Abstract
Context:
The combination of peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) has been proposed as a potential treatment for diabetes and obesity. However, the combined effects of these hormones, PYY3–36 and GLP-17–36 amide, on glucose homeostasis are unknown.
Objective:
This study sought to investigate the acute effects of PYY3–36 and GLP-17–36 amide, individually and in combination, on insulin secretion and sensitivity.
Setting and Design:
Using a frequently sampled iv glucose tolerance test (FSIVGTT) and minimal modeling, this study measured the effects of PYY3–36 alone, GLP-17–36 amide alone, and a combination of PYY3–36 and GLP-17–36 amide on acute insulin response to glucose (AIRg) and insulin sensitivity index (SI) in 14 overweight human volunteers, studied in a clinical research facility.
Results:
PYY3–36 alone caused a small but nonsignificant increase in AIRg. GLP-17–36 amide alone and the combination of PYY3–36 and GLP-17–36 amide did increase AIRg significantly. No significant differences in SI were observed with any intervention.
Conclusions:
PYY3–36 lacks any significant acute effects on first-phase insulin secretion or SI when tested using an FSIVGTT. Both GLP-17–36 amide alone and the combination of PYY3–36 and GLP-17–36 amide increase first-phase insulin secretion. There does not seem to be any additive or synergistic effect between PYY3–36 and GLP-17–36 amide on first-phase insulin secretion. Neither hormone alone nor the combination had any significant effects on SI.
The obesity pandemic has become a priority global health concern. By 2015, the World Health Organization predicts that four billion adults will be overweight and more than 700 million will be obese. This has predictably resulted in an increase in prevalence of the comorbidities of obesity, including type 2 diabetes, cardiovascular disease, hypertension, cancer, and obstructive sleep apnea, all resulting in a reduced life expectancy (1, 2). Bariatric surgery, for example the Roux-en-Y gastric bypass, is currently the most effective treatment, leading to a sustained 25–30% weight loss (3–5). It is well documented that gastric bypass surgery also induces a rapid and prolonged improvement in glucose levels, with greater than 40% of operated subjects achieving a complete remission of their diabetes (6). At present, the mechanisms for the favorable changes in body weight and glycemia are unclear, although alterations in peptide gut hormone secretion—in particular elevated postprandial levels of peptide YY (PYY) and glucagon-like peptide-1 (GLP-1)—are thought to play an important role (7, 8). As a result, analogs of PYY and GLP-1 are being developed as treatments for obesity and diabetes, although the doses that can be given are limited by adverse effects, principally nausea (9, 10). Low-dose combinations of PYY and GLP-1 represent an attractive route to achieving better weight-lowering efficacy without nausea: we have previously shown that PYY3–36 coinfused with GLP-17–36 amide reduces appetite and food intake in an additive fashion without adverse effects (11, 12).
GLP-1 is well established as an incretin hormone with insulinotropic effects (13). In contrast, the PP-fold (pancreatic polypeptide-fold) peptides neuropeptide Y (NPY) and PYY have differing effects on insulin secretion. NPY, acting at the neuropeptide Y1 receptor, inhibits insulin release from islets (14, 15), and sympathetic nerve terminals on pancreatic islets release both NPY and norepinephrine to produce inhibition of insulin secretion (16). Consistent with this, Y1 receptor knockout mice exhibit a basal hyperinsulinemia (17). PYY1–36, the full-length version of PYY that is able to activate Y1 receptors, similarly has insulinostatic effects in rodents and dogs (18–21), although it seems to increase insulin secretion after an ad libitum meal when infused into humans at 1.6 pmol/kg/min (22). Unlike NPY and PYY1–36, relatively little is known about the effects of PYY3–36 on glucose metabolism. PYY3–36 is considerably less active at the Y1 receptor but fully active at the neuropeptide Y2 receptor. Given that the Y2 receptor has been shown to act as a presynaptic auto-inhibitor of sympathetic transmission (23), Y2 activation might not affect or could even cause disinhibition of insulin release. In animal studies, administration of PYY3–36 was associated with increased glucose disposal under hyperinsulinaemic conditions, ie, an increase in insulin sensitivity (24). In humans, Sloth et al (22) reported that an acute iv infusion of PYY3–36 (at a dose of 0.2 pmol/kg/min, achieving mean levels of 76 ± 23 pmol/L) was able to increase the postprandial insulin response to an ad libitum meal, as judged by area under the curve (AUC) for insulin concentration.
If low-dose combinations of PYY3–36 and GLP-17–36 amide are to be used as future treatments for obesity and/or diabetes, it is important to establish their effects on glucose homeostasis. We therefore decided to investigate the effects of PYY3–36 and GLP-17–36 amide, individually and in combination, on insulin secretion and sensitivity in fasted overweight humans.
