Abstract
Background
While inflow occlusion techniques have given surgeons the ability to carry out increasingly complex liver resections, ischemia-reperfusion (IR) injury continues to be a source of morbidity. Efforts to ameliorate IR injury have been hindered in absence of adequate pre-clinical models. The goal of the present study was to develop a simple, efficient, and cost-effective means of studying hepatic IR injury.
Methods
Liver cubes were procured from normal (C57BL/6) mice. Following hepatectomy, 4 mm punch biopsies were taken for individual placement in culture wells containing hepatocyte media. Experimental cubes underwent hypoxia for 60 minutes, while controls remained normoxic. Supernatants were collected from individual wells following 0, 6 and 12 hours of rediffusion for transaminase and cytokine measurement. Histologic examination was performed on individual cubes.
Results
Extensive histologic injury was seen in the experimental cubes compared to controls with greater staining for activated caspase-3 and TUNEL at 6 and 24 hours, respectively. Changes consistent with ischemic injury occurred more centrally in liver cubes whereas markers for rediffusion injury were appreciated along the periphery. Transaminases were significantly higher at 6 hours following rediffusion in experimental cubes compared to controls, p = 0.02. TNF-α and IL-1β were significantly higher in the media of experimental cubes compared to controls at 12 hours rediffusion, p = 0.05 and 0.03 respectively.
Conclusions
In vitro IR of cubes produces a significant injury whose pattern is reflective of hepatic lobular architecture. This novel technique may open new avenues for uncoupling the mechanisms of IR while facilitating rapid screening of potential therapies.
INTRODUCTION
Accentuated damage to a hypoxic organ upon its reperfusion is a ubiquitous phenomenon encountered in a variety of clinical settings 1. While IR injury is unavoidable in liver transplantation, vascular inflow occlusion and total vascular occlusion techniques are increasingly important for liver resection surgery. IR injury plays a key role in morbidity following these procedures 2. In addition to local hepatocellular effects of IR, clinical studies have found hepatic IR also influences remote organ dysfunction, including Adult Respiratory Distress Syndrome (ARDS) 3-4. Efforts to ameliorate the clinical effects of hepatic IR have been hindered by the absence of comparable pre-clinical models to efficiently develop and test potential therapies.
Most evidence for the underlying molecular mechanisms of hepatic IR has been obtained through whole animal, in vivo experimentation. However, these methods often utilize only partial or segmental hepatic ischemia due to the poor tolerance of animals to total hepatic ischemia 5-6. Models utilizing partial ischemia are limited in scope as they do not accurately reflect the clinical scenario of total vascular occlusion. In addition, these techniques are limited by animal usage, cost, and the experimental conditions under which in vivo studies may be carried out.
While hepatocyte isolation has yielded successful in vitro models for studying hepatic IR, they fail to account for the role of non-parenchymal cells in hepatic IR. Mounting evidence suggests Kupffer cells contribute to IR by releasing proinflammatory cytokines that cause delayed hepatocellular injury following reperfusion 7. Both TNF-α and IL-1β are important early mediators of IR generated by Kupffer cell activation. In addition, meaningful outcome measures to assess for IR in isolated hepatocytes are lacking. Traditional measures, such as transaminase release and histopathology, can not be assessed reliably with the hepatocyte model. An in vitro alternative is needed to study the effects of total hepatic ischemia at the organ level.
In 1968 Wicks and colleges published a sentinel paper describing hepatic organ culture 8. Livers from rat fetuses were procured, cut into cubes, and placed in culture media in order to study the induction of liver enzymes by glucocorticoids. Histological and biochemical analysis confirmed the liver tissue remained healthy over a 3 day period. This method was later utilized for toxicology purposes where it remains an invaluable in vitro technique for studying the dose-effect of many pharmaceutical agents 9.
The goal of the present study was to adapt the technique of hepatic organ culture in order to develop an in vitro model for studying hepatic IR. We procured viable liver tissue from mice in the form of cubes in order to stage a totally in vitro IR event.
