Abstract
The role of brain-derived neurotrophic factor (BDNF) in recognition memory was investigated by locally infusing oligodeoxynucleotides (ODNs) into perirhinal cortex, a region of the temporal lobe essential for familiarity discrimination. Antisense but not sense BDNF ODN impaired consolidation of long-term (24h) but not shorter-term (20min) recognition memory.
Keywords: familiarity discrimination, perirhinal cortex, neurotrophin, oligodeoxynucleotide, consolidation
Brain-derived neurotrophic factor (BDNF) is a neurotrophin involved in synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD) (Pang et al., 2004; Bramham and Messaoudi, 2005; Woo et al., 2005; Bekinschtein et al., 2008; Lu et al., 2008). As synaptic plasticity is believed to underlie memory processes, it is interesting to test links between BDNF and memory. Indeed, interfering with BDNF actions in hippocampus, amygdala or parietal cortex impairs fear-conditioning, inhibitory avoidance learning or spatial memory (Ma et al. 1998; Mu et al., 1999; Mizuno et al, 2000, 2003; Alonso et al. 2002, 2005; Lee et al., 2004; Rattiner et al., 2004, 2005; Bekinschtein et al., 2007; Heldt et al., 2007). Recently, an increase in BDNF mRNA has been reported in perirhinal cortex 2h after exposure to novel objects (Romero-Granados et al., 2009). However, the necessity for recognition memory of BDNF activity within perirhinal cortex remains to be tested.
The familiarity discrimination component of recognition memory for individual items relies on the integrity of the perirhinal cortex of the temporal lobe (Brown and Aggleton, 2001). We investigated the role of BNDF in the acquisition, retrieval and consolidation of recognition memory processes at long and short delays using a novel object preference task (Ennaceur and Delacour, 1988). Infusion of an antisense oligodeoxynucleotide (ODN) into perirhinal cortex was used to interfere with BDNF expression, as previously employed in hippocampus (Ma et al., 1998; Lee et al. 2004; Bekinschtein et al., 2007).
Two groups (n = 9 and n = 10) of adult male pigmented Dark Agouti rats (220-250g; Bantin and Kingman, UK) were maintained on a 12h light/dark cycle, with the dark phase during normal daylight. All experiments were performed in accordance with the UK Animals Scientific Procedures Act (1986) and had the approval of the University of Bristol Ethical Review Committee. Oligodeoxynucleotides (ODNs) were PAGE-purified phosphotioate end-capped 18-mer sequences (Sigma Genosys Ltd., Haverhill, UK): BDNF antisense ODN: 5′-TCTTCCCCTTTTAATGGT-3′; BDNF sense ODN (control sequence): 5′-AGAAGGGGAAAATTACCA-3′. Antisense or sense ODN was infused locally into the perirhinal cortex through bilaterally implanted cannulae at 1nmol in 1μl normal saline, the dose following that used by Lee et al. (2004).
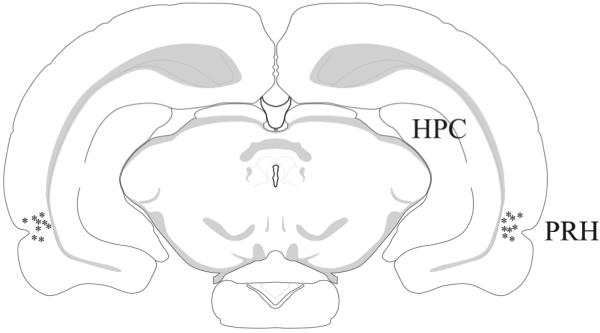
Cannula implantation into perirhinal cortex was carried out stereotaxically in rats deeply anaesthetized with Isoflurane (Merial Animal Health Ltd., Harlow, UK). Details of procedures have been published previously (Warburton et al, 2003; Barker et al., 2006a, b, 2008). Two stainless-steel guide cannulae (26 gauge, Plastics One Inc., Roanoke, Virginia, USA, via Semat in UK) were implanted through holes in the skull, at an angle of 20° to the vertical (coordinates relative to bregma: AP −5.6mm, L ± 4.5mm, and V −6.7mm relative to the skull surface) and anchored to the skull. Behavioural testing began >20d later. Infusions through a cannula in each hemisphere were made by inserting a 33 gauge cannula (Plastics One Inc.) which protruded 1mm beyond the guide cannula tip and which was connected by PVC tubing (Barloworld Scientific Ltd., Berkshire, UK) to a Hamilton syringe (Hamilton Bonaduz, Bonaduz, Switzerland). To minimise the amount of ODN used, the PVC tubing was pre-filled with a non-water soluble solution of 0.3mg/ml Oil Red O (Sigma-Aldrich Chemie, Steinheim, Germany) in paraffin oil (Fluka, Steinheim, Germany). The syringe was advanced with an infusion pump (Harvard Bioscience, Holliston, MA, USA) at a rate of 0.7μl/min for 1min and 30 sec; 5min later the injection cannulae were withdrawn. Cannula locations were checked, as previously (Warburton et al, 2003; Barker et al., 2006a, b; 2008) using coronal (40μm), cresyl violet sections. All reported data are from animals where the tips of the cannulae were within perirhinal cortex (areas 35 and 36) from bregma −5.5 to −4.5 (Paxinos and Watson, 1998; Shi & Cassell, 1999); Fig.1.