Materials and Methods
Peptides
PYY3–36 and GLP-17–36 amide were purchased from Bachem. Following initial high-fidelity synthesis, the peptide hormones underwent purification by high-resolution, high-performance liquid chromatography. Peptide compositions and purity were verified by quantitative amino acid analysis.
Sterile 0.9% (w/v) saline was purchased from Bayer. Using an aseptic technique in a laminar flow cabinet, PYY3–36 and GLP-17–36 amide were separately dissolved in 0.9% saline, aliquoted into vials, and freeze dried. Representative PYY3–36 and GLP-17–36 amide vials were sterile after culture for 7 days (Department of Microbiology, Hammersmith Hospital, London, United Kingdom), and endotoxin levels as measured by the Limulus Amoebocyte Lysate test (Associates of Cape Cod) were within the safe range for human infusion. Further representative vials of both PYY3–36 and GLP-17–36 amide were randomly selected and sent for amino acid analysis by Alta Bioscience to calculate the actual peptide content of the vials. The bioactivity of the peptides was verified by measuring the suppression of food intake over 24 hours when injected sc into C57/BL6 mice (12), and by receptor-binding affinity assays using membranes prepared from HEK293 cells overexpressing recombinant human Y2 or GLP-1 receptor (25).
Subjects
Fourteen overweight and obese volunteers, 11 men and 3 women, of mean age 34.5 ± 2.7 years (range, 21–50 years), and mean body mass index 30.1 ± 0.9 kg/m2 (range, 26.8–35.9 kg/m2), were recruited by advertisement. All volunteers underwent a standardized 75 g oral glucose tolerance test to exclude both diabetes and impaired glucose tolerance, to reduce variability in insulin secretion and sensitivity. Inclusion criteria were: age, 18 years and older; sex, male or female; body mass index, 25–40 kg/m2; and nonsmokers with stable weight for at least 3 months. Exclusion criteria were: diabetes mellitus or impaired glucose tolerance according to World Health Organization 2006 and 2011 criteria; history of alcoholism or substance abuse; and history of any major illness or use of any medications including over-the-counter products, which, in the opinion of the investigator, would either interfere with the study or potentially cause harm to the volunteer. Women who were pregnant, breastfeeding, or unable to maintain adequate contraception for the duration of the study and for 1 month afterward were also excluded.
All volunteers were screened and determined to be in normal health by medical history, physical examination, 12-lead electrocardiogram, and routine biochemistry and hematology. Women of child-bearing age were advised to avoid pregnancy during the study and for 1 month after completion. The study was approved by the Hammersmith & Queen Charlotte's Research Ethics Committee (Reference No. 09/H0707/77). All volunteers gave written informed consent, and the study was planned and performed in accordance with the Declaration of Helsinki.
Study protocol
Each volunteer attended five study visits. The first visit was to acclimatize the volunteer to the clinical environment and to experimental procedures. This acclimatization visit was run in identical fashion to subsequent, randomized, single-blinded visits, except that the infusion always consisted only of vehicle. Data from the acclimatization visit was not included in the analysis. The subsequent four visits followed a randomized, single-blind, placebo-controlled crossover design comparing four different infusions: 1) Vehicle alone (Gelofusine; B. Braun Medical); 2) PYY3–36 alone (0.15 pmol/kg/min); 3) GLP-17–36 amide alone (0.2 pmol/kg/min); 4) PYY3–36 + GLP-17–36 amide together (0.15 pmol/kg/min and 0.2 pmol/kg/min, respectively). The infused doses of the peptide hormones were selected after a preliminary dose-finding phase to achieve plasma concentrations of PYY3–36 at 80–120 pmol/L, a level that has previously been shown to increase postprandial insulin AUC values after an ad libitum meal (22). For GLP-17–36 amide, we aimed to achieve 100–140 pmol/L, a level that has previously been shown to increase insulin secretion rate in response to a graded glucose infusion (26). Randomization was carried out by an independent clinician not otherwise involved in the study.
