Abstract
Background:
Iron supplementation can decrease the absorption of zinc and influence other antioxidants levels such as vitamin C. This study aimed to investigate the effect of iron supplements alone and in combination with vitamin C on zinc and vitamin C status in iron deficient female students.
Methods:
In a double-blind randomized clinical trail, 60 iron deficient students were selected from 289 volunteers residing in dormitory. After matching, subjects were randomly assigned into two groups: Group I (50 mg elemental iron supplements) and Group II (50 mg elemental iron + 500 mg ascorbic acid). Serum ferritin, iron, serum zinc, and plasma vitamin C concentrations were measured by using enzyme-linked immunosorbent assay, spectrophotometer, atomic absorption spectrometer, and colorimeter, respectively after 6 and 12 weeks supplementation. Student's t-test and repeated measures analysis of variance were applied to analyze the data using SPSS software.
Results:
Serum zinc levels had no significant differences between 2 groups at the baseline; however, its concentration decreased from 80.9 ± 4.2-68.9 ± 2.7 μg/dl to 81.2 ± 4.5-66.1 ± 2.9 μg/dl (P < 0.001) in Groups I and II, respectively after 6 weeks of supplementation. Continuous supplementation increased serum zinc concentration to baseline levels (79.0 ± 2.9 μg/dl; P < 0.01) in Group I and 70.5 ± 3.1 μg/dl in Group II following 12 weeks of supplementation. Plasma vitamin C increased from 3 ± 0/1-3.3 ± 0.2 mg/dl to 2.7 ± 0. 1-4.2 ± 0.2 mg/dl (P < 0.01) in Groups I and II, respectively. At the end of study, plasma vitamin C significantly increased from 3.3 ± 0.3-4.7 ± 0.3 (P < 0.01) to 4.2 ± 0.2-7.1 ± 0.2 (P < 0.001) in Groups I and II, respectively.
Conclusions:
Iron supplementation with and without vitamin C led to reduction in serum Zn in iron-deficient female students after 6 weeks. However, the decreasing trend stops after repletion of iron stores and Zn levels returned to the approximately baseline values after 12 weeks.
Keywords: Female, iron deficiency, iron supplementation, serum zinc, vitamin C
INTRODUCTION
Iron deficiency anemia (IDA) and zinc deficiency are the widespread nutritional problems with high prevalence in women of reproductive age living in developing countries such as Iran.[1,2,3] The prevalence of IDA was found to be 16.6% in Iranian women of childbearing age and was not significantly different between rural and urban settings.[4,5] In humans, iron is an essential component of proteins involved in oxygen transport. It is also essential for the regulation of cell growth and differentiation.[6,7] Zinc presents in the zinc fingers of DNA, RNA, and enzymes structures and is required for the essential body's biochemical reactions.[8,9,10,11] Iron supplementation is suggested by health organizations as an effective strategy to prevent iron deficiency and anemia specially in developing world, whereas there is no regulated policy to prevent other elements’ deficiency such as zinc.[12,13] On the other hand, interventions to combat mild Fe deficiency in women of childbearing age may affect Zn nutriture.[14] Interaction between iron and zinc has been shown during intestinal absorption in animal models.[15,16,17] Therefore, excess iron supplementation interfere intestinal absorption or plasma diffusion of bivalent elements presenting in foods (such as zinc) and results in deficit of other trace elements. It could cause impaired zinc absorption, too.[18,19,20,21]
Currently, it has been suggested that in addition to possible interaction between iron and other elements like zinc, accumulation of iron in tissues could increase oxidative stress. Moreover, higher level of iron has been known as a major source of free radicals production[22,23] and the measurement of plasma antioxidants like vitamin C determines the effects of iron on free radicals production.[24,25] Until now, some studies have been shown that ascorbic acid acts as an antioxidant in the presence of high level of iron and traps free radicals, and prevents the diffusion of these components to membrane of red blood cells and low density lipoprotein particles.[26,27]
Regarding the importance of zinc and its interaction with iron, the effect of excess iron on elevation of oxidative stress, and production of free radicals, we aimed to evaluate the effect of iron supplementation alone and iron with vitamin C on zinc and vitamin C status in iron deficient female students.
