Abstract
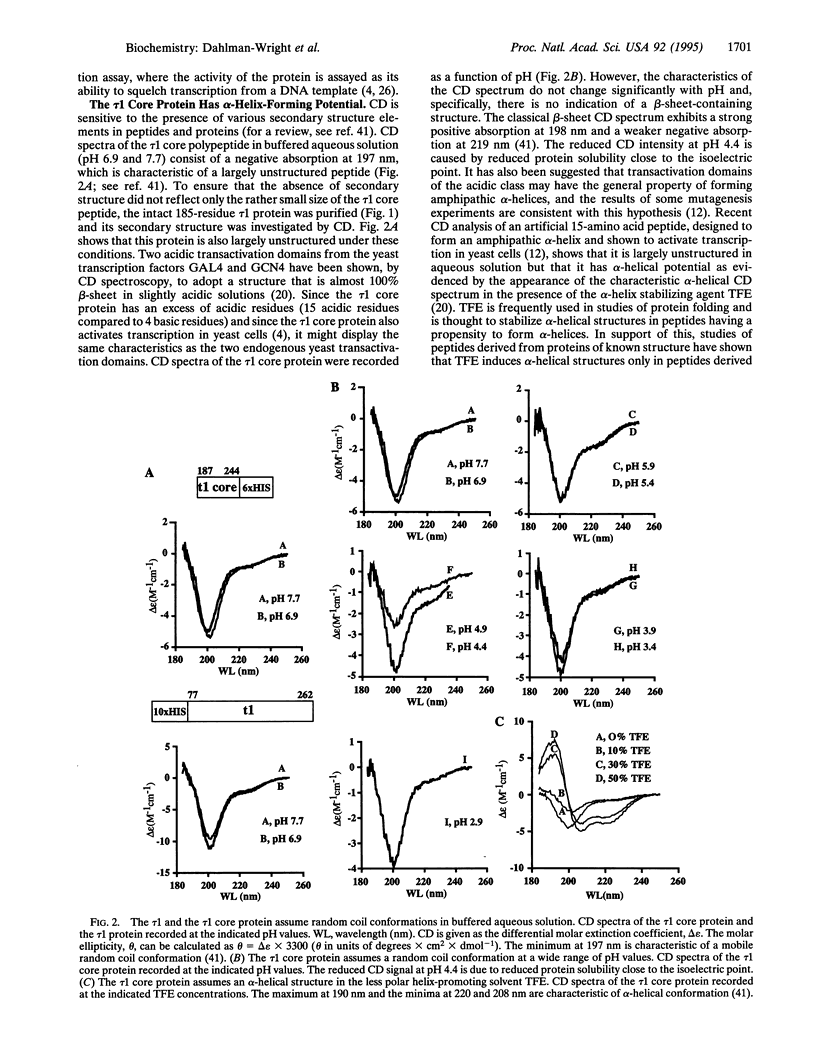
A 58-amino acid polypeptide containing the functional core region, the tau 1 core, of the major transactivation domain of the human glucocorticoid receptor has been expressed in Escherichia coli and purified to homogeneity. The polypeptide retains 60-70% of the activity of the intact domain when assayed in vivo or in vitro. This report describes a structural characterization of the tau 1 core peptide fragment. Circular dichroism spectroscopy shows that the tau 1 core and a larger fragment encompassing the intact tau 1 domain are largely unstructured in water solution under a variety of pH conditions. The tau 1 core, however, acquires a significant alpha-helical structure when analyzed in the presence of trifluoroethanol, an agent that favors secondary structure formation in regions that have propensity for alpha-helical conformation. Two- and three-dimensional NMR spectroscopy of 15N-labeled tau 1 core, in the presence of trifluoroethanol, has allowed sequential assignment of 1H and 15N resonances and identification of three protein segments with alpha-helical character. Potentially helix-breaking proline substitutions, in proposed alpha-helical regions, lead to reduced activity, suggesting that alpha-helices are important for transactivation in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bax A. Two-dimensional NMR and protein structure. Annu Rev Biochem. 1989;58:223–256. doi: 10.1146/annurev.bi.58.070189.001255. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. A bacterial repressor protein or a yeast transcriptional terminator can block upstream activation of a yeast gene. Nature. 1984 Dec 13;312(5995):612–615. doi: 10.1038/312612a0. [DOI] [PubMed] [Google Scholar]
- Cress W. D., Triezenberg S. J. Critical structural elements of the VP16 transcriptional activation domain. Science. 1991 Jan 4;251(4989):87–90. doi: 10.1126/science.1846049. [DOI] [PubMed] [Google Scholar]
- Dahlman-Wright K., Almlöf T., McEwan I. J., Gustafsson J. A., Wright A. P. Delineation of a small region within the major transactivation domain of the human glucocorticoid receptor that mediates transactivation of gene expression. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1619–1623. doi: 10.1073/pnas.91.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson L., Capone J. P. Purification and characterization of the carboxyl-terminal transactivation domain of Vmw65 from herpes simplex virus type 1. J Biol Chem. 1992 Jan 25;267(3):1411–1414. [PubMed] [Google Scholar]
- Dyson H. J., Merutka G., Waltho J. P., Lerner R. A., Wright P. E. Folding of peptide fragments comprising the complete sequence of proteins. Models for initiation of protein folding. I. Myohemerythrin. J Mol Biol. 1992 Aug 5;226(3):795–817. doi: 10.1016/0022-2836(92)90633-u. [DOI] [PubMed] [Google Scholar]
- Dyson H. J., Rance M., Houghten R. A., Wright P. E., Lerner R. A. Folding of immunogenic peptide fragments of proteins in water solution. II. The nascent helix. J Mol Biol. 1988 May 5;201(1):201–217. doi: 10.1016/0022-2836(88)90447-0. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G., Ptashne M. Mutants of GAL4 protein altered in an activation function. Cell. 1987 Oct 9;51(1):121–126. doi: 10.1016/0092-8674(87)90016-x. [DOI] [PubMed] [Google Scholar]
- Giniger E., Ptashne M. Transcription in yeast activated by a putative amphipathic alpha helix linked to a DNA binding unit. Nature. 1987 Dec 17;330(6149):670–672. doi: 10.1038/330670a0. [DOI] [PubMed] [Google Scholar]
