Abstract
The main purpose of this study was to perform a treatment planning study for lung cancer comparing 2-field (2F) versus 3-field (3F) techniques in uniform scanning proton therapy (USPT). Ten clinically approved lung cancer treatment plans delivered using USPT at our proton center were included in this retrospective study. All 10 lung cases included 4D computed tomography (CT) simulation. The delineation of target volumes was done based on the maximum intensity projection (MIP) images. Both the 3F and 2F treatment plans were generated for the total dose of 74 cobalt-gray-equivalent (CGE) with a daily dose of 2 CGE. 3F plan was generated by adding an extra beam in the 2F plan. Various dosimetric parameters between 2F and 3F plans were evaluated. 3F plans produced better target coverage and conformality as well as lower mean dose to the lung, with absolute difference between 3F and 2F plans within 2%. In contrast, the addition of third beam led to increase of low-dose regions (V20 and V5) in the lung in 3F plans compared to the ones in 2F plans with absolute difference within 2%. Maximum dose to the spinal cord was lower in 2F plans. Mean dose to the heart and esophagus were comparable in both 3F and 2F plans. In conclusion, the 3F technique in USPT produced better target coverage and conformality, but increased the low-dose regions in the lung when compared to 2F technique.
Keywords: Dosimetric, lung cancer, proton therapy, treatment planning
Introduction
The most recent statistics on lung cancer provides an estimation of 224,210 new cases and 159,260 deaths in 2014.[1] Lung cancer continues to be the leading cause of cancer-related deaths in the US.[1] Proton therapy is one of the external beam radiation therapy (EBRT) techniques used for the lung cancer treatment. Since proton beams have finite range with no exit dose, most of the proton dose can be deposited in the tumor volume and spare the critical structures beyond the distal end of spread-out Bragg Peak (SOBP) region.
Clinical results of lung cancer treatment using proton therapy have been reported in the literature.[2,3,4] Nihei et al.,[2] reported 84% survival rate and 80% local control rate at 2 years for stage I non-small-cell lung cancer (NSCLC); whereas Nakayama et al.,[3] reported 97.8% survival rate and 97.0% local control rate at 2 years for stage I NSCLC. Several treatment planning studies[5,6,7,8,9,10,11,12,13] have investigated the use of proton therapy for lung cancer treatment. One of the similarities among previous studies[5,6,7,8,9,10,11,12,13] is the use of passive scattering beam delivery system. However, there is no common consensus on the number of treatment fields used to generate lung treatment plans in proton therapy. For instance, Seco et al.[5] used either 2 fields (2F) or 3 fields (3F), whereas Wang et al.,[6] used 2-4 fields in their studies. Nichols et al.,[7] used no more than 3F, whereas only 3F were used by Hoppe et al.,[8] Chang et al.,[9] and Zhang et al.[10] Dosimetric results in proton therapy may also depend on the beam arrangement and the number of treatment fields used in the treatment planning. Macdonald et al.,[12] used the passive scattering and intensity modulated proton therapy (IMPT) techniques to perform dosimetric analysis of lung cancer planning comparing 1F versus 2F versus 3F. Macdonald et al.,[12] reported better target coverage in the lung plan with the addition of beams. However, an increase in beam number in passive scattering increased the mean dose to the organs at risk (OARs), whereas the addition of beams in IMPT did not produce substantial change in the mean and maximum doses to the OARs.[12]
The majority of the studies mentioned above used either passive scattering only[5,6,7,8,9,10,11] or passive scattering and IMPT.[12] Recently, a bilateral lung cancer case study[14] comparing proton therapy and photon therapy was published. However, the dosimetric impact of number of treatment fields in lung plans generated using uniform scanning proton therapy (USPT), which is different from passive scattering, is yet to be investigated. At our center, we currently use IBA Cyclotron (IBA, Louvain-la-Neuve, Belgium) for the uniform scanning proton beam delivery [Figure 1], and we use USPT to treat all of our cancer patients. In our UPST system, proton beam is scanned laterally in a zigzag pattern by vertical and horizontal scanning magnets, which have different virtual source to isocenter distance (SAD). The beam scanning is done with a constant frequency in order to deliver a uniform proton dose for a near rectangular scanning area.[15,16]
Figure 1.