METHODS
This study was a double-blinded clinical trial. The required sample size was determined using serum levels of ferritin as a key dependent variable.[10] The study power was considered 80%. Because of dropping samples during the study, 30 subjects were considered for each group at the start of the study. Two hundreds eighty-nine students enrolled from the Al-Zahra Dormitory Complex affiliated to Shahid Beheshti University of Medical Sciences. All participants agreed to attend in the screening phase of the study in written consent form. Detailed information about age, height, weight, body mass index (BMI), history of disease, menstrual status, medication, and supplement use were collected by completing questionnaire. Venous blood in fasting state (5 ml) was drown and divided into parts: 1 ml transferred into tubes (containing 0.2 cc ethylenediaminetetraacetic acid 5%) and 4 ml was collected in hemolysis tubes and was transferred to the Nutritional Research Lab of the National Nutrition and Food Technology Research Institute. Hemoglobin, hematocrit, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and ferritin were measured in this phase. Sixty nonanemic iron deficient volunteers with hemoglobin levels higher than 12.5 mg/dl and serum ferritin levels < 23 ng/ml[28] were selected in our study. Subjects were attended in information session and signed written consent form. Participants with history of thalassemia, diabetes, gastrointestinal, liver, kidney, inflammatory, and infectious diseases were excluded from the study. Furthermore, participants who were not interested to continue consuming less 75 out of 90 capsules during the study period, were withdrawn from the study. After matching, 60 subjects based on their age, BMI, serum ferritin were allocated in 2 groups: Receiving iron (50 mg/d elemental iron; Group I) and iron + vitamin C (50 mg/d elemental iron + 500 mg/d vitamin C; Group II) for 12 weeks. Supplements were ferrous fumarate with or without vitamin C made in Iran Daru Company and similar in shape, color, and taste.
To determine serum levels of iron, zinc and plasma levels of vitamin C, 10 ml venous blood samples were drown at the beginning, week 6 and 12 of intervention. Hemoglobin, ferritin, iron, vitamin C, and zinc were quantified using cell counter, enzyme-linked immunosorbent assay (commercial kits, RADIM, Italy), spectrophotometry (commercial kit, Zeist Chemistry), colorimeter (using DNPH 4, 2),[29] and atomic absorption spectrometer, respectively.
Energy, iron, zinc, vitamin C, and other macro and micronutrients intake were estimated using three 24-h dietary recalls (at baseline, week 6 and 12) throughout the study. Dietary data was analyzed using a widely used nutritional software package (Food Processor II Windows v. 7.6; ESHA Research, Salem, OR). Physical activity of participants was assessed using validated questionnaire about time spent on physical activities and leisure time activities at baseline and at the end of the study.[30]
Statistical analysis
Statistical analysis was performed using SPSS for Windows (version 17; SPSS Inc., Chicago, IL, USA). Kolmogorov-Smirnov test was applied to ensure normal distribution of variables. Quantitative variables (type of supplements and period of the study) were compared using two-way repeated measures analysis of variance. In addition, quantitative variables of each group were compared using paired t-test at the beginning, week 6 and 12 of the study. To manage any interfering variables, BMI, physical activity, iron, and vitamin C intake were considered as a covariance in the model. P < 0.05 was considered as significant.