Figure 1.
Infusion sites. The location of cannula tips, as observed in cresyl violet stained sections, for all implanted animals used in object recognition test (n = 9 and n = 10) fell within the dark grey area, which was located within the perirhinal cortex (PRH) from bregma −5.5 to −4.5 (Paxinos and Watson, 1998; Shi & Cassell, 1999).
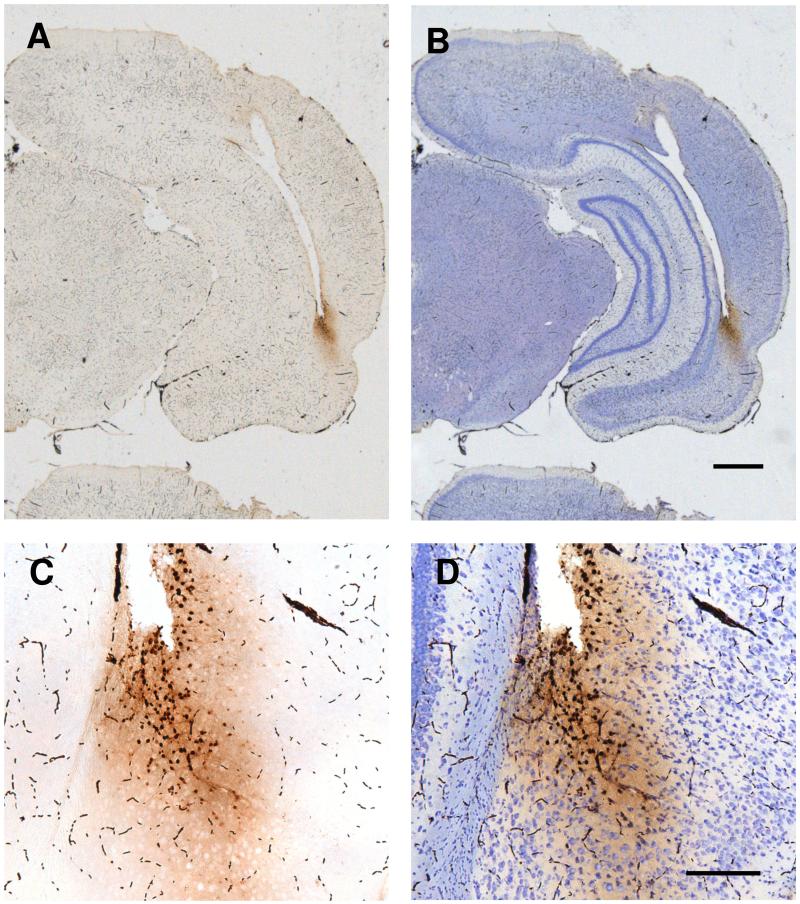
To visualise the extent of ODN diffusion within the brain, 1nmol of biotinylated antisense BDNF ODN (ODN sequence labelled with biotin on 5′end) in 1μl saline was infused into the PRH via cannula 1h (n = 2) before being anaesthetised with Euthatal. The brain was removed and fixed in 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) for at least 6d before being immersed in 30% sucrose in 0.1M phosphate buffer for at least 2d. The right hemisphere of the brain was marked with an incision before being sectioned (40μm) coronally on a cryostat. Floating sections were collected and washed in 0.1M phosphate buffer saline containing 0.2% Triton X-100 (PBST; pH 7.4), and processed with avidin-biotinylated horseradish peroxidase complex (Vectastain ABC Kit; Vector Laboratories Inc.) in PBST for 1h at room temperature, and the reaction was visualized using 3,3′-diaminobenzidine (Sigma Fast™, Sigma-Aldrich). As the brain needed to be immersion-fixed, the sections also contain black blood cells, identifiable by their size and configuration. Some of the sections were counter-stained with cresyl violet. Biotinylated antisense BDNF ODN labelling was chiefly restricted to the PRH and was clearly visible within neurons (see Fig. 2). Staining in the entorhinal cortex or area Te2 was estimated to be < 5% of these structures in each case. No stained neurons were found in the hippocampus. The spread in the anterior-posterior axis was 900 ± 80 μm (mean ± SEM for 4 infusion sites) and estimates indicated that approximately 31% of the total perirhinal cortex volume contained biotinylated antisense BDNF ODN. In the central 500μm approximately 45% of the perirhinal cortex contained such BDNF staining. It should be noted that as deep cortical layers were chiefly involved in area 36 and superficial layers in area 35, more of perirhinal cortex’s processing capacity was likely to have been affected by the ODN infusions than the above quoted percentage figures might imply. In previous studies, biotinylated BDNF ODN spread was shown at 90 min, 2h or 24h after a local infusion in the hippocampus using doses of 1nmol or 2nmol ODN in 1μl of saline (Lee et al., 2004; Bekinschtein et al., 2007). A wide ODN spread was found at 90 min or 2 h, but by 24h the presence of biotinylated ODN in the hippocampus was greatly reduced. This suggests that at time points later than 24h, such as 1 week, BDNF ODN may be expected to have disappeared.
