Abstract
In computed tomography (CT), some superficial organs which have increased sensitivity to radiation, receive doses that are significant enough to be matter of concern. Therefore, in this study, the effects of using shields on the amount of dose reduction and image quality was investigated for pediatric imaging. Absorbed doses of breasts, eyes, thyroid and testes of a series of pediatric phantoms without and with different thickness of bismuth and lead were calculated by Monte Carlo simulation. Appropriate thicknesses of shields were chosen based on their weights, X-ray spectrum, and the amount of dose reduction. In addition, the effect of lead shield on image quality of a simple phantom was assessed quantitatively using region of interest (ROI) measurements. Considering the maximum reduction in absorbed doses and X-ray spectrum, using a lead shield with a maximum thickness of 0.4 mm would be appropriate for testes and thyroid and two other organs (which are exposed directly) should be protected with thinner shields. Moreover, the image quality assessment showed that lead was associated with significant increases in both noise and CT attenuation values, especially in the anterior of the phantom. Overall, the results suggested that shielding is a useful optimization tool in CT.
Keywords: Image quality, lead and bismuth, Monte Carlo simulation, superficial organs' doses
Introduction
Computed tomography (CT) scanning technology is a valuable tool to diagnose many diseases; however, the level of radiation dose is a source of concerns. Given that high effective dose (ED) is delivered in CT examinations, an effort to minimize it, is critically important. This is especially important in children, because the younger the patient is at the time of exposure to radiation the greater is this risk.[1] Due to higher radiosensitivity of children cells, the lifetime cancer risk associated with an individual CT examination is higher in children than in adults[2] and there is an increased risk for thyroid, skin, brain, and breast cancer in children.[3]
A long-accepted method of dose minimization during radiographic examinations is the use of shielding to protect superficial organs from scatter radiation. Increasing research has gone into the development of shields that can be utilized within the CT scan range.[4,5] These shields allow meaningful reduction in dose to superficial organs through the absorption of lower energy dose contributing photons, while not degrading image quality. At general diagnostic imaging energies (60–140 kVp) in soft tissues and bone, a large fraction of the attenuation occurs by Compton scatter rather than by photoelectric absorption, chiefly because of the low atomic number of the tissues. X-ray scatter reduces subject contrast by adding background signals that are not representative of the anatomy. But, in the photoelectric absorption there are no additional nonprimary photons to degrade the image. Therefore, it can be said that image contrast decreases when higher X-ray energies are used in the imaging process.[6] In addition, photons with lower energies are absorbed in the superficial tissues of the body and do not contribute in image construction and just increase received doses especially in sensitive organs such as breast, thyroid, eyes and gonads.
Despite the advantages of using protective shields, concerns are increasingly being raised as to its efficacy especially in relation to its impact on image quality.[7] In spite of some discouraging statements about using shields.[8] some professionals reported that using shielding technique reduces the surface dose to patients with no appreciable loss in diagnostic quality.[9,10,11,12,13]
Another technique for radiation dose management is automatic exposure control (AEC),[11,14] which adjusts scanner output based on patient attenuation to deliver a user-specified level of image noise. According to AAPM statement, users can change AEC parameters to more aggressively decrease the tube current. But, it should be noted that since AEC systems on CT scanners can be complex and involve adjustments of several parameters, they are urged to consult with a medical physicist and/or applications specialist when making changes to the AEC parameters.[15] In some countries, AEC systems are not implemented in all CT scanner and are not commonly used in radiology departments. On the other hand, it was reported that good radiographic technique includes standard use of lead or equivalent shielding of child's body in the immediate proximity of the diagnostic field and if shields are placed appropriately with enough distance to minimize the subjacent artifact, they can be used to protect superficial organs from direct or scattered radiation.[16] The AAPM stated that the use of bismuth shielding along AEC can have unpredictable and undesirable effects on both image quality and dose,[15] but recently it was reported that if shield is not positioned on the patient during acquiring the CT radiograph, no increase is observed in tube current and dose despite the existence of shields during scan. This means that placing the bismuth on the phantom prior to the CT radiograph would be counterproductive; the system would detect the increased attenuation of the shield and increase the tube current to compensate for the increased attenuation, negating potential dose reductions.[11,17,18,19,20,21] Thus, users must trick the CT system into “not seeing” the shield by placing it on the phantom or patient after the CT radiograph. This trick also works for AEC systems that adapt the tube current during the scan and for AEC systems that do not adapt the tube current during the scan. In adaptive systems that “see” the increased attenuation during the scan, the distribution of dose around the patient per rotation is adjusted to match the actual patient attenuation profile. So, the generic models of patient shape provide a first estimate of attenuation for planning the scan, and the real-time feedback loop adjusts the tube current for each patient's specific shape. However, the system constrains the total tube current that can be applied during each rotation to avoid reaching tube or system limits during the scan due to a large unanticipated increase in patient attenuation.[18]
Given the importance of dose reduction in CT examinations,[22] the purpose of this study is to determine the efficiency of shielding the superficial organs as a mean of dose reduction. Its impact on both organ dose and image quality will be compared with those of non-shielded routine CT scanning.
Materials and Methods
Monte Carlo dose calculation
In this study, all CT simulations and organ dose calculations were performed using Monte Carlo simulation, which is the most reliable way to obtain accurate values of dose under CT imaging.[23,24] For this work, the simulation was operated by Monte Carlo N-Particle eXtended (MCNPX) code in photon mode with a low-energy cutoff of 1 keV. The photon transport model creates electrons but assumes that they travel in the direction of the primary photon and that the electron energy is deposited at the photon interaction site, creating a condition of charged particle equilibrium (CPE). Under conditions of CPE, collision kerma is valid to be equal or very approximate to absorbed dose and is recorded using the type 6 (F6: p) tally of the MCNPX code.[25] The simulations provide dose in MeV g−1, that is, energy deposition (MeV) per unit mass (g), per emitted particle, therefore some factors are applied to provide absorbed dose in unit of Gy (J kg−1). By definition, this tally provides the average dose to entire organ or tissue. In all simulations, 2E9 particles were tracked to obtain statistically meaningful results with relative errors less than or equal to 2% in most organs.