To limit adsorption of peptide to the infusion apparatus, Gelofusine was used as the vehicle for all peptide infusions to dissolve the contents of the randomized vials of peptide and to prime all syringes and infusion lines (27). Each peptide was drawn up under sterile conditions in a separate 50-mL syringe, and to enable the use of two different infusion rates, was delivered by separate syringe drivers (Graseby 3100, SIMS Graseby or Asena GH Mk III, Alaris Medical Systems). Thus, on a visit when the volunteer received only one peptide, the second syringe delivered vehicle only, set at the delivery rate calculated for the other hormone. Study visits for each volunteer were at least 3 days apart to allow for washout of peptides and peptide effects. During the 24-hour period prior to each study visit, volunteers refrained from strenuous exercise and alcohol consumption. They fasted from 2200 h the night before the study, drinking only water. On the morning of each study visit, volunteers attended a dedicated Clinical Investigation Unit at the Hammersmith Hospital. Female volunteers underwent a urine β-hCG test to exclude pregnancy before the peptide infusion was started. Two cannulae were inserted into the volunteer's peripheral veins. One cannula was used for sampling and the other one was used to administer peptide infusion and iv glucose bolus (via a multiport connector). The infusion containing the peptide hormone(s) was started at 0 minutes. For evaluation of the acute insulin response to glucose (AIRg) and insulin sensitivity index (SI), a frequently sampled iv glucose tolerance test (FSIVGTT) was performed at +60 minutes with an iv glucose bolus of 0.3 g/kg administered manually over 2 minutes (28). Augmentation of iv glucose tolerance test plasma insulin concentrations by tolbutamide or insulin injection was not undertaken because volunteers were normoglycaemic and insulin release was, in any case, likely to be amplified by the GLP-1 infusions. The peptide infusion was stopped at +240 minutes. Volunteers completed a series of visual analog scales that rated hunger, satiety, prospective food consumption, and nausea throughout the study. These consisted of 100-mm lines with text expressing the most positive and the most negative rating for each variable anchored at either end (29). Pulse and blood pressure were regularly monitored.
Blood samples were taken for glucose into fluoride oxalate tubes, and insulin into plain serum tubes (Becton, Dickinson) at −30, 0, 20, 40, 60, 62, 63, 64, 65, 66, 68, 70, 72, 74, 78, 80, 82, 85, 90, 100, 110, 130, 160, 200, and 240 minutes. Larger samples were taken at 0, 20, 40, 60, 80, 100, 160, and 240 minutes for plasma gut hormone analysis in lithium heparin coated tubes (International Scientific Supplies) containing 2000 kallikrein inhibitor units (0.2 ml) aprotinin (Trasylol, Bayer Schering Pharma). The insulin samples were allowed to clot for 10 minutes at room temperature, after which they were centrifuged and separated and stored at −20°C until analysis. All other samples underwent immediate centrifugation for 10 minutes, 4000 rpm at 4°C, after which plasma was promptly separated and stored at –20°C until analysis.
Plasma gut hormone assays
All samples were assayed in duplicate and within a single assay to eliminate interassay variation. Serum insulin was assayed using the Siemens Immulite 2000 immunoassay, which is a solid-phase, two-site chemiluminescent immunoassay with an analytical range of 2–300 mIU/L and an intra-assay coefficient of variation of 3.3–5.5%. Plasma glucose was assayed using an Abbott Architect automated analyzer using a hexokinase-glucose-6-phosphate dehydrogenase method. The analytical range was 0.278–44.4 mmol/L with an intra-assay coefficient of variation of 0.65–1.98% and an interassay coefficient of variation of 0.84–0.93%. Plasma total PYY and amidated GLP-1 were measured using established in-house RIAs (30, 31). The PYY assay's functional detection limit was 16.8 pmol/L (95% confidence interval [CI] 14.4–19.3) with an intra-assay coefficient of variation of 7.4%. The GLP-1 assay's functional detection limit was 13.4 pmol/L (95% CI, 12.5–14.2) with an intra-assay coefficient of variation of 3.1%.
Data analysis
Data are expressed as mean ± SEM except where noted. Statistical analysis was carried out using Prism 5.0 (GraphPad Software). The acute plasma insulin concentration response to glucose (AIRg, 0–10 min), a sensitive index of beta cell function and first-phase insulin response (32), was calculated as the area under the FSIVGTT insulin concentration profile (AUC) from 0–10 minutes following glucose administration, calculated using the trapezoid rule (33). The insulin sensitivity index SI, a measure of the ability of insulin to enhance glucose disposal, was determined from FSIVGTT glucose and insulin concentrations using the minimal model of glucose disappearance (34) implemented as previously described (35). The FSIVGTT-derived measures, AIRg and SI provide the so-called disposition index (DI), calculated as SI × AIRg (36). This widely used, dimensionless measure of beta cell function quantifies beta cell adaptation to variation in SI according to the hyperbolic relationship between insulin resistance and insulin secretion.
Results
Table 1 summarizes the average plasma hormone concentrations achieved in each of the study arms. PYY exposures were similar between the two arms that included PYY3–36 in the infusion (Figure 1A), as were GLP-1 exposures comparing the arms that included GLP-17–36 amide in the infusion (Figure 1B). There were no variations in pulse and blood pressure across infusions and analysis of visual analog scale scores revealed no nausea in response to the gut hormone infusions nor any differences in subjective ratings of hunger or pleasantness to eat between any of the interventions (data not shown).
Table 1.