- Gronenborn A. M., Bax A., Wingfield P. T., Clore G. M. A powerful method of sequential proton resonance assignment in proteins using relayed 15N-1H multiple quantum coherence spectroscopy. FEBS Lett. 1989 Jan 16;243(1):93–98. doi: 10.1016/0014-5793(89)81224-4. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Evans R. M. Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell. 1988 Dec 2;55(5):899–906. doi: 10.1016/0092-8674(88)90145-6. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope I. A., Mahadevan S., Struhl K. Structural and functional characterization of the short acidic transcriptional activation region of yeast GCN4 protein. Nature. 1988 Jun 16;333(6174):635–640. doi: 10.1038/333635a0. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr Protein secondary structure and circular dichroism: a practical guide. Proteins. 1990;7(3):205–214. doi: 10.1002/prot.340070302. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrman S. R., Tuls J. L., Lund M. Peptide alpha-helicity in aqueous trifluoroethanol: correlations with predicted alpha-helicity and the secondary structure of the corresponding regions of bovine growth hormone. Biochemistry. 1990 Jun 12;29(23):5590–5596. doi: 10.1021/bi00475a025. [DOI] [PubMed] [Google Scholar]
- Ma J., Ptashne M. A new class of yeast transcriptional activators. Cell. 1987 Oct 9;51(1):113–119. doi: 10.1016/0092-8674(87)90015-8. [DOI] [PubMed] [Google Scholar]
- Manavalan P., Johnson W. C., Jr Variable selection method improves the prediction of protein secondary structure from circular dichroism spectra. Anal Biochem. 1987 Nov 15;167(1):76–85. doi: 10.1016/0003-2697(87)90135-7. [DOI] [PubMed] [Google Scholar]
- McClary J. A., Witney F., Geisselsoder J. Efficient site-directed in vitro mutagenesis using phagemid vectors. Biotechniques. 1989 Mar;7(3):282–289. [PubMed] [Google Scholar]
- McEwan I. J., Wright A. P., Dahlman-Wright K., Carlstedt-Duke J., Gustafsson J. A. Direct interaction of the tau 1 transactivation domain of the human glucocorticoid receptor with the basal transcriptional machinery. Mol Cell Biol. 1993 Jan;13(1):399–407. doi: 10.1128/mcb.13.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Williams G. Structural studies of the acidic transactivation domain of the Vmw65 protein of herpes simplex virus using 1H NMR. Biochemistry. 1992 Apr 28;31(16):4150–4156. doi: 10.1021/bi00131a035. [DOI] [PubMed] [Google Scholar]
- Rance M., Sørensen O. W., Bodenhausen G., Wagner G., Ernst R. R., Wüthrich K. Improved spectral resolution in cosy 1H NMR spectra of proteins via double quantum filtering. Biochem Biophys Res Commun. 1983 Dec 16;117(2):479–485. doi: 10.1016/0006-291x(83)91225-1. [DOI] [PubMed] [Google Scholar]
- Regier J. L., Shen F., Triezenberg S. J. Pattern of aromatic and hydrophobic amino acids critical for one of two subdomains of the VP16 transcriptional activator. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):883–887. doi: 10.1073/pnas.90.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruden D. M., Ma J., Li Y., Wood K., Ptashne M. Generating yeast transcriptional activators containing no yeast protein sequences. Nature. 1991 Mar 21;350(6315):250–252. doi: 10.1038/350250a0. [DOI] [PubMed] [Google Scholar]
- Sigler P. B. Transcriptional activation. Acid blobs and negative noodles. Nature. 1988 May 19;333(6170):210–212. doi: 10.1038/333210a0. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoy M., Leuther K. K., Kodadek T., Johnston S. A. The acidic activation domains of the GCN4 and GAL4 proteins are not alpha helical but form beta sheets. Cell. 1993 Feb 26;72(4):587–594. doi: 10.1016/0092-8674(93)90077-4. [DOI] [PubMed] [Google Scholar]
- Van Hoy M., Leuther K. K., Kodadek T., Johnston S. A. The acidic activation domains of the GCN4 and GAL4 proteins are not alpha helical but form beta sheets. Cell. 1993 Feb 26;72(4):587–594. doi: 10.1016/0092-8674(93)90077-4. [DOI] [PubMed] [Google Scholar]
- Wright A. P., Carlstedt-Duke J., Gustafsson J. A. Ligand-specific transactivation of gene expression by a derivative of the human glucocorticoid receptor expressed in yeast. J Biol Chem. 1990 Sep 5;265(25):14763–14769. [PubMed] [Google Scholar]
- Wu C. S., Ikeda K., Yang J. T. Ordered conformation of polypeptides and proteins in acidic dodecyl sulfate solution. Biochemistry. 1981 Feb 3;20(3):566–570. doi: 10.1021/bi00506a019. [DOI] [PubMed] [Google Scholar]
- Zilliacus J., Carlstedt-Duke J., Gustafsson J. A., Wright A. P. Evolution of distinct DNA-binding specificities within the nuclear receptor family of transcription factors. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4175–4179. doi: 10.1073/pnas.91.10.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]