CT scanner modeling
A Siemens Somatom Sensation 16 (Siemens Medical Systems, Germany) was simulated within the MCNPX Monte Carlo radiation transport code. The CT scanner had a fan beam originating from the focal spot with a fan beam angle of 52° and a focal spot-to-axis distance of 57 cm. The information about X-ray spectra and scanner's characteristics were provided by the manufacturer. These spectra following passage through different thickness of lead were calculated by compiling a FORTRAN program.
In the MCNPX code, there are at least three ways to define the specific shape of the fan beam.[26,27,28] In this research, in the same method as Khursheed et al., CT imaging was simulated by exposing a series of contiguous transverse slices of 1 cm thickness in each phantom to X-rays emitted from sources lying on a circle around the phantom in the same plane as each slice.[26]
Validation of CT model
A common way to validate the CT scanner model is through comparisons between experimentally measured and simulated computed tomography dose index (CTDI) values.[24,28,29,30,31] For this purpose, a set of CTDI data was simulated for head and body CTDI phantoms with diameters of 16 and 32 cm, respectively, and compared with the measured CTDI values reported by Lee et al.,[24,29] under the same radiation exposure condition. Moreover, peripheral CTDI values at 12 o′clock were measured with and without shield and were compared with the results of simulation. Therefore, the chamber was positioned at the most anterior chamber location directly above the centre and on the periphery of the phantom at a distance of 1.0 cm from the surface, because this position most closely represents the location of superficial organs in a patient. A 100 mm pencil shaped Radcal® ion chamber model 10 × 5-3 CT (Radcal Corporation, Monrovia, CA), which has an excellent response in diagnostic energy range,[32] and a Radcal 9015 dosimeter (Radcal Corporation, Monrovia, CA) were used to determine the CTDI values. To perform the comparison, the CTDI phantoms were modeled as a cylinder having a diameter of 16 cm for head and 32 cm for body phantoms, with a length of 15 cm each. The material composition of the CTDI phantoms was simulated as polymethylmethacrylate with a density of 1.19 g cm−3. The ion chamber was modeled as three 10 cm long concentric cylinders. The innermost cylinder with a diameter of 6.7 mm defined the active air volume. The second cylinder with a diameter of 10.2 mm defined the chamber wall, which is C552 air-equivalent material with a density of 1.76 g cm−3. The third cylinder with a diameter of 13.7 mm defined a build-up cap, which was modeled as polyacetal plastic with a density of 1.43 g cm−3.[24]
Stylized models of children
The series of stylized computational phantoms developed at the Oak Ridge National Laboratory (ORNL) in the early 1980's have been used extensively in the study of organ doses. These phantoms utilize 3D surface equations to represent both internal organ structure and external body shape. The ORNL series are hermaphrodites (inclusive of both male and female organs and tissues), and include mathematical representations of a newborn, 1-year-old, 5-year-old, 10-year-old, 15-year-old and an adult male. Han et al., were made some revisions to these phantoms such as; developing stylized models (e.g. the head, brain, kidneys, and etc), incorporating new models of some organs such as salivary glands, and using reference values of elemental tissue compositions and mass densities from ICRP Publication 89 and ICRU Report 46.[33] In this study the revised model of ORNL pediatric phantoms (newborn, 1-year, 5-year, 10-year and 15-year olds) were employed.
Organ dose estimations
For determination of the amount of dose reduction achievable by shielding the superficial organs, the absorbed doses for organs, which were irradiated directly (breasts in chest scan and eye lenses in head scan) or were mainly exposed by scattered radiation (thyroid in chest scan and testes in abdomen-pelvis scan) were calculated with and without bismuth and lead shields. To compare the absorbed doses, the same parameters were used for both steps. Considering the linear relationship between the mAs and dose,[22] the results were normalized to mAs.
ED estimations
ED is the tissue-weighted sum of the equivalent doses (radiation weighted of absorbed doses) in all specified tissues and organs of the body. Therefore, in addition to the organs under investigation, the absorbed doses of all organs and tissues, which were necessary for ED calculations, were estimated. In this study, EDs were calculated based on tissue weighting factors reported in ICRP 103. Since the ORNL phantoms are hermaphrodites, the average value of ED was used for both sexes.[2]
Appropriate in-plane shields
As mentioned, shields should remove the lower energy part of the spectrum, so materials with high attenuation coefficients in diagnostic energy range (lead and bismuth), decrease the amount of required thickness for dose reduction, and therefore reduce the image artifacts. Therefore, lead and bismuth were selected as protective shield to cover the anterior surface of the phantom, so that they did not significantly exceed the width of the anterior surface. Almost 2-cm-thick spacer was used below the shield to decrease streak artifacts observed in the regions adjacent to the surface in contact with the shield. It should be noted that shields should be lightweight, and should not disturb the patient's comfort during a CT examination nor should they interfere with the patient's respiration. Considering the densities of bismuth and lead (9.78 g cm−3 and 11.34 g cm−3, respectively), the effect of bismuth and lead up to 0.5 mm thickness was investigated in this research. For instance, the maximum weight of lead shield on newborn breasts and thyroid was almost 53 and 37 gram, respectively, while these amounts on 15-year-old phantom were about 461 and 145 gram, separately.
Image quality analysis
To investigate the effects of shield on image quality, a simple calibration phantom with 0.15 mm lead shield over its anterior surface was used [Figure 1]. Two scans were performed without and with lead shield at two different protocols (80 kVp, 192 mAs and 130 kVp, 260 mAs) and the differences between image contrasts of shielded and unshielded images were studied.
Figure 1.