Figure 2.
Visualisation of biotinylated antisense BDNF ODN (1nmol in 1μl saline) 1h after infusion into the perirhinal cortex. Top, photomicrographs showing a section stained for biotinylated BDNF ODN (A) and counterstained with cresyl violet (B). The section is approximately −6.0 relative to bregma (Paxinos and Watson, 1998) and immediately posterior to the cannula track. The great majority of biotinylated BDNF ODN labelling was restricted to perirhinal cortex. Bottom, a magnification of the same section showing incorporation of biotinylated BDNF ODN into neurons (C and D). Scale bars: A, B = 1mm; C, D = 200μm.
Recognition memory was tested using the novel object preference test as previously (Warbuton et al., 2003; Barker et al., 2006a, b, 2007). In brief, this task took place in an arena (90 × 100 × 50cm) surrounded to a height of 1.5m with black curtains, and with sawdust on the floor. Rat behaviour was monitored using a camera and a video recorder. The objects were made of Duplo bricks (Lego Produktion A.G., Baar, Switzerland) and varied in size (range: 10 × 5 × 15cm to 20 × 20 × 30cm), colour and shape, and were placed near the two corners at either end of one side of the arena (15cm from each adjacent wall). Prior to the start of memory testing, each rat was habituated to the empty arena for 5min daily for 4d. The novel object preference test comprised two phases, acquisition and test, separated by a delay of 20min or 24h. In the acquisition phase, an animal was allowed to explore two identical objects for 40s of exploration or a maximum of 4min spent in the arena. In the test phase, the rat was allowed to explore an identical third copy of the previously explored object and a novel object for 3min from the start of the objects’ exploration. In both acquisition and test phases, the time spent exploring each of the objects was recorded. Exploration was scored only when the animal’s nose was directed towards the object at a distance of less than 1cm. If the time of exploration was less than 15s in the acquisition phase or less than 10s in the test phase, the animal was discarded from the analysis of that experiment. The objects used as novel or familiar and their position in the arena were counterbalanced between animals receiving the same treatment, and between control and drug-treated animals. Each experiment used a cross-over design with half the animals receiving sense and half receiving antisense ODN infusions followed a week later by infusions of the opposite compounds. The scorer was blind as to the treatment of each animal. The role for BDNF in recognition memory was tested using experimental conditions differing both in the time of infusion of antisense or sense ODN and in the delay between the acquisition and test phases (see Table 1).
