Abstract
Previous work has shown that immunohistochemical imaging of Fos protein is a reliable marker for changes in activity related to recognition memory in the perirhinal cortex of the medial temporal lobe; however, whether perirhinal Fos expression is necessary for recognition memory had not been established. To investigate this potential requirement, antisense Fos oligodeoxynucleotide (ODN) was infused locally into perirhinal cortex to interfere with Fos production. As in previous studies, differential Fos expression produced by viewing novel or familiar visual stimuli was measured by immunohistochemistry: antisense Fos ODN infusion into perirhinal cortex disrupted the normal pattern of differential Fos expression in perirhinal cortex. The effect of antisense Fos ODN infusion into perirhinal cortex was therefore sought on recognition memory. Infusion before or immediately after acquisition impaired recognition memory for objects when the memory delay was 3 h or 24 h, but not when the delay was 20 min, nor when the ODN was infused before retrieval after a 24 h delay. The findings indicate a role for Fos in consolidation processes underlying long-term recognition memory for objects and establish that interfering with its expression impairs recognition memory. Antisense Fos ODN infusion also impaired object-in-place recognition memory. The results demonstrate that Fos is necessary for neuronal mechanisms in perirhinal cortex essential to recognition memory.
Keywords: familiarity, immediate early gene, medial temporal lobe, oligodeoxynucleotide, perirhinal cortex
Introduction
Fos protein, product of the immediate-early gene (IEG) c-fos, is a transcription factor associated with neuronal plasticity and learning (Herdegen & Leah 1998; Herrera & Robertson 1996; Tischmeyer & Grimm 1999). Interfering with Fos expression impairs long-term hippocampal-dependent memory (Countryman et al 2005; Fleischmann et al 2003; Grimm et al 1997; Guzowski 2002; He et al 2002; Yasoshima et al 2006) and medial prefrontal cortex-dependent memory (Morrow et al 1999). However, whether Fos expression within perirhinal cortex (PRH) of the temporal lobe is necessary for recognition memory is undetermined.
The issue is important because previous research has provided strong grounds for expecting perirhinal Fos expression to be critical for recognition memory. Perirhinal cortex is essential for recognition memory for objects (Brown & Aggleton 2001; Brown et al 2010). Lesions of rat perirhinal cortex impair such memory (Aggleton et al 1997; Albasser et al 2009; Barker et al 2007; Bussey et al 1999; Ennaceur et al 1996; Mumby & Pinel 1994; Mumby et al 2007; Winters et al 2004). Moreover, interfering with neurotransmission in perirhinal cortex (Barker et al 2006b; Wan et al 2004; Warburton et al 2003) also impairs recognition memory. More particularly, perirhinal Fos protein is a reliable marker of changes in neuronal activity related to recognition memory: viewing novel visual stimuli produces an increase in the number of perirhinal Fos-stained neurons compared to viewing familiar stimuli (Albasser et al 2010; Wan et al 1999; Wan et al 2004; Warburton et al 2005; Warburton et al 2003; Zhu et al 1995b; Zhu et al 1996). This lower Fos signal for familiar compared to novel stimuli parallels the reduction in single neuronal responses found when previously novel stimuli are seen again (Brown et al 2010; Brown et al 1987; Brown & Xiang 1998; Zhu et al 1995a). Moreover, such response reduction is thought to be dependent on synaptic weakening (Brown & Bashir 2002; Brown et al 1987; Brown & Xiang 1998; Griffiths et al 2008). Importantly, Fos expression has been linked to mechanisms of synaptic weakening such as long-term depression (LTD) (Lindecke et al 2006; Nakazawa et al 1993) and to perirhinal intracellular signalling pathways involved in recognition memory (Seoane et al 2010; Tinsley et al 2009; Warburton et al 2005).
Therefore, first, the effects of perirhinal infusion of an antisense oligodeoxynucleotide (ODN) were compared to those of a sense Fos ODN on the Fos produced by viewing novel stimuli, using the same task as in previous published work (e.g. Wan et al. 1999; Warburton et al 2003, 2005; Zhu et al 1996). Second, to determine whether Fos production is necessary for recognition memory as opposed to being a non-specific correlate, the effects on such memory of the antisense oligodeoxynucleotide (ODN) was sought using the same tasks as in previous published work (e.g. Barker et al 2006b; Griffiths et al 2008; Warburton et al 2005; Winters et al. 2008). As perirhinal lesions impair recognition memory for the association between an object and a location (object-in-place memory) (Barker et al 2007; Bussey et al 2001; Bussey et al 2000), the effect of antisense Fos ODN was tested on object-in-place as well as object recognition memory. Antisense, but not sense, Fos ODN will bind to and hence prevent translation of c-fos mRNA, so preventing Fos production. It was hypothesised that the antisense Fos ODN would therefore interfere with the cascade of consolidation processes resulting in long-term recognition memory storage.
Methods
Animals and drugs
Adult male pigmented Dark Agouti rats (220-250g; Bantin and Kingman, UK) were maintained on a 14h light/10h dark cycle, with the dark phase during normal daylight. Rats with implanted cannulae were housed in pairs. All rats had 24 h access to food and water, except those used for the paired-viewing test when water was restricted to two hours a day for a maximum of 6 consecutive days. All experiments were performed in accordance with the UK Animals Scientific Procedures Act (1986) and had been approved by the University of Bristol Ethical Review Group.
Oligodeoxynucleotides (ODNs) were PAGE-purified phosphotioate end-capped 15-mer sequences (Sigma Genosys Ltd., Haverhill, UK). Fos antisense ODN: 5′-GAACATCATGGTCGT-3′; Fos sense ODN (control sequence): 5′-CTTGTAGTACCAGCA-3′. Previous work has indicated that the antisense Fos ODN has high selectivity and is maximally effective at ~1 h after administration with the effects not lasting longer than several hours (e.g. Hebb et al 1997; Sommer et al 2000); however, others have reported a slower time course of action (Lamprecht and Dudai 1996 and Countryman et al 2005). For this reason pilot studies were conducted. These investigations resulted in a period of 1 h being selected as sufficient to allow time for the Fos ODN to be effective. To visualise the ODN spread within the PRH biotinylated Fos antisense ODN was used. Both antisense and sense ODNs were infused locally into the PRH through bilaterally implanted cannulae at 1nmol in 1μl normal saline, a dose similar to that used by Yasoshima et al. (2006) and following the methodology previously employed for the hippocampus (Chiasson et al 1992; Countryman et al 2005; Grimm et al 1997; Guzowski 2002; He et al 2002; Yasoshima et al 2006) and medial prefrontal cortex (Morrow et al 1999).
Cannula implantation into PRH
Cannula implantation was carried out in rats deeply anaesthetised with Isoflurane (Merial Animal Health Ltd., Harlow, UK) and placed in a stereotaxic frame where the skull was held in a flat position (the height difference between bregma and lambda was < 0.1mm). Two stainless-steel guide cannulae (26 gauge, Plastics One Inc., Roanoke, Virginia, USA, via Semat in UK) were implanted through holes in the skull, at an angle of 20° to the vertical and according to the following coordinates (relative to bregma): AP −5.6mm, L ± 4.5mm and V −6.7mm (relative to the skull surface) (Paxinos and Watson, 1998). The guide cannulae were anchored to the skull with two stainless steel screws, epoxy-resin (Araldite, Bostik Ltd., Leicester, UK) and dental cement (CMW1 Radiopaque with gentamycin, DePuy International Ltd., Blackpool, UK). Cannulae were protected with dummy inserts (Plastics One Inc.) except at the time of the infusion. The rats were allowed to recover for at least 20d before the experiment began. Sense or antisense ODN infusions through a cannula in each hemisphere were made by inserting a 33 gauge cannula (Plastics One Inc.) which protruded 1mm beyond the guide cannula tip and which was connected by PVC tubing (Barloworld Scientific Ltd., Berkshire, UK) to a Hamilton syringe (Hamilton Bonaduz, Bonaduz, Switzerland). To minimise the amount of ODN used, the PVC tubing was pre-filled with a non-water soluble solution of 0.3mg/ml Oil Red O (Sigma-Aldrich Chemie, Steinheim, Germany) in paraffin oil (Fluka, Steinheim, Germany). The syringe was advanced with an infusion pump (Harvard Bioscience, Holliston, MA, USA) at a rate of 0.7μl/min for 1min and 30 sec; 5 min later the injection cannulae were withdrawn.
Paired-viewing test
To study the interference with Fos expression induced by antisense Fos ODN, Fos immunohistochemistry was performed following a paired-viewing test, as in previous work (Wan et al 1999; Wan et al 2004; Warburton et al 2005; Warburton et al 2003; Zhu et al 1996).