Calibration phantom without (a), and with (b) lead shield and spacer
For a more detailed study, five rectangular ROIs (~ 6 cm2) within a homogenous region of the calibration phantom (a cylinder contains water) were selected to measure both the average Hounsfield units (HU) and the standard deviation (SD) in each region. SD measures can be used as a quantitative measure of noise within CT images and collecting the average HU will check for variations caused by the presence of shields.[21] ROIs were placed in the cylinder (one in anterior, two in lateral, one in middle and one in posterior) on six consecutive slices. ROIs within the inhomogeneous region were precluded from the image quality analysis, as this region cannot be accurately compared between slices.
Results
Model validation
Four different point doses (central dose and doses at 12, 3, and 6 o′clock positions) were determined within the CTDI head and body phantoms by using the ion chamber with the collimation of 10 mm under the three tube potentials of 80, 100, and 120 kVp. The weighted CTDI (CTDIw), which is defined as the summation of one-third of CTDIcenter and two-thirds of CTDIperiphery, was 6.2, 11.6 and 16.4 mGy, for CTDI head phantom at tube voltages of 80, 100 and 120 kVp, respectively. The simulated doses of this study agreed with the measured ones with maximum error of almost 9% for all tube potentials. These results were comparable with those given in other published studies.[24,29,30,31]
In addition, it was observed that there is a good agreement between the results of peripheral CTDI values obtained by simulation and measurement (less than 8%). For instance, the measured values of peripheral CTDI at 12:00 without and with 0.1 mm lead shield at tube voltage of 80 kVp were 7.25 and 2.67 mGy (63% reduction), respectively, while the simulated CTDI values without and with shield were 6.81 and 2.48 mGy (64% reduction), separately. For further validation, the CTDI at 12:00 without and with shield calculated in this study were compared with those obtained by Midgley et al., under the condition considered in their investigation. The CTDI values calculated in this study and those reported by Midgley et al., at 12:00 were 2.27E-02 and 2.5E-02 mGy/mAs, whereas by using bismuth shield, they reduced to 1.18E-02 and 1.3E-02 mGy/mAs, respectively.[20]
Dose estimation
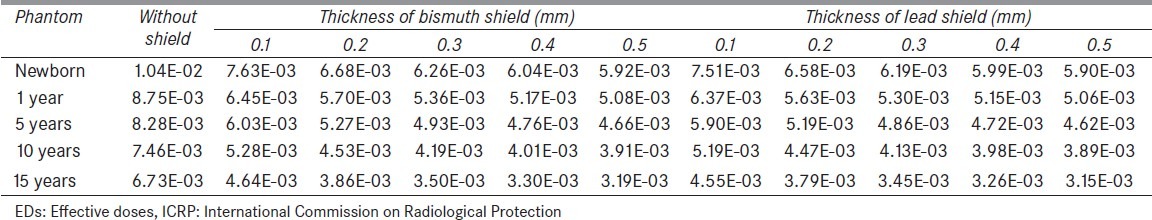
Figure 2 displays breasts, eyes, thyroid, and testes doses in mGy/mAs for different thickness of lead shield at tube voltages of 80 kVp. From the figure, it is obvious that even a small thickness of shield reduces the received doses significantly. As expected, by increasing the shield thickness, the value of absorbed dose decreases. The values of ED based on ICRP 103 weighting factors in chest examination at tube voltage of 80 kVp for different thicknesses of lead and bismuth shield on breasts are provided in Table 1. The same data are available for the other three tube voltages.
Figure 2.
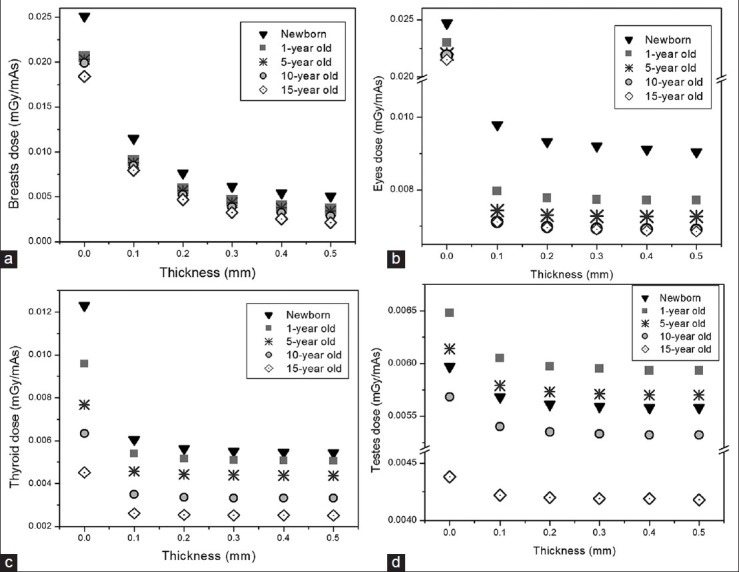
Reduction in absorbed dose by using different thickness of lead shields at tube voltages of 80 kVp for breasts (a), eye lenses (b), thyroid (c) and testes (d), calculated by Monte Carlo simulation
Table 1.
EDs in (mSv/mAs) calculated by tissue-weighted sum of the equivalent doses (mGy/mAs) for chest examinations on five anthropomorphic phantoms at tube voltage of 80 kVp without and with five different thicknesses of bismuth and lead shields (EDs were calculated based on ICRP103 weighting factors)
X-ray spectra
X-ray spectra following passage through different thickness of lead at tube voltages 80 kVp is illustrated in Figure 3.
Figure 3.