Table 1.
Infusion times relative to and delays between acquisition and test phases for each condition.
| Condition Abbreviation | Infusion time | Delay between acquisition and test |
|---|---|---|
| Acquisition 20min | 1h before acquisition | 20min |
| Acquisition 24h | 1h before acquisition | 24h |
| Consolidation 24h | 2min after acquisition | 24h |
| Retrieval 24h | 1h before test | 24h |
| 6h Post-acquisition 24h | 6h after acquisition | 30h (test done 24h after infusion) |
There were no significant differences between antisense- and sense-treated rats in the time spent in exploration in either acquisition or test phases in any of the experiments (see Table 2). Thus, there was no evidence that the memory impairments were due to problems with general exploration. Moreover, in all the experiments sense ODN treatment produced discrimination and total exploration values similar to those of previous studies for rats injected or infused with saline (Warburton et al., 2003; Barker et al., 2006b). Accordingly, there was no evidence that sense ODN infusion affected familiarity discrimination.
Table 2.
Exploration time (mean ± SEM, in s) in acquisition and test phases of the novel object preference task. The times are given for the groups receiving sense or anti-sense BDNF ODNs and for each experiment. No significant difference was found between sense BDNF ODN and anti-sense BDNF ODN exploration times.
| Acquisition 20min | Acquisition 24h | |||
|---|---|---|---|---|
| sense | antisense | sense | antisense | |
| acquisition | 32.4 ± 2.1 | 32.5 ± 1.5 | 22.5 ± 1.4 | 25.5 ± 2.0 |
| test | 27.5 ± 2.6 | 28.7 ± 4.4 | 18.9 ± 2.4 | 19.6 ± 2.3 |
| Retrieval 24h | Consolidation 24h | |||
|---|---|---|---|---|
| sense | antisense | sense | antisense | |
| acquisition | 28.4 ± 3.0 | 23.6 ± 2.1 | 28.6 ± 1.4 | 28.1 ± 2.9 |
| test | 33.4 ± 4.8 | 30.1 ± 2.7 | 38.5 ± 3.9 | 36.3 ± 1.5 |
| 6h Post-acquisition 24h | ||
|---|---|---|
| sense | antisense | |
| acquisition | 31.5 ± 2.7 | 30.8 ± 2.0 |
| test | 29.7 ± 2.0 | 24.7 ± 2.1 |
The discrimination ratio (DR) was calculated as the time in the test phase spent exploring the novel object minus that spent exploring the previously experienced object divided by the total exploration time. Comparisons with other studies (Warburton et al., 2003; Barker et al., 2006b) established that the performance of rats under control conditions (sense ODN infusion) showed no evidence of residual effects from the antisense ODN infusion one week earlier. Comparisons between treatment conditions were analysed by within-subject ANOVAs. One-sample t-tests were used to determine whether the DR for each condition was significantly different from zero (no discrimination between novel and familiar objects). All tests used a significance level of p = 0.05 and were two-tailed.