Procedure
The paired-viewing procedure is designed to allow measurement of differential neuronal activation produced by novel and familiar visual stimuli. The methodology has been described in detail in previous studies (Warburton et al 2003; Zhu et al 1995b; Zhu et al 1996). In brief, each rat, lured by the availability of juice, viewed two pictures presented simultaneously on video monitors positioned so that each picture was seen only within the monocular visual field of one eye (Fig. 1B). The visual information was therefore chiefly processed by the opposite cerebral hemisphere, making possible the comparison of within rat differences in activation between the two hemispheres. Across 6d, the rat was repeatedly shown a ‘repeat set’ of pictures together with sets of novel pictures; 30 pictures were shown to each eye in the morning and 30 in the afternoon. This period of training familiarised the rat with the whole procedure, including the occurrence of new pictures, and allowed the repeatedly shown pictures to become familiar. At the end of the 6th day, both eyes had had equal exposure to the pictures of the repeat set and to novel pictures. On the 7th day, 2h after viewing the repeat set with both eyes, rats were bilaterally infused with sense or antisense ODN (n = 6 and n = 5, respectively) via cannulae implanted into the PRH. One hour later (i.e. the total memory delay was 3 h), this repeat set of pictures was seen again by one eye, while the other eye viewed a set of 30 novel pictures (the ‘novel set’). The set of pictures (novel or repeat set), and the eye exposed to novel or to repeat pictures were counterbalanced across rats.
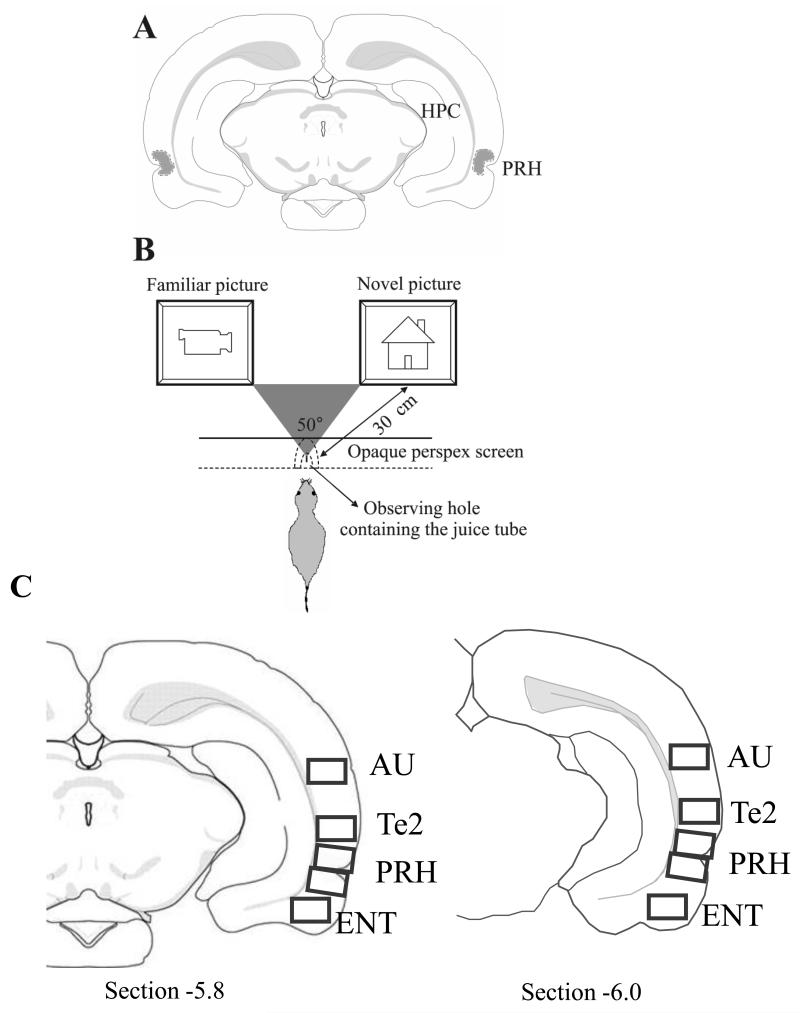
Figure 1. Procedures.
A: the location of cannula tips, as observed in cresyl violet stained sections, for all implanted animals used in behavioural tests (n = 24), ODN spread study (n = 2) and paired-viewing test (n = 11) fell within the dotted outlined area, which was located within the PRH from bregma −5.5 to −4.5. B: Paired-viewing apparatus: a rat, lured by the availability of juice, places its head in a hole in a Perspex barrier. The central partition is placed so that each eye can see the picture only on the computer monitor on that side. Pictures were presented simultaneously on both monitors. C: Fos-labelled cell counting was performed in rectangular areas (shown in grey) from two sections from bregma −5.5 to −6.0 in the following regions: perirhinal cortex (PRH), temporal association cortex (Te2), entorhinal cortex (ENT) and auditory cortex (AU) (Paxinos & Watson 1998; Shi & Cassell 1999).
Fos immunohistochemistry
Each rat was anaesthetised with Euthatal (Merial Animal Health Ltd.) and perfusion-fixed with 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) 1.5h after the final sets of pictures were presented. The brain was removed and post-fixed in the same fixative for at least 24 h before being immersed in 30% sucrose in 0.1M phosphate buffer for at least 2 d. The right hemisphere of the brain was marked with an incision and the brain was sectioned (40μm) coronally on a cryostat. Two series of floating sections were collected. The first series was stained with cresyl violet to determine the location of the cannula tracks. For all rats, the infusion sites were within the PRH (bregma −5.5 to −4.5); Fig. 1A. The second series were immunohistochemically processed for Fos. The sections were washed in 0.1M phosphate buffer saline containing 0.2% Triton X-100 (PBST; pH 7.4), immersed in 0.3% hydrogen peroxidase for 20 min, washed again before being incubated with primary Fos antibody (anti-c-Fos (sc-52) rabbit polyclonal IgG; Santa Cruz Biotechnology, Heidelberg, Germany) diluted 1:2000 in PBST for 48h at 4°C. After washes with PBST, the sections were incubated with secondary antibody (biotinylated anti-rabbit IgG, BA-1000; Vector Laboratories Inc., Burlingame, USA) diluted 1:200 in PBST with 1.5% of normal goat serum for 2h at room temperature. After several washes, sections were processed with an ABC horseradish peroxidase complex (Vectastain ABC Kit; Vector Laboratories Inc.) in PBST for 1 h at room temperature, and the reaction was visualized using 3,3′-diaminobenzidine (Sigma Fast™, Sigma-Aldrich).
Fos counting
The Fos-labelled cells were counted using an automated image analysis system using in-house software (CellCountMainv.3.1, J. Leendertz, University of Bristol) that counted round or oval objects 10-22μm diameter and above a darkness threshold relative to the smoothed surrounding background level. The threshold was preset in preliminary experiments so that clearly stained neurons were counted. Cell counts were obtained in both hemispheres for rectangular areas (0.94 × 0.67mm) from two sections from bregma −5.5 to −6.0 (sections caudal to the cannula track at −5.5 to − 4.5). Counts were made close to the cannula tip so as to be within the area affected by the infusions but not immediately adjacent to it so as to avoid any distortions by produced by the implant itself. The rectangular area included all cortical layers. Stereological corrections were not used as relative changes were all that were sought. The regions where counting was performed were the PRH, temporal association cortex (here termed Te2), entorhinal cortex (ENT) and auditory cortex (AU) (Paxinos & Watson 1998; Shi & Cassell 1999); Fig. 1C. Two sections each containing two counting frames (upper and lower) were sampled for perirhinal cortex to ensure coverage of both areas 35 (lower) and 36 (upper). Their counts were averaged before further analysis. The frames approximately rather than exactly sampled areas 35 and 36. There was no significant difference in the findings for the ‘upper’ and ‘lower’ frames (e.g. both showed a significant novel vs. familiar difference for the sense infusions). In some brains the lower of the two perirhinal sections may have included a small part of the adjacent entorhinal cortex; however, this was a small (<10%) proportion of the total area. All processing and counting was blind as to the treatment of each animal.
Behavioural testing
Procedure
The methodology of both behavioural tests, novel object preference task and object-in-place task, have been described in detail in previous studies (Barker et al 2006a; Barker et al 2007; Barker et al 2006b; Warburton et al 2003). In brief, these tasks took place in an arena (100 × 90 × 50cm) surrounded with black curtains to a height of 1.5m and with sawdust on the floor. The rat’s behaviour was monitored using a camera (FC-30, Ganz, NY) and a video/DVD recorder (DR-MV5, JVC, UK). The objects were made of Duplo bricks (Lego Produktion A.G., Baar, Switzerland) or were junk objects, and varied in size (range: 10 × 5 × 15cm to 20 × 20 × 30cm), colour and shape, and were placed near the corners at either end of one side of the arena (15cm from each adjacent wall). The objects used and their position in the arena were counterbalanced between animals in a group, and between sense and antisense-treated animals. Prior to the start of memory testing, each rat was habituated to the empty arena for 5 min daily for 4d. The experimenter was blind as to the treatment of each animal.