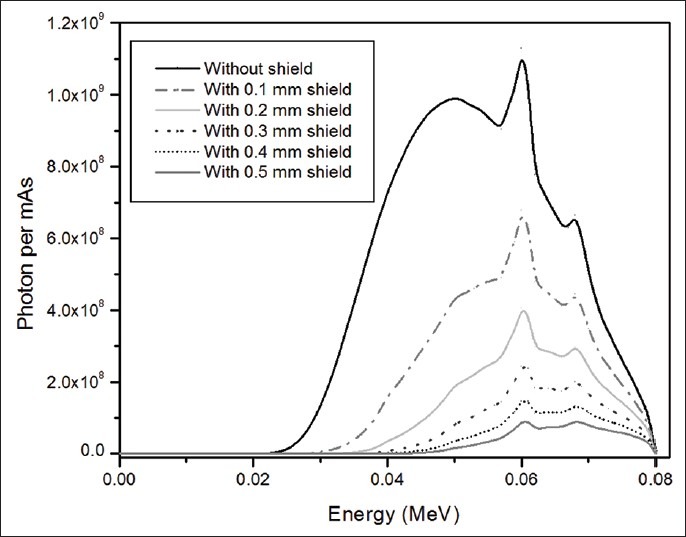
X-ray spectrum following passage through different thickness of lead at tube voltage of 80 kVp
Image quality assessment
Shielded and unshielded CT images of one slice of calibration phantom at tube voltage of 80 kVp and tube loading of 192 mAs are indicated in Figure 4. In this figure, the variations in HUs of consequent image, due to applying lead shield, are observed to some extent, especially in the anterior aspect of the phantom. For a quantitative analysis on the variations of HUs, in addition, Figure 5 details the results of total 30 ROI measurements made at the cylinder in each of the two experimental techniques. In this figure, error bars indicate SD from the mean. As observed, when shielding is used noises increase throughout, and significant increase is noted in the anterior ROI. In unshielded CT images, the variations in HU (SD), at anterior, middle, left lateral, right lateral and posterior were almost 36, 35, 27, 31 and 30, respectively; while these values in shielded CT images increased to 45, 37, 38, 35 and 30, separately. Figure 6 displays mean noise recorded in each ROI at tube voltage of 80 kVp and tube loading of 192 mAs.
Figure 4.
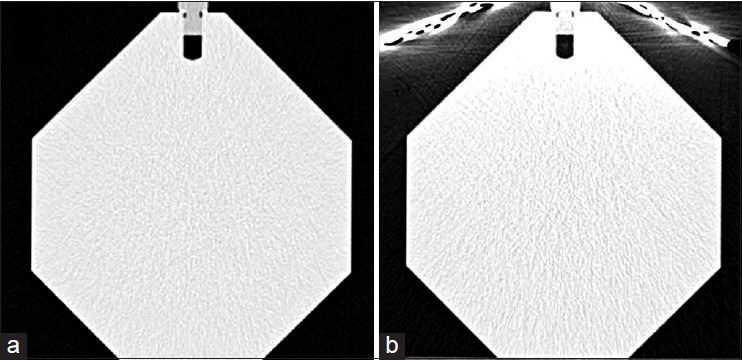
Unshielded (a) and shielded (b) CT images of one slice of the calibration phantom (In both images the grayscale ranges from 0–1211)
Figure 5.
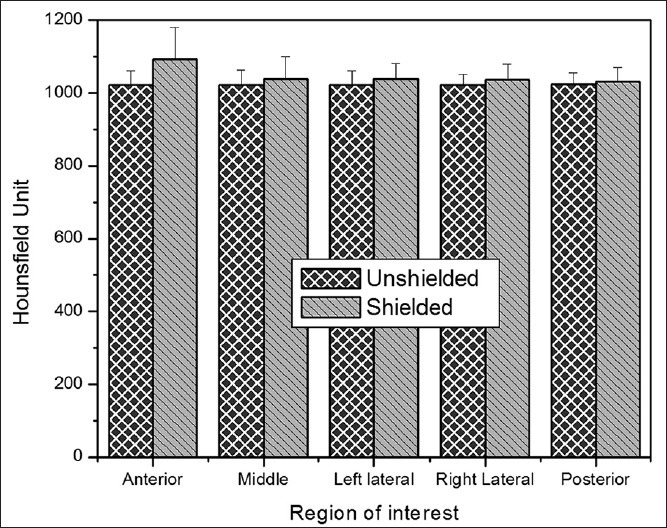
Mean HUs units recorded in each ROI at tube voltage of 80 kVp and tube loading of 192 mAs
Figure 6.
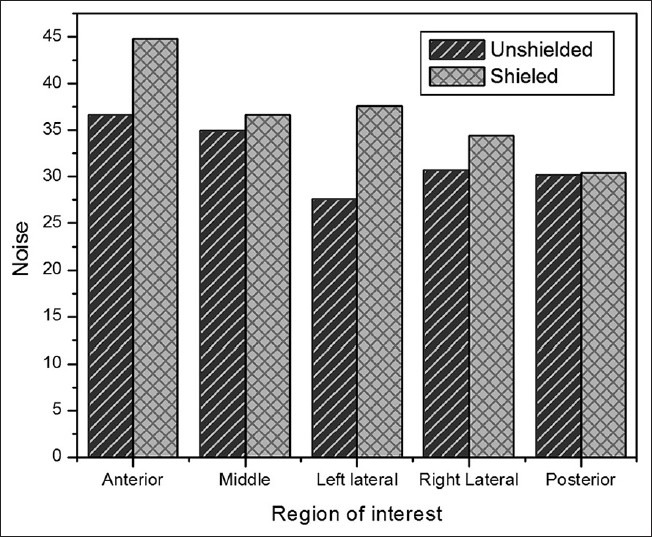
Mean noise (SD of HU) recorded in each ROI at tube voltage of 80 kVp and tube loading of 192 mAs
Discussion
Radiation dose remains a concern within CT, given the dramatic rise in its use worldwide.[34] Despite the introduction of newer technologies, there has been a reported increase in average CT dose with the advent of multidetector technology;[35] therefore, any efforts to reduce dose to the sensitive organs will be of particular benefit in lowering the risks of CT examinations.[36]
Results of this study show that absorbed doses to breasts, eyes, thyroid and testes are high enough to be matter of concern. These tissues usually are not even the target organs during CT imaging, and they receive radiation doses as a by-product of their anatomical locations. These scales of the absorbed doses reinforce the need for using any technique, which reduces doses to these radiosensitive tissues (in compliance with the ALARA principle) and does not affect image quality.[21]
Organ dose estimation
This study quantified the amount of dose reduction using Monte Carlo simulation. The modifications applied to ORNL series caused differences in results of ED estimations, which were more significant in smaller phantoms. But, considering the fact that the effect of discrepancies (in shape, size and location of the organs) on absorbed dose is more substantial in small size phantoms, these disagreements seem reasonable.