MATERIALS AND METHODS
The study was conducted with approval from the Animal Studies Committee (ASC) at Washington University in Saint Louis. Eight adult, male C57BL/6 mice (28.3 ± 1.00 g) were obtained from Jackson Laboratories and maintained on standard chow ad lib in our institution’s animal facility.
Surgery
At 11-14 weeks of age, each animal underwent hepatectomy for liver cube procurement. Following induction with 3% isoflurane, the mice were shaved and placed on the operating table. Their abdomens were prepped with betadine while maintenance isoflurane was administered at 1.5% via nose cone. A midline laparotomy was then performed for liver exposure. Following dissection of the ligamentous attachments to the liver, the animals underwent euthanasia via cardiac puncture. The hepatectomy was then completed for liver cube procurement. The time between cardiac puncture and culture did not exceed 20 minutes.
Liver Cube Procurement
Livers were splayed onto a sterile metal plate in their anatomic configuration. Then, using a standard 4 mm punch biopsy tool, liver cubes were procured. This method of procurement reliably produced liver cubes of similar size and weight (mean ± SD = 0.022 ± 0.002 g) (Figure 1). Approximately six cubes could be procured from each liver.
Figure 1. Liver Cube Procurement and Culture.
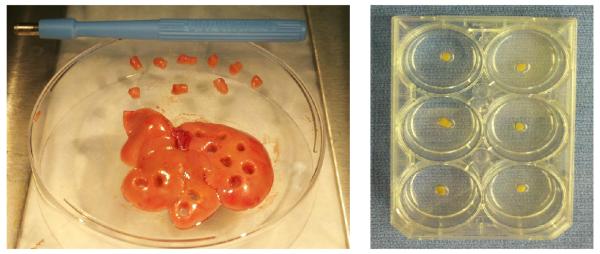
Left- A 4mm standard punch biopsy tool was utilized to procure liver cubes following hepatectomy. Right- Liver cubes were individually cultured in wells containing 1 ml of hepatocyte basal medium.
After procurement, cubes were transferred into a metal strainer where they were washed with PBS containing a pen-strep antibiotic admixture. Six-well tissue culture plates containing 1mL of hepatocyte basal medium (Clonetics) per well were then utilized to individually culture each cube.
Incubation
All tissue culture plates containing liver cubes were initially placed in a CO2 water-jacketed incubator (NuAire™) set at 5% CO2 and 37° C. Arterial blood gases drawn from the media confirmed that liver cubes were maintained under normoxic conditions (dissolved p O2 132.2 mmHg). In anticipation of a transaminase leak from the procurement procedure, liver cubes were transferred into new, individual culture wells containing 1mL of fresh hepatocyte basal media per well after 24 hours of incubation. Experiments were then carried out.
In Vitro Model of Total Hepatic IR
Experimental plates of liver cubes were placed into a hypoxic chamber containing 85% N2, 10% H2, and 5% CO2 while controls remained normoxic in the incubator. Dissolved oxygen content from media inside the hypoxic chamber was 33.7 mmHg. After 60 minutes of hypoxia, experimental plates were removed from the chamber and placed back in the incubator (dissolved p O2 157.1 mmHg). Assessments were made at specific time intervals from “rediffusion,” which was marked by the removal of the experimental plates from the hypoxic chamber.
Assessment
At 0, 6, 12, and 24 hours following rediffusion, assessments were made in order to characterize the nature and severity of hepatic injury as a result of in vitro hypoxia and rediffusion. Towards this, we collected media supernatants at 0, 6, and 12 hours following rediffusion from individual wells of both experimental and control plates of liver cubes. Aliquots from these samples were examined for hepatic transaminase content (Vitros DT chemistry analyzer, Kodak) as well as inflammatory cytokines (ELISA, Invitrogen). In addition, H&E, activated caspase-3, and TUNEL staining were performed on the cubes to characterize the nature of injury following in vitro hypoxia and rediffusion.