Novel object preference task
For the novel object preference test, the arena had four grey walls with black curtains behind each wall. This task comprised two phases, acquisition and test, separated by a delay of 20 min, 3 h or 24 h. In the acquisition phase, an animal was allowed to explore two identical objects for 40s of exploration or a maximum of 4min spent in the arena. In the test phase, the rat was allowed to explore an identical third copy of the object explored in the acquisition phase and a novel object for 3min from the start of the objects’ exploration.
Object-in-place task
For the object-in-place task, the arena had three grey walls and one black wall with the black curtains behind only two walls. This task comprised two phases, acquisition and test, separated by a delay of 3 h. In the acquisition phase, an animal was allowed to explore four different objects for 5 min (time spent in the arena from the start of exploration). During the delay period, objects were cleaned with alcohol to remove olfactory cues. For the test phase, the positions of two objects on either the right or the left of the arena were interchanged, with the other two objects remaining in their same positions. The rat was allowed to explore the objects for 3min (time spent in the arena from the start of exploration of the objects).
In both behavioural tasks, the time spent exploring each of the objects was recorded in both acquisition and test phases. Exploration was measured only when the animal’s nose was directed towards the object at a distance of less than 1cm. If the time of exploration was less than 15s in the acquisition phase or less than 10s in the test phase, the animal was excluded from the analysis of that experiment.
Experimental design
One group of rats (n = 15) was used to assess the effects of antisense Fos ODN infused before acquisition of object recognition memory. Antisense or sense Fos ODN was administered locally into the PRH through the bilaterally implanted cannulae 1 h before the acquisition phase. To evaluate shorter-term and longer-term memory, delays between acquisition and test phases of 20 min, 3 h and 24 h were used. A second group of rats (n = 9) was used to study the effects on retrieval and consolidation of object recognition memory. To assess the effects on long-term memory retrieval, antisense or sense Fos ODN was administered 1 h before the test phase, with a 24 h delay period. To evaluate whether antisense Fos ODN had an effect on the consolidation of long-term memory, antisense or sense ODN was administered within 2 min after the acquisition phase, with the 24 h delay period. This second group was also used to study effects on object-in-place memory: antisense or sense Fos ODN was administered 1 h before the acquisition phase, with the 3 h delay period. Table 1 shows the animal groups included in the experiments. As stated previously some rats were excluded because their exploratory levels were too low: 2 rats were excluded from the acquisition trial of the object recognition 20 min delay experiment and 6 rats were excluded from the acquisition trial of the object recognition 3 h delay experiment.
Table 1. Groups of animals used in each experimental condition.
| Group of animals 1 (n = 15) |
| acquisition 20 min |
| acquisition 3 h |
| acquisition 24 h |
| Group of animals 2 (n = 9) |
| retrieval 24 h |
| consolidation 24 h |
| object-in-place 3 h |
| Group of animals 3 (n = 6) |
| paired-viewing procedure bilaterally infused with sense ODN into PRH |
| Group of animals 4 (n = 5) |
| paired-viewing procedure bilaterally infused with antisense ODN into PRH |
| Group of animals 5 (n = 2) |
| histology to visualize the extent of ODN diffusion in the brain |
Each experiment was performed in a counterbalanced cross-over design: thus rats treated with the antisense ODN in one experimental trial were administered the sense ODN on the following trial, and vice versa. The animals were allowed to rest between experimental trials for at least 1 week to ensure the ODNs had cleared (Hebb et al 1997; Sommer et al 2000).
Histology
Cannula locations were checked to confirm that infusions were in PRH. 2 brain sections stained with cresyl violet were obtained using the same techniques as described for the immunohistochemistry. All animals had the tips of their cannulae within the PRH (dorsal and ventral; Shi & Cassell 1999) from bregma −5.5 to −4.5 (Paxinos & Watson 1998); Fig. 1A.
ODN diffusion
To visualise the extent of ODN diffusion within the brain, 1 nmol of biotinylated antisense ODN in 1 μl saline was infused into the PRH via cannula 15 min (n = 1) or 1 h (n = 1) before the rat was anaesthetised with Euthatal. The brain was removed and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for at least 6 d (to ensure thorough immersion fixation) before being immersed in 30% sucrose in 0.1 M phosphate buffer for at least 2 d. The brain was sectioned (40μm) coronally on a cryostat. Floating sections were collected and washed in 0.1 M phosphate buffer saline containing 0.2% Triton X-100 (PBST; pH 7.4), immersed in 0.3% hydrogen peroxidase for 20 min, washed again before being processed with avidin-biotinylated horseradish peroxidase complex (Vectastain ABC Kit; Vector Laboratories Inc.) in PBST for 1 h at room temperature, and the reaction was visualized using 3,3′-diaminobenzidine (Sigma Fast™, Sigma-Aldrich).
To confirm that antisense infusions of ODNs reduced Fos levels at the infusion site, antisense and sense ODNs were infused. Rats (n=2) received an infusion of antisense ODN to one side of the brain and an infusion of sense ODN to the other side. One rat received ODN infusions at a dose of 1nmol in 1μl and the other received infusions at a dose of 5nmol in 1μl. The rat brains were perfused and processed for Fos imaging 2.5h after the infusions of ODNs using the immunohistochemical procedures described below.
Statistical analysis
Novel object preference test
The discrimination ratio (DR) was calculated as the time in the test phase spent exploring the novel object minus that spent exploring the previously experienced object divided by the total exploration time. Comparisons between treatments within the same group of animals were analysed by within-subject ANOVAs. One-sample t-tests were used to determine whether the DR for each group was significantly different from zero, as zero corresponds to a lack of discrimination between novel and familiar objects. All tests used a significance level of p = 0.05 and were two-tailed.
Fos-labelled cell counts
Each count was normalised by dividing it by the mean of all the counts for that area across both hemispheres for each rat. The normalised and absolute (non-normalised) counts were analysed by ANOVA with repeat measures, with the factor novel/familiar pictures being measured between subjects, and the factors treatment (sense or antisense) and area (PRH, Te2 and AU) being measured within subjects.
Results
Localised spread after ODN infusion
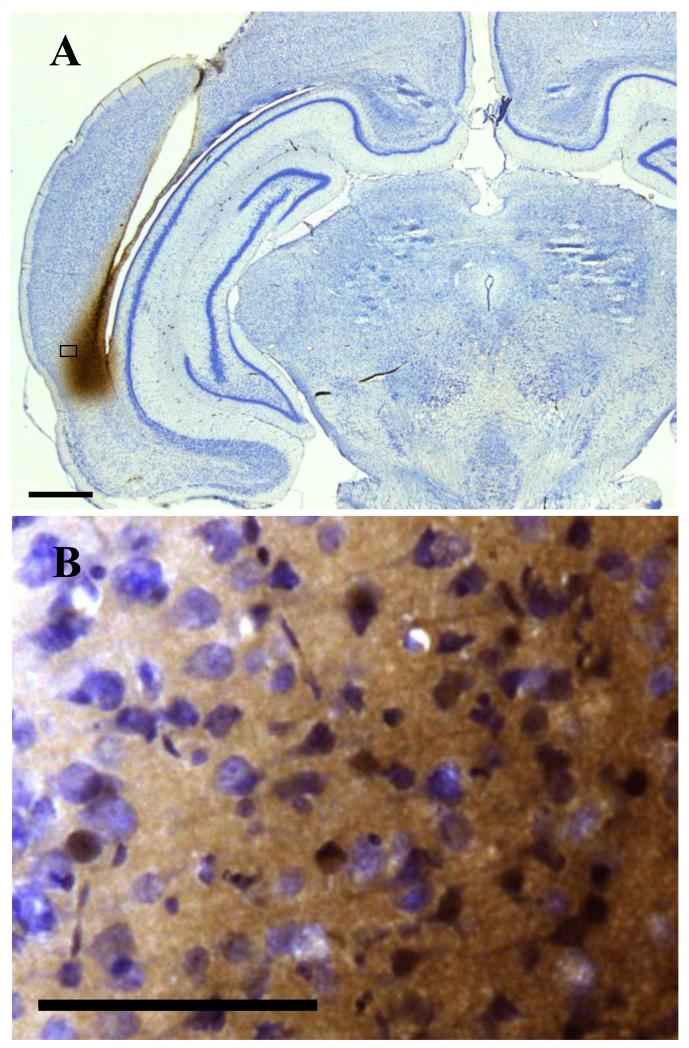
The spread of infusate was first checked in two rats. Biotinylated antisense Fos ODN was visualised within the PRH 15 min or 1 h after its infusion (1nmol in 1μl). Stained cells were found from bregma −6.2 to −4.7 (1.5mm length; 15 min delay; Fig. 2A) or from bregma −6.7 to −5.4 (1.3mm length, 1 h delay). The staining was found both between and inside the cells at both time points (Fig. 2B). The volume of stained tissue encompassed 80% of the PRH as defined by Shi & Cassell (1999), where PRH includes both areas 35 and 36, or dorsal and ventral PRH, and corresponds approximately to caudal PRH of Burwell, et al. (1995). Less than 5% of the volume of either area Te2 or ENT was stained. A small amount of ODN staining was seen in the neuropil of the hippocampus, corresponding to approximately 0.5% of the total hippocampal volume. The staining was confined to a small region of the neuronally-sparse stratum oriens of the CA1 region. Within this region a few isolated stained cells were seen.
