Abstract
Radiation therapy is widely used with curative or palliative intent in the clinical management of multiple cancers. Although mainly aimed at direct tumor cell killing, mounting evidence suggests that radiation can alter the tumor to become an immunostimulatory milieu. Data suggest that the immunogenic effects of radiation can be exploited to promote synergistic antitumor effects in combination with immunotherapeutic agents. Here we review concepts associated with the immunogenic consequences of radiation therapy, and highlight how preclinical findings are translating into clinical benefit for patients receiving combination regimens of radiation therapy and therapeutic cancer vaccines.
Introduction
Radiation therapy (RT), the standard of care for multiple cancers, aims to eliminate malignant lesions through direct killing of tumor cells. The success of RT as an anticancer treatment modality lies in its ability to cause DNA double-strand breaks in irradiated tumor cells, ultimately leading to cell death. However, the presence of systemic disease, development of treatment resistance, or the need for sublethal doses of radiation in order to reduce toxicity to normal tissue can result in surviving tumor cell populations and subsequent disease progression or recurrence.1,2
Tumors are often weakly immunogenic and mount multiple mechanisms to evoke immune evasion and/or tolerance.3-5 Although RT was initially thought to be immunosuppressive, multiple clinical observations in recent years have indicated that certain cancer patients obtain greater clinical benefit from immunotherapy regimens if they have been previously treated with RT.2 These observations are supported by increasing evidence demonstrating that, through modulation of the immune system and/or direct effects on malignant cells, RT can modulate the tumor to become an immunostimulatory milieu.1,6-8 Upon exposure to RT, both dying and surviving tumor cell populations undergo a spectrum of immunogenic modifications (Fig. 1). This immunogenic milieu can then be exploited in a combined regimen with therapeutic cancer vaccines to promote a more robust immune response against the tumor and maximize clinical benefit.
Figure 1.
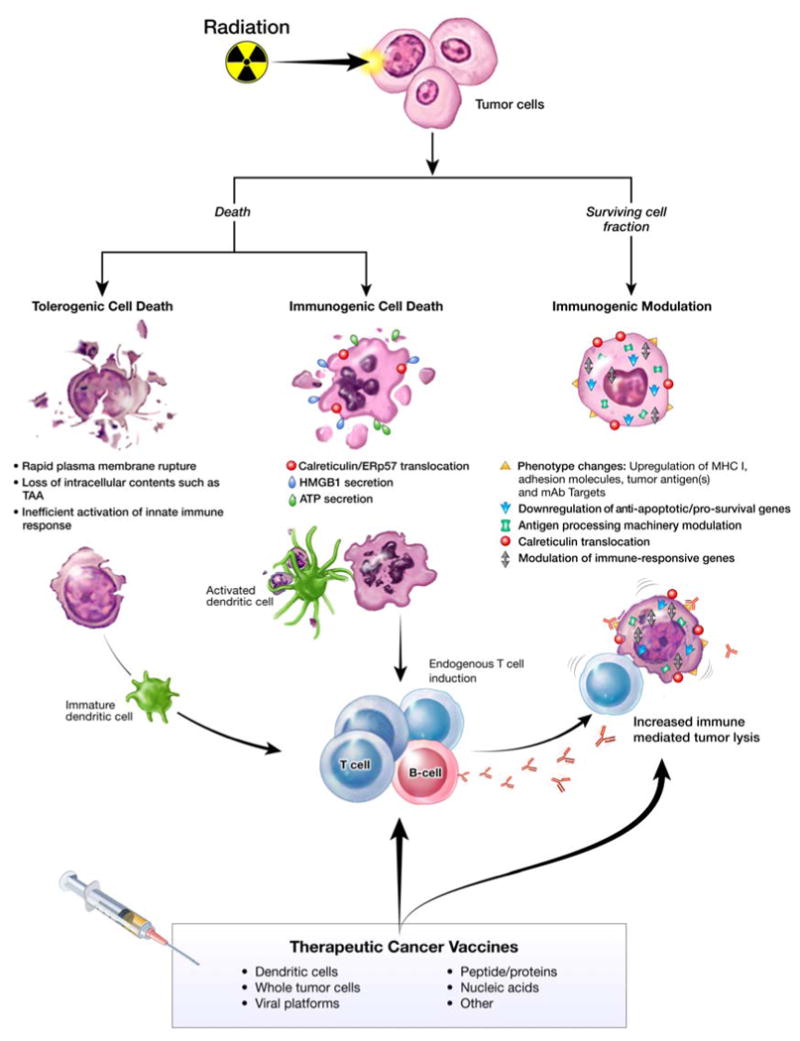
Multiple immunogenic consequences of radiation therapy that can be exploited to promote anti-tumor synergy in combination regimens with therapeutic cancer vaccines.