Summary of PYY and GLP-1 Plasma Concentrations (in pmol/L) and Area Under The Curve Values (pmol · L−1 · min) for Each of the Infusion Arms: Vehicle, PYY3–36, GLP-17–36 amide, and Combination
| Infusion Arm | Baseline Plasma Concentration, pmol/L | End-Infusion Plasma Concentration, pmol/L | AUC Plasma Concentrations Over Infusion Period, pmol · L−1 · min |
|---|---|---|---|
| PYY levels | |||
| Vehicle | 47.7 ± 8.7 | 26.7 ± 15.8 | 2766 ± 423.7 |
| PYY | 45.8 ± 8.1 | 113.5 ± 13.7 | 6091 ± 861.2 |
| GLP-1 | 34.1 ± 5.3 | 21.3 ± 13.9 | 3395 ± 575.9 |
| Combination | 52.2 ± 10.9 | 97.8 ± 37.2 | 7297 ± 1460 |
| GLP-1 levels | |||
| Vehicle | 43.7 ± 6.2 | 44.0 ± 8.5 | 3614 ± 344.2 |
| PYY | 43.8 ± 7.7 | 33.4 ± 2.6 | 3813 ± 458.7 |
| GLP-1 | 55.6 ± 9.7 | 142.2 ± 22.3 | 9084 ± 1134 |
| Combination | 52.6 ± 15.2 | 140.4 ± 22.0 | 8639 ± 1495 |
Figure 1.
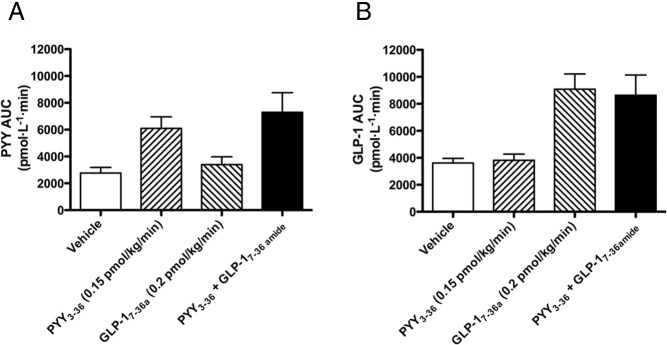
A, PYY; and B, GLP-1 exposure during the FSIVGTT. Integrated area AUC for 0–100 min, from the start of the infusion to the end of the intensive minimal modeling period, is plotted on the y-axis. The x-axis indicates infusion given. Mean ± SEM plotted. Baseline plasma PYY levels (at 0 min) were vehicle, 47.7 ± 8.7 pmol/L; PYY3–36, 45.8 ± 8.1 pmol/L; GLP-17–36 amide, 34.1 ± 5.3 pmol/L; PYY3–36 + GLP-17–36 amide, 52.2 ± 10.9 pmol/L). End-infusion (+240 min: steady state) levels were vehicle, 26.7 ± 15.8 pmol/L; PYY3–36, 113.5 ± 13.7 pmol/L; GLP-17–36 amide, 21.3 ± 13.9 pmol/L; PYY3–36 + GLP-17–36 amide, 97.8 ± 37.2 pmol/L. To estimate the exposure of volunteers to PYY3–36 from 0–100 min, the respective AUC for each infusion arm was calculated as follows: vehicle, 2766 ± 423.7 pmol · L−1 · min; PYY3–36, 6091 ± 861.2 pmol · L−1 · min; GLP-17–36 amide, 3395 ± 575.9 pmol · L−1 · min; PYY3–36 + GLP-17–36 amide, 7297 ± 1460 pmol · L−1 · min. Baseline plasma GLP-1 levels (at 0 min) across different infusion arms were vehicle, 43.7 ± 6.2 pmol/L; PYY3–36, 43.8 ± 7.7 pmol/L; GLP-17–36 amide, 55.6 ± 9.7 pmol/L; PYY3–36 + GLP-17–36 amide, 52.6 ± 15.2 pmol/L). End-infusion (+240 min: steady state) levels were vehicle, 44.0 ± 8.5 pmol/L; PYY3–36, 33.4 ± 2.6 pmol/L; GLP-17–36 amide, 142.2 ± 22.3 pmol/L; PYY3–36 + GLP-17–36 amide, 140.4 ± 22.0 pmol/L. To estimate the exposure of volunteers to GLP-17–36 amide from 0–100 min, the respective AUC for each infusion arm was calculated as follows: vehicle, 3614 ± 344.2 pmol · L−1 · min; PYY3–36, 3813 ± 458.7 pmol · L−1 · min; GLP-17–36 amide, 9084 ± 1134 pmol · L−1 · min; PYY3–36 + GLP-17–36 amide, 8639 ± 1495 pmol · L−1 · min.