Figure 2. Diffusion of 1nmol of biotinylated antisense ODN in 1μl saline 15 min after being infused into the PRH via cannula.
A: Spread was found within PRH from bregma −6.2 to −4.7. Scale bar: 1mm. B: Staining was found between and inside the cells. Scale bar: 100μm.
Effect of ODNs on Fos expression
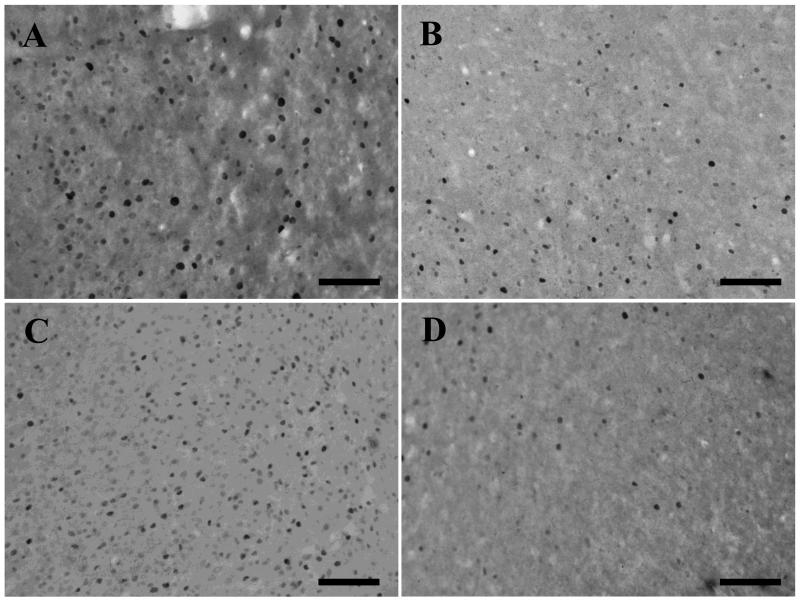
The efficacy of the infusion procedure was then checked in two rats, antisense and sense Fos ODNs (1nmol or 5nmol in 1μl) were infused into perirhinal cortex and the tissue immunohistochemically processed for Fos 2.5h after the infusions. In counting frames adjacent to the infusion when 1nmol ODN was infused, counts of Fos stained nuclei were reduced by 40% near the antisense compared to near the sense infusion (n=1) (Fig. 3 A and B). When 5nmol ODN was infused, there was a clearly visible reduction in Fos stained nuclei in the region infused with the antisense ODN; counts of Fos stained nuclei were 68% lower in the antisense compared to sense counting frames (n=1) (Fig. 3. C and D). Thus the higher dose of antisense ODN reduced Fos counts by two-thirds while the lower dose (1nmol) produced a reduction of more than one-third in the infused region. The lower dose (1nmol) was used in the experiments below.
Figure 3. Reduction in Fos staining following infusion of antisense Fos ODNs.
Photomicrographs show Fos staining following infusion (2.5h earlier) of sense Fos ODN (A and C) or antisense Fos ODN (B and D). The amount infused was 1nmol in 1μl (A and B) and 5nmol in 1μl (C and D). Cell counts confirmed a reduction in Fos staining following the antisense infusions (1nmol: sense count = 260; antisense count = 156); the 5nmol antisense infusion produced a readily visible reduction (sense count = 397; antisense count = 127). Scale bar = 100 μm.
Effect of ODNs on differential Fos expression
The effect of the ODNs was sought on the different neuronal activations produced by viewing novel and familiar pictures imaged using immunohistochemistry for Fos, as in previous work indicating a correlation between perirhinal Fos activation and recognition memory processes (e.g. Wan et al. 1999; Warburton et al 2003, 2005; Zhu et al 1996). This paired viewing procedure allows the measurement of differential Fos expression by using multiple stimuli under closely controlled conditions that are not possible in the preferential object exploration task that is standardly used to measure behaviour. (Besides the difficulty of providing adequate control groups, the low number of stimuli that a rat will explore in the preferential object exploration task in a short time period makes achieving potential group sizes for adequate statistical power in comparisons of immunohistochemical differences infeasible.) The activations were compared between hemispheres after a rat had viewed novel pictures with one eye and familiar pictures with the other eye using the paired-viewing procedure (Fig. 1B). This procedure allows the measurement of differential Fos expression produced by multiple stimuli presented under closely controlled conditions. In normal rats, the hemisphere primarily processing novel information (the ‘novel hemisphere’) has higher Fos counts in PRH and Te2 than that primarily processing familiar information (the ‘familiar hemisphere’), whereas no changes are normally found in auditory (AUD) or entorhinal (ENT) cortex (Wan et al 1999; Warburton et al 2005; Warburton et al 2003; Zhu et al 1996). Antisense or sense ODN was bilaterally infused locally into PRH on the final training day 1 h before the viewing of novel and familiar pictures so that the ODN was present during acquisition and early consolidation of the novel pictures. The familiar pictures had last been seen 3 h previously, giving a memory delay of at least 3 h.
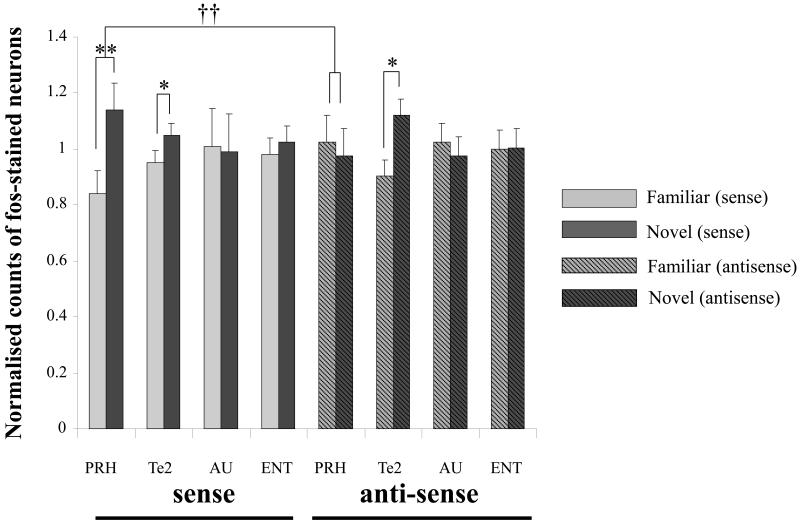
Antisense ODN treatment disrupted the normal pattern of differential Fos expression in PRH, without changing Fos expression in Te2 and control areas of auditory (AU) and entorhinal (ENT) cortices (Fig. 4). For the normalised Fos counts (counts were normalised to remove batch differences), a repeated measures ANOVA with factors for area, novelty/familiarity and treatment (antisense or sense) revealed a significant three-way interaction (F3,27 = 6.42; p < 0.01). The analysis was therefore continued by analysing the data for each region separately. For PRH, there was a significant interaction between novelty/familiarity and treatment (F1,9 = 15.92; p < 0.01). For the sense-treated group, Fos counts were significantly higher in the novel than the familiar hemisphere (F1,5 = 18.97; p < 0.01); Fig. 4. In contrast, for the antisense-treated group, no difference was found between the familiar and novel hemispheres (F1,4 = 3.04; p > 0.1). In confirmation of the analysis of the normalised counts, a significant interaction was found between novelty/familiarity and treatment for the absolute (non-normalised) Fos counts in PRH (F1,9 = 17.49; p < 0.01): the novel hemisphere counts were significantly higher than the familiar for the sense but not the antisense ODN infusions (Table 3). Returning to the analysis of the normalised counts, in Te2, there was no significant interaction between novelty/familiarity and treatment (F1,9 = 2.77; p > 0.1). In both sense and antisense groups, Fos counts in Te2 were higher in the novel than the familiar hemisphere (sense: F1,5 = 7.06; p < 0.05; antisense: F1,4 = 10.64; p < 0.05); Fig. 4. A significant interaction involving area, treatment and novelty/familiarity was found when the PRH and Te2 data were analysed together (F1,9 = 19.66; p < 0.01), establishing that there were different effects of the infusions in PRH and Te2. In control areas, AU and ENT, no significant interaction was found between novelty and treatment; and no difference was found between Fos counts in the novel and familiar hemispheres for either the sense or antisense groups.