Radiation Triggers Immunogenic Modulation of Tumor Through Multiple Mechanisms
Preclinical murine studies have demonstrated that tumor cell death resulting from exposure to lethal irradiation elicits significant antitumor immune responses, defined as immunogenic cell death (ICD).9 Although cancer patients receiving RT alone mount weak to null antitumor immune responses, accumulating evidence indicates that the immunogenic effects of RT can be exploited to promote synergistic clinical benefit for patients receiving combination regimens with therapeutic cancer vaccines (Fig. 1).1,2,7,10
RT evokes a spectrum of molecular alterations in the biology of surviving tumor cells, defined as immunogenic modulation, that render tumor cells more sensitive to attack by antigen-specific CD8+ cytotoxic T lymphocytes (CTL). The molecular mechanisms associated with immunogenic modulation include (a) changes in tumor cell-surface phenotype, (b) modulation of antiapoptotic/survival and/or immune-responsive genes, (c) modulation of antigen-processing machinery components, and (d) translocation of calreticulin (CRT) to the tumor cell surface (Fig. 1).1,11-14 Recent findings strongly suggest that immunogenic modulation, and the resulting heightened sensitivity to CTL lysis observed in tumor cells recovering from radiation exposure, stems from a survival response to radiation-induced endoplasmic reticulum stress.15 However, as knowledge of the immunologic consequences of RT increases, other mechanisms may also be identified.
Tumor cells that survive RT undergo a spectrum of phenotypic changes on the cellular surface that render them more recognizable by the immune system, including increased expression of death receptors such as Fas/CD95, intercellular adhesion molecule-1 (ICAM-1/CD54), tumor-associated antigens (TAAs) (i.e., carcinoembryonic antigen [CEA] and MUC1), and MHC class I.15-19 Increased expression of effector T-cell costimulatory molecules in irradiated human tumor cells, such as OX-40L and 4-1BBL, has also been reported.20,21 These costimulatory molecules have been shown to reduce levels of regulatory T cells (Tregs), and their altered expression may play an additional role in modulating immune suppression.22-24 Production of chemoattractant factors and increased trafficking of T cells to the tumor have also been reported.25,26 Functionally, RT-induced modulation of tumor phenotype has been shown to enhance antitumor T-cell activity in both in vitro15,16,21,27,28 in vivo tumor models.29,30 These and other findings have created a paradigm shift in recent years. It is now widely accepted that local RT not only damages DNA and induces distinct forms of tumor cell death, but also modifies surviving tumor cells' phenotype, making them better targets for immune attack.
It is well established that the most effective cancer immunotherapy strategies generate TAA-specific CTLs capable of killing tumor cells. Available data support the idea that RT can be a powerful adjuvant to active therapeutic cancer vaccines, either independently or as a complement to its ability to induce ICD. For example, RT-induced ICD can function as an in situ boost to antitumor T cells that persist post-vaccination. In addition, some in vivo models indicate that RT may temporarily curb immunosuppressive cell populations, allowing vaccine-induced T cells to function more efficiently.31 These observations provide a rationale for using RT to modulate tumors and the tumor microenvironment, allowing for heightened functionality of TAA-specific, vaccine-induced CTLs.
Numerous preclinical and clinical studies have demonstrated that RT can be successfully combined with active immunotherapeutic regimens. The following is a review of current evidence of RT's ability to enhance the antitumor efficacy of diverse therapeutic cancer vaccine strategies currently under investigation.32
Combination Therapy with Radiation Plus Therapeutic Cancer Vaccines
Dendritic-Cell Vaccines
Preclinical
Preclinical studies have demonstrated enhanced antitumor efficacy in mice treated with RT plus dendritic cell (DC)-based vaccines. In a murine model of MCA-102 fibrosarcoma, intratumoral injection of DCs following 15 Gy of external-beam radiation therapy (EBRT) induced tumor-specific CTL activity and efficient antitumor immunity not observed with either modality alone.33 Antitumor activity against an established tumor at a distant site was also observed in mice receiving the combination therapy, but not with either modality alone. In a murine model of CT-26 colon carcinoma expressing prostate-specific antigen (PSA), the combination of radiation with interleukin-2/granulocyte-macrophage colony-stimulating factor (IL-2/GM-CSF) plus intratumoral injection of DCs resulted in significantly reduced tumor burden.34 Tumors were exposed to 8 Gy of EBRT 1 or 2 days post-vaccination. Four weeks after combination therapy, mice were refractory to tumor re-challenge to the contralateral flank, suggesting that RT modulated the tumor milieu, making it a more favorable environment for immune attack.
Clinical
Sipuleucel-T (Provenge®; Dendreon) is an autologous DC vaccine for the treatment of metastatic castration-resistant prostate cancer. Approval by the U.S. Food and Drug Administration (FDA) of sipuleucel-T, the first therapeutic cancer vaccine to be approved, has spurred considerable interest in combining therapeutic cancer vaccines with standard-of-care therapies, including RT. For instance, a phase I study evaluated the combination of RT plus injection of autologous immature DCs in 14 patients with advanced-stage/metastatic hepatoma.35 Patients received a single fraction of 8 Gy conformal RT followed by 2 intratumoral injections, given 3 weeks apart, of autologous immature DCs in 4 dose cohorts. This combination resulted in tumor-specific immune responses in 7/10 assessable patients. Two partial responses and 4 minor responses were also reported.