Absorbed doses of directly exposed organs
Results of the dosimetric analysis show that for 5-year-old phantom the amounts of breasts and eyes doses range from 3.55E-03 - 2.03E-02 mGy/mAs and from 7.26E-03-2.2E-02 mGy/mAs, respectively for different thickness of bismuth at tube voltage of 80 kVp. As expected, dose savings is evident for these organs. Moreover, owing to its increased density, larger dose reduction is achieved when lead shield is used.
Absorbed doses of sensitive adjacent tissues of scan region
From Figure 2c and d, the maximum reductions in thyroid and testes doses of 5-year-old are almost 50% and 9%, respectively. As observed, the amount of dose reduction in thyroid is more than that in testes. This is because in ORNL phantoms, the thyroid center of mass is closer to the scan region. It should be noted that, the amount of dose reduction for organs exposed by scattered radiation, is not as significant as organs exposed directly in the scan range.
Internal scattering has the most contribution (almost 98%) in the absorbed doses of these organs. Considering that, thickness of body in the trunk region is more than that in the neck and legs region, testes and thyroid have less distance with central of the body. In this condition, numbers of X-ray beams, which are scattered internally in the trunk region, enter the body through the skin of the neck (for thyroid) or skin of genitalia (for testes). Therefore, when shield is used to protect testes and thyroid, lead partially absorbs these internally scattered radiations, which could reach to the desired organ through the skin. So, it could be said that lead reduces the doses of internally scattered radiation for testes and thyroid, which have depth differences with the trunk. Although, shielding superficial organs, which are not included in the scan range, has less effect on radiation dose reduction, but the amounts of dose reduction, are still significant as stated by the other authors.[37,38,39]
For all organs (exposed directly or indirectly), there is an exponential relationship between absorbed doses and the thickness of shield, which means that in all the graphs there is a maximum thickness, which causes the maximum reduction in absorbed dose and thicknesses higher than that would not significantly change organ absorbed doses. Moreover, it is observed that lead shield is more effective in dose reduction, so it could be used as a protective shield for superficial organs.
For further validation, the results of absorbed doses calculated in this study were compared with those measured in the literature. Results of the dosimetric analysis show that, shielding results in a reduction in mean absorbed dose to the breast tissue of almost 56% and to the thyroid of almost 50% in chest scan. Midgley et al., stated that the amount of breast dose reduction at chest scan using a bismuth shield on a phantom varies between 53–63% at different tube voltages.[20] The results of dose measurements of McLaughlin and Mooney showed that applying shield could reduce the amount of thyroid dose in chest scan by 56%.[40] The similar results were reported by Hopper et al.,[4] and Vollmar et al.[41] As observed, the results of this study are in agreement with other studies.[12]
ED estimations
Considering Table 1, shielding the organs exposed directly causes meaningful reduction in ED especially using lead as protection. By increasing the shield thickness, ED decreases. Compared to the calculated values of ED for directly exposed organs, shielding thyroid and testes does not reduce ED significantly. Nevertheless, employing shields on these sensitive organs partly protects them and because shields are not covered in the scan range, probably, they do not affect image quality.
X-ray spectra analysis
As shown, lead shield is more efficient for protecting superficial organs. However, it should be noted that using lead shield causes changes in X-ray spectra, which could affect the image quality. In order to find the effect of different thickness of lead shield on image, the X-ray spectra could be studied. As mentioned earlier, to optimize the X-ray spectra, the higher and lower energy parts of the spectrum should be removed, whilst the characteristic peaks of the spectra (at 60 keV and 68 keV) should not change. In addition, as the thickness of lead increases, more incident photon energies are removed from the spectrum and the abundance of each peak reduces. For instance, the abundance of the peak located at 60 keV without applying shield is 6.68E8, and it reduces to 4.46E8 (33% reduction), 2.99E8 (55% reduction), 2.01E8 (70% reduction), 1.34E8 (80% reduction) and 9.01E7 (86% reduction), for 0.1, 0.2, 0.3, 0.4, and 0.5 mm lead shield, respectively. As observed in the Figure 3, there is significant reduction in each peak, especially for higher thicknesses, which causes the peak located at 68 keV disappears gradually (this peak is not recognizable for thicknesses higher than 0.2 mm). Thus, considering the role of characteristic peaks in the contrast of the images (due to their photoelectric interaction), the excessive increase in thickness of shield decreases the probability of photoelectric interactions (especially for higher thicknesses) and causes undesirable effects on the image. Therefore, the optimum thickness of shield is the one, which does not change the abundance of characteristic peaks significantly, while it removes the higher and lower energy parts of spectrum as possible. From the spectrum point of view, the lead shield with thicknesses higher than 0.2 mm degrade the image contrast, so for shielding the superficial organs, which are directly exposed to the radiation the maximum lead thickness of 0.2 mm seems be appropriate to remove the lower energies. Although the amount of dose reduction with higher thickness of lead is more considerable, but the destructive effects of thicker layer on the spectrum restrain us to suggest higher thickness of lead as appropriate shields for breasts and eyes tissues. There are no such restrictions for two other organs, which receive dose from scattered radiation. Therefore, for thyroid and testes at tube voltage of 80 and 100 kVp a lead shield with a thickness of 0.4 mm and at tube voltage of 120 and 140 kVp a lead shield with a thickness of 0.5 mm would be proper for significant reduction in dose.