RESULTS
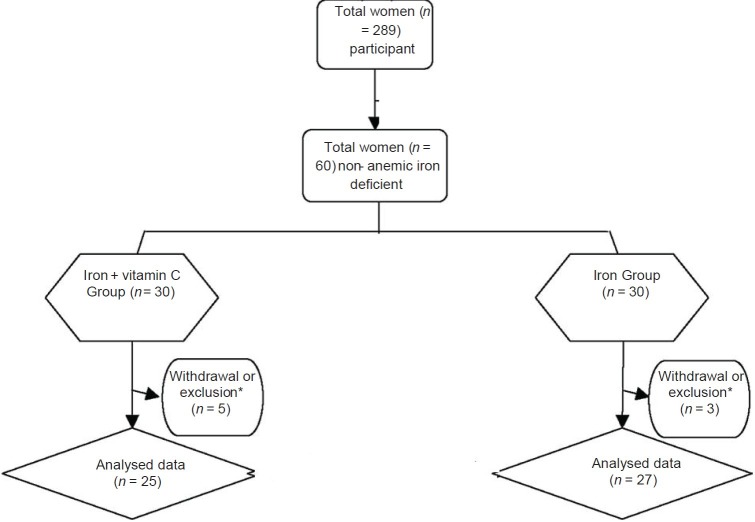
From 30 subjects allocated to each group, 3 subjects from Group I (iron) and 5 subjects from Group II (iron + vitamin C) were excluded due to the irregular use of supplements or health complications. Therefore, final analyses were done on 27 and 25 subjects in intervention groups, respectively [Figure 1].
Figure 1.
Recruitment, withdrawals, exclusions, and intervention group numbers
Mean of age, height, weight, BMI, and selected nutrient intakes of the subjects at the baseline are presented in Table 1. There were no significant differences for these variables between Groups I and II.
Table 1.
Baseline characteristics and selected nutrient intakes of participants in each intervention group
Mean of physical activity were not different between Groups I and II at the beginning of the study, too (10.16 ± 1.9 and 14.6 ± 2.8 h/week, respectively). Baseline relative frequencies of subjects with serum zinc levels lower than 70 μg/dl are shown in Figure 2. Serum levels of zinc lower than normal status were found in 14.3% and 16.7% of subjects, respectively.
Figure 2.
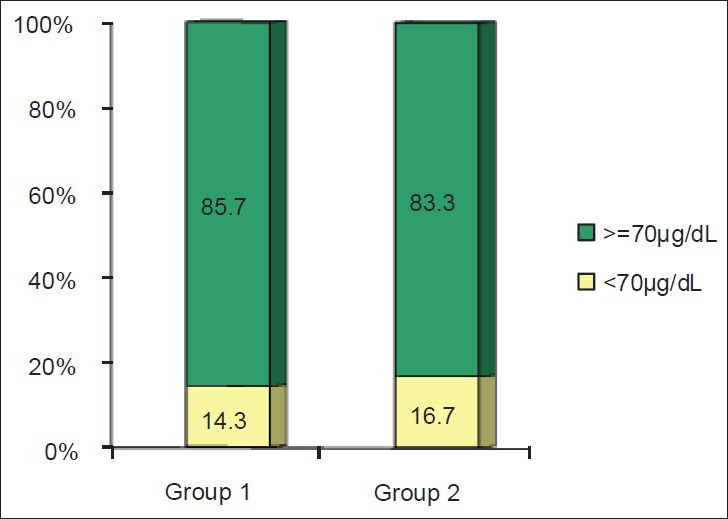
Relative frequency of subjects with serum zinc levels lower than normal at the beginning of the study
The mean and standard error of mean for hemoglobin, serum iron, ferritin, zinc and vitamin C before and after intervention were shown in Table 2. Mean levels of hemoglobin in week 6 significantly increased compared with the beginning of the study in both groups; however, its level decreased at the end study compared with mid-term of the study. Hemoglobin level at the end of the study was significantly higher than baseline levels (P < 0.001) and was not significantly different between two groups.
Table 2.