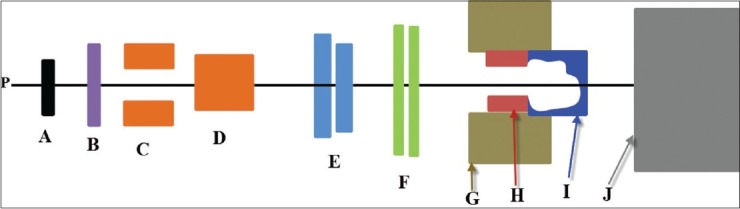
A schematic diagram of the IBA uniform scanning nozzle: Proton beams (p), a first scatterer (a), a range modulator wheel (b), two scanning magnets (c) and (d), variable collimators (e), monitor unit ionization chambers (f), a snout (g), an aperture (h), a range compensator (i), and patient or phantom surface (j). Figure not to scale
Since beam delivery technique of USPT is different from that of passive scattering and IMPT, it is essential to address if the addition of uniform scanning proton beam has significant impact on the dosimetric results in the lung plans. The main purpose of this lung treatment planning study is to compare 2F versus 3F approach in USPT. The comparative analysis was done using various dosimetric parameters.
Materials and Methods
Simulation and contouring
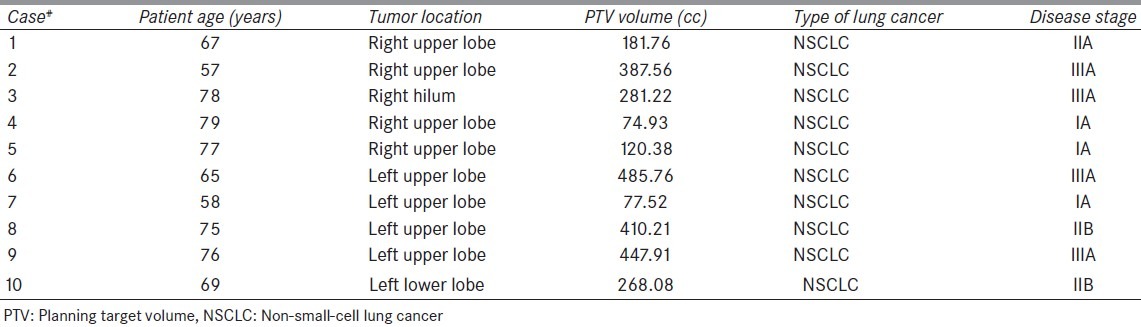
Ten lung cancer patients treated with USPT at our proton center were included in this retrospective study. Among 10 cases, 5 of them had tumor in the right lung and the remaining 5 cases had tumor in the left lung. The age of the patients ranged from 57–79 years. The location of the tumor and clinical stage of the disease for each case is provided in Table 1. All 10 lung cancer patients underwent 4-dimensional computed tomography (4DCT) simulation in a head first supine position on a General Electric CT Scanner. Patients were immobilized using wing board, knee roll, and Vac-lok system (CIVCO Medical Solutions, Kalona, Iowa). The CT images were acquired using a slice thickness of 1.25 mm. The delineation of target volumes was done based on the institutional protocol. Specifically, internal gross tumor volume (IGTV) was contoured on the maximum intensity projection (MIP) images such that IGTV encompasses the GTV on all phases of respiration. From the MIP images, the average 4DCT was constructed for the treatment planning purpose. The clinical target volume (CTV) was generated by an isotropic expansion of 5–8 mm from the IGTV. The planning target volume was generated by expanding 5 mm from the CTV. The normal lung tissue was defined as the total lung volume excluding the CTV. Other OARs such as esophagus, spinal cord, and heart were contoured too.
Table 1.