Figure 4. Infusion of antisense Fos ODN disrupts differential Fos expression in the perirhinal cortex.
Fos counts normalised across both brain hemispheres for each brain region are shown for the novel and the familiar hemispheres for sense or antisense ODN-treated rats for the following regions: perirhinal cortex (PRH), temporal association cortex (Te2), auditory cortex (AU) and entorhinal cortex (ENT). Following infusions of sense Fos ODN (control) into the perirhinal cortex there is a differential expression of Fos in response to viewing novel or familiar images in the perirhinal cortex (in agreement with previous studies): repeated measures ANOVA effect of novelty/familiarity: * p < 0.05, ** p < 0.01. After infusion of antisense Fos ODN the differential expression of Fos within the perirhinal cortex is disrupted: repeated measures ANOVA interaction of treatment and novelty/familiarity: †† p < 0.01. In PRH, a significant interaction between novelty/familiarity and ODN treatment was found. In both sense and antisense groups, Fos counts in Te2 were higher in the novel than the familiar hemisphere.
Table 3. Non-normalised Fos-labelled cell counts (mean ± SEM) in sample areas.
| Sense Fos ODN |
Antisense Fos ODN |
|||
| Areas |
Familiar |
Novel |
Familiar |
Novel |
| PRH | 100 ± 23 | 136 ± 24 | 153 ± 38 | 145 ± 42 |
| TE | 141 ± 31 | 158 ± 47 | 152 ± 42 | 192 ± 72 |
| AU | 292 ± 85 | 291 ± 96 | 300 ± 74 | 292± 104 |
| ENT | 191 ± 36 | 203 ± 43 | 243 ± 32 | 265 ± 29 |
Behavioural effects of ODNs
Recognition memory for objects
The task chosen to determine the effects of antisense Fos ODN on object recognition memory was preferential exploration of a novel rather than a familiar object, the task used in previously published work demonstrating the necessity of perirhinal cortex for recognition memory (see for reviews, Brown et al. 2010; Winters et al. 2008). As Fos production following stimulation occurs over a time course of approximately 30min to 3 h (Herdegen & Leah, 1998), the expectation was that impairment would be seen in consolidation processes and would be apparent at longer rather than shorter time delays. Experiments were performed using infusions (1nmol sense or antisense Fos ODN in 1μl) at different times (either before or immediately after acquisition), with memory tested after different memory delay periods (20 min, 3 h, 24 h); see Fig. 5. For each rat, the time in the test phase spent exploring the novel object minus that spent exploring the previously experienced object divided by the total exploration time was calculated as the discrimination ratio (DR); a DR of zero represents no discrimination.
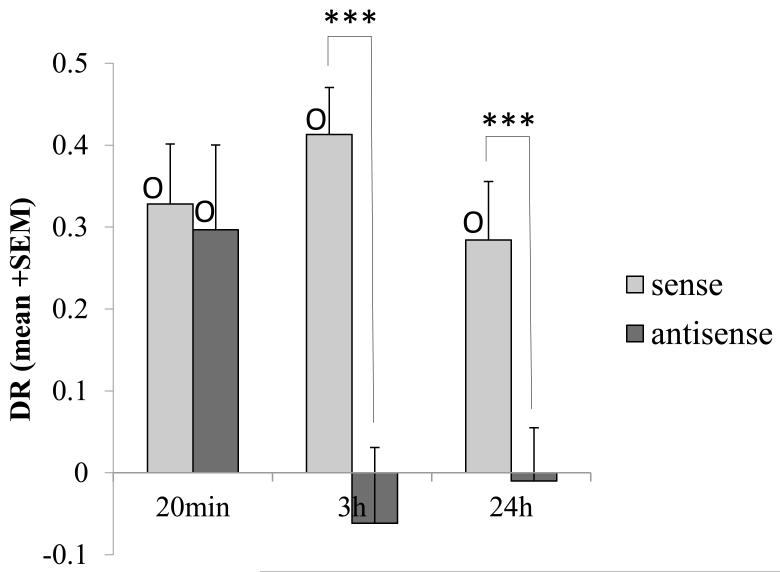
Figure 5. Antisense Fos ODN impairs object recognition memory.
Bilateral perirhinal infusion of sense Fos (control) ODN (light grey) or antisense Fos ODN (dark grey) were made prior to object recognition memory testing. Antisense ODN impaired familiarity discrimination when it was administered before acquisition and the memory delay period was 3 h or 24 h (long-term memory) but not if the memory delay period was 20 min (shorter-term memory). All groups receiving sense Fos ODN infusions showed significant discrimination (DR>0) denoted by O. Within subject ANOVA treatment difference: *** p < 0.001. One-sample t-test of DR value against zero: O p < 0.05. Following administration of sense Fos ODN there was no significant difference between discrimination levels (DR values: ANOVA, p>0.05) for the acquisition experiments described above. The order that the experiments were run was (1) 24h, (2) 20min and (3) 3h. The finding that memory was unimpaired in the second experiment even though it was impaired in the first indicates that the ability to acquire new recognition memories was not permanently impaired by the previous antisense Fos ODN infusion.
Previous work indicates that Fos production is required for consolidation rather than acquisition mechanisms (Bertaina-Anglade et al. 2000; Katche et al. 2010; Doron et al. 2010). However, in inital experiments, sense or antisense Fos ODN was administered 1 h before the acquisition phase so that the infusate had time to diffuse into neurons and influence their intracellular processing for Fos during early consolidation (studies with similar ODNs indicate that uptake is significant by 1 h post-infusion: Seoane et al. 2011; Lee et al. 2004). Three memory delays were tested: 20 min, 3 h or 24 h. DR values were subjected to a two-factor ANOVA (treatment, delay) with treatment as a repeated measure. This analysis revealed a significant main effect of treatment (F1,34 = 16.6, p<0.0001) and a significant treatment by delay interaction (F2,34 = 3.64, p <0.04).
The interaction arose because the antisense ODN caused impairment of familiarity discrimination when the memory delay was 3 h or 24 h, but not when it was 20 min (see Fig. 5), whereas the sense ODN produced no impairment at any delay. In detail, at the 3 h delay, the antisense-treated rats were significantly impaired compared to the sense group control (F1,8 = 18.7; p = 0.003); the antisense group failed to discriminate between the novel and familiar objects, whereas the sense controls did (DR ~ 0: antisense, t8 < 1, p > 0.5; sense, t8 = 6.78, p < 0.001); Fig. 5. Similarly, at the 24 h delay, antisense-treated rats were significantly impaired compared to the sense controls (F1,14 = 14.7; p = 0.02); the antisense group did not show significant discrimination, whereas the sense controls did (DR ~ 0: antisense, t8 < 1, p > 0.1; sense, t8 = 6.95, p < 0.0001); Fig. 5. In contrast, with a 20 min delay, both antisense- and sense-treated rats spent a significantly greater proportion of time exploring the novel rather than the familiar object (DR > 0: sense: t12 = 4.31, p = 0.001; antisense: t12 = 2.76, p < 0.02) and the two groups did not differ statistically; Fig. 5. Hence antisense Fos ODN given before acquisition to be active during early consolidation impaired object recognition memory tested after delays of 3 h or 24 h, but not at a delay of 20 min.
The next experiment was designed to test the hypothesis that the effect of antisense Fos ODN in impairing long-term recognition memory when infused after acquisition would be similar to that when administered well before acquisition, in spite of the reduced length of time available for the infusion to become effective. Sense or antisense ODN was administered < 2min after the acquisition phase so that it was active during consolidation (but not acquisition), the delay period being 24 h. Consistent with the consolidation hypothesis, when so infused immediately after acquisition, rats treated with the antisense ODN did not show significant discrimination, whereas the sense controls did (DR ~ 0: antisense, t8 < 1, p > 0.1; sense, t8 = 2.55, p = 0.03); Fig. 6. Moreover, analysis of the data from the two experiments with a memory delay of 24 h, with the infusion given either before (data from experiments above) or immediately after acquisition, indicated that the effect of treatment was highly significant (F1,22 = 13.72; p = 0.001) and that there was no significant difference between the effects of treatment for the two infusion times (interaction between treatment and experiment: ANOVA F1,22 < 1; p > 0.1). In particular, there was no significant effect of infusion time (pre-acquisition versus immediately post-acquisition; designed comparisons) for groups receiving either the sense (ANOVA: F1,22 = <1; p = 0.9) or antisense (ANOVA: F1,22 = <1; p = 0.6) ODN, i.e. the behavioural effects were similar for ODNs given before and after acquisition. Accordingly, the impairment produced by antisense Fos ODN is equivalent whether given before or after acquisition. Hence, in agreement with the initial hypothesis, the effect is most readily explained as on early consolidation rather than on acquisition of long-term recognition memory.