Mean and standard error of hemoglobin, serum iron, ferritin, zinc and vitamin C of subjects during different periods between groups
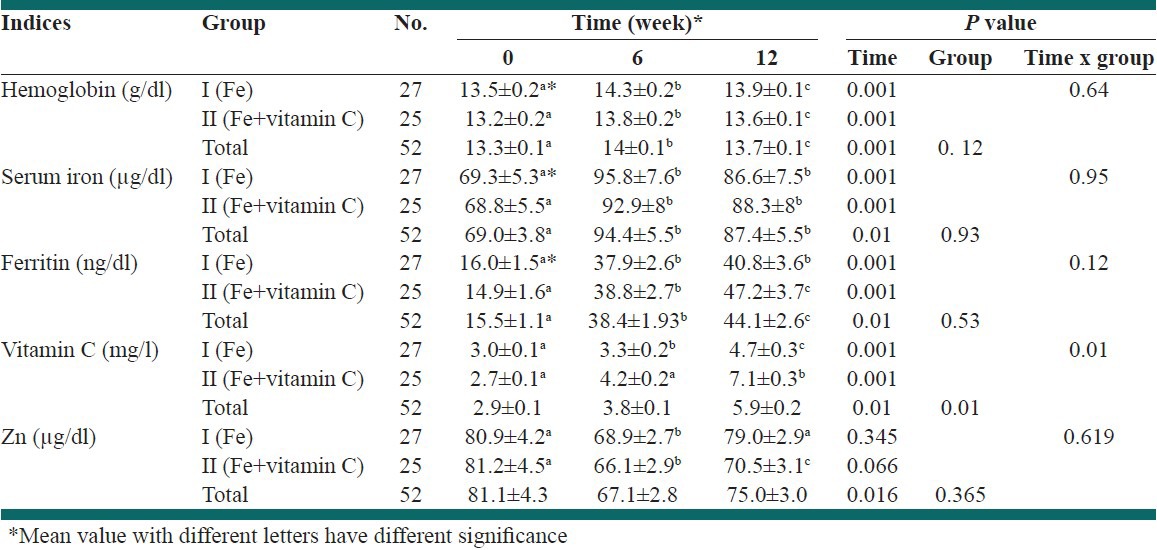
Serum levels of iron after 6 weeks supplementation was significantly higher than baseline levels in both groups. Serum levels of ferritin significantly increased after 6 weeks supplementation compared to baseline (P < 0.001). Continuous supplementation for 12 weeks significantly increased serum ferritin level of Group II, but not in Group I.
Mean plasma vitamin C values increased significantly in Group I after 6 weeks supplementation (P < 0.01), but this increase was not significant in Group II. At the end of the study, plasma vitamin C was significantly increased compared to week 6 in both groups (P < 0.001). There was no significant difference at the beginning of study in terms of vitamin C.
Serum zinc levels had no significant differences at baseline in both groups. Its level decreased significantly after 6 weeks supplementation (P < 0.001), however, after 12 weeks supplementation significantly increased to baseline levels in Group I, but not in Group II [Table 2].
DISCUSSION
Baseline anthropometric profile and age of subjects had no significant differences in both groups. In addition, mean of energy, protein, iron, and vitamin C intake were not different between groups. Therefore, we were able to compare interested variables in our study.
We found a significant reduction of serum zinc levels after 6 weeks of supplementation. Haidar et al. showed that serum zinc of pregnant women significantly decreased when they received iron supplements for 3 months.[31] In agreement with our findings Seyyed Shariat- Doust et al. have reported that iron supplementation of healthy women (hemoglobin 13.2 g/dl) caused significant decrease of serum zinc levels.[32] In addition, in a study by Ziaei et al., iron supplementation in pregnant women with hemoglobin > 13.2 g/dl reduces serum levels of copper and zinc.[33] O’Brien et al. observed similar findings, too.[18] In contrast, Harvey and his colleagues’ findings showed no reduction in serum zinc of pregnant women after iron supplementation.[34] In addition, Falahi et al. have reported iron supplementation for 4 months increased serum levels of zinc.[35] However, in most of the studies, iron supplementation did not affect the biochemical status of zinc, but the data are not clear regarding morbidity outcomes.[36,37,38] On the other hand, the addition of zinc to iron folic acid supplements did not modify efficacy on iron status or improve zinc status.[39]
It has been suggested that decreased absorption of zinc after iron supplementation is the outcome of higher affinity of bivalent ions transporters (divalent metal ion transporter) to iron ion. Furthermore, it has been shown that iron could prevent diffusion and uptake of zinc from intestinal cells. Studies using animal models have shown that the higher ratio of iron to zinc has negative impact on absorbance of zinc from jejunum cells of intestine. Furthermore, there is no adverse interaction between iron and zinc within normal range of them. But at higher ratios, such as the present study, it could cause inhibitory effect on the serum zinc levels.[18]
In our study, zinc absorption improved in the presence of relatively higher levels of iron after 6 weeks of supplementation. The level of iron in Group I recovered to the baseline and in Group II increased compared to the beginning of the study. This elevation was higher in Group II rather than Group I. It has been suggested that iron uptake increases across intestinal cells at the lower iron levels and its absorption decreases when its level is normal. Consequently, less binding of iron to its receptor on intestinal cell membranes and less competition between zinc and iron in binding to their shared receptors results in higher zinc uptake.