Another recent trial reported results from 40 patients treated with an autologous DC-based vaccine in combination with conformal RT.36 Patients had recurrent, metastatic, or locally advanced tumors of the head and neck, pancreas, lung, esophagus, or uterus. Matured DCs were pulsed with autologous tumor-cell lysates or tumor-specific peptides. Patients were treated with intensity-modulated RT using tomotherapy, stereotactic body RT (SBRT), or 3-dimensional conformal RT. The total doses were 30 Gy and 60 Gy (at standard 2 Gy/fraction in 38/40 patients) for patients with and without previous RT treatment, respectively. DC vaccines were administered every other week thereafter, up to 7 times. The 31 patients receiving full-dose RT had a response rate of 61%. The 9 patients who had previously received RT had a response rate of 55%. Tumor response outside the RT target volume was evaluable in 9 patients. Of these, 22% had a partial response, 33% had stable disease, and 44% had progressive disease according to the response evaluation criteria in solid tumors (RECIST). These findings indicate that the combination of DC-based vaccine and RT induces evaluable clinical responses.
Several ongoing clinical trials are investigating this combination strategy,37 including a phase II study of sipuleucel-T plus EBRT,38 a phase II study of sipuleucel-T plus stereotactic ablative body radiation,39 and a pilot study of sipuleucel-T plus high-dose single-fraction RT40 in hormone-refractory prostate cancer. A phase II study of EBRT alone or in combination with an intratumoral DC vaccine in soft tissue sarcoma is ongoing.41 In this 2-arm study, conventionally fractionated RT is followed by autologous DCs administered prior to surgical resection. A phase II study in glioblastoma is also underway that combines EBRT with surgery and temozolomide with or without DCs pulsed with tumor-cell lysates.42
Whole Tumor-Cell Vaccines
Preclinical
In preclinical studies, whole tumor-cell vaccines (WTCVs) such as GVAX, a cellular vaccine secreting GM-CSF, have been shown to promote DC maturation and enhance TAA presentation and T-cell activation.43,44 Whole-brain RT enhanced the effectiveness of immunotherapy with irradiated GL261 cells secreting GM-CSF as a WTCV.45 In this model, RT induced β2-microglobulin expression in vivo 48 h after 2 fractions of 4 Gy, and upregulated MHC class I protein expression in vitro after 4 Gy, suggesting that enhanced antigen presentation was a major factor.
Clinical
Current studies are investigating RT's ability to enhance the antitumor efficacy of WTCV strategies. A phase I study in patients with resected adenocarcinoma of the pancreas46 is comparing GVAX vaccine, fractionated SBRT (6.6 Gy), and FOLFIRINOX chemotherapy with and without low-dose cyclophosphamide. SBRT (6.6 Gy) will be administered over 5 days starting either 6 to 10 weeks after surgery (arm 1), or 13 to 17 days after the first vaccine (arm 2). The inclusion of chemotherapy will make the contribution of RT difficult to identify; however, the variant scheduling of RT between study arms may highlight key differences.
Viral Vaccines
Preclinical
The biological synergy between viral-based vaccines and RT has been thoroughly investigated in preclinical and clinical studies. Using mice transgenic for human CEA and a murine carcinoma cell expressing CEA, Chakraborty et al. demonstrated that local tumor irradiation in combination with active specific vaccine therapy elicited durable antitumor responses to established tumors.29 The vaccine regimen consisted of a prime with vaccinia and boosts with a recombinant avipoxvirus expressing CEA and 3 T-cell costimulatory molecules (TRICOM).47 Neither RT (8 Gy) nor vaccine alone inhibited the growth of 8-day established tumors. However, the combination of vaccine therapy and local RT achieved a significant number of cures. This result was mediated by the engagement of the Fas/Fas ligand pathway, as CEA+ tumor cells expressing dominant-negative Fas were not susceptible to the combination therapy. Synergy between this vaccination strategy and RT was also evident when radiolabeled antibodies were used.48 A single dose of yttrium-90-labeled anti-CEA monoclonal antibody (mAb) in combination with prime-boost vaccine therapy resulted in a statistically significant increase in survival in tumor-bearing mice over vaccine or mAb alone. Mice receiving the combination therapy also showed a significant increase in the percentage of viable tumor-infiltrating CEA-specific CD8+ T cells compared to vaccine alone. In vitro studies indicated that exposing human tumor cells to palliative doses of 153Sm-EDTMP (Quadramet®, an FDA-approved radiopharmaceutical targeting bone metastasis) could alter the phenotype of tumor cells to render them more susceptible to T cell-mediated killing.27 In a murine model of colon carcinoma (MC38), RT plus a vaccine consisting of recombinant vaccinia/fowlpox-CEA/TRICOM induced a significant influx of CTLs into the tumor milieu and a subsequent slowing of tumor growth.49 Combination therapy also mediated the regression of CEA− metastases at nonirradiated sites by promoting antigen cascade.49 This abscopal effect has also been observed in mice given a single dose of yttrium-90-labeled anti-CEA mAb with vaccine.48 Antigen cascade as a result of EBRT, brachytherapy, or yttrium-90-labeled mAb induced T lymphocytes specific for antigens not encoded by the poxviral vaccine, but that are expressed in MC38 tumors.48,49 Thus, it seems likely that diverse radiation protocols, including EBRT, radiolabeled antibodies, and radiopharmaceutical agents, may enhance the efficacy of this vaccine platform.