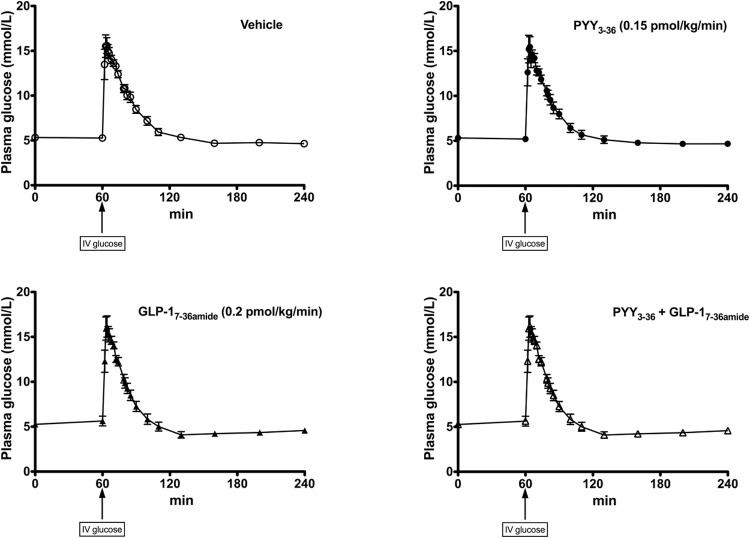
Fasting glucose levels were very similar between all infusion arms (Figure 2). With the administration of the iv glucose bolus, glucose levels peaked at 15.5–16.2 mmol/L (64 minutes) and decreased back to baseline by 110 minutes. In no case did any volunteer experience a biochemical or symptomatic hypoglycemia as a result of the endogenous insulin release in response to the large iv glucose bolus.
Figure 2.
Plasma glucose levels during the FSIVGTT. Y-axis shows plasma glucose levels (mmol/L). X-axis shows time (min). iv glucose bolus (0.3 g/kg) given at 60 min. Mean ± SEM plotted. Open circles, dashed line placebo infusion arm; closed circles, solid line: PYY3–36 infusion (0.15 pmol/kg/min). Closed triangles, solid line: GLP-17–36 amide infusion (0.2 pmol/kg/min). Open triangles, solid line: combined PYY3–36 + GLP-17–36 amide infusion. Fasting glucose values for vehicle, 5.3 ± 0.1 mmol/L; PYY3–36, 5.3 ± 0.2 mmol/L; GLP-17–36 amide, 5.3 ± 0.1 mmol/L; combined PYY3–36 + GLP-17–36 amide, 5.4 ± 0.1 mmol/L.
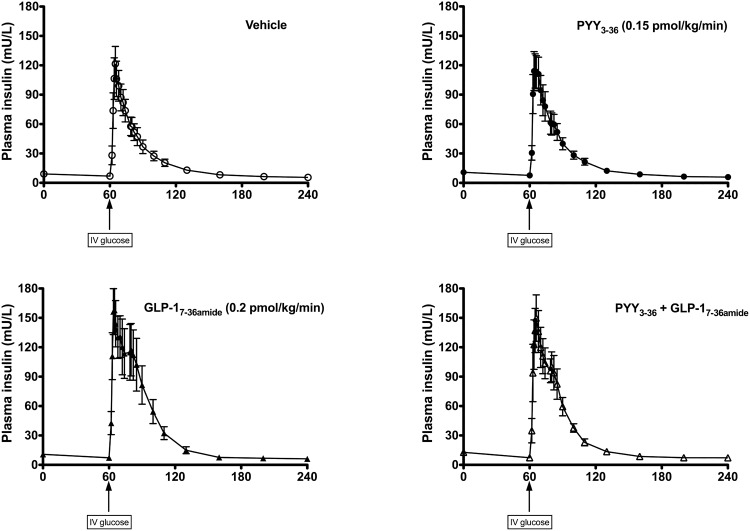
The insulin response to the iv glucose bolus is shown in Figure 3. Infusion of GLP-17–36 amide, either alone or in combination with PYY3–36, augmented the insulin-secretory response following the iv glucose bolus compared with either vehicle or PYY alone. In line with this, the AIRg during each infusion showed a significant difference in means (P = .005; Figure 4A). No significant difference was detected on post-hoc testing between vehicle and PYY3–36 (mean difference in AIRg, 60.71 mU · L−1 · min, 95% CI for difference, −210.2–331.7). A significant difference was detected between vehicle and GLP-17–36 amide (P < .01: mean difference in AIRg, 341.7 mU · L−1 · min; 95% CI for difference, 70.77–612.7). The PYY3–36 + GLP-17–36 amide combination also significantly increased AIRg compared with vehicle, similar to GLP-17–36 amide alone (P < .05: mean difference, 275.4 mU · L−1 · min; 95% CI for difference, 4.48–546.4). Comparison of AIRg in GLP-17–36 amide alone vs PYY3–36 + GLP-17–36 amide showed no significant difference (mean difference, −66.29 mU · L−1 · min; 95% CI for difference, −337.2- 204.7).
Figure 3.