Patient information for 10 lung cancer cases
Treatment planning
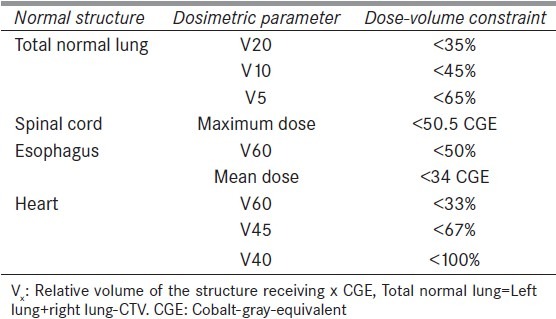
This retrospective study included treatment plans for the total dose of 74 cobalt-gray-equivalent (CGE) with a daily dose of 2 CGE. Furthermore, all ten lung plans were generated in the XiO treatment planning system (CMS Inc., St. Louis, MO). For this study, 2F plans were generated such that both proton fields have clinically feasible gantry and couch angles for the treatment delivery at our proton center. Additionally, maximum effort was made to select the appropriate beams with an objective of maximizing the target coverage while meeting our normal tissue dose constraints provided in Table 2. For a given case, 3F plan were regenerated from the corresponding 2F plan by adding an extra beam to it. Each treatment field was designed with an aperture of 0.8–1.0 cm margin around the PTV and a range compensator of 1.0 cm smearing radius. For the same proton beam in a given case, the apertures and compensators in 2F plan were identical to the ones in 3F plan. Dose calculations were performed using pencil beam algorithm[17] with a grid size of 3 × 3 × 3 mm.
Table 2.
Dose constraints used for the treatment planning. (Total prescribed dose to the planning target volume was 74.0 CGE with a daily dose of 2 CGE per fraction)
Dosimetric analysis
2F and 3F plans were compared using the dosimetric results obtained from dose-volume histogram (DVH). The PTV was compared for coverage (defined in equation 1), ratio of 100% prescription isodose volume to PTV (defined in equation 2), and ratio of 50% prescription isodose volume to PTV (defined in equation 3). Normal lung was evaluated for the mean dose and the relative lung volume receiving dose equal to or greater than 5 and 20 Gy (V5 and V20, respectively). Esophagus and heart were evaluated for the mean dose, whereas the spinal cord was evaluated for the maximum dose.

Statistical analysis
Paired two-sided student's t-test was carried out to observe the statistical differences between 2F and 3F plans. The statistical analysis was done using Microsoft Excel spreadsheet. The difference was considered statistically significant if the P < 0.05.
Results
Table 3 provides the summary of the dosimetric results, and the values presented in Table 3 are averaged over 10 analyzed cases. Figure 2 shows the difference (D) in various dosimetric parameters between 2F and 3F plans in ten cases, and D is calculated as
Table 3.
Dosimetric results of the planning target volume, lung, esophagus, spinal cord, and heart in 3-field and 2-field proton plans. The values are averaged over ten analyzed cases
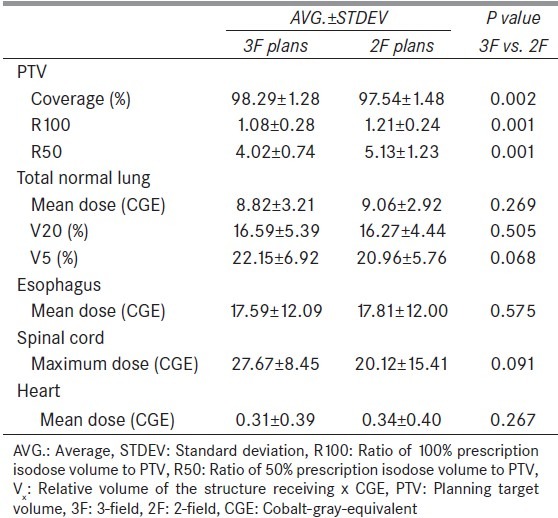
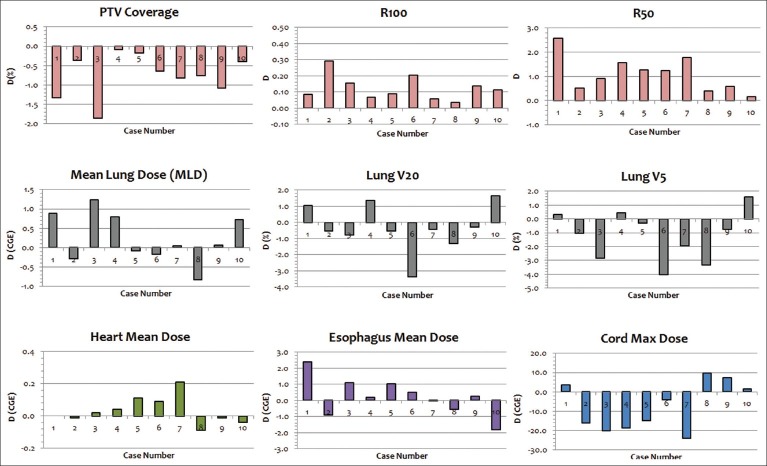
Figure 2.