Clinical
The preclinical findings outlined above are being translated into clinical evaluation of RT combined with poxviral-based therapeutic vaccines. In a phase I study, patients with localized prostate cancer who were treated with EBRT and a poxviral-based vaccine had a significant increase in PSA-specific T-cell responses compared to patients receiving EBRT alone.50 Patients were given a priming vaccination of an admixture of recombinant vaccinia (rV)-PSA/rV-B7.1, followed by 7 monthly boosts with recombinant fowlpox (rF)-PSA. All vaccines were given on day 2 of each 28-day cycle, with GM-CSF given s.c. at the vaccination site on days 1 to 4. IL-2 was given s.c. in the abdomen on days 8 to 12. Standard EBRT (≥ 70 Gy, with 1.8 to 2.0 Gy per fraction) was given between the fourth and sixth vaccinations. Twenty-eight patients completed the therapy. Thirteen of the 17 who received EBRT plus vaccine had a ≥ 3-fold increase in PSA-specific T cells, a result not observed in the 11 patients treated with RT alone.51 A follow-up report on 26 patients treated with EBRT plus vaccine revealed that the combination did not appear to induce significant differences in PSA control compared to standard treatment.52 There was also limited evidence of long-term immune response following vaccine therapy. In all, of 12 patients evaluated for PSA-specific immune responses, one demonstrated a response 66 months post-enrollment.
It is important to note that these early clinical studies used RT in combination with poxviral vaccines that contained a single costimulatory molecule. Subsequent preclinical studies demonstrated that a triad of costimulatory molecules (TRICOM) more effectively generated antitumor immune responses.1,10,53 A pilot study evaluated the tolerability of rV/F-CEA/TRICOM vaccine in combination with RT in 12 patients with advanced gastrointestinal malignancies metastatic to the liver.54 Patients received rVCEA/TRICOM on day 1, followed by biweekly boosts with rF-CEA/TRICOM and split-course radiation (total 32 Gy) starting on day 21. All vaccines were given with rF-GM-CSF. No grade ≥ 3 toxicities were observed, and 2 patients had stable disease for 5 months, showing that RT plus TRICOM vaccine was safe even in heavily pretreated patients with high tumor burden (median time since last chemotherapy: 2 months). Patients with locally recurrent or progressive prostate cancer were also treated with PSA-TRICOM after definitive RT.55 Examination of patient biopsies demonstrated an increase in T cells infiltrating the tumor microenvironment post-treatment. Furthermore, the majority of patients had improved serum PSA kinetics.
Although the primary endpoint of these initial trials was safety, some data suggested enhanced immune activity. Evidence of immune responses to antigens not included in the vaccine but present within the tumor tissue (antigen cascade) has been reported. Gulley et al. detected immune response to prostate-specific membrane antigen, prostatic acid phosphatase, prostate stem cell antigen, and MUC1 following vaccination with a PSA poxviral-based vaccine in patients with localized prostate cancer treated with RT.50 This result has also been reported in preclinical studies combining a poxviral-based vaccine and RT.56 Additionally, induction of antigen cascade was associated with regression of tumors not expressing the vaccinating antigen after local irradiation of the antigen-positive tumor.49
These findings have led to promising clinical benefits for patients receiving RT plus immunotherapy.1,2,10 In a multicenter phase II study, patients with metastatic castration-resistant prostate cancer (n = 44) were randomized to receive 153Sm-EDTMP alone or in combination with PSA-TRICOM vaccine. Time to progression significantly improved (P = 0.03) with combination therapy (3.7 months) compared to 153Sm-EDTMP alone (1.7 months).10
Clinical studies are also underway to evaluate the safety57 and efficacy58 of adenoviral vectors containing the herpes simplex thymidine kinase gene in combination with RT for diverse cancers.
Peptide/Protein Vaccines
Preclinical
Several studies have investigated RT as an adjuvant to peptide- or protein-based therapeutic cancer vaccines. A cervical cancer animal model was used to evaluate the potential benefits of combining RT with human papillomavirus (HPV) E7 subunit vaccines.59 Although the vaccine targets a viral gene responsible for tumorigenesis, the therapeutic synergy of the combination was mediated by CD8+ CTLs and was concomitant with histological changes, including heavy infiltration of lymphocytes. Phenotypic changes in irradiated tumors (i.e., increased MHC class I and Fas) and increased sensitivity to CTL-mediated killing also appeared to be responsible for therapeutic synergy. Mice with 6- to 8-mm tumors were cured following RT (28 Gy) to the tumor in combination with E7 subunit vaccine given 3 times at weekly intervals. Moreover, these mice were refractory to tumor rechallenge. A recent innovative approach combining RT with Hsp70 peptide complexes obtained from radioresistant tumor cells and DC fusions (Hsp70.PC-F) demonstrated that specific immunity to radioresistant tumor cells could be induced.60 In these studies, mice received 6- and 9-Gy RT to the tumor on days 8 and 10 post-tumor implant, followed by Hsp70.PC-F s.c. vaccination on days 12, 19, and 26. Vaccination plus RT inhibited the growth of primary tumors as well as the number of tumor cells metastasizing to lung.