Statistical analyses were conducted using GraphPad software, San Diego CA. Differences between groups were compared using a paired Student’s t test. All data is expressed as mean ± SEM. P value ≤ 0.05 was considered significant.
RESULTS
Histologic Findings
Pattern of Injury
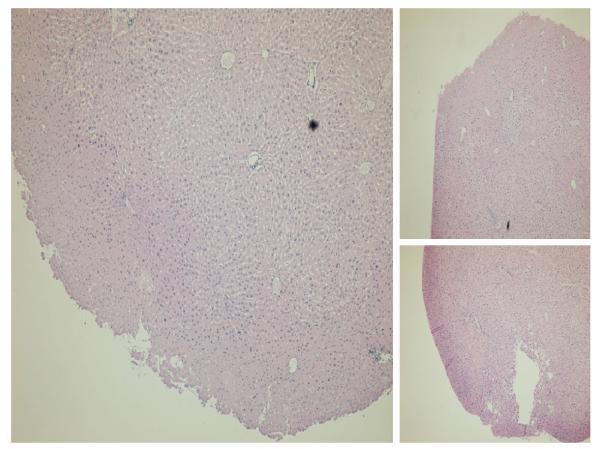
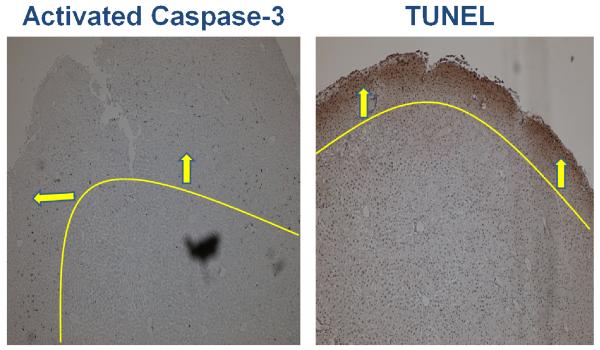
Relative to the periphery, greater histologic injury was seen toward the center of liver cubes subjected to in vitro ischemia-alone. Increased vacuolization with loss of cell-cell junctions were appreciated on H&E staining toward the center of liver cubes whereas the cytoarchitecture of the periphery remained relatively intact (Figure 2). Interestingly, this pattern of distribution was reversed when assessing for apoptotic markers 6 hours following rediffusion. Both Activated Caspase-3 and TUNEL stained with greater intensity toward the periphery of liver cubes relative to the center (Figure 3).
Figure 2. Distribution of Injury Following In Vitro ischemia.
Following 60 minutes of ischemia, H&E stain reveals a centralized pattern of injury. There is peripheral preservation of cytoarchitecture relative to the core. Images are at 4x magnification.
Figure 3. Distribution of Injury Following In Vitro Rediffusion.
Following 6 hours of rediffusion, there is a peripheral pattern of apoptosis. Both activated caspase-3 and TUNEL stain with greater intensity toward the periphery of liver cubes subjected to IR. This pattern of rediffusion injury is in contrast to the centralization of ischemic injury. Lines / arrows have been superimposed to demarcate the peripheral pattern of injury. Images are at 4x magnification.