Other studies have reported that iron supplementation to iron deficient individuals caused lower uptake of zinc.[40] Iron deficiency increased the inhibitory effect of iron on zinc absorption from 26 to 39% to 59-82%. It occurs after adaptation of intestinal cells in response to lower body stores of iron. Obviously, the effect of iron on uptake of zinc mostly has been reported in pregnant, breast feeding women, and athletes. As expected when iron deficiency status improves, this inhibitory effect is reduced and the serum level of zinc yields to its baseline level.[41] In addition, it has been suggested that significant increase of serum vitamin C levels after 6 weeks of iron + vitamin C supplementation could improve iron uptake and yield to lower uptake of zinc. In addition, lack of significant increase in zinc level could be the attributed to direct interaction of vitamin C and zinc. In this regard, Oladip et al. showed that supplementation of 200 mg vitamin C caused significant reduction of zinc uptake.[42]
The significant changes in both hemoglobin and serum iron concentrations in both groups are within normal limits, but do not seem clinically meaningful. These changes might be due to homeostatic mechanisms of iron absorption, including dietary regulator, which limits iron absorption and stores excess iron as ferritin.[43,44] Iron supplementation with iron alone did not increase ferritin levels after 6 weeks, which can be attributed to decrease in iron absorbtion with duration of supplementation; However, it seems that co supplementation with iron and vitamin C had further effects on repletion of iron stores between 6 and 12 weeks supplementation.
The results of this study revealed that plasma vitamin C levels of both groups (Group I after 12 weeks and Group II after 6 and 12 weeks supplementation) increased. To the best of our knowledge, oxidative stress is associated with both iron deficiency and accumulation of iron.[22,23] Then, we quantified vitamin C level to consider the possibility of peroxidase activity of iron and accordingly reduction of vitamin C as an antioxidant. First, our findings indicated that iron supplementation improves iron deficiency and secondary did not cause excess iron accumulation. As a result, antioxidant activity of vitamin C was increased.[45] Therefore, elevation of vitamin C concentration of Group II has no conflict with our findings and it seems higher level of vitamin C of Group I relates to seasonal eating habits. First sampling was done on March, the period that vitamin C intake from foods are in its lowest level, while second sampling was done on August that consumption of vegetables and fruit (as main sources of vitamin C) is high. However, as a limitation of this study, our 24-h dietary recalls did not confirm it.
CONCLUSIONS
Overall finding of this study confirmed that iron supplementation alone and combined with vitamin C causes reduction of serum zinc levels after 6 weeks; however, by repairing the iron stores, its absorption improves. In addition, iron supplementation for 12 weeks has no adverse effect on plasma vitamin C.
ACKNOWLEDGMENT
This study was supported by a funding from the National Nutrition and Food Technology Research Institute. The authors appreciate the valuable assistance of all participants. We also would like to thank the authorities of Shaheed Beheshti University of Medical Sciences, Al-Zahra dormitory complex for their cooperation. The contributions of Mr. Kalayi, Hossieni, and Mrs. Shariyatzadeh in data collection are greatly appreciated.
Footnotes
Source of Support: This study was supported by a funding from the National Nutrition and Food Technology Research Institute
Conflict of Interest: None declared.