Clinical
The combination of peptide vaccine and RT has been evaluated clinically. In studies using chemoradiation, investigators have found it difficult to evaluate the direct effect of RT on the peptide vaccination strategy.61 An ongoing phase I trial is evaluating the toxicity profile of vaccine given concurrently with temozolomide and RT in patients with newly diagnosed glioblastoma multiforme.62 A pilot study in patients with low-grade gliomas is evaluating the effects of vaccination with HLA-A2-restricted glioma antigen peptides in combination with poly-IC.63 Patients are enrolled in 1 of 2 cohorts, based on whether they have received RT ≥ 6 months prior to enrollment, and induction of antigen-specific immune response is compared between cohorts 1 (no RT) and 2 (prior RT).
Nucleic-Acid Vaccines
Preclinical
The combination of low-dose RT and DNA vaccine has been explored preclinically using epithelial cells transformed with HPV E6/E7 and activated ras oncogene as a cervical cancer model.64 The DNA vaccine consists of CRT linked to the mutated form of the HPV-16 E7 antigen. Tumor-bearing mice treated with RT combined with CRT/E7 DNA vaccine generated significant antitumor responses. The highest frequency of E7-specific CD8+ T cells was seen in the tumors of treated mice. Therapeutic vaccines were given on days 8, 12, and 16, and the effect of administering RT on the day of the first, second, or third vaccination was evaluated. RT (16 Gy) given with the second DNA vaccination generated the highest frequency of E7-specific CD8+ T cells in the tumors and spleen, demonstrating that RT can enhance the effect of therapeutic DNA-based vaccines. Furthermore, investigators found that the timing of RT can greatly affect the magnitude of antitumor immune responses generated.
Unmethylated cytosine-phosphorothioate-guanine (CpG) oligodeoxynucleotide, a ligand of toll-like receptor 9, has also been used in combination with RT.65 Mice bearing Lewis lung carcinoma cells expressing ovalbumin (OVA) were irradiated twice with 14 Gy at intervals of 24 h when tumors became palpable (7 to 8 mm). After the second irradiation, mice received CpG + OVA liposome intradermally near the draining lymph node. Tumor growth was greatly inhibited, and 60% of mice were cured following treatment with the combination. This combination therapy also increased OVA-specific CTLs in tumor-bearing mice. While such preclinical studies demonstrate the feasibility of combining nucleic-acid vaccines with RT for the treatment of cancer, the research has not been translated into clinical studies.
Additional Vaccine Approaches
Preclinical
Novel immunotherapeutic approaches are also being evaluated in combination with RT. Human cancers frequently overexpress a high-affinity cell-surface receptor for folic acid. Tumor cells can be made more recognizable by the immune system when highly immunogenic haptens are targeted to folate receptor-expressing cell surfaces. Folate-targeted hapten immunotherapy (FTHI) eliminates medium-sized tumors in antihapten-immunized mice.66 Radiotherapy (3 Gy/dose on days 1, 5, and 12) synergizes with FTHI in antihapten-immunized mice, curing animals bearing tumors > 300 mm3. Moreover, in animals receiving local RT, nonirradiated distal tumor masses showed increased response to hapten immunotherapy, indicating an immune-mediated abscopal effect. In contrast, combination FTHI plus paclitaxel chemotherapy had no enhanced antitumor efficacy.
Immunotherapy with live, attenuated Listeria monocytogenes-based vaccine is another innovative approach being evaluated preclinically in combination with RT.67 The combination of RT and a Listeria monocytogenes-based PSA vaccine (ADXS31-142) was recently evaluated in a mouse model of prostate cancer. Mice bearing PSA-expressing TPSA23 tumors received no treatment, ADXS31-142, RT, control Listeria vector, and the combination of ADXS31-142 plus RT. The Listeria-based vaccine was given on days 10, 17, and 21 post-tumor implant and single-fraction EBRT of 10 Gy was delivered on day 12. Combination therapy with RT and vaccine induced significant tumor regression compared to either modality alone, resulted in complete regression of tumors in 60% of treated mice, and augmented PSA-specific immune responses.
Conclusion
The preclinical studies and early clinical trials described here demonstrate that RT can enhance the efficacy of therapeutic cancer vaccines. While these outcomes provide the rationale for current clinical trials employing both modalities, further investigation will be required to achieve synergy and realize the full potential of the combination. So far, the combination of poxviral-based vaccines with RT has been the subject of the most intense preclinical and clinical scrutiny.