Image quality
The main concern with the use of shielding is the potential negative effects on image quality caused by the preferential absorption of lower energy photons. Fricke et al.,[9] reported no increase with the use of bismuth shielding in pediatric patients, Midgley et al.,[20] also reported preservation of image quality with mild streaking seen. Gelejins et al.,[7] reported increases in image noise, while Kalra et al.,[42] reported increasing attenuation values as well as image noise. The results of this work support the latter, so that both image noise and attenuation values were slightly increased. Noise and mean attenuation value (and consequently HUs) were increased in all ROIs, which was most evident in the anterior, directly below the shield [Figures 5 and 6]. This confirms the results from other researches; that shield results in an increase in noise[18,43] and mean attenuation.[18,42] Applying shields affects the values of HU, because the effective energy of X-ray is changed and HU is directly depends on energy. As declared by Kalra et al., and according to the results of this study, the changes in HU of different ROIs were more significant in the anterior surface of the phantom. The differential increase in CT numbers, particularly at the shielded surface of the phantom, is most likely due to increased attenuation of the incident X-ray beam at the shielded surface compared to the opposite non-shielded aspect of the phantom. On the contrary, less pronounced but definite increase in the central CT numbers may be explained by the greater contribution of X-ray beams from the non-shielded aspect of the phantom compared to that from the shielded aspect. The increase in image noise was likely related to increased beam hardening and scattering associated with the shield.[42]
Although it seems that tube current reduction is a better technique for decreasing dose, but there is another important point in selecting the best dose reduction method: Acceptable levels of noise and image quality within CT images, which can be different even within one scan range, depending on the anatomy included. Tube current reduction increases the noise, and noise may affect the diagnostic ability in low contrast regions. Alternatively, use of superficial shielding can offer a solution to this predicament, as shielding is applied only to body surface and dose can be maintained at optimal levels outside of the region of interest.[21]
In the study of Foley et al.,[21] it was reported that applying shield raises mean noise about 27%, and the maximum HU increases more than five-fold. Kalra et al.,[42] showed that the mean noise increases about 43%, while the HU increases about 2.5 fold. The results of this study showed that the maximum increase in noise is about 22%, while the maximum increase in HU is 19%. Considering the comparability of the results of this study with those reported by Foley et al.,[21] and Kalra et al.,[42] it could be said that the level of noise is acceptable in several clinical situations as discussed below. User needs to remember that pretty pictures are not needed for all diagnostic tasks, but, rather, a choice can be made between low noise and a low dose, depending on the diagnostic task.[44]
Considering that the maximum increase in the image noise is in the anterior aspect of the phantom, in particular, shields should be avoided when a CT is being performed for the evaluation of abnormalities located in the vicinity of the surface over which the shield will be placed. Moreover, radiologist should avoid the use of shields when the absolute CT numbers need to be used for clinical interpretation such as for coronary calcium scoring, renal cyst, or adrenal mass characterization.[42] It was stated that, in abdominal studies, low-contrast areas are severely affected by an increase in image noise, while pelvis, with its greater inherent contrast, is usually not noticeably affected.[45] A much higher level of noise may be tolerated in the high-contrast region of the central lung than in the region of the liver where noise may affect the detection of low-contrast lesions.[21] A low dose CT is unlikely to compromise the required information such as for a pulmonary nodule follow up, evaluation of a bony thorax, and other follow up chest CT studies. Another situation where shields may be beneficial would be for shielding breasts in abdominal CT studies as well as shielding the thyroid gland for chest CT studies. In addition, for children, who are typically scanned at a low radiation dose and have a higher image noise, there is no evidence against applying shield.[42] Further studies into optimization of dose and noise values for various patient types are necessary to identify acceptable thresholds of image quality with minimum radiation doses.
Conclusions
In this study, the absorbed doses of breasts, eye lenses, thyroid and testes together with the EDs were estimated for pediatric phantoms undergoing CT examinations without and with different thicknesses of bismuth and lead shields at tube potentials of 80, 100, 120 and 140 kVp. In addition, the effects of using shield on the X-ray spectrum and image quality of a simple phantom were studied. It was observed that applying a small thickness of bismuth or lead shield caused significant reduction in absorbed dose and the amount of dose reduction achievable using lead shields were larger than using bismuth. Assessing the X-ray spectra showed that using a lead shield with thickness of 0.4 mm for tube voltage of 80 kVp is proper for meaningful dose reduction in thyroid and testes and thinner slice of bismuth or lead (with maximum thickness of 0.2 mm) should be applied for shielding breasts and eye lenses.
It could be said that, shielding the superficial organs can play an important role in dose optimization during CT scanning. The artifacts and noises produced by the shield are found to be somewhat distracting and they are most evident in the material beneath the shield. However, if superficial organs are not the target of CT imaging, shields cannot interfere with the interpretation of the image and as the organ's shield is excluded from the imaging field, the presence of shield is inconsequential in terms of image quality. Therefore, when it is used in an appropriate setting, bismuth or lead shielding technique is a valid and valuable tool to reduce radiation risk in children.