Plasma insulin levels during the FSIVGTT. Y-axis shows insulin levels (mU/L). X-axis shows time (min). iv glucose bolus (0.3 g/kg) given at 60 min. Mean ± SEM plotted. Open circles, dashed line: placebo infusion arm. Closed circles, solid line: PYY3–36 infusion (0.15 pmol/kg/min). Closed triangles, solid line: GLP-17–36 amide infusion (0.2 pmol/kg/min). Open triangles, solid line: combined PYY3–36 + GLP-17–36 amide infusion.
Figure 4.
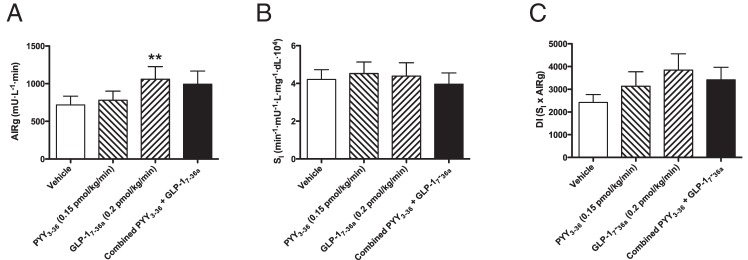
A, AIRg response to iv glucose. Means plotted ± SEM, one-way repeated measures ANOVA (P = .0046). AIRg means for vehicle 718.1 ± 115.7 mU · L−1 · min; GLP-17–36 amide infusion, 1060 ± 167.6 mU · L−1 · min; PYY3–36, 778.8 ± 122.4 mU · L−1 · min; combined PYY3–36 + GLP-17–36 amide, 993.5 ± 173.3 mU · L−1 · min. *, = P < .05 and **, P < .01 for comparison of combined and GLP-1 (respectively) to vehicle for AIRg by Bonferroni's multiple correction test. B, SI. Means plotted ± SEM, one-way repeated measures ANOVA (P = .7929). SI for vehicle, 4.21 ± 0.51 min−1 · mU−1 · L · mg−1 · dL · 104; PYY3–36, 4.52 ± 0.61 min−1 · mU−1 · L · mg−1 · dL · 104; GLP-17–36 amide, 4.39 ± 0.71 min−1 · mU−1 · L · mg−1 · dL · 104; combined PYY3–36 + GLP-17–36 amide, 3.96 ± 0.59 min−1 · mU−1 · L · mg−1 · dL · 104. C, DI. Means plotted ± SEM, one-way repeated measures ANOVA (P = .07). DI for vehicle, 2417 ± 350; PYY3–36, 3131 ± 638; GLP-17–36 amide, 3844 ± 717; combined PYY3–36 + GLP-17–36 amide, 3414 ± 554.
No significant differences in SI index were discerned between infusion arms (P = .79; Figure 4B). There was no significant difference in mean DI between infusion arms (P = .07; Figure 4C).
Discussion
In this study, we measured the changes in first-phase insulin secretion and SI in response to an acute infusion of PYY3–36 and GLP-17–36 amide in healthy, overweight, nondiabetic humans using an FSIVGTT. As expected from its known action as an incretin hormone, GLP-17–36 amide infusion significantly increased first-phase insulin secretion in response to the iv glucose compared with vehicle. Similarly, we noted a significant elevation in AIRg with combination PYY3–36 + GLP-17–36 amide infusion. PYY3–36 infusion alone resulted in a slight, nonsignificant increase in AIRg compared with vehicle. There seems to be no additive or synergistic effect between PYY3–36 and GLP-17–36 amide on insulin secretion because the combination caused an elevation in AIRg of similar magnitude to GLP-17–36 amide alone. Furthermore, neither hormone had any significant acute effect on measures of SI in this cohort, hence the changes in disposition indices mirrored the changes in AIRg across all infusion arms.
We have therefore shown that acute, low-dose administration of PYY3–36 to overweight humans has no effect on SI and no significant effect on β cell secretory function. In the study by Sloth et al (22), which did report an insulinotropic effect of exogenously administered PYY3–36 at 0.2 pmol/kg/min, the insulin response was examined after an ad libitum lunch, but the PYY3–36 group surprisingly ate slightly more than the placebo group, perhaps because the PYY infusion day always followed the placebo day, allowing acclimatization to the study environment and a potential order effect. Thus, the increased insulin response with PYY3–36 observed by Sloth et al may be merely a response to an increased energy intake at the meal, and also because of an increase in endogenous incretin secretion. In this study, volunteers were acclimatized to experimental conditions, infusions were given in a random order, and we used a standardized method to examine insulin secretion in response to a fixed iv glucose stimulus. Moreover, the use of an iv glucose stimulus avoids any confounding by endogenous incretin secretion, unlike the meal stimulus employed by Sloth et al.