The molecular-level effects of combining RT with immunotherapy are just beginning to be elucidated. Radiation induces a spectrum of immunogenic alterations in tumor biology, ranging from immunogenic modulation to immunogenic cell death. These may be harnessed to achieve optimal synergy with therapeutic cancer vaccines, mAb, and other immunotherapy regimens to maximize clinical benefit, even for patients who have failed RT or who have limited treatment options. Further investigation into the molecular mechanisms that result in RT's ability to enhance antitumor immune responses will be required to capitalize on these biological changes and reduce the morbidity and mortality of cancer. For immunogenic modulation by RT to translate into clinical success, the optimal administration of RT must be based on defined cellular and molecular changes within irradiated tumors that directly enhance therapeutic vaccine approaches. It is our hope that the next phase of research will generate data that will drive the clinical implementation of combination therapies employing RT plus therapeutic cancer vaccines. Further research may provide relevant information on the dose, delivery, and timing of radiation that will best capitalize on the immunomodulatory activities of RT in combination with each of the diverse vaccine approaches.
Acknowledgments
The authors thank Dr. Jeffrey Schlom for his helpful suggestions and Bonnie L. Casey for editorial assistance in the preparation of this manuscript.
Grant support: This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. This research was supported by a Research Initiation Grant from Georgia State University and R21CA162235 from the National Cancer Institute.
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kwilas AR, Donahue RN, Bernstein MB, et al. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Front Oncol. 2012;2:104. doi: 10.3389/fonc.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangia N, Ferrone S. Antigen presentation machinery (APM) modulation and soluble HLA molecules in the tumor microenvironment: do they provide tumor cells with escape mechanisms from recognition by cytotoxic T lymphocytes? Immunol Invest. 2006;35(3-4):485–503. doi: 10.1080/08820130600808246. [DOI] [PubMed] [Google Scholar]
- 4.Leone P, Shin EC, Perosa F, et al. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J Natl Cancer Inst. 2013;105(16):1172–1187. doi: 10.1093/jnci/djt184. [DOI] [PubMed] [Google Scholar]
- 5.Poggi A, Musso A, Dapino I, et al. Mechanisms of tumor escape from immune system: Role of mesenchymal stromal cells. Immunol Lett. 2014;159(1-2):55–72. doi: 10.1016/j.imlet.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed MM, Hodge JW, Guha C, et al. Harnessing the potential of radiation-induced immune modulation for cancer therapy. Cancer Immunol Res. 2013;1(5):280–284. doi: 10.1158/2326-6066.CIR-13-0141. [DOI] [PubMed] [Google Scholar]
- 7.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draghiciu O, Walczak M, Hoogeboom BN, et al. Therapeutic immunization and local low-dose tumor irradiation, a reinforcing combination. Int J Cancer. 2014;134(4):859–872. doi: 10.1002/ijc.28418. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 10.Heery CR, Madan RA, Bilusic M, et al. A phase II randomized clinical trial of samarium-153 EDTMP (Sm-153) with or without PSA-tricom vaccine in metastatic castration-resistant prostate cancer (mCRPC) after docetaxel. J Clin Oncol. 2013;31(suppl 6):102. abstr. [Google Scholar]
- 11.Hodge JW, Kwilas A, Ardiani A, et al. Attacking malignant cells that survive therapy: Exploiting immunogenic modulation. Oncoimmunology. 2013;2(12):e26937. doi: 10.4161/onci.26937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makinde AY, John-Aryankalayil M, Palayoor ST, et al. Radiation survivors: understanding and exploiting the phenotype following fractionated radiation therapy. Mol Cancer Res. 2013;11(1):5–12. doi: 10.1158/1541-7786.MCR-12-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des. 2002;8(19):1765–1780. doi: 10.2174/1381612023394089. [DOI] [PubMed] [Google Scholar]
- 14.Janssens S, Tschopp J. Signals from within: the DNA-damage-induced NF-kappaB response. Cell Death Differ. 2006;13(5):773–784. doi: 10.1038/sj.cdd.4401843. [DOI] [PubMed] [Google Scholar]
- 15.Gameiro SR, Jammeh ML, Wattenberg MM, et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5(2):403–416. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garnett CT, Palena C, Chakraborty M, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64(21):7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 17.Ifeadi V, Garnett-Benson C. Sub-lethal irradiation of human colorectal tumor cells imparts enhanced and sustained susceptibility to multiple death receptor signaling pathways. PLoS One. 2012;7(2):e31762. doi: 10.1371/journal.pone.0031762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santin AD, Hermonat PL, Hiserodt JC, et al. Effects of irradiation on the expression of major histocompatibility complex class I antigen and adhesion costimulation molecules ICAM-1 in human cervical cancer. Int J Radiat Oncol Biol Phys. 1997;39(3):737–742. doi: 10.1016/s0360-3016(97)00372-6. [DOI] [PubMed] [Google Scholar]
- 19.Klein B, Loven D, Lurie H, et al. The effect of irradiation on expression of HLA class I antigens in human brain tumors in culture. J Neurosurg. 1994;80(6):1074–1077. doi: 10.3171/jns.1994.80.6.1074. [DOI] [PubMed] [Google Scholar]