Comparison to controls
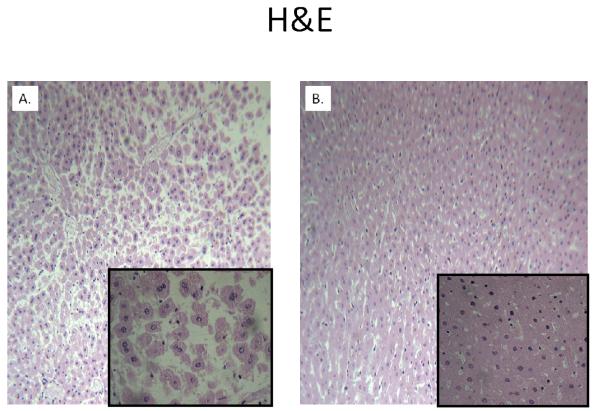
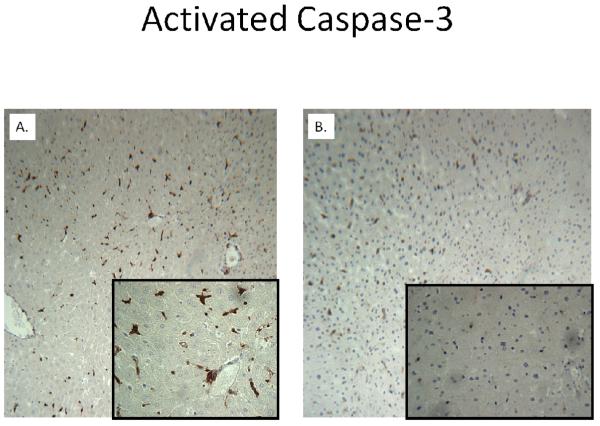
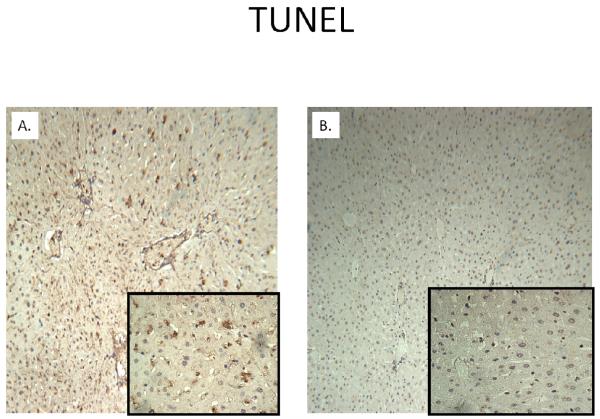
H&E staining of liver cubes at 6 hours following IR injury revealed extensive vacuolar degeneration in experimental cubes (Figure 4). The cytoarchitecture remained largely intact in control cubes without evidence of significant injury. Activated caspase-3 was considerably greater at 6 hours following rediffusion in experimental cubes compared to controls (Figure 5). Similarly, TUNEL positive cells were more abundant in cubes subjected to IR after 24 hours (Figure 6).
Figure 4. Comparison of H&E Staining at 6 Hours of Rediffusion.
Extensive vacuolar degeneration of an experimental liver cube (A) is seen relative to control (B), whose cytoarchitecture remains intact. Background images are at 10x magnification, whereas the inset is at 40x.
Figure 5. Activated Caspase-3 Staining at 6 hours of Rediffusion.
Activity for caspase-3 is considerably greater in an experimental liver cube (A) compared to control (B). Background images are at 10x magnification, whereas the inset is at 40x.
Figure 6. TUNEL Staining at 24 Hours of Rediffusion.
TUNEL activity is seen with greater intensity in an experimental liver cube (A) compared to control (B). Background images are at 10x magnification, whereas the inset is at 40x.
Media Transaminase Levels
Comparison following ischemia-alone
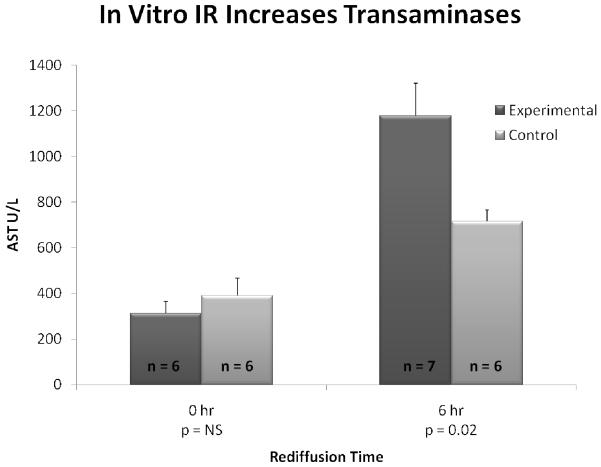
Following 24 hours in culture, experimental liver cubes underwent 60 minutes of hypoxia whereas controls remained normoxic. Media supernatants were collected immediately upon removal from the hypoxic chamber and compared to controls. Media transaminase levels were not statistically different among liver cubes subjected to 60 minutes of ischemia (AST 313.3 ± 53.4 U/L) relative to normoxic controls (AST 390.8 ± 76.4 U/L, p = 0.44) (Figure 7).