Difference (D) in dosimetric parameter (e.g., PTV coverage, R100, etc.) between 2-field (2F) and 3-field. D is defined in equation 4
D(x)=F2(x)-F3(x) (4)
where F2 and F3 are the dosimetric results in F2 and F3 plans, respectively, for the dosimetric parameter x (e.g. mean dose, R100, etc.). Figure 3 shows the dose color wash of the 3F and 2F plan for case number 7.
Figure 3.
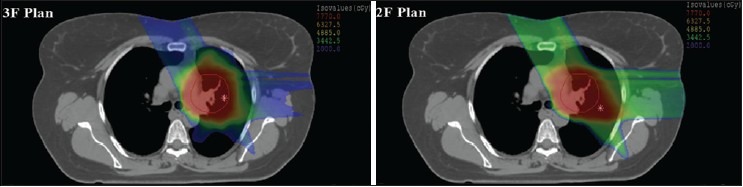
Dose color wash of 3-field (3F) plan and 2-field (2F) plan in XiO treatment planning system
The PTV results showed that the averaged coverage was slightly lower in 2F plans (97.54%) than in 3F plans (98.29%) with statistical difference (P = 0.002). Also, Figure 2 demonstrates that the PTV coverage was lower in 2F plans for all ten cases, with D ranging from -0.08% to -1.34%. Both the R100 and R50 were lower in 3F plans than in 2F plans showing that 3F plans had better plan conformality. Specifically, on average, the R100 was 1.08 in 3F plans and 1.21 in 2F plans, whereas the R50 was 4.02 and 5.13 in 3F and 2F plans, respectively. The D ranged from 0.04-0.29 for R100 and from 0.18-2.59 for R50.
For the lung, the averaged mean dose was slightly lower in 3F plans than in 2F plans (8.82 CGE vs. 9.06 CGE; P = 0.269) with D ranging from -0.83-1.23 CGE. In contrast, the averaged V20 and V5 were slightly higher in 3F plans than in 2F plans (V20: 16.59% vs. 16.27%; P = 0.505 and V5: 22.15% vs. 20.96%; P = 0.068). For both the esophagus and heart, the averaged mean dose was comparable in 3F and 2F plans and no statistical significance was observed (esophagus: 17.59 CGE vs. 17.81 CGE; P = 0.575, and heart: 0.31 CGE vs. 0.34 CGE; P = 0.267). For the spinal cord, the averaged maximum dose was lower in 2F plans (20.12 CGE) than in 3F plans (27.67 CGE), but the difference did not produce statistical significance (P = 0.091).
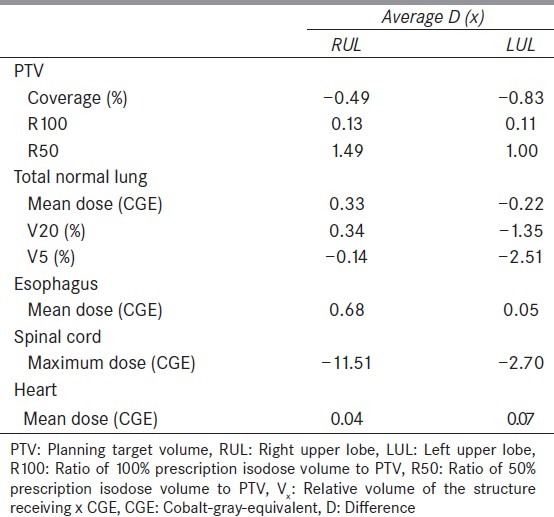
Table 4 provides the summary of the dosimetric results that are analyzed based on the tumor location in the lung. Specifically, the average D (x) is calculated for the right upper lobe (RUL) cases (N = 4) and left upper lobe (LUL) cases (N = 4). For PTV coverage, lung V20, and lung V5, the impact of number of treatment fields was slightly higher (larger absolute value of D in Table 4) in the LUL than in the RUL. For PTV R50, lung mean dose, esophagus mean dose, and cord maximum dose, the impact of number of treatment fields was found to be higher in the RUL than in the LUL. Other parameters (PTV R100 and heart mean dose) had very minimal difference in D (x) between RUL and LUL.