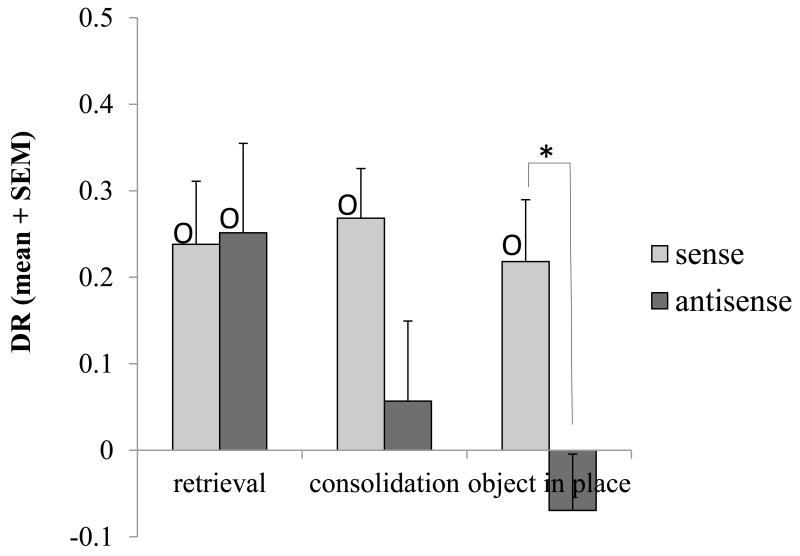
Figure 6. Antisense Fos ODN tested on consolidation and retrieval of object recognition memory, and object-in-place recognition memory.
Recognition memory was tested after bilateral perirhinal infusion of sense Fos (control) ODN (light grey) or antisense Fos ODN (dark grey). With a 24 h memory delay period, antisense Fos ODN impaired familiarity discrimination when it was administered just after acquisition (consolidation) but not if the infusion was before the test phase (retrieval). Antisense ODN also impaired object-in-place performance at a 3 h delay. All groups receiving sense Fos ODN infusions showed significant discrimination (DR>0) denoted by O. Within subject ANOVA treatment difference: * p < 0.05. One-sample t-test of DR value against zero: O p < 0.05. Following administration of sense Fos ODN there was no significant difference between discrimination levels (DR values: ANOVA, p>0.05) for each of experiments shown. The order that the experiments were run was (1) object-in-place, (2) consolidation and (3) retrieval. The finding that memory was unimpaired in the last experiment even though it was impaired in the first two experiments indicates that the ability to acquire new recognition memories was not permanently impaired by the previous antisense Fos ODN infusions.
In the fifth experiment, sense or antisense ODN was administered 23 h after acquisition and 1 h before the test phase (total memory delay 24 h) so that it was active during retrieval. Comparison of the results for the experiment where the ODNs were given before retrieval with that where they were given before acquisition and memory tested after a 24 h delay (data from first experiments) revealed a significant interaction of treatment by injection time (two-factor ANOVA with repeated measures for treatment: F1,22 = 6.92, p<0.02) as well as a significant main effect of treatment (F1,22 = 5.77, p<0.03). The interaction arose because, in contrast to when infusion was before acquisition, neither sense nor anti-sense Fos ODN produced an impairment in recognition memory when infused before retrieval (ANOVA F1,8 < 1; p > 0.1): both groups of rats discriminated (DR > 0: sense: t8 = 2.37, p < 0.05; antisense: t8 = 3.56, p = 0.007); Fig. 6. Antisense Fos ODN was without effect on retrieval.
Object-in-place recognition memory
Preferential exploration of objects that had been moved to a different location rather than objects that were in the same location was used to determine the effects of antisense Fos oligonucleotide on object-in-place associative recognition memory. This task is also impaired by perirhinal lesions (Barker et al. 2007; Bussey et al. 2000).
Sense or antisense ODN was administered 1 h before the acquisition phase so that it was active during acquisition and early consolidation; the delay period was 3 h. Antisense ODN caused an impairment in object-in-place performance. The antisense-treated rats were significantly impaired compared to the sense controls (F1,8 = 8.74; p = 0.02); the antisense group failed to discriminate between objects with a changed or unchanged location, whereas the sense controls did (DR ~ 0: antisense, t8 < 1, p > 0.1; sense, t8 = 2.51, p = 0.04); Fig. 6.
Time of exploration in behavioural tests
In all of the object recognition and object-in-place experiments, the times of exploration in both acquisition and test phases were recorded to confirm that the discrimination impairments observed could not be ascribed to changes in overall exploration (see Table 2). In all the measures, there was only one significant difference between antisense and sense groups in the time spent exploring and this was not such as to explain an impairment (see Table 2 and legend). Thus, there was no evidence that the memory impairments were due to problems with general exploration. In addition, the mean DR values for the rats infused with sense Fos ODN were similar to those of rats treated with saline in other experiments using the similar procedures (Barker et al 2006b; Warburton et al 2003). Accordingly, there was no evidence to suggest that the sense ODN infusions affected exploration or recognition memory.
Table 2. Exploration time (mean ± SEM; in s) in acquisition and test phases (behavioural tasks).
| Group of animals 1 (n = 15) |
||||||
| acquisition 20min |
acquisition 3h |
acquisition 24h |
||||
| sense |
antisense |
sense |
antisense |
sense |
antisense |
|
| acquisition test | 29.3 ± 1.9 | 27.1 ± 2.0 | 29.1 ± 2.0 | 27.6 ± 2.4 | 30.6 ± 2.2 | 29.5 ± 2.1 |
| 23.3 ± 2.6 | 19.7 ± 2.8 | 18.3 ± 1.7 | 27.2 ± 3.0 | 20.3 ± 2.0 | 22.5 ± 1.7 | |
| Group of animals 2 (n = 9) |
||||||
| retrieval 24h |
consolidation 24h |
object-in-place 3h |
||||
| sense |
antisense |
sense |
antisense |
sense |
antisense |
|
| acquisition test | 34.0 ± 2.7 | 30.5 ± 3.0 | 32.7 ± 2.5 | 27.5 ± 2.3 | 44.2 ± 5.4 | 44.4 ± 6.5 |
| 28.9 ± 4.5 | 31.2 ± 2.2 | 26.6 ± 2.3 | 26.9 ± 3.6 | 25.9 ± 3.5 | 25.0 ± 2.4 | |
Discussion
The results establish that Fos production is essential for perirhinal-based long-term recognition memory. Antisense, but not sense, Fos ODN will bind to and hence prevent translation of c-fos mRNA, so preventing Fos protein production. Familiarity discrimination both for objects and for object-place associations was impaired when antisense Fos ODN was locally infused into PRH. In the object recognition memory task, impairments were found when infusions of antisense Fos ODN were either before or immediately after acquisition and memory measured after a 3 h or 24 h delay. Infusions of sense Fos ODN were without effect. There was no effect of sense or antisense Fos ODN infusion on memory after a 20 min delay, nor on retrieval, nor any effect on general behaviour as measured by total exploration times. The known actions of Fos lead to the expectation that its effects will be upon consolidation mechanisms (Yoneda et al 2001; Guzowski et al 2001; Kubik et al 2007). Thus the impairments seen when infusion of antisense Fos ODN was made before acquisition are most readily explicable as being due to the prevention of Fos production during consolidation – although infused earlier, the antisense ODN would still be active during early consolidation (effects maximal at ~ 1 h after infusion but not detectable after several hours: Hebb et al 1997; Sommer et al 2000). Indeed, rats infused with antisense Fos ODN after acquisition were similarly amnesic at a memory delay of 24 h, even though the antisense ODN may not have had time to be maximally efficacious. Thus the results are consistent with the initial hypothesis that the action of antisense Fos ODN is on consolidation. This action on consolidation is necessary by for memory tested 3 h after acquisition; however, it is not necessary for memory tested 20 min after acquisition. Such a conclusion is in accord with impairment of hippocampally dependent memory by antisense Fos ODN (Countryman et al 2005; Fleischmann et al 2003; Grimm et al 1997; Guzowski 2002; He et al 2002; Yasoshima et al 2006).
The memory impairment was in parallel to the disruption by antisense but not sense Fos ODN of the normally greater Fos expression produced by novel than familiar stimuli in PRH, this normal difference having been reported in several previous studies (Albasser et al 2010; Wan et al 1999; Wan et al 2004; Warburton et al 2005; Warburton et al 2003; Zhu et al 1995b; Zhu et al 1996). This disruption of the normal pattern of evoked Fos expression is consistent with the behavioural findings of impaired recognition memory by the antisense ODN (the effective memory delay was ≥3 h). Notably, the necessity of Fos for recognition memory processes provides a basis for the reliability of perirhinal Fos expression as a marker of activity related to recognition memory. Furthermore, this necessity supplies explanation for the disruption of the normal perirhinal pattern of Fos expression produced by manipulations that impair recognition memory, as reported in the above cited studies.