Figure 7. Transaminases are Increased Following In Vitro IR of Liver Cubes.
Following 60 minutes of either ischemia (experimental) or normoxia (control), there are no differences in media transaminase levels of liver cubes. At 6 hours of rediffusion, AST levels in the media of experimental liver cubes are significantly higher than in controls, p = 0.02.
Comparison following ischemia-rediffusion
Media transaminase levels were significantly higher at 6 hours following rediffusion in experimental cubes compared to controls (AST 1178.3 ± 165.3 U/L vs. 715.5 ± 49.6 U/L, p = 0.02) (Figure 7). The trend continued at 12 hours of rediffusion; however, the difference was not statistically significant (AST 1469.0 ± 94.6 U/L vs. 1285.5 ± 92.6 U/L, p = 0.30).
Cytokine Profile
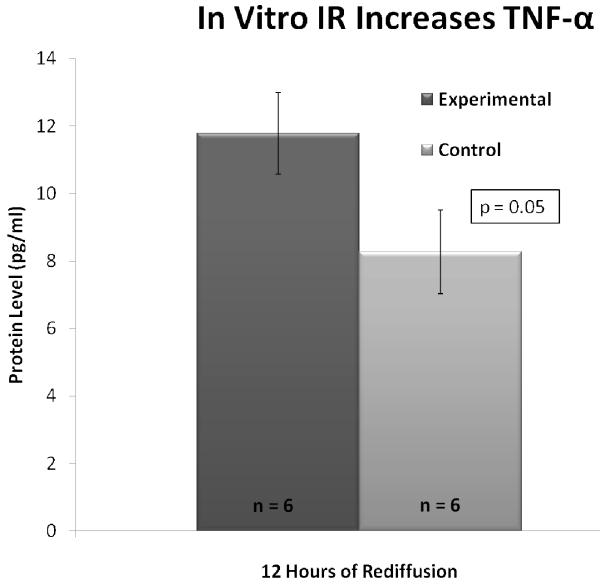
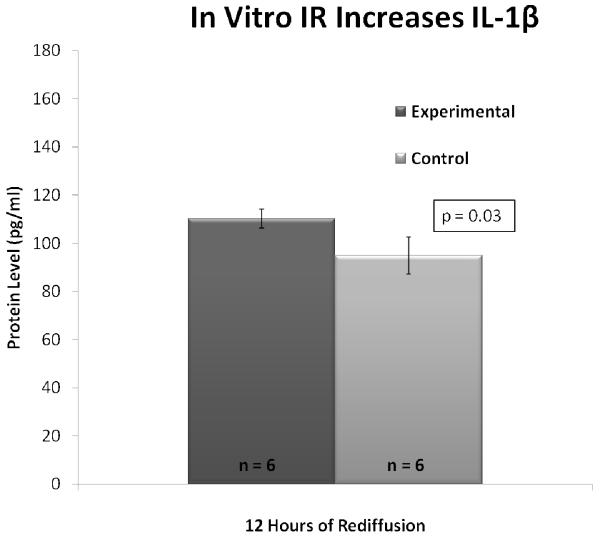
Inflammatory cytokines TNF-α and IL-1β were assessed in the media of experimental and control liver cubes. At 12 hours rediffusion, the level of TNF-α was significantly higher in the media of experimental cubes compared to controls (11.78 ± 1.21 pg/mL vs. 8.27 ± 1.25 pg/mL, p = 0.05) (Figure 8). Likewise, IL-1β was significantly elevated in experimental cubes versus controls at 12 hours following rediffusion (110.25 ± 3.93 pg/mL vs. 94.96 ± 7.69 pg/mL, p = 0.03) (Figure 9).
