Cord blood transplantation (CBT) from an unrelated donor has been increasingly used as an alternative transplant method for adult patients without human leukocyte antigen (HLA)-compatible related or unrelated donors.1–4 However, the main disadvantage of CBT is still the limited cell dose, especially in adults, and this might contribute to a higher incidence of graft failure and delayed hematopoietic recovery, leading to higher transplant-related mortality (TRM) or overall mortality after CBT.
The purpose of a conditioning regimen prior to allogeneic hematopoietic stem cell transplantation (allo-HSCT) for hematologic malignancies is disease eradication and immunosuppression to overcome graft rejection. Although the standard myeloablative conditioning regimen prior to allo-HSCT has been total body irradiation (TBI) or busulfan combined with cyclophosphamide (CY) for patients with adult acute myeloid leukemia (AML), the role of an intensified conditioning regimen has been analyzed extensively in order to reduce the rate of post-transplant relapse and improve survival.5–7 However, the majority of these studies analyzed patients receiving allo-HSCT using bone marrow (BM) or mobilized peripheral blood (PB) as a stem cell source. Therefore, an optimal myeloablative conditioning regimen prior to CBT for adult AML still has to be determined.
Granulocyte colony-stimulating factor (G-CSF) stimulates proliferation, differentiation, and functional activation of neutrophils. In clinical use, G-CSF is most commonly used for reducing the duration of neutropenia after chemotherapy and HSCT, and for the mobilization of hematopoietic stem/progenitor cells from the BM into PB for HSCT. Furthermore, since administration of G-CSF increases the susceptibility to cytarabine arabinoside (Ara-C) through induction of cell cycle entry of dormant leukemia cells,8,9 the efficacy of concomitant use of G-CSF and chemotherapy has been analyzed.10,11 Several studies, as well as our own single institute studies, have demonstrated that G-CSF combined with myeloablative conditioning prior to allo-HSCT could be safely and effectively used for patients with myeloid malignancies in a single arm trial.9,12,13 However, there has been no comparative study of transplant outcomes for AML after allo-HSCT following a conditioning regimen with or without G-CSF. This retrospective study is the first to assess the effect of a G-CSF combination in a myeloablative conditioning regimen for CBT on the transplant outcome in adult AML patients in Japan. Patients and study methods are described in the Online Supplementary Appendix.