Table 4.
The difference in dosimetric results between 3-field and 2-field plans for right upper lobe and left upper lobe. The values for RUL are averaged for four cases and the values for LUL are also averaged for 4 cases
Discussion
In this study, we have evaluated the dosimetric impact of 3F versus 2F in USPT for lung cancer planning. The difference in the PTV coverage between 3F and 2F plans produced a clear trend, which showed that the addition of an extra beam in 2F plan produced better target coverage. This finding is consistent with previous study[12], which demonstrated better target coverage using greater number of beams. The magnitude of absolute difference in the PTV coverage between 3F and 2F plans in our study, however, remains below 2%. Additionally, the results of both the R100 and R50 also produced a clear pattern showing that 3F technique has resulted slightly better plan conformality.
Although the difference in the PTV values (coverage, R100, and R50) produced clear trends between 3F and 2F plans, no such clear trend was observed for the OARs. [Figure 2: Second and third rows] For instance, 3F technique produced lower mean lung dose for the majority of the cases (N = 6), whereas 2F technique produced higher number of cases with lower V20 (N = 7) and V5 (N = 7). Similar result was observed by Macdonald et al.[12] for both the IMPT and passive scattering, and the authors reported that low-dose regions were incrementally increased or not changed. Furthermore, the addition of beam in IMPT did not have substantial change in the mean dose to the OAR, whereas in passive scattering, the mean dose to the OAR was increased due to the addition of the beam.[12] In our study, the addition of third beam tends to lower the mean lung dose with average difference of 0.24 CGE. In contrast, the addition of third beam led to increase the low-dose regions in the lung with average differences of -0.32% for V20 and -1.19% for V5. Studies have reported increasing risk of lung toxicity with increase in low-dose regions such as V5 and V20 of the lung.[18,19,20,21] Although the absolute differences in lung V5 and V20 between 3F and 2F plans are not very large in this study, the use of 2F technique in USPT could be beneficial for lung cancer patients with pulmonary morbidities such as interstitial pneumonitis. The use of two proton beams for lung cancer planning in USPT could decrease the low-dose regions, thus reducing the probability of radiation-related lung injury. The 2F technique is also likely to reduce the total treatment time when compared to 3F technique. However, the use of less number of proton beams could lead to increase in lung dose due to uncertainties in the increased relative biological effectiveness (RBE) at the distal end of SOBP region.[22] The clinical impact of using 3F versus 2F for lung cancer planning in proton therapy can be the subject of future research.
One of the drawbacks of uniform scanning proton therapy planning is the lack of inverse planning technique, and all the plans in this study were generated using 3D conformal approach without plan optimization. Currently, IMPT has the feature of inverse planning, and by applying dose constraints during the optimization in IMPT, it is possible to obtain better dosimetric results than the ones presented in this study. We have attempted to analyze the results based on tumor location in RUL versus LUL. The tumor location, tumor volume, and patient anatomy typically vary among a group of patients. Hence, the number of beams required to obtain the optimal plan for the treatment of a lesion in the same region (e.g. RUL) may vary from one case to another. Nevertheless, based on the results presented in this study, we did not notice a clear trend showing the addition of third beam will produce either better or worse dosimetric results in the RUL than in the LUL (or vice-versa). Although dosimetric results for lung cancer proton planning may vary depending on the number of beams, treatment planner experience, treatment planning system, and beam delivery techniques, the primary objective of this study was to investigate the dosimetric differences in lung planning due to use of 2 beams versus 3 beams in XiO TPS for uniform scanning proton therapy. In conventional photon therapy, several investigators have reported the dependency of lung planning results on the type of dose calculation algorithm and treatment planning system.[23,24,25,26,27,28] Recently, Zhuang et al.,[29] investigated the volume and location dependence on the differences between the Monte Carlo and pencil beam dose calculations, and reported that the differences were dependent on the PTV volume and location of the tumor. In our study, we did not use multiple dose calculation algorithms for treatment plan calculations, and it would be interesting to investigate if proton dose calculations for lung cancer depend on the proton dose calculation algorithm.