It is plausible that the lack of effect on recognition memory with a 20 min delay is because the stage of consolidation for which Fos expression is necessary has not yet been reached, with other, shorter-term mechanisms supporting the memory at this time. Other results have established that shorter-term (20 min) and longer-term (≥1 h) perirhinal-dependent recognition memory have independent underlying mechanisms (Barker et al 2006b; Brown & Xiang 1998; Tinsley et al 2011), so it is also possible that any early effects of antisense Fos ODN are masked by a second, Fos-independent system responsible for shorter-term memories. The difference in the effects at delays of 20 min and 24 h is not explained by state-dependency, that is by need for the active agent to be present at both acquisition and retrieval. Although antisense Fos ODN given before acquisition is likely to have been active at both acquisition and retrieval at the 20 min delay and not the 24 h delay, a similar memory impairment was seen with the 24 h delay when infusion was after acquisition so that the ODN would not have been present at either acquisition or retrieval.
Measurement of the spread of the infusion indicated that the tissue involved was very largely confined to PRH as defined in Shi & Cassell (1999), and approximately equivalent to caudal PRH (Burwell et al. 1995). This region receives inputs from sensory association cortices, notably including visual association cortices (Witter et al. 2000; Furtak et al. 2007; Agster & Burwell, 2009). It is the region in which recognition-related Fos changes have been found in previous work (Albasser et al 2010; Wan et al 1999; Wan et al 2004; Warburton et al 2005; Warburton et al 2003; Zhu et al 1995b; Zhu et al 1996). In particular, very little staining was detected in either the hippocampus or area Te2. Results from this and previous studies in Bristol (Seoane et al 2011) indicate that infused biotinylated ODNs are clearly visible in the perirhinal cortex 15 min and 1 h after infusion and that this induces knockdown 2.5 h later. Studies elsewhere indicate that maximal knockdown occurs approximately 1 h after administration (Hebb et al 1997; Sommer et al 2000) and ODNs are largely dispersed by 24 h after local infusion (Lee et al 2004).
Interestingly, Fos produced by viewing novel and familiar images was still differentially expressed in area Te2 following infusion of antisense Fos ODN into neighbouring PRH (where such differential expression was lost). An unsolved issue is the extent to which recognition memory processes in PRH and Te2 are independent of each other or, alternatively, are dependent on feed forward (Te2 to PRH) or feedback (PRH to Te2) signals. Evidence for the feedback of perirhinal information has been found in experiments on paired associate learning in the monkey: there was backward propagation of learned information at retrieval from perirhinal cortex to adjacent visual association cortex (Naya et al 2001). The present findings indicate that differential Fos expression in Te2 is not dependent on the unimpaired operation of Fos-dependent consolidation processes in PRH. The lack of effect of PRH infusions on Fos expression in Te2 is in contrast to the effect of the localised inhibition of either the phosphorylation of calcium-calmodulin dependent protein kinase II (CAMKII) or calcium-calmodulin dependent protein kinase kinase (CAMKK) in PRH: such inhibition in PRH is accompanied by loss of the differential phosphorylation of CAMKII or CAMKK by novel and familiar stimuli in Te2, i.e. for CAMKII and CAMKK the change in Te2 is dependent on PRH activity (Tinsley et al 2009). Further experiments are needed to determine whether activity differences in Te2 are related to a signal that passes from PRH to Te2 after the time that CAMKII has been activated, i.e. >20 min after acquisition (Tinsley et al 2009), but before further processing has been disrupted by preventing Fos expression, i.e. <3 h after acquisition. Nevertheless even given such possible feedback signals to Te2, the effects on behaviour of disruption of intracellular signal processing within perirhinal cortex indicate that any differential activity in Te2 is insufficient to support recognition memory when processing in PRH is compromised.
Several inter- and intra- cellular signalling mechanisms have now been linked to perirhinal recognition memory processes, for example, activation within PRH of different types of glutamate receptors (Barker et al 2006a; Barker et al 2006b; Winters & Bussey 2005), L-type voltage dependent calcium channels (Seoane et al 2009), CAMKII (Tinsley et al 2009) and the transcription factor cAMP responsive element-binding protein (CREB) (Warburton et al 2005). In particular, phosphorylation of CREB within PRH is essential for long-term plasticity and recognition memory mechanisms, and phospho-CREB stained neurons occur in increased numbers in the perirhinal cortex 90min after exposure to novel compared to familiar images (Warburton et al 2005). Moreover, CREB phosphorylation can lead to Fos production (Ahn et al 1998; Silva et al 1998) and Fos expression has been linked to LTD mechanisms (Lindecke et al 2006; Nakazawa et al 1993). Prevention of LTD expression mechanisms in PRH impairs recognition memory (Griffiths et al 2008).
Infusion of antisense Fos ODN also impaired object-in-place memory. This associative memory is impaired by both hippocampal and perirhinal lesions and by disconnection of the perirhinal and hippocampal cortices (Warburton and Brown 2010). Differences in Fos expression are not seen during performance of a radial arm maze task where familiar stimuli are rearranged to form a new arrangement compared to the same familiar stimuli in a familiar arrangement (Jenkins et al. 2004); however, this lack of difference is explicable because all individual stimuli are similarly familiar in the two situations compared. Nevertheless, infusions of the NMDA receptor antagonist AP5 into perirhinal cortex produces object-in-place impairment at delays of ≥ 2h, suggesting that this task may rely on some plastic process in perirhinal cortex. However, such memory is not impaired by viral transduction of perirhinal cortex that prevents activity-dependent removal of AMPA receptors and hence prevents synaptic weakening such as occurs in the expression of processes such as long-term depression (Griffiths et al 2008). Hence Fos may play a role in perirhinal cortex in other plastic processes that are necessary for object-in-place memory but which do not rely on such synaptic weakening; such processes might be related to those of long-term potentiation and possibly of reconsolidation for all the familiar stimuli. If disruption of such a plastic process resulted in the prior occurrence of the stimuli being forgotten, the object-in-place task would be impaired because the previous associations between the stimuli and their locations would be likely to be disrupted.
In summary, infusion of antisense (but not sense) Fos ODN locally into PRH disrupted consolidation of object recognition memory measured after a 3 h or 24 h (but not 20 min) delay. Such infusion also prevented the greater Fos expression produced by viewing novel rather than familiar objects in PRH but not in neighbouring Te2. In addition, antisense Fos ODN infusions into PRH impaired object-in-place recognition memory with a 3 h delay.
Acknowledgements
We are grateful to the Medical Research Council and Wellcome Trust for financial support, and to J. Robbins, K. Narduzzo and K. Sanders for technical assistance.