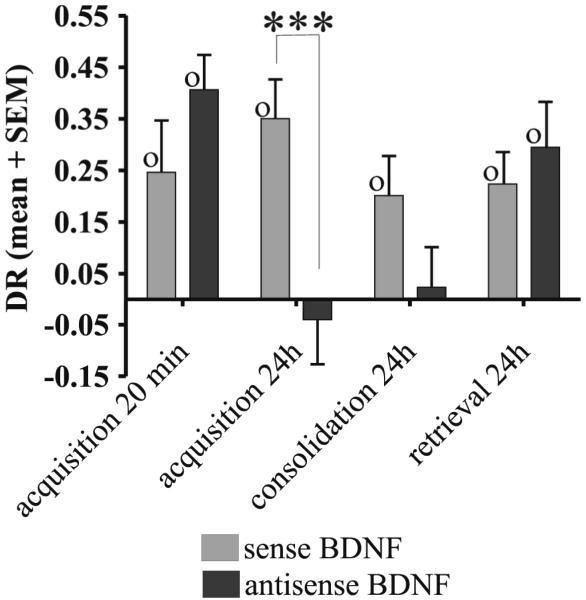
The effects of BDNF ODNs were determined on the difference in exploration of a novel rather than a familiar object (DR values). In three experiments with a 24h memory delay period (n = 9), sense or anti-sense ODN was administered either 1h before the acquisition phase so that it was active during and after acquisition, 1h before the test phase so that it was active during retrieval, or within 2min after the acquisition phase so that it was active at the start of consolidation. That anti-sense ODN impaired early consolidation was indicated by the following findings. The effect of treatment (sense or anti-sense ODN) was significant (ANOVA F(1,7) = 23.94; p = 0.002) but the interaction between treatment and experiment (time of infusion) was also significant (ANOVA F(2,14) = 3.92; p < 0.05). Further analysis of the data in the two experiments for infusions given before or immediately after acquisition indicated that the effect of treatment was highly significant (F(1,7) = 15.14; p = 0.006) and that there was no significant difference between the effects of treatment for the two infusion times (interaction between treatment and experiment: ANOVA F(1,7) = 3.63; p = 0.1). With infusion before acquisition, rats given the antisense ODN failed to discriminate the familiarity of objects, whereas when given the sense ODN (controls) they did discriminate (DR ~ 0: antisense, t(8) < 1, p > 0.1; sense, t(8) = 4.36, p = 0.002); Fig. 3. Similarly, with infusion immediately after acquisition, rats treated with antisense ODN did not show significant discrimination, whereas when treated with sense ODN they did (DR ~ 0: antisense, t(8) < 1, p > 0.1; sense, t(8) = 6.95, p < 0.0001); Fig. 3. As there was impairment when the antisense-ODN was not present at acquisition and might be expected to be no longer active at test at the 24 h delay, the impairment could not be ascribed to a difference in the presence against absence of the compound at acquisition and retrieval (i.e. impairment was not due to state-dependency). Neither sense nor anti-sense BDNF ODN produced an impairment in recognition memory when infused before retrieval (ANOVA F(1,8) < 1; p > 0.1): rats discriminated in both conditions (DR > 0: antisense: t(8) = 3.15, p = 0.01; sense: t(8) = 3.38, p = 0.01); Fig. 3.
Figure 3.
Effects of anti-BDNF ODN on familiarity discrimination. With a 24h memory delay period, anti-sense ODN impaired familiarity discrimination when it was administered before or just after acquisition but not if the infusion was before the test (retrieval) phase or if administration was before acquisition but the memory delay period was 20min. DR: discrimination ratio (difference in time exploring the novel and familiar objects divided by the total exploration time in the test phase). Within subject ANOVA treatment difference: *** p < 0.001. One-sample t-test of DR value against zero: O p < 0.05.
In contrast, when the memory delay was 20min rather than 24h (n = 9, same animals as used in the previous experiments), antisense BDNF ODN administered 1h before acquisition did not cause an impairment in familiarity discrimination; rats when given sense or antisense ODN spent a significantly greater proportion of time exploring the novel rather than the familiar object (DR > 0: antisense: t(8) = 5.681, p < 0.0001; sense: t(8) = 2.31, p < 0.01); Fig. 3.
To determine whether the familiarity discrimination impairment found at the 24h delay was because of an effect on early but not late memory consolidation processes, sense or anti-sense ODN was administered to an additional group of rats (n = 10) 6h after the acquisition phase, with the test phase 24h after the infusion. Rats preferred the novel to the familiar object for both the sense and antisense-treated conditions (antisense: mean DR = 0.36 ± 0.1; DR> 0, t(9) = 3.46, p < 0.01; sense: mean DR = 0.30 ± 0.09; DR> 0, t(9) = 3.22, p = 0.01), and their mean DRs did not differ (t(18) = <1, p > 0.1). Thus antisense ODN infusion did not impair long-term familiarity discrimination when the antisense ODN was administered 6h after acquisition. This lack of impairment additionally indicates that under these circumstances long-term recognition memory is normal 24h after infusion of the antisense ODN; this lack of impairment excludes a long-term non-specific action of the ODN as an explanation for the difference in its effects on memory at 20min and 24h delays.