One of the major challenges with proton therapy for lung cancer is tumor motion resulting from patient's breathing.[9,30,31] In the study by Liu et al.,[31] it was reported that about 50% of lung tumors have motion from 0.5 cm to 1 cm and 10% of lung tumors have motion more than 1 cm. The results from Liu et al.,[31] also suggest that tumor motion may depend on the size of the GTV, disease stage, and diaphragm motion. Motion control strategies such as breath holding are typically used for a large tumor motion. Proton therapy planning for the moving tumor and quantification of its movement can be further challenging. Engelsman et al.,[32] compared various treatment planning strategies of proton therapy for lung cancer, and authors showed that 4DCT simulation is necessary in order to evaluate the tumor motion. At our proton center, lung treatment planning protocol requires tumor motion to be less than 1 cm and the magnitude of tumor motion is obtained from the 4DCT scans. For the treatment planning, we generate range compensator based on the IGTV, which is obtained from the 4DCT scans. Since IGTV includes the tumor location in different breathing phases, the whole tumor will get treated regardless of its position during the breathing cycle. However, normal lung tissue will get slightly more dose than expected when tumor is not within the treatment field. Such strategy of proton planning for lung cancer has been previously reported too.[9]
Another challenge in proton therapy is the range uncertainty. At present, proton range uncertainty values used by different proton centers in the US are not the same.[33] The uncertainty in the proton range calculations can be dependent on several factors (e.g. dose calculation, beam delivery, etc.) and the reduction of the range uncertainty can lead to decrease in the treatment volume, thus reducing dose to the normal tissues.[33] For lung treatment planning in proton therapy, the change in internal density along the proton beam path as well as tumor shrinkage [Figure 4] can significantly affect the range, and the change in range during the course of treatment can cause the loss of target coverage and overdose of normal tissues. Additionally, treatment plans with larger aperture margins, which are based on the lateral penumbra, may result increased dose to the OARs. Limitation of treatment planning system in heterogeneous media must be investigated too. Future work involves the range and penumbra calculations in the presence of inhomogeneities and comparisons of calculated results with the measurements or Monte Carlo.
Figure 4.
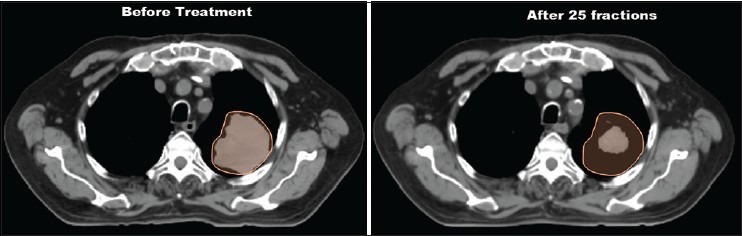
Planning 4DCT Scan before the treatment and QA 4DCT scan after 25 fractions (QA scan performed during the course of treatment). The orange contour in above picture is the IGTV, which was delineated on the MIP images before the treatment. The IGTV was superimposed on the QA scan by registering the QA scan to the planning scan. The QA scan showed significant reduction in the target volume (tumor shrinkage)
Conclusion
3F technique in USPT increased the PTV coverage by average difference of 0.75%, whereas the lung V5 was smaller using 2F technique by average difference of 1.19%. Maximum dose to the spinal cord was lower in 2F plans. Mean dose to the heart and esophagus were comparable in both 3F and 2F plans.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Cancer Facts and Figures 2014. Atlanta: American Cancer Society; 2014. American Cancer Society. [Google Scholar]
- 2.Nihei K, Ogino T, Ishikura S, Nishimura H. High-dose proton beam therapy for Stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:107–11. doi: 10.1016/j.ijrobp.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama H, Sugahara S, Tokita M, Satoh H, Tsuboi K, Ishikawa S, et al. Proton beam therapy for patients with medically inoperable stage I non-small-cell lung cancer at the university of tsukuba. Int J Radiat Oncol Biol Phys. 2010;78:467–71. doi: 10.1016/j.ijrobp.2009.07.1707. [DOI] [PubMed] [Google Scholar]