Figure 8. TNF-α is Increased Following In Vitro IR of Liver Cubes.
At 12 hours of rediffusion, media levels of TNF-α are significantly higher in experimental liver cubes than controls, p = 0.05.
Figure 9. IL-1β is Increased Following In Vitro IR of Liver Cubes.
At 12 hours of rediffusion, media levels of IL-1β are significantly higher in experimental liver cubes than controls, p = 0.03.
DISCUSSION
Progress towards ameliorating the clinical effects of IR depends upon the adequacy of pre-clinical models. An accurate reflection of the clinical scenario is paramount to translating hypotheses from the lab into the clinic. The present study demonstrates a simple and efficient in vitro method of IR using liver cubes that reliably produces a pattern of hepatocellular injury characteristic of IR.
The differential susceptibility among hepatocytes to hypoxia is a reflection of zonal oxygen gradients in a perfused system. Whereas zone 1 (periportal) hepatocytes are relatively tolerant to hypoxia, zone 3 (perivenous) hepatocytes are particularly susceptible to low oxygen tension and suffer worse injury 10. Liver cubes in culture have a diffusion capacity that mimics the in vivo hepatic lobular organization. Zone 1 hepatocytes in vivo have the greatest supply of oxygen and nutrients as a result of perfusion. Similarly, the peripheral zone of liver cubes adjacent to the media has the greatest supply of oxygen and nutrients. Just as the sinusoidal distribution capacity deceases toward zone 3 in vivo, the diffusion capacity decreases toward the center of liver cubes in vitro. Centralization of the hypoxia-related injury in liver cubes reflects in vivo patterns of hepatic lobules.
Apoptosis is recognized as the predominate mode of cell death from reperfusion injury, which is carried out by caspase-dependent mechanisms 11-13. Whereas zone 3 hepatocytes have the greatest susceptibility to hypoxia, zone 1 hepatocytes (periportal) are particularly vulnerable following reperfusion 14-15. Increased delivery of reactive oxygen species and other direct cytotoxins are thought to mediate intralobular heterogeneity. While H&E staining of liver cubes subjected to ischemia-alone revealed a centralized pattern of injury, staining for markers of apoptosis clearly delineate a predilection for the periphery following rediffusion. Seemingly paradoxical, these findings are actually in keeping with patterns seen in vivo. Given the diffusion capacity of liver cubes is greatest toward the periphery adjacent to the media, so must the rediffusion capacity. Peripheral staining for markers of apoptosis reveals a true “rediffusion” phenomenon distinct from the centralized hypoxia-related areas of injury. Furthermore, increased levels of TNF-α and IL-1β following in vitro hypoxia and rediffusion establish a proinflammatory mediation of that injury, which is characteristic of IR 1,3,16.
Diffusion of oxygen and nutrients does not fully support the metabolic activity of liver cubes. It is clear that even normoxic controls suffer from some ongoing hypoxia as evidenced by the time dependent increase in transaminases (Figure 7). Although there is some ongoing injury inherent within the model, its utility in the study of IR injury is evident. Using a small number of animals, many cubes may be utilized and efficiently manipulated under controlled experimental conditions. Liver cubes in culture are a model for the hepatic lobule, which uncouples ischemia from reperfusion through differences in the distribution of injury. This novel technique will assist in understanding the underlying molecular mechanisms that both separate and unite ischemia-reperfusion. Further, this technique can be utilized to rapidly and effectively screen potential interventions for hepatic IR before moving to the in-vivo model. In summary, the present study demonstrates an efficient, simple, cost-effective, and reliable method for studying hepatic IR. Whereas its validity has been demonstrated, future efforts will focus on developing the utility of in vitro IR using liver cubes.