The results establish a role for BDNF in long-term recognition memory: familiarity discrimination was impaired when antisense BDNF ODN was locally infused into perirhinal cortex either before or immediately after acquisition (but not 6h after acquisition) and memory measured after a 24h delay. Additionally, antisense BDNF ODN did not affect retrieval. By contrast, memory was not impaired at a 20min delay. The antisense, but not the sense, BDNF ODN will bind to and hence prevent translation of BDNF mRNA, so preventing BDNF production. Thus these findings suggest a role for BDNF in early (<7h) consolidation processes underlying perirhinal familiarity discrimination.
The BDNF impairment of long-term but not shorter-term recognition memory found here is in accord with the impairment in fear conditioning following anti-sense BDNF ODN or anti-BDNF antibody infusions into the hippocampus (Alonso et al., 2002; Lee et al., 2004) or parietal cortex (Alonso et al., 2005); these impairments were also interpreted as arising from interference with consolidation. For example, infusion of anti-BDNF antibody into the hippocampus 15 min prior or 1h or 4h after inhibitory avoidance training caused memory impairment in one-trial performance carried out at 24h delay (Alonso et al., 2002). Impairment of long-term but not shorter-term perirhinal recognition memory has been found previously for antagonism of either L-type voltage-dependent calcium channels (Seoane and Brown, 2006), NMDA (Barker et al., 2006a) or metabotropic (Barker et al., 2006b) glutamate receptors in PRH. By contrast, perirhinal kainate receptor antagonism resulted in the opposite pattern of memory loss (amnesia after a 20min but not a 24h delay), indicating that there must be more than one underlying mechanism that supports perirhinal familiarity discrimination (Barker et al., 2006a).
BDNF receptor TrkB colocalises with proteins of the NMDA-receptor complex (Aoki et al., 2000; Yoshii and Constantine-Paton, 2007; Lu et al., 2008). Moreover, BDNF can facilitate glutamate release and increase phosphorylation of NR1 and NR2B subunits of the NMDA-receptor complex (Suen et al., 1997; Levine et al., 1998; Lin et al., 1998; Carvalho et al., 2007). Glutamate receptor transmission and plasticity are essential for perirhinal recognition memory (Massey et al., 2004; Winters and Bussey, 2005; Barker et al., 2006a,b; Griffiths et al., 2008). However, it seems unlikely that the impairments reported here arise from a direct effect on glutamatergic transmission because memory was normal when antisense BDNF ODN was infused before retrieval or memory was measured at a 20 min delay.
One possible route through which BDNF might influence consolidation of perirhinal recognition memory is via activation of the transcription factor cAMP responsive element-binding protein (CREB). Phosphorylation of CREB within perirhinal cortex is essential for long-term plasticity and recognition memory mechanisms (Warburton et al., 2005). Exposure of neurons to BDNF can lead to activation of CREB (Finkbeiner et al., 1997; Alonso et al., 2005; Bekinschtein et al., 2008); and the activation of CREB transcriptional processes has been linked with learning and memory, and synaptic plasticity (Bourtchuladze et al., 1994; Deisseroth et al., 1996; Silva et al., 1998; Genoux et al., 2002). Moreover, CREB phosphorylation can lead to Fos production (Ahn et al., 1998; Silva et al., 1998) and oligonucleotide antagonism of Fos production in perirhinal cortex impairs familiarity discrimination (Seoane and Brown, 2007).
In conclusion, the results indicate that BDNF plays a role in the early (<7h) consolidation of long-term (24h) perirhinal recognition memory.
Acknowledgements
We are grateful to the Medical Research Council for financial support, and to J. Robbins and K. Narduzzo for technical assistance.