REFERENCES
- 1.World Health Organization. Geneva, Switzerland: World Health Organization; 2001. Iron Deficiency: Assessment, Prevention and Control. [Google Scholar]
- 2.Badii A, Nekouei N, Fazilati M, Shahedi M, Badiei S. Effect of consuming zinc-fortified bread on serum zinc and iron status of zinc-deficient women: A double blind, randomized clinical trial. Int J Prev Med. 2012;(3 Suppl 1):S124–30. [PMC free article] [PubMed] [Google Scholar]
- 3.Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–6. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 4.Sheikholeslam R, Abdollahi Z, Jamshidbeygi E, Salehian P, Malekafzali H. Prevalence of iron deficiency, anemia and iron deficiency anemia among Iranian women of child bearing age (15-49) in urban and rural areas. Teb va Tazkiyeh. 2003;11:37. [Google Scholar]
- 5.Safavi M, Sheikholeslam R, Abdollahi Z, Naghavi M, Sadeghian-Sharif S, Sadeghzadeh E, et al. Prevalence of iron deficiency anemia among Iranian pregnant women, Spring 2001. Iran J Epidemiol. 2006;2:1–10. [Google Scholar]
- 6.West CE. Iron deficiency: The problem and approaches to its solution. Food Nutr Bull. 1996;17:37–41. [Google Scholar]
- 7.Tontisirin K, Nantel G, Bhattacharjee L. Food-based strategies to meet the challenges of micronutrient malnutrition in the developing world. Proc Nutr Soc. 2002;61:243–50. doi: 10.1079/PNS2002155. [DOI] [PubMed] [Google Scholar]
- 8.Mahmoodi MR, Kimiagar M. Epidemiological assessment of zinc deficiencies in youth: Correlation among zinc status indices. Biol Trace Elem Res. 2001;10:71–9. [Google Scholar]
- 9.Navai L, Kimiagar M, Abolhasanzadeh A, Lashgari M. Sci Res J Army Univ Med Sci I.R. Vol. 8. Iran: 2010. Survey of serum iron, zinc, and copper deficiency prevalence in urban and rural areas in Tehran district; pp. 20–7. [Google Scholar]
- 10.Nishiyama S, Kiwaki K, Miyazaki Y, Hasuda T. Zinc and IGF-I concentrations in pregnant women with anemia before and after supplementation with iron and/or zinc. J Am Coll Nutr. 1999;18:261–7. doi: 10.1080/07315724.1999.10718861. [DOI] [PubMed] [Google Scholar]
- 11.Salgueiro MJ, Zubillaga MB, Lysionek AE, Caro RA, Weill R, Boccio JR. The role of zinc in the growth and development of children. Nutrition. 2002;18:510–9. doi: 10.1016/s0899-9007(01)00812-7. [DOI] [PubMed] [Google Scholar]
- 12.Mora JO, Mora OL. Washington, DC: USAID/WHO; 1998. Micronutrient Deficiencies in Latin America and the Caribbean: Iodine, Calcium and Zinc. [Google Scholar]
- 13.Viteri FE. Iron supplementation for the control of iron deficiency in populations at risk. Nutr Rev. 1997;55:195–209. doi: 10.1111/j.1753-4887.1997.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 14.Prosser NR, Heath AL, Williams SM, Gibson RS. Influence of an iron intervention on the zinc status of young adult New Zealand women with mild iron deficiency. Br J Nutr. 2010;104:742–50. doi: 10.1017/S0007114510001091. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Ayala AE, Campos MS, López-Aliaga I, Pallarés I, Hartiti S, Barrionuevo M, et al. Effect of source of iron on duodenal absorption of iron, calcium, phosphorous, magnesium, copper and zinc in rats with ferropoenic anaemia. Int J Vitam Nutr Res. 1997;67:106–14. [PubMed] [Google Scholar]