- 20.Kumari A, Cacan E, Greer S, et al. Turning T cells on: epigenetically enhanced expression of effector T-cell costimulatory molecules on irradiated human tumor cells. J Immunother Cancer. 2013;1:1–17. doi: 10.1186/2051-1426-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernstein MB, Garnett CT, Zhang H, et al. Radiation-induced modulation of costimulatory and coinhibitory T-cell signaling molecules on human prostate carcinoma cells promotes productive antitumor immune interactions. Cancer Biother Radiopharm. 2014 Apr 2; doi: 10.1089/cbr.2013.1578. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi BK, Bae JS, Choi EM, et al. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol. 2004;75(5):785–791. doi: 10.1189/jlb.1003491. [DOI] [PubMed] [Google Scholar]
- 23.Vu MD, Xiao X, Gao W, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110(7):2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valzasina B, Guiducci C, Dislich H, et al. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105(7):2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 25.Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 26.Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181(5):3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakraborty M, Wansley EK, Carrasquillo JA, et al. The use of chelated radionuclide (samarium-153-ethylenediaminetetramethylenephosphonate) to modulate phenotype of tumor cells and enhance T cell-mediated killing. Clin Cancer Res. 2008;14(13):4241–4249. doi: 10.1158/1078-0432.CCR-08-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa E, Tsuboi K, Saijo K, et al. X-irradiation to human malignant glioma cells enhances the cytotoxicity of autologous killer lymphocytes under specific conditions. Int J Radiat Oncol Biol Phys. 2004;59(5):1505–1512. doi: 10.1016/j.ijrobp.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 29.Chakraborty M, Abrams SI, Coleman CN, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64(12):4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 30.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Xiong S, Zhang L, et al. Enhancement of antitumor immunity by low-dose total body irradiation is associated with selectively decreasing the proportion and number of T regulatory cells. Cell Mol Immunol. 2010;7(2):157–162. doi: 10.1038/cmi.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104(8):599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim KW, Kim SH, Shin JG, et al. Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. Int J Cancer. 2004;109(5):685–690. doi: 10.1002/ijc.20036. [DOI] [PubMed] [Google Scholar]
- 34.Wang YS, Tsang YW, Chi CH, et al. Synergistic anti-tumor effect of combination radio- and immunotherapy by electro-gene therapy plus intra-tumor injection of dendritic cells. Cancer Lett. 2008;266(2):275–285. doi: 10.1016/j.canlet.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 35.Chi KH, Liu SJ, Li CP, et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother. 2005;28(2):129–135. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 36.Shibamoto Y, Okamoto M, Kobayashi M, et al. Immune-maximizing (IMAX) therapy for cancer: Combination of dendritic cell vaccine and intensity-modulated radiation. Mol Clin Oncol. 2013;1(4):649–654. doi: 10.3892/mco.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vacchelli E, Vitale I, Tartour E, et al. Trial Watch: Anticancer radioimmunotherapy. Oncoimmunology. 2013;2(9):e25595. doi: 10.4161/onci.25595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sipuleucel-T With or Without Radiation Therapy in Treating Patients With Hormone-Resistant Metastatic Prostate Cancer. 2014 May 5; Available from: http://clinicaltrials.gov/ct2/show/NCT01807065?term=NCT01807065&rank=1.
- 39.Sipuleucel-T and Stereotactic Ablative Body Radiation (SABR) for Metastatic Castrate-resistant Prostate Cancer (mCRPC) 2014 May 5; Available from: http://clinicaltrials.gov/ct2/show/NCT01818986?term=NCT01818986&rank=1.
- 40.Radiation Therapy in Treating Patients With Metastatic Hormone-Resistant Prostate Cancer Receiving Sipuleucel-T. 2014 May 5; Available from: http://clinicaltrials.gov/ct2/show/NCT01833208?term=NCT01833208&rank=1.
- 41.Radiation Therapy and Intratumoral Autologous Dendritic Cells in Soft Tissue Sarcomas (STS) 2014 May 5; Available from: http://clinicaltrials.gov/ct2/show/NCT01347034?term=NCT01347034&rank=1.
- 42.Study of DC Vaccination Against Glioblastoma. 2014 May 5; Available from: http://clinicaltrials.gov/ct2/show/NCT01567202?term=NCT01567202&rank=1.
- 43.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mach N, Gillessen S, Wilson SB, et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60(12):3239–3246. [PubMed] [Google Scholar]
- 45.Newcomb EW, Demaria S, Lukyanov Y, et al. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res. 2006;12(15):4730–4737. doi: 10.1158/1078-0432.CCR-06-0593. [DOI] [PubMed] [Google Scholar]
- 46.Pancreatic Tumor Cell Vaccine (GVAX), Low Dose Cyclophosphamide, Fractionated Stereotactic Body Radiation Therapy (SBRT), and FOLFIRINOX Chemotherapy in Patients With Resected Adenocarcinoma of the Pancreas. 2014 May 5; Available from: http://clinicaltrials.gov/ct2/show/NCT01595321?term=NCT01595321&rank=1.