Grant sponsor: Medical Research Council; Programme Grant No. G9713086
References
- Ahn S, Olive M, Aggarwal S, Krylov D, Ginty DD, Vinson C. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol. 1998;18:967–977. doi: 10.1128/mcb.18.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M, Vianna MR, Depino AM, Mello e Souza T, Pereira P, Szapiro G, Viola H, Pitossi F, Izquierdo I, Medina JH. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12:551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- Alonso M, Bekinschtein P, Cammarota M, Vianna MR, Izquierdo I, Medina JH. Endogenous BDNF is required for long-term memory formation in the rat parietal cortex. Learn Mem. 2005;12:504–510. doi: 10.1101/lm.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Wu K, Elste A, Len G, Lin S, McAuliffe G, Black IB. Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. J Neurosci Res. 2000;59:454–463. doi: 10.1002/(SICI)1097-4547(20000201)59:3<454::AID-JNR21>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Barker GR, Bashir ZI, Brown MW, Warburton EC. A temporally distinct role for group I and group II metabotropic glutamate receptors in object recognition memory. Learn Mem. 2006a;13:178–186. doi: 10.1101/lm.77806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC, Koder T, Dolman NP, More JC, Aggleton JP, Bashir ZI, Auberson YP, Jane DE, Brown MW. The different effects on recognition memory of perirhinal kainate and NMDA glutamate receptor antagonism: implications for underlying plasticity mechanisms. J Neurosci. 2006b;26:3561–3566. doi: 10.1523/JNEUROSCI.3154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. NMDA receptor plasticity in the perirhinal and prefrontal cortices is crucial for the acquisition of long-term object-in-place associative memory. J Neurosci. 2008;28:2837–2844. doi: 10.1523/JNEUROSCI.4447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neuroscientist. 2008;14:147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol. 2008;153(Suppl 1):S310–324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Griffiths S, Scott H, Glover H, Bienemann A, Ghorbel MT, Uney J, Brown MW, Warburton EC, Bashir ZI. Expression of long-term depression underlies visual recognition memory. Neuron. 2008;58:186–94. doi: 10.1016/j.neuron.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci U S A. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res Mol Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–967. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2a and NR2b-containing NMDA receptors in cortical long-term potentiation and long-term depression. Journal of Neuroscience. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, He J, Nakajima A, Nabeshima T. Involvement of BDNF receptor TrkB in spatial memory formation. Learn Mem. 2003;10:108–115. doi: 10.1101/lm.56003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999;835:259–265. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. Neuroscientist. 2005;11:323–333. doi: 10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- Romero-Granados R, Fontán-Lozano A, Delgado-García JM, Carrión AM. From learning to forgetting: behavioral, circuitry, and molecular properties define the different functional states of the recognition memory trace. Hippocampus. 2009 doi: 10.1002/hipo.20669. e-print. [DOI] [PubMed] [Google Scholar]
- Seoane A, Brown MW. The role of L-type voltage-gated calcium channels in recognition memory in rats. 5th Forum of European Neuroscience; Vienna, Austria: 2006. [Google Scholar]
- Seoane A, Brown MW. Interfering with Fos expression impairs recognition memory in rats. British Neuroscience Association; Harrogate, UK: 2007. [Google Scholar]
- Shi CJ, Cassell MD. Perirhinal cortex projections to the amygdaloid complex and hippocampal formation in the rat. J Comp Neurol. 1999;406:299–328. doi: 10.1002/(sici)1096-9861(19990412)406:3<299::aid-cne2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Suen PC, Wu K, Levine ES, Mount HT, Xu JL, Lin SY, Black IB. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-D-aspartate receptor subunit 1. Proc Natl Acad Sci U S A. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, Aggleton JP, Bashir ZI, Brown MW. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38:987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Glover CP, Massey PV, Wan H, Johnson B, Bienemann A, Deuschle U, Kew JN, Aggleton JP, Bashir ZI, Uney J, Brown MW. cAMP responsive element-binding protein phosphorylation is necessary for perirhinal long-term potentiation and recognition memory. J Neurosci. 2005;25:6296–6303. doi: 10.1523/JNEUROSCI.0506-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Glutamate receptors in perirhinal cortex mediate encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 2005;25:4243–4251. doi: 10.1523/JNEUROSCI.0480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]