Characteristics of patients and cord blood units are shown in Table 1. There was a significant difference in cumulative incidence of neutrophil recovery among the four groups in univariate analysis (P<0.001) (Figure 1A). In the multivariate analysis, the hazard risk of neutrophil engraftment was significantly higher in the TBI≥10Gy+Ara-C/G-CSF+CY group (P<0.001) and lower in the TBI≥10Gy+other group (P=0.03) and TBI<10Gy+other or non-TBI group (P<0.001) compared with the TBI≥10Gy+Ara-C+CY group (Table 2). Among patients achieving neutrophil engraftment, neutrophil recovery times were significantly shorter in the TBI≥10Gy+Ara-C/G-CSF+CY group compared with the TBI≥10Gy+Ara-C+CY group (P<0.001). There was a significant difference in cumulative incidence of platelet recovery among the four groups in univariate analysis (P<0.001) (Figure 1B). Multivariate analysis showed no significant difference between the TBI≥10Gy+Ara-C+CY group and TBI≥10Gy+Ara-C/G-CSF+CY group (P=0.14). However, the hazard risk of platelet engraftment was significantly lower in the TBI≥10Gy+other group (P<0.001) and TBI<10Gy+other or non-TBI group (P<0.001) compared with the TBI≥10Gy+Ara-C+CY group (Table 2). Among patients achieving platelet engraftment, there was no significant difference in platelet recovery times among the four groups (P=0.32).
Table 1.
Characteristics of patients, cord blood units, and transplantation.
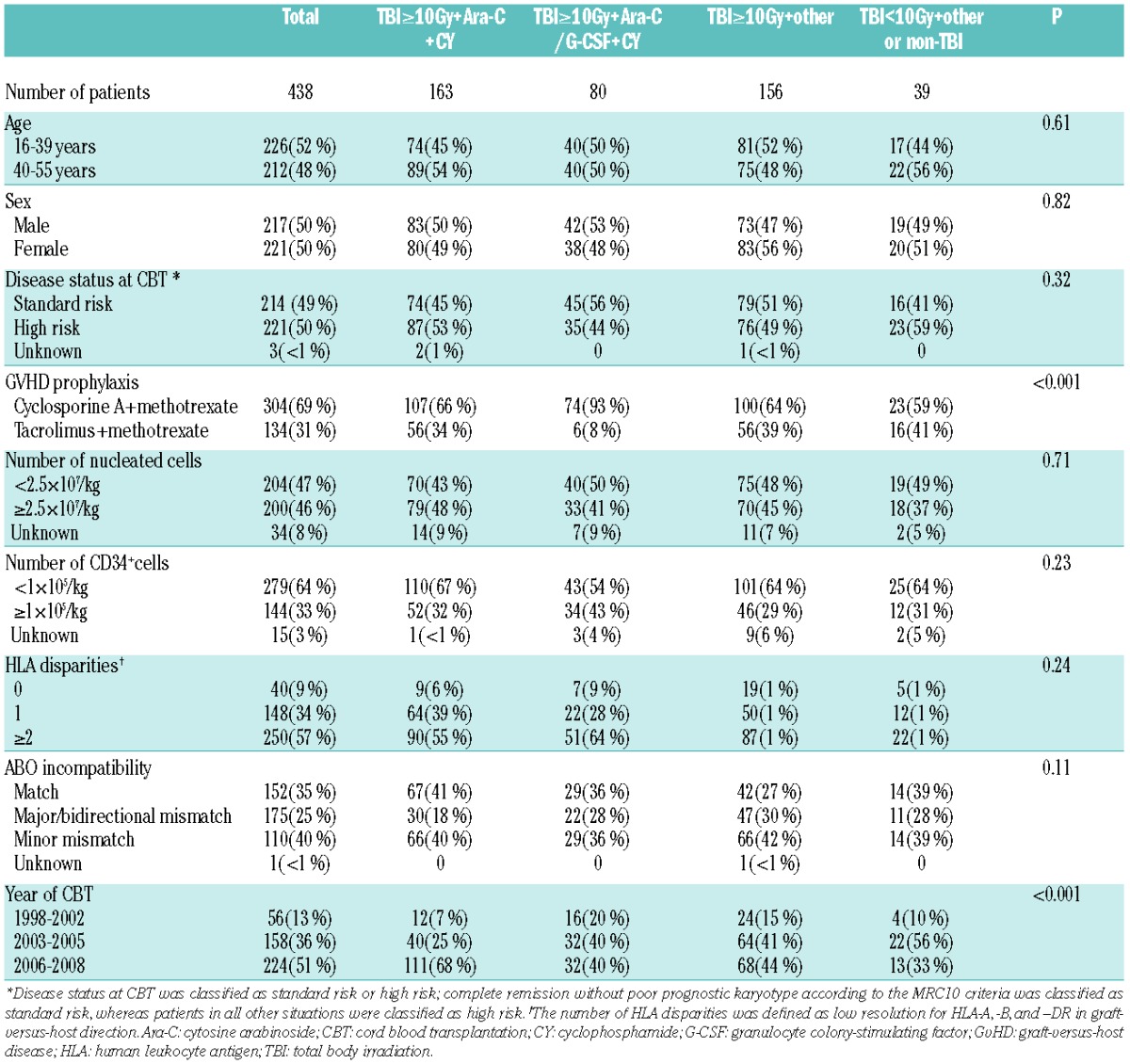
Figure 1.
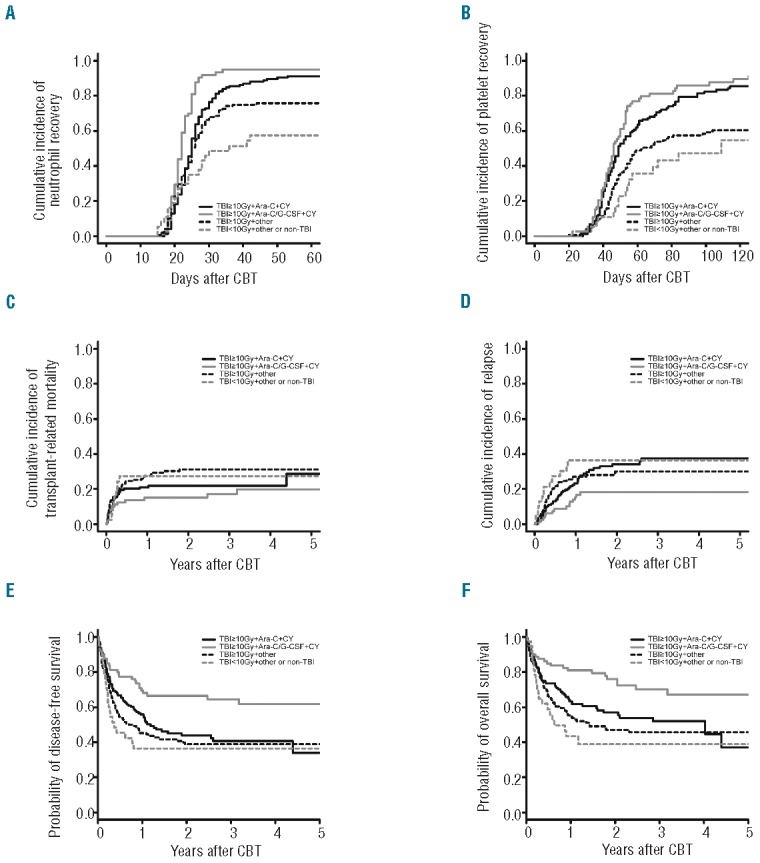
Cumulative incidences of neutrophil (A) and platelet (B) recovery, transplant-related mortality (TRM) (C) and relapse (D), probabilities of disease-free survival (E) and overall survival (F) after cord blood transplantation according to conditioning regimen. (A) Cumulative incidence of neutrophil recovery 42 days after CBT was 88% [95% confidence interval (CI): 81%–92%] in the TBI≥10Gy+Ara-C+CY group, 95% (95%CI: 85%–98%) in the TBI≥10Gy+Ara-C/G-CSF+CY group, 74% (95%CI: 66%–81%) in the TBI≥10Gy+other group, and 57% (95%CI: 37%–70%) in the TBI<10Gy+other or non-TBI group. Median times to neutrophil recovery were 24 days (range 17–53 days) in the TBI≥10Gy+Ara-C+CY group, 22 days (range 16–34 days) in the TBI≥10Gy+Ara-C/G-CSF+CY group, 23 days (range 15–65 days) in the TBI≥10