- 47.Hodge JW, McLaughlin JP, Kantor JA, et al. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15(6-7):759–768. doi: 10.1016/s0264-410x(96)00238-1. [DOI] [PubMed] [Google Scholar]
- 48.Chakraborty M, Gelbard A, Carrasquillo JA, et al. Use of radiolabeled monoclonal antibody to enhance vaccine-mediated antitumor effects. Cancer Immunol Immunother. 2008;57(8):1173–1183. doi: 10.1007/s00262-008-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm. 2012;27(1):12–22. doi: 10.1089/cbr.2012.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11(9):3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 51.Lechleider RJ, Arlen PM, Tsang KY, et al. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res. 2008;14(16):5284–5291. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamrava M, Kesarwala AH, Madan RA, et al. Long-term follow-up of prostate cancer patients treated with vaccine and definitive radiation therapy. Prostate Cancer Prostatic Dis. 2012;15(3):289–295. doi: 10.1038/pcan.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garnett CT, Greiner JW, Tsang KY, et al. TRICOM vector based cancer vaccines. Curr Pharm Des. 2006;12(3):351–361. doi: 10.2174/138161206775201929. [DOI] [PubMed] [Google Scholar]
- 54.Gulley JL, Madan RA, Tsang KY, et al. A pilot safety trial investigating a vector-based vaccine targeting carcinoembryonic antigen in combination with radiotherapy in patients with gastrointestinal malignancies metastatic to the liver. Expert Opin Biol Ther. 2011;11(11):1409–1418. doi: 10.1517/14712598.2011.615741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gulley JL, Heery CR, Madan RA, et al. Phase I study of intraprostatic vaccine administration in men with locally recurrent or progressive prostate cancer. Cancer Immunol Immunother. 2013;62(9):1521–1531. doi: 10.1007/s00262-013-1448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res. 2005;11(6):2416–2426. doi: 10.1158/1078-0432.CCR-04-1380. [DOI] [PubMed] [Google Scholar]
- 57.Phase 1b Study of AdV-tk + Valacyclovir Combined With Radiation Therapy for Malignant Gliomas (BrTK01) 2014 May 5; Available from: http://clinicaltrials.gov/ct2/show/NCT00751270?term=NCT00751270&rank=1.
- 58.Phase 3 Study of ProstAtak™ With Standard Radiation Therapy for Localized Prostate Cancer (PrTK03) 2014 May 5; Available from: http://clinicaltrials.gov/ct2/show/NCT01436968?term=NCT01436968&rank=1.
- 59.Ye GW, Park JB, Park YJ, et al. Increased sensitivity of radiated murine cervical cancer tumors to E7 subunit vaccine-driven CTL-mediated killing induces synergistic anti-tumor activity. Mol Ther. 2007;15(8):1564–1570. doi: 10.1038/sj.mt.6300149. [DOI] [PubMed] [Google Scholar]
- 60.Weng D, Song B, Koido S, et al. Immunotherapy of radioresistant mammary tumors with early metastasis using molecular chaperone vaccines combined with ionizing radiation. J Immunol. 2013;191(2):755–763. doi: 10.4049/jimmunol.1203286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iinuma H, Fukushima R, Inaba T, et al. Phase I clinical study of multiple epitope peptide vaccine combined with chemoradiation therapy in esophageal cancer patients. J Transl Med. 2014;12(1):84. doi: 10.1186/1479-5876-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaccine Therapy, Temozolomide, and Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma Multiforme. 2014 May 5; Available from: http://clinicaltrials.gov/ct2/show/NCT01222221?term=NCT01222221&rank=1.
- 63.Effects of Vaccinations With HLA-A2-Restricted Glioma Antigen-Peptides in Combination With Poly-ICLC for Adults With High-Risk WHO Grade II Astrocytomas and Oligo-Astrocytomas. 2014 May 5; Available from: http://clinicaltrials.gov/ct2/show/NCT00795457?term=NCT00795457&rank=1.
- 64.Tseng CW, Trimble C, Zeng Q, et al. Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. Cancer Immunol Immunother. 2009;58(5):737–748. doi: 10.1007/s00262-008-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chamoto K, Takeshima T, Wakita D, et al. Combination immunotherapy with radiation and CpG-based tumor vaccination for the eradication of radio- and immunoresistant lung carcinoma cells. Cancer Sci. 2009;100(5):934–939. doi: 10.1111/j.1349-7006.2009.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sega EI, Lu Y, Ringor M, et al. Low-dose radiation potentiates the therapeutic efficacy of folate receptor-targeted hapten therapy. Int J Radiat Oncol Biol Phys. 2008;71(2):559–566. doi: 10.1016/j.ijrobp.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 67.Hannan R, Zhang H, Wallecha A, et al. Combined immunotherapy with Listeria monocytogenes-based PSA vaccine and radiation therapy leads to a therapeutic response in a murine model of prostate cancer. Cancer Immunol Immunother. 2012;61(12):2227–2238. doi: 10.1007/s00262-012-1257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