References
- Aggleton JP, Keen S, Warburton EC, Bussey TJ. Extensive cytotoxic lesions involving both the rhinal cortices and area TE impair recognition but spare spatial alternation in the rat. Brain Res Bull. 1997;43:279–87. doi: 10.1016/s0361-9230(97)00007-5. [DOI] [PubMed] [Google Scholar]
- Agster KL, Burwell RD. Cortical efferents of the perirhinal, postrhinal and entorhinal cortices of the rat. Hippocampus. 2009;19(12):1159–86. doi: 10.1002/hipo.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Olive M, Aggarwal S, Krylov D, Ginty DD, Vinson C. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Molecular and cellular biology. 1998;18:967–77. doi: 10.1128/mcb.18.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser MM, Davies M, Futter JE, Aggleton JP. Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: Effects of varying the lesion extent and the duration of the sample period. Behav Neurosci. 2009;123:115–24. doi: 10.1037/a0013829. [DOI] [PubMed] [Google Scholar]
- Albasser MM, Poirier GL, Aggleton JP. Qualitatively different modes of perirhinal-hippocampal engagement when rats explore novel vs. familiar objects as revealed by c-Fos imaging. Eur J Neurosci. 2010;31:134–47. doi: 10.1111/j.1460-9568.2009.07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Bashir ZI, Brown MW, Warburton EC. A temporally distinct role for group I and group II metabotropic glutamate receptors in object recognition memory. Learn Mem. 2006a;13:178–86. doi: 10.1101/lm.77806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–57. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC, Koder T, Dolman NP, More JC, et al. The different effects on recognition memory of perirhinal kainate and NMDA glutamate receptor antagonism: implications for underlying plasticity mechanisms. J Neurosci. 2006b;26:3561–6. doi: 10.1523/JNEUROSCI.3154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brown MW, Bashir ZI. Evidence concerning how neurons of the perirhinal cortex may effect familiarity discrimination. Philos Trans R Soc Lond B Biol Sci. 2002;357:1083–95. doi: 10.1098/rstb.2002.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Warburton EC, Aggleton JP. Recognition memory: material, processes and substrates. Hippocampus. 2010 doi: 10.1002/hipo.20858. [DOI] [PubMed] [Google Scholar]
- Brown MW, Wilson FA, Riches IP. Neuronal evidence that inferomedial temporal cortex is more important than hippocampus in certain processes underlying recognition memory. Brain Res. 1987;409:158–62. doi: 10.1016/0006-8993(87)90753-0. [DOI] [PubMed] [Google Scholar]
- Brown MW, Xiang JZ. Recognition memory: neuronal substrates of the judgement of prior occurrence. Prog Neurobiol. 1998;55:149–89. doi: 10.1016/s0301-0082(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Witter MP, Amaral DG. Perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus. 1995;5(5):390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Dias R, Amin E, Muir JL, Aggleton JP. Perirhinal cortex and place-object conditional learning in the rat. Behav Neurosci. 2001;115:776–85. doi: 10.1037//0735-7044.115.4.776. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Duck J, Muir JL, Aggleton JP. Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behav Brain Res. 2000;111:187–202. doi: 10.1016/s0166-4328(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Aggleton JP. Functionally dissociating aspects of event memory: the effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J Neurosci. 1999;19:495–502. doi: 10.1523/JNEUROSCI.19-01-00495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson BJ, Hooper ML, Murphy PR, Robertson HA. Antisense oligonucleotide eliminates in vivo expression of c-fos in mammalian brain. European journal of pharmacology. 1992;227:451–3. doi: 10.1016/0922-4106(92)90167-t. [DOI] [PubMed] [Google Scholar]
- Countryman RA, Kaban NL, Colombo PJ. Hippocampal c-fos is necessary for long-term memory of a socially transmitted food preference. Neurobiol Learn Mem. 2005;84:175–83. doi: 10.1016/j.nlm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav Brain Res. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Hvalby O, Jensen V, Strekalova T, Zacher C, et al. Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J Neurosci. 2003;23:9116–22. doi: 10.1523/JNEUROSCI.23-27-09116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus. 2007;17(9):697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus. 2005;15:347–55. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- Griffiths S, Scott H, Glover C, Bienemann A, Ghorbel MT, et al. Expression of long-term depression underlies visual recognition memory. Neuron. 2008;58:186–94. doi: 10.1016/j.neuron.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Grimm R, Schicknick H, Riede I, Gundelfinger ED, Herdegen T, et al. Suppression of c-fos induction in rat brain impairs retention of a brightness discrimination reaction. Learn Mem. 1997;3:402–13. doi: 10.1101/lm.3.5.402. [DOI] [PubMed] [Google Scholar]
- Guzowski JF. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 2002;12:86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- He J, Yamada K, Nabeshima T. A role of Fos expression in the CA3 region of the hippocampus in spatial memory formation in rats. Neuropsychopharmacology. 2002;26:259–68. doi: 10.1016/S0893-133X(01)00332-3. [DOI] [PubMed] [Google Scholar]
- Hebb MO, Robertson HA. Coordinate suppression of striatal ngfi-a and c-fos produces locomotor asymmetry and up-regulation of IEGs in the globus pallidus. Brain Res Mol Brain Res. 1997;48(1):97–106. doi: 10.1016/s0169-328x(97)00086-7. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain research. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Dudai Y. Transient expression of c-Fos in rat amygdala during training is required for encoding conditioned taste eversion memory. Learn Mem. 1996;3:31–41. doi: 10.1101/lm.3.1.31. [DOI] [PubMed] [Google Scholar]
- Lindecke A, Korte M, Zagrebelsky M, Horejschi V, Elvers M, et al. Long-term depression activates transcription of immediate early transcription factor genes: involvement of serum response factor/Elk-1. Eur J Neurosci. 2006;24:555–63. doi: 10.1111/j.1460-9568.2006.04909.x. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Inglis FM, Roth RH. An antisense oligonucleotide reverses the footshock-induced expression of fos in the rat medial prefrontal cortex and the subsequent expression of conditioned fear-induced immobility. J Neurosci. 1999;19:5666–73. doi: 10.1523/JNEUROSCI.19-13-05666.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Pinel JP. Rhinal cortex lesions and object recognition in rats. Behav Neurosci. 1994;108:11–8. doi: 10.1037//0735-7044.108.1.11. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Piterkin P, Lecluse V, Lehmann H. Perirhinal cortex damage and anterograde object-recognition in rats after long retention intervals. Behav Brain Res. 2007;185:82–7. doi: 10.1016/j.bbr.2007.07.026. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Karachot L, Nakabeppu Y, Yamamori T. The conjunctive stimuli that cause long-term desensitization also predominantly induce c-Fos and Jun-B in cerebellar Purkinje cells. Neuroreport. 1993;4:1275–8. doi: 10.1097/00001756-199309000-00017. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Seoane A, Massey PV, Keen H, Bashir ZI, Brown MW. L-type voltage-dependent calcium channel antagonists impair perirhinal long-term recognition memory and plasticity processes. J Neurosci. 2009;29:9534–44. doi: 10.1523/JNEUROSCI.5199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane A, Tinsley CJ, Brown MW. Interfering with perirhinal brain-derived neurotrophic factor expression impairs recognition memory in rats. Hippocampus. 2011;21:121–126. doi: 10.1002/hipo.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Perirhinal cortex projections to the amygdaloid complex and hippocampal formation in the rat. J Comp Neurol. 1999;406:299–328. doi: 10.1002/(sici)1096-9861(19990412)406:3<299::aid-cne2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annual review of neuroscience. 1998;21:127–48. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Sommer W, Hebb MO, Heilig M. Pharmacokinetic properties of oligonucleotides in brain. Methods Enzymol. 2000;314:261–75. doi: 10.1016/s0076-6879(99)14109-0. [DOI] [PubMed] [Google Scholar]
- Tinsley CJ, Narduzzo KE, Ho JW, Barker GR, Brown MW, Warburton EC. A role for calcium-calmodulin-dependent protein kinase II in the consolidation of visual object recognition memory. Eur J Neurosci. 2009;30:1128–39. doi: 10.1111/j.1460-9568.2009.06917.x. [DOI] [PubMed] [Google Scholar]
- Tinsley CJ, Fontaine-Palmer NS, Vincent M, Endean EPE, Aggleton JP, Brown MW, Warburton EC. Differing time dependencies of object recognition memory impairments produced by nicotinic and muscarinic antagonism in perirhinal cortex. Learn Mem. 2011;18:484–492. doi: 10.1101/lm.2274911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischmeyer W, Grimm R. Activation of immediate early genes and memory formation. Cell Mol Life Sci. 1999;55:564–74. doi: 10.1007/s000180050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Aggleton JP, Brown MW. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J Neurosci. 1999;19:1142–8. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Warburton EC, Zhu XO, Koder TJ, Park Y, et al. Benzodiazepine impairment of perirhinal cortical plasticity and recognition memory. Eur J Neurosci. 2004;20:2214–24. doi: 10.1111/j.1460-9568.2004.03688.x. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Glover CP, Massey PV, Wan H, Johnson B, et al. cAMP responsive element-binding protein phosphorylation is necessary for perirhinal long-term potentiation and recognition memory. J Neurosci. 2005;25:6296–303. doi: 10.1523/JNEUROSCI.0506-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, et al. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38:987–96. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Glutamate receptors in perirhinal cortex mediate encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 2005;25:4243–51. doi: 10.1523/JNEUROSCI.0480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–8. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10(4):398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Yasoshima Y, Sako N, Senba E, Yamamoto T. Acute suppression, but not chronic genetic deficiency, of c-fos gene expression impairs long-term memory in aversive taste learning. Proc Natl Acad Sci U S A. 2006;103:7106–11. doi: 10.1073/pnas.0600869103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XO, Brown MW, Aggleton JP. Neuronal signalling of information important to visual recognition memory in rat rhinal and neighbouring cortices. Eur J Neurosci. 1995a;7:753–65. doi: 10.1111/j.1460-9568.1995.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Zhu XO, Brown MW, McCabe BJ, Aggleton JP. Effects of the novelty or familiarity of visual stimuli on the expression of the immediate early gene c-fos in rat brain. Neuroscience. 1995b;69:821–9. doi: 10.1016/0306-4522(95)00320-i. [DOI] [PubMed] [Google Scholar]
- Zhu XO, McCabe BJ, Aggleton JP, Brown MW. Mapping visual recognition memory through expression of the immediate early gene c-fos. Neuroreport. 1996;7:1871–5. doi: 10.1097/00001756-199607290-00037. [DOI] [PubMed] [Google Scholar]