Acknowledgments
Funding Sources: This work was supported in part by the American Society of Transplant Surgeons-Astellas Faculty Development Award (CDA), and National Institute of Health Grants: P30 DK056341 and L30 DK082350 (CDA)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klune JR, Tsung A. Molecular biology of liver ischemia/reperfusion injury: established mechanisms and recent advancements. Surg Clin North Am. 2010;90(4):665–77. doi: 10.1016/j.suc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Brancatisano R, Isla A, Habib N. Is radical hepatic surgery safe? Am J Surg. 1998;175(2):161–3. doi: 10.1016/S0002-9610(97)00265-1. [DOI] [PubMed] [Google Scholar]
- 3.Wanner GA, Ertel W, Muller P, Hofer Y, Leiderer R, Menger MD, et al. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock. 1996;5(1):34–40. doi: 10.1097/00024382-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Matuschak GM, Rinaldo JE. Organ interactions in the adult respiratory distress syndrome during sepsis. Role of the liver in host defense. Chest. 1988;94(2):400–6. doi: 10.1378/chest.94.2.400. [DOI] [PubMed] [Google Scholar]
- 5.Kanoria S, Glantzounis G, Jalan R, Davies NA, Seifalian AM, Williams R, et al. A model to study total hepatic ischemia-reperfusion injury. Transplant Proc. 2004;36(9):2586–9. doi: 10.1016/j.transproceed.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Sankary HN, Yin DP, Chong AS, Ma LL, Blinder L, Shen JK, et al. The portosystemic shunt protects liver against ischemic reperfusion injury. Transplantation. 1999;68(7):958–63. doi: 10.1097/00007890-199910150-00010. [DOI] [PubMed] [Google Scholar]
- 7.Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA., Jr. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85(6):1936–43. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wicks WD. Induction of tyrosine-alpha-ketoglutarate transaminase in fetal rat liver. J Biol Chem. 1968;243(5):900–6. [PubMed] [Google Scholar]
- 9.Zimmerman HJ. Hepatotoxicity : the adverse effects of drugs and other chemicals on the liver. 2nd ed Lippincott Williams & Wilkins; Philadelphia: 1999. [Google Scholar]
- 10.Broughan TA, Naukam R, Tan C, Van De Wiele CJ, Refai H, Teague TK. Effects of hepatic zonal oxygen levels on hepatocyte stress responses. J Surg Res. 2008;145(1):150–60. doi: 10.1016/j.jss.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Kohli V, Selzner M, Madden JF, Bentley RC, Clavien PA. Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver. Transplantation. 1999;67(8):1099–105. doi: 10.1097/00007890-199904270-00003. [DOI] [PubMed] [Google Scholar]
- 12.Natori S, Selzner M, Valentino KL, Fritz LC, Srinivasan A, Clavien PA, et al. Apoptosis of sinusoidal endothelial cells occurs during liver preservation injury by a caspase-dependent mechanism. Transplantation. 1999;68(1):89–96. doi: 10.1097/00007890-199907150-00018. [DOI] [PubMed] [Google Scholar]
- 13.Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32(6):1280–8. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- 14.Taniai H, Hines IN, Bharwani S, Maloney RE, Nimura Y, Gao B, et al. Susceptibility of murine periportal hepatocytes to hypoxia-reoxygenation: role for NO and Kupffer cell-derived oxidants. Hepatology. 2004;39(6):1544–52. doi: 10.1002/hep.20217. [DOI] [PubMed] [Google Scholar]
- 15.Kato Y, Tanaka J, Koyama K. Intralobular heterogeneity of oxidative stress and cell death in ischemia-reperfused rat liver. J Surg Res. 2001;95(2):99–106. doi: 10.1006/jsre.2000.5831. [DOI] [PubMed] [Google Scholar]
- 16.Shirasugi N, Wakabayashi G, Shimazu M, Oshima A, Shito M, Kawachi S, et al. Up-regulation of oxygen-derived free radicals by interleukin-1 in hepatic ischemia/reperfusion injury. Transplantation. 1997;64(10):1398–403. doi: 10.1097/00007890-199711270-00004. [DOI] [PubMed] [Google Scholar]