- 4.Bush DA, Slater JD, Shin BB, Cheek G, Miller DW, Slater JM. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest. 2004;126:1198–203. doi: 10.1378/chest.126.4.1198. [DOI] [PubMed] [Google Scholar]
- 5.Seco J, Panahandeh HR, Westover K, Adams J, Willers H. Treatment of non-small cell lung cancer patients with proton beam-based stereotactic body radiotherapy: Dosimetric comparison with photon plans highlights importance of range uncertainty. Int J Radiat Oncol Biol Phys. 2012;83:354–61. doi: 10.1016/j.ijrobp.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Nakayama H, Sugahara S, Sakae T, Tokuuye K. Comparisons of dose-volume histograms for proton-beam versus 3-D conformal x-ray therapy in patients with stage I non-small cell lung cancer. Strahlenther Onkol. 2009;185:231–4. doi: 10.1007/s00066-009-1923-x. [DOI] [PubMed] [Google Scholar]
- 7.Nichols RC, Huh SN, Henderson RH, Mendenhall NP, Flampouri S, Li Z, et al. Proton radiation therapy offers reduced normal lung and bone marrow exposure for patients receiving dose-escalated radiation therapy for unresectable stage iii non-small-cell lung cancer: A dosimetric study. Clin Lung Cancer. 2011;12:252–7. doi: 10.1016/j.cllc.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Hoppe BS, Huh S, Flampouri S, Nichols RC, Oliver KR, Morris CG, et al. Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: A dosimetric comparison with photon-based stereotactic body radiotherapy. Radiother Oncol. 2010;97:425–30. doi: 10.1016/j.radonc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Chang JY, Zhang X, Wang X, Kang Y, Riley B, Bilton S, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1087–96. doi: 10.1016/j.ijrobp.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Li Y, Pan X, Xiaoqiang L, Mohan R, Komaki R, et al. Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non-small-cell lung cancer: A virtual clinical study. Int J Radiat Oncol Biol Phys. 2010;77:357–66. doi: 10.1016/j.ijrobp.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auberger T, Seydl K, Futschek T, Sztankay A, Sweeney RA, Lukas P. Photons or protons: Precision radiotherapy of lung cancer. Strahlenther Onkol. 2007;183:3–6. doi: 10.1007/s00066-007-2002-9. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald OK, Kruse JJ, Miller JM, Garces YI, Brown PD, Miller RC, et al. Proton beam radiotherapy versus three-dimensional conformal stereotactic body radiotherapy in primary peripheral, early-stage non-small-cell lung carcinoma: A comparative dosimetric analysis. Int J Radiat Oncol Biol Phys. 2009;75:950–8. doi: 10.1016/j.ijrobp.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Register SP, Zhang X, Mohan R, Chang JY. Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;80:1015–22. doi: 10.1016/j.ijrobp.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rana S, Pokharel S, Zheng Y, Zhao L, Risalvato D, Vargas C, et al. Treatment planning study comparing proton therapy, RapidArc and intensity modulated radiation therapy for a synchronous bilateral lung cancer case. Int J Cancer Ther Oncol. 2014;2:020216. [Google Scholar]
- 15.Zheng Y, Ramirez E, Mascia A, Ding X, Okoth B, Zeidan O, et al. Commissioning of output factors for uniform scanning proton beams. Med Phys. 2011;38:2299–306. doi: 10.1118/1.3569581. [DOI] [PubMed] [Google Scholar]
- 16.Rana S, Singh H. Impact of heterogeneities on lateral penumbra in uniform scanning proton therapy. Int J Cancer Ther Oncol. 2013;1:01026. [Google Scholar]
- 17.Hong L, Goitein M, Bucciolini M, Comiskey R, Gottschalk B, Rosenthal S, et al. A pencil beam algorithm for proton dose calculations. Phys Med Biol. 1996;41:1305–30. doi: 10.1088/0031-9155/41/8/005. [DOI] [PubMed] [Google Scholar]