Nevertheless, a limitation of our study is that it does not entirely exclude a modest insulinotropic effect of PYY3–36, within the setting of the validated FSIVGTT protocol, which incorporated a large glycemic excursion as standard. A second limitation is that we only studied the first-phase insulin response because the FSIVGTT incorporates a transient glucose stimulus and not the sustained hyperglycaemic stimulus necessary to observe the second-phase insulin response (37). It therefore remains possible that the second-phase insulin response is modulated by PYY3–36, and this remains to be tested. Third, it should be noted that we studied low doses of PYY3–36 and GLP-1, selected on the basis of prior evidence of being just sufficient to affect insulin secretion. It is possible that higher doses of the combination could have effects on SI. This could be explored in future studies. A final limitation of note with our study is that it only examined the effects of PYY3–36 and GLP-1 in an acute setting. We speculate that longer-term treatment with the combination of PYY3–36 and GLP-1 may ultimately improve SI over the hormones given individually through additive reductions in food intake and therefore greater weight loss.
Importantly, we have shown that the combination of GLP-1 and PYY3–36 retains the glucose-lowering insulinotropic effect observed with GLP-1, which adds further support to the concept of multiple gut hormone therapy as a treatment for diabetes and obesity. Future studies should focus on measuring the effects of chronic administration of these gut hormones on weight loss, insulin sensitivity and secretion.
Acknowledgments
We thank the staff of the NIHR/Wellcome Trust Clinical Research Facility at Hammersmith Hospital, without whom this project would not have been possible.
Author Contributions: T.M.T., B.C.T.F., and S.R.B. designed the study. T.M.T., V.S., R.C.T., A.A., B.C.T.F., A.D.S., S.M., N.P., and K.C.R.B. conducted the study. I.F.G. performed the minimal modeling analysis. M.D., J.M., and M.A.G. advised on the assays used in the study. T.M.T. and V.S. wrote the manuscript. All authors reviewed and commented on the manuscript. S.R.B. is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis.
This work was supported by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (P26288). Investigative Medicine is funded by the Medical Research Council (MRC), Biotechnology and Biological Sciences Research Council (BBSRC), NIHR, an Integrative Mammalian Biology (IMB) Capacity Building Award, and a FP7-HEALTH-2009-241592 EuroCHIP grant, and by funding from the NIHR Imperial Biomedical Research Centre within the Academic Health Sciences Centre. S.R.B. is supported by an NIHR Senior Investigator Award and the MRC. T.M.T. is supported by grants from the MRC. A.D.S. is a recipient of a Wellcome Trust Clinical Research Training Fellowship. R.C.T., V.S., and B.C.T.F. are recipients of MRC Clinical Research Training Fellowships, and B.C.T.F. received an NIHR Clinical Lectureship. A.A. is an NIHR Academic Foundation Year 2 trainee. S.M. is an NIHR Academic Clinical Fellow.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AIRg
- acute insulin response to glucose
- AUC
- area under the curve
- CI
- confidence interval
- DI
- disposition index
- FSIVGTT
- frequently sampled iv glucose tolerance test
- GLP-1
- glucagon-like peptide-1
- NPY
- neuropeptide Y
- PYY
- peptide YY
- SI
- insulin sensitivity index.
References
- 1. WHO Consultation. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1–253. [PubMed] [Google Scholar]
- 2. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. [DOI] [PubMed] [Google Scholar]
- 3. Sjöström L. Bariatric surgery and reduction in morbidity and mortality: Experiences from the SOS study. Int J Obes (Lond). 2008;32 Suppl 7:S93–S97. [DOI] [PubMed] [Google Scholar]
- 4. Sjöström L, Gummesson A, Sjöström CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): A prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–662. [DOI] [PubMed] [Google Scholar]
- 5. Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. [DOI] [PubMed] [Google Scholar]
- 6. Pournaras DJ, Osborne A, Hawkins SC, et al. Remission of type 2 diabetes after gastric bypass and banding: Mechanisms and 2 year outcomes. Ann Surg. 2010;252:966–971. [DOI] [PubMed] [Google Scholar]
- 7. le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bueter M, Miras AD, Chichger H, et al. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav. 2011;104:709–721. [DOI] [PubMed] [Google Scholar]
- 9. le Roux CW, Borg CM, Murphy KG, Vincent RP, Ghatei MA, Bloom SR. Supraphysiological doses of intravenous PYY3–36 cause nausea, but no additional reduction in food intake. Ann Clin Biochem. 2008;45:93–95. [DOI] [PubMed] [Google Scholar]
- 10. Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. [DOI] [PubMed] [Google Scholar]
- 11. De Silva A, Salem V, Long CJ, et al. The gut hormones PYY 3–36 and GLP-1 7–36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14:700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neary NM, Small CJ, Druce MR, et al. Peptide YY3–36 and glucagon-like peptide-17–36 inhibit food intake additively. Endocrinology. 2005;146:5120–5127. [DOI] [PubMed] [Google Scholar]
- 13. Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7–36: A physiological incretin in man. Lancet. 1987;2:1300–1304. [DOI] [PubMed] [Google Scholar]
- 14. Schwetz TA, Ustione A, Piston DW. Neuropeptide Y and somatostatin inhibit insulin secretion through different mechanisms. Am J Physiol Endocrinol Metab. 2013;304:E211–E221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang ZL, Bennet WM, Wang RM, Ghatei MA, Bloom SR. Evidence of a paracrine role of neuropeptide-Y in the regulation of insulin release from pancreatic islets of normal and dexamethasone-treated rats. Endocrinology. 1994;135:200–206. [DOI] [PubMed] [Google Scholar]
- 16. Skoglund G, Gross R, Ahrén B, Loubatières-Mariani MM. Different mechanisms are involved in neuropeptide Y-induced pancreatic vasoconstriction and inhibition of insulin secretion. Eur J Pharmacol. 1993;236:69–74. [DOI] [PubMed] [Google Scholar]
- 17. Kushi A, Sasai H, Koizumi H, Takeda N, Yokoyama M, Nakamura M. Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice. Proc Natl Acad Sci U S A. 1998;95:15659–15664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szecówka J, Tatemoto K, Rajamäki G, Efendic S. Effects of PYY and PP on endocrine pancreas. Acta Physiol Scand. 1983;119:123–126. [DOI] [PubMed] [Google Scholar]
- 19. Böttcher G, Ahrén B, Lundquist I, Sundler F. Peptide YY: Intrapancreatic localization and effects on insulin and glucagon secretion in the mouse. Pancreas. 1989;4:282–288. [PubMed] [Google Scholar]
- 20. Nieuwenhuizen AG, Karlsson S, Fridolf T, Ahrén B. Mechanisms underlying the insulinostatic effect of peptide YY in mouse pancreatic islets. Diabetologia. 1994;37:871–878. [DOI] [PubMed] [Google Scholar]
- 21. Greeley GH, Jr, Lluis F, Gomez G, Ishizuka J, Holland B, Thompson JC. Peptide YY antagonizes beta-adrenergic-stimulated release of insulin in dogs. Am J Physiol. 1988;254:E513–E517. [DOI] [PubMed] [Google Scholar]
- 22. Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab. 2007;292:E1062–E1068. [DOI] [PubMed] [Google Scholar]
- 23. Malmström RE, Lundberg JO, Weitzberg E. Autoinhibitory function of the sympathetic prejunctional neuropeptide Y Y(2) receptor evidenced by BIIE0246. Eur J Pharmacol. 2002;439:113–119. [DOI] [PubMed] [Google Scholar]
- 24. van den Hoek AM, Heijboer AC, Corssmit EP, et al. PYY3–36 reinforces insulin action on glucose disposal in mice fed a high-fat diet. Diabetes. 2004;53:1949–1952. [DOI] [PubMed] [Google Scholar]
- 25. Dakin CL, Gunn I, Small CJ, et al. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142:4244–4250. [DOI] [PubMed] [Google Scholar]
- 26. Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: Effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52:380–386. [DOI] [PubMed] [Google Scholar]
- 27. Kraegen EW, Lazarus L, Meler H, Campbell L, Chia YO. Carrier solutions for low-level intravenous insulin infusion. BMJ. 1975;3:464–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pacini G, Mari A. 2007. Assessment of insulin sensitivity from steady-state and dynamic tests. In: Roden M, ed. Clinical diabetes research: Methods and techniques. Chichester, England: John Wiley, Sons; 27–41. [Google Scholar]
- 29. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. [DOI] [PubMed] [Google Scholar]
- 30. Adrian TE, Savage AP, Sagor GR, et al. Effect of peptide YY on gastric, pancreatic, and biliary function in humans. Gastroenterology. 1985;89:494–499. [DOI] [PubMed] [Google Scholar]
- 31. Ghatei MA, Uttenthal LO, Bryant MG, Christofides ND, Moody AJ, Bloom SR. Molecular forms of glucagon-like immunoreactivity in porcine intestine and pancreas. Endocrinology. 1983;112:917–923. [DOI] [PubMed] [Google Scholar]
- 32. Kahn SE, Carr DB, Faulenbach MV, Utzschneider KM. An examination of beta-cell function measures and their potential use for estimating beta-cell mass. Diab Obes Metab. 2008;10 Suppl 4:63–76. [DOI] [PubMed] [Google Scholar]
- 33. Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–E677. [DOI] [PubMed] [Google Scholar]
- 35. Godsland IF, Agbaje OF, Hovorka R. Evaluation of nonlinear regression approaches to estimation of insulin sensitivity by the minimal model with reference to Bayesian hierarchical analysis. Am J Physiol Endocrinol Metab. 2006;291:E167–E174. [DOI] [PubMed] [Google Scholar]
- 36. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. [DOI] [PubMed] [Google Scholar]
- 37. Gerich JE. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes. 2002;51 Suppl. 1:S117–S121. [DOI] [PubMed] [Google Scholar]