Acknowledgment
The authors would like to thank Miss Fatemeh Akbari, Mrs Sara Abdollahi, and CT radiologists of Reza Radiotherapy and Oncology Center in Mashhad City for their cooperation in CT imaging of the calibration phantom.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.BEIR VII, Phase 2. Washington: The National Academies Press; 2005. Committee on the Biological Effects of lonizing Radiation; National Research Council of the National Academies, Health risks from exposure to low levels of ionizing radiation; pp. 21–172. [Google Scholar]
- 2.International Commission on Radiological Protection (ICRP), The 2007 recommendations of the international commission on radiological protection. ICRP Publication. 2007;21:1–328. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 3.United Nation Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), Epidemiological evaluation of radiation-induced cancer. Annex I. UNSCEAR 2000, Report. 2000;Vol II:297–450. [Google Scholar]
- 4.Hopper KD, King SH, Lobell ME, TenHave TR, Weaver JS. The breast: In-plane X-ray protection during diagnostic thoracic CT–shielding with bismuth radioprotective garments. Radiology. 1997;205:853–8. doi: 10.1148/radiology.205.3.9393547. [DOI] [PubMed] [Google Scholar]
- 5.Parker MS, Kelleher NM, Hoots JA, Chung JK, Fatouros PP, Benedict SH. Absorbed radiation dose of the female breast during diagnostic multidetector chest CT and dose reduction with a tungsten-antimony composite breast shield: Preliminary results. Clin Radiol. 2008;63:278–88. doi: 10.1016/j.crad.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Bushberg JT, Siebert JA, Leidholdt EM, Boone JM. In: The essential physics of medical imaging. 2nd ed. John JR, Snyder A, DeGeorge T, editors. Vol. 1. Philadelphia: Lippincott Williams and Wilkins; 2002. [Google Scholar]
- 7.Geleijns J, Salvado Artells M, Veldkamp WJ, Lopez Tortosa M, Calzado Cantera A. Quantitative assessment of selective in-plane shielding of tissues in computed tomography through evaluation of absorbed dose and image quality. Eur Radiol. 2006;16:2334–40. doi: 10.1007/s00330-006-0217-2. [DOI] [PubMed] [Google Scholar]
- 8.Halliburton SS, Abbara S, Chen MY, Gentry R, Mahesh M, Raff GL, et al. SCCT guidelines on radiation dose and dose optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr. 2011;5:198–224. doi: 10.1016/j.jcct.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fricke BL, Donnelly LF, Frush DP, Yoshizumi T, Varchena V, Poe SA, et al. In-plane bismuth breast shields for pediatric CT: Effects on radiation dose and image quality using experimental and clinical data. AJR Am J Roentgenol. 2003;180:407–11. doi: 10.2214/ajr.180.2.1800407. [DOI] [PubMed] [Google Scholar]
- 10.Perisinakis K, Raissaki M, Theocharopoulos N, Damilakis J, Gourtsoyiannis N. Reduction of eye lens radiation dose by orbital bismuth shielding in pediatric patients undergoing CT of the head: A Monte Carlo study. Med Phys. 2005;32:1024–30. doi: 10.1118/1.1881852. [DOI] [PubMed] [Google Scholar]
- 11.Coursey C, Frush DP, Yoshizumi T, Toncheva G, Nguyen G, Greenberg SB. Pediatric chest MDCT using tube current modulation: Effect on radiation dose with breast shielding. AJR Am J Roentgenol. 2008;190:W54–61. doi: 10.2214/AJR.07.2017. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Frush DP, Yoshizumi TT. Bismuth shielding in CT: Support for use in children. Pediatr Radiol. 2010;40:1739–43. doi: 10.1007/s00247-010-1807-3. [DOI] [PubMed] [Google Scholar]
- 13.Gbelcova L, Nikodemova D, Horvathova M. Dose reduction using bismuth shielding during pediatric CT examinations in Slovakia. Radiat Prot Dosimetry. 2011;147:160–3. doi: 10.1093/rpd/ncr336. [DOI] [PubMed] [Google Scholar]
- 14.Paterson A, Frush DP. Dose reduction in pediatric MDCT: General principles. Clin Radiol. 2007;62:507–17. doi: 10.1016/j.crad.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 15.The American Association of Physicists in Medicine (AAPM) AAPM position statement on the use of bismuth shielding for the purpose of dose reduction in CT scanning. [Last accessed on 15 February 2014]. Available from: http://www.aapm.org/publicgeneral/BismuthShielding.pdf .
- 16.ICRP. Khong PL, Ringertz H, Donoghue V, Frush D, Rehani M, et al. ICRP publication 121: Radiological protection in paediatric diagnostic and interventional radiology. Ann ICRP. 2013;42:1–63. doi: 10.1016/j.icrp.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Leswick DA, Hunt MM, Webster ST, Fladeland DA. Thyroid shields versus z-axis automatic tube current modulation for dose reduction at neck CT. Radiology. 2008;249:572–80. doi: 10.1148/radiol.2492071430. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Duan X, Christner JA, Leng S, Yu L, McCollough CH. Radiation dose reduction to the breast in thoracic CT: Comparison of bismuth shielding, organ-based tube current modulation, and use of a globally decreased tube current. Med Phys. 2011;38:6084–92. doi: 10.1118/1.3651489. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Duan X, Christner JA, Leng S, Grant KL, McCollough CH. Bismuth shielding, organ-based tube current modulation, and global reduction of tube current for dose reduction to the eye at head CT. Radiology. 2012;262:191–8. doi: 10.1148/radiol.11110470. [DOI] [PubMed] [Google Scholar]
- 20.Midgley SM, Einsiedel PF, Langenberg F, Lui EH, Heinze SB. Assessment of patient dose and image quality for cardiac CT with breast shields. Radiat Prot Dosimetry. 2012;151:463–8. doi: 10.1093/rpd/ncs038. [DOI] [PubMed] [Google Scholar]
- 21.Foley SJ, McEntee MF, Rainford LA. An evaluation of in-plane shields during thoracic CT. Radiat Prot Dosimetry. 2013;155:439–50. doi: 10.1093/rpd/nct030. [DOI] [PubMed] [Google Scholar]