- 18.Allen AM, Czerminska M, Jänne PA, Sugarbaker DJ, Bueno R, Harris JR, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys. 2006;65:640–5. doi: 10.1016/j.ijrobp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Oshiro Y, Sakurai H. The use of proton-beam therapy in the treatment of non-small-cell lung cancer. Expert Rev Med Devices. 2013;10:239–45. doi: 10.1586/erd.12.81. [DOI] [PubMed] [Google Scholar]
- 20.Hernando ML, Marks LB, Bentel GC, Zhou SM, Hollis D, Das SK, et al. Radiation-induced pulmonary toxicity: A dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:650–9. doi: 10.1016/s0360-3016(01)01685-6. [DOI] [PubMed] [Google Scholar]
- 21.Stephans KL, Djemil T, Reddy CA, Gajdos SM, Kolar M, Machuzak M, et al. Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol. 2009;4:838–44. doi: 10.1097/JTO.0b013e3181a99ff6. [DOI] [PubMed] [Google Scholar]
- 22.Grün R, Friedrich T, Krämer M, Zink K, Durante M, Engenhart-Cabillic R, et al. Physical and biological factors determining the effective proton range. Med Phys. 2013;40:111716. doi: 10.1118/1.4824321. [DOI] [PubMed] [Google Scholar]
- 23.Rana S. Clinical dosimetric impact of Acuros XB and analytical anisotropic algorithm (AAA) on real lung cancer treatment plans: Review. Int J Cancer Ther Oncol. 2014;2:02019. [Google Scholar]
- 24.Latifi K, Oliver J, Baker R, Dilling TJ, Stevens CW, Kim J, et al. Study of 201 non-small cell lung cancer patients given stereotactic ablative radiation therapy shows local control dependence on dose calculation algorithm. Int J Radiat Oncol Biol Phys. 2014;88:1108–13. doi: 10.1016/j.ijrobp.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 25.Lu L. Dose calculation algorithms in external beam photon radiation therapy. Int J Cancer Ther Oncol. 2013;1:01025. [Google Scholar]
- 26.Oyewale S. Dose prediction accuracy of collapsed cone convolution superposition algorithm in a multi-layer inhomogenous phantom. Int J Cancer Ther Oncol. 2013;1:01016. [Google Scholar]
- 27.Ojala JJ, Kapanen MK, Hyödynmaa SJ, Wigren TK, Pitkänen MA. Performance of dose calculation algorithms from three generations in lung SBRT: Comparison with full Monte Carlo-based dose distributions. J Appl Clin Med Phys. 2014;15:4662. doi: 10.1120/jacmp.v15i2.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaikh A, Giraud J, Balosso J. A method to quantify and assess the dosimetric and clinical impact resulting from the heterogeneity correction in radiotherapy for lung cancer. Int J Cancer Ther Oncol. 2014;2:020110. [Google Scholar]
- 29.Zhuang T, Djemil T, Qi P, Magnelli A, Stephans K, Videtic G, et al. Dose calculation differences between Monte Carlo and pencil beam depend on the tumor locations and volumes for lung stereotactic body radiation therapy. J Appl Clin Med Phys. 2013;14:4011. doi: 10.1120/jacmp.v14i2.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang JY, Dong L, Mohan R, Liao Z, Cox J, Komaki R. Image-guided proton radiotherapy for medically inoperable stage I non-small cell lung cancer. Lung Cancer. 2005;49:S93. [Google Scholar]
- 31.Liu HH, Balter P, Tutt T, Choi B, Zhang J, Wang C, et al. Assessing respiration-induced tumor motion and internal target volume using 4DCT for radiation therapy of lung cancer. Int J Radiat Oncol Biol Phys. 2007;68:531–40. doi: 10.1016/j.ijrobp.2006.12.066. [DOI] [PubMed] [Google Scholar]
- 32.Engelsman M, Rietzel E, Kooy HM. Four-dimensional proton treatment planning. Int J Radiat Oncol Biol Phys. 2006;64:1589–95. doi: 10.1016/j.ijrobp.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 33.Paganetti H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys Med Biol. 2012;57:R99–117. doi: 10.1088/0031-9155/57/11/R99. [DOI] [PMC free article] [PubMed] [Google Scholar]