- 16.Isfaoun A, Bureau F, Mouly-Boudey M, Drosdowsky M, Arhan P, Bouglé D. Relationships between iron and zinc metabolism: Predictive value of digestive absorption on tissue storage. J Trace Elem Med Biol. 1997;11:23–7. doi: 10.1016/s0946-672x(97)80005-3. [DOI] [PubMed] [Google Scholar]
- 17.King JC. Determinants of maternal zinc status during pregnancy. Am J Clin Nutr. 2000;71(5 Suppl):1334S–43. doi: 10.1093/ajcn/71.5.1334s. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien KO, Zavaleta N, Caulfield LE, Wen J, Abrams SA. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. J Nutr. 2000;130:2251–5. doi: 10.1093/jn/130.9.2251. [DOI] [PubMed] [Google Scholar]
- 19.Stein ML, Gunston KD, May RM. Iron dextran in the treatment of iron-deficiency anaemia of pregnancy. Haematological response and incidence of side-effects. S Afr Med J. 1991;79:195–6. [PubMed] [Google Scholar]
- 20.Mills CF. Dietary interactions involving the trace elements. Annu Rev Nutr. 1985;5:173–93. doi: 10.1146/annurev.nu.05.070185.001133. [DOI] [PubMed] [Google Scholar]
- 21.Bloxam DL, Williams NR, Waskett RJ, Pattinson-Green PM, Morarji Y, Stewart SG. Maternal zinc during oral iron supplementation in pregnancy: A preliminary study. Clin Sci (Lond) 1989;76:59–65. doi: 10.1042/cs0760059. [DOI] [PubMed] [Google Scholar]
- 22.Götz ME, Künig G, Riederer P, Youdim MB. Oxidative stress: Free radical production in neural degeneration. Pharmacol Ther. 1994;63:37–122. doi: 10.1016/0163-7258(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 23.Chen OS, Schalinske KL, Eisenstein RS. Dietary iron intake modulates the activity of iron regulatory proteins and the abundance of ferritin and mitochondrial aconitase in rat liver. J Nutr. 1997;127:238–48. doi: 10.1093/jn/127.2.238. [DOI] [PubMed] [Google Scholar]
- 24.Niki E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am J Clin Nutr. 1991;54:1119S–24. doi: 10.1093/ajcn/54.6.1119s. [DOI] [PubMed] [Google Scholar]
- 25.Berger TM, Polidori MC, Dabbagh A, Evans PJ, Halliwell B, Morrow JD, et al. Antioxidant activity of vitamin C in iron-overloaded human plasma. J Biol Chem. 1997;272:15656–60. doi: 10.1074/jbc.272.25.15656. [DOI] [PubMed] [Google Scholar]
- 26.Fuller CJ, Jialal I. Effects of antioxidants and fatty acids on low-density-lipoprotein oxidation. Am J Clin Nutr. 1994;60:1010S–3S. doi: 10.1093/ajcn/60.6.1010S. [DOI] [PubMed] [Google Scholar]
- 27.Lehr HA, Frei B, Olofsson AM, Carew TE, Arfors KE. Protection from oxidized LDL-induced leukocyte adhesion to microvascular and macrovascular endothelium in vivo by vitamin C but not by vitamin E. Circulation. 1995;91:1525–32. doi: 10.1161/01.cir.91.5.1525. [DOI] [PubMed] [Google Scholar]
- 28.Alonso Cotoner C, Casellas Jordá F, Chicharro Serrano ML, de Torres Ramírez I, Malagelada Benaprés JR. Iron deficiency: Not always blood losses. An Med Interna. 2003;20:227–31. [PubMed] [Google Scholar]
- 29.Neyestani TR, Fereydouni Z, Hejazi S, Salehi-Nasab F, Nateghifard F, Maddah M, et al. Vitamin C status in Iranian children with acute lymphoblastic leukemia: Evidence for increased utilization. J Pediatr Gastroenterol Nutr. 2007;45:141–4. doi: 10.1097/MPG.0b013e31804c5047. [DOI] [PubMed] [Google Scholar]
- 30.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 31.Haidar J, Umeta M, Kogi-Makau W. Effect of iron supplementation on serum zinc status of lactating women in Addis Ababa, Ethiopia. East Afr Med J. 2005;82:349–52. [PubMed] [Google Scholar]
- 32.Seyyed Shariat-Doust S, Zeyaei S, Faghehzadeh S, Kashanizadeh N, Shamasnorani A. The effect of iron supplementation on serum zinc element levels of pregnant women with hemoglobin of >13.2 g/dL. J Kosar. 2004;10:285–91. [Google Scholar]
- 33.Ziaei S, Janghorban R, Shariatdoust S, Faghihzadeh S. The effects of iron supplementation on serum copper and zinc levels in pregnant women with high-normal hemoglobin. Int J Gynaecol Obstet. 2008;100:133–5. doi: 10.1016/j.ijgo.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 34.Harvey LJ, Dainty JR, Hollands WJ, Bull VJ, Hoogewerff JA, Foxall RJ, et al. Effect of high-dose iron supplements on fractional zinc absorption and status in pregnant women. Am J Clin Nutr. 2007;85:131–6. doi: 10.1093/ajcn/85.1.131. [DOI] [PubMed] [Google Scholar]
- 35.Falahi E, Seifi M, Hasanvand MA. The effect of iron and zinc supplementation alone and combined on iron and zinc status in elementary level students. J ShahreKord Univ Med Sci. 2005;8:1–10. [Google Scholar]
- 36.Shid Far F, Ameri A, Keshavarz A, Jalai M. The effect of iron supplementation on serum zinc status in pregnant women in Islam Shahar City. Iran J Endocrinol Metab. 2001;4:249–54. [Google Scholar]
- 37.Fischer Walker C, Kordas K, Stoltzfus RJ, Black RE. Interactive effects of iron and zinc on biochemical and functional outcomes in supplementation trials. Am J Clin Nutr. 2005;82:5–12. doi: 10.1093/ajcn.82.1.5. [DOI] [PubMed] [Google Scholar]
- 38.Mello-Neto J, Rondó PH, Oshiiwa M, Morgano MA, Zacari CZ, dos Santos ML. Iron supplementation in pregnancy and breastfeeding and iron, copper and zinc status of lactating women from a human milk bank. J Trop Pediatr. 2013;59:140–4. doi: 10.1093/tropej/fms055. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen P, Grajeda R, Melgar P, Marcinkevage J, Flores R, Ramakrishnan U, et al. Effect of zinc on efficacy of iron supplementation in improving iron and zinc status in women. J Nutr Metab 2012. 2012:216179. doi: 10.1155/2012/216179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wien EM, Glahn RP, Van Campen DR. Ferrous iron uptake by rat duodenal brush border membrane vesicles: Effects of dietary iron level and competing mineral (Zn + 2, Mn + 2 and Ca + 2) J Nutr Biochem. 1994;5:571–7. [Google Scholar]
- 41.Pérès JM, Bureau F, Neuville D, Arhan P, Bouglé D. Inhibition of zinc absorption by iron depends on their ratio. J Trace Elem Med Biol. 2001;15:237–41. doi: 10.1016/S0946-672X(01)80039-0. [DOI] [PubMed] [Google Scholar]
- 42.Oladipo A, Falade MS, Otemuyiwa IO, Adewusi SR. Ascorbic acid and mineral availability in two Nigerian plant foods. Afr J Med Med Sci. 2004;33:171–5. [PubMed] [Google Scholar]
- 43.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–95. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 44.Muñoz M, García-Erce JA, Remacha AF. Disorders of iron metabolism. Part 1: Molecular basis of iron homoeostasis. J Clin Pathol. 2011;64:281–6. doi: 10.1136/jcp.2010.079046. [DOI] [PubMed] [Google Scholar]
- 45.Khoshfetrat MR, Mohammadi F, Mortazavi S, Rashidi A, Neyestani T, Kalantari N, et al. The effect of iron-vitamin C co-supplementation on biomarkers of oxidative stress in iron-deficient female youth. Biol Trace Elem Res. 2013;153:171–7. doi: 10.1007/s12011-013-9695-7. [DOI] [PubMed] [Google Scholar]