- 22.Akhlaghi P, Miri Hakimabad H, Rafat Motavalli L. An overview of exposure parameters, dose measurements and strategies for dose reduction in pediatric CT examinations. Radioprotection. 2014;49:9–15. [Google Scholar]
- 23.DeMarco JJ, Cagnon CH, Cody DD, Stevens DM, McCollough CH, O′Daniel J, et al. A Monte Carlo based method to estimate radiation dose from multidetector CT (MDCT): Cylindrical and anthropomorphic phantoms. Phys Med Biol. 2005;50:3989–4004. doi: 10.1088/0031-9155/50/17/005. [DOI] [PubMed] [Google Scholar]
- 24.Lee C, Kim KP, Long DJ, Fisher R, Tien C, Simon SL, et al. Organ doses for reference adult male and female undergoing computed tomography estimated by Monte Carlo simulations. Med Phys. 2011;38:1196–206. doi: 10.1118/1.3544658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MCNPX User's Manual, Version 2.4. New Mexico: 2002. Los Alamos National Laboratory. [Google Scholar]
- 26.Khursheed A, Hillier MC, Shrimpton PC, Wall BF. Influence of patient age on normalized effective doses calculated for CT examinations. Br J Radiol. 2002;75:819–30. doi: 10.1259/bjr.75.898.750819. [DOI] [PubMed] [Google Scholar]
- 27.Gu J, Bednarz B, Xu XG, Jiang SB. Assessment of patient organ doses and effective doses using the VIP-MAN adult male phantom for selected cone-beam CT imaging procedures during image guided radiation therapy. Radiat Prot Dosimetry. 2008;131:431–43. doi: 10.1093/rpd/ncn200. [DOI] [PubMed] [Google Scholar]
- 28.Gu J, Bednarz B, Caracappa PF, Xu XG. The development, validation and application of a multi-detector CT (MDCT) scanner model for assessing organ doses to the pregnant patient and the fetus using Monte Carlo simulations. Phys Med Biol. 2009;54:2699–717. doi: 10.1088/0031-9155/54/9/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee C, Kim KP, Long DJ, Bolch WE. Organ doses for reference pediatric and adolescent patients undergoing computed tomography estimated by Monte Carlo simulation. Med Phys. 2012;39:2129–46. doi: 10.1118/1.3693052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarry G, DeMarco JJ, Beifuss U, Cagnon CH, McNitt-Gray MF. A Monte Carlo-based method to estimate radiation dose from spiral CT: From phantom testing to patient-specific models. Phys Med Biol. 2003;48:2645–63. doi: 10.1088/0031-9155/48/16/306. [DOI] [PubMed] [Google Scholar]
- 31.Staton RJ, Lee C, Lee C, Williams MD, Hintenlang DE, Arreola MM, et al. Organ and effective doses in newborn patients during helical multislice computed tomography examination. Phys Med Biol. 2006;51:5151–66. doi: 10.1088/0031-9155/51/20/005. [DOI] [PubMed] [Google Scholar]
- 32.Radcal corporation. [Last accessed on 1 August 2014]. Available from: http://www.radcal.com/10×5.3ct .
- 33.Han EY, Bolch WE, Eckerman KF. Revisions to the ORNL series of adult and pediatric computational phantoms for use with the MIRD schema. Health Phys. 2006;90:337–56. doi: 10.1097/01.HP.0000192318.13190.c4. [DOI] [PubMed] [Google Scholar]
- 34.United Nation Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), Sources and effects of ionizing radiation, Report to the general assembly with scientific annexes. UNSCEAR 2008, Report Vol I and II. 2008:1–776. [Google Scholar]
- 35.Moore WH, Bonvento M, Olivieri-Fitt R. Comparison of MDCT radiation dose: A phantom study. AJR Am J Roentgenol. 2006;187:W498–502. doi: 10.2214/AJR.05.1491. [DOI] [PubMed] [Google Scholar]
- 36.Berrington de Gonzalez A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–7. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brinc Z, Vekic B, Hebrang A, Anic P. Efficiency of breast shielding during CT of the head. Eur Radiol. 2003;13:2436–40. doi: 10.1007/s00330-003-1945-1. [DOI] [PubMed] [Google Scholar]
- 38.Hohl C, Mahnken AH, Klotz E, Das M, Stargardt A, Mühlenbruch G, et al. Radiation dose reduction to the male gonads during MDCT: The effectiveness of a lead shield. AJR Am J Roentgenol. 2005;184:128–30. doi: 10.2214/ajr.184.1.01840128. [DOI] [PubMed] [Google Scholar]
- 39.Ngaile JE, Uiso CB, Msaki P, Kazema R. Use of lead shields for radiation protection of superficial organs in patients undergoing head CT examinations. Radiat Prot Dosimetry. 2008;130:490–8. doi: 10.1093/rpd/ncn095. [DOI] [PubMed] [Google Scholar]
- 40.McLaughlin DJ, Mooney RB. Dose reduction to radiosensitive tissues in CT. Do commercially available shields meet the users′ needs? Clin Radiol. 2004;59:446–50. doi: 10.1016/j.crad.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Vollmar SV, Kalender WA. Reduction of dose to the female breast in thoracic CT: A comparison of standard-protocol, bismuth-shielded, partial and tube-current-modulated CT examinations. Eur Radiol. 2008;18:1674–82. doi: 10.1007/s00330-008-0934-9. [DOI] [PubMed] [Google Scholar]
- 42.Kalra MK, Dang P, Singh S, Saini S, Shepard JA. In-plane shielding for CT: Effect of off-centering, automatic exposure control and shield to surface distance. Korean J Radiol. 2009;10:156–63. doi: 10.3348/kjr.2009.10.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurwitz LM, Yoshizumi TT, Goodman PC, Nelson RC, Toncheva G, Nguyen GB, et al. Radiation dose savings for adult pulmonary embolus 64-MDCT using bismuth breast shields, lower peak kilovoltage, and automatic tube current modulation. AJR Am J Roentgenol. 2009;192:244–53. doi: 10.2214/AJR.08.1066. [DOI] [PubMed] [Google Scholar]
- 44.McCollough CH, Bruesewitz MR, Kofler JM., Jr CT dose reduction and dose management tools: Overview of available options. Radiographics. 2006;26:503–12. doi: 10.1148/rg.262055138. [DOI] [PubMed] [Google Scholar]
- 45.Kalra MK, Maher MM, Toth TL, Hamberg LM, Blake MA, Shepard J, et al. Strategies for CT radiation dose optimization. Radiology. 2004;230:619–28. doi: 10.1148/radiol.2303021726. [DOI] [PubMed] [Google Scholar]


