhonghua Fangshe Zhongliuxue Zazhi. 2003;12:30–32. [Google Scholar]
- 24.Lan DQ, Gong XH, Wei XL. The efficacy analysis of transcatheter hepatic arterial chemoembolization combined with radiotherapy for primary liver cancer. Zhonghua Fangshe Zhongliuxue Zazhi. 2005;14:152–153. [Google Scholar]
- 25.Chia-Hsien Cheng J, Chuang VP, Cheng SH, Lin YM, Cheng TI, Yang PS, Jian JJ, You DL, Horng CF, Huang AT. Unresectable hepatocellular carcinoma treated with radiotherapy and/or chemoembolization. Int J Cancer. 2001;96:243–252. doi: 10.1002/ijc.1022. [DOI] [PubMed] [Google Scholar]
- 26.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Yu YQ, Xu DB, Zhou XD, Lu JZ, Tang ZY, Mack P. Experience with liver resection after hepatic arterial chemoembolization for hepatocellular carcinoma. Cancer. 1993;71:62–65. doi: 10.1002/1097-0142(19930101)71:1<62::aid-cncr2820710111>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Wang N, Guan Q, Wang K, Zhu B, Yuan W, Zhao P, Wang X, Zhao Y. TACE combined with PEI versus TACE alone in the treatment of HCC: a meta-analysis. Med Oncol. 2011;28:1038–1043. doi: 10.1007/s12032-010-9620-2. [DOI] [PubMed] [Google Scholar]
- 29.Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19:3872–3882. doi: 10.3748/wjg.v19.i24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao H, Xu Z, Long H, Zhang LL, Zhang J, Peng ZP, Li SL. Transcatheter arterial chemoembolization in combination with high-intensity focused ultrasound for unresectable hepatocellular carcinoma: a systematic review and meta-analysis of the chinese literature. Ultrasound Med Biol. 2011;37:1009–1016. doi: 10.1016/j.ultrasmedbio.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Gu L, Liu H, Fan L, Lv Y, Cui Z, Luo Y, Liu Y, Li G, Li C, Ma J. Treatment outcomes of transcatheter arterial chemoembolization combined with local ablative therapy versus monotherapy in hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2014;140:199–210. doi: 10.1007/s00432-013-1528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao M, Huang J, Zhang T, Wu H. Transarterial chemoembolization in combination with local therapies for hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8:e68453. doi: 10.1371/journal.pone.0068453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2013;25:187–194. doi: 10.1097/MEG.0b013e32835a0a07. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Shi J, Xie WF. Transarterial chemoembolization in combination with percutaneous ablation therapy in unresectable hepatocellular carcinoma: a meta-analysis. Liver Int. 2010;30:741–749. doi: 10.1111/j.1478-3231.2010.02221.x. [DOI] [PubMed] [Google Scholar]
- 35.Yan S, Xu D, Sun B. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci. 2013;58:2107–2113. doi: 10.1007/s10620-013-2570-8. [DOI] [PubMed] [Google Scholar]
- 36.Tesdal IK, Wikström M, Flechtenmacher C, Filser T, Dueber C. Percutaneous treatment of hepatocellular carcinoma in patients with transjugular intrahepatic portosystemic shunts. Cardiovasc Intervent Radiol. 2006;29:778–784. doi: 10.1007/s00270-005-0063-7. [DOI] [PubMed] [Google Scholar]
- 37.Seong J, Kim SH, Suh CO. Enhancement of tumor radioresponse by combined chemotherapy in murine hepatocarcinoma. J Gastroenterol Hepatol. 2001;16:883–889. doi: 10.1046/j.1440-1746.2001.02533.x. [DOI] [PubMed] [Google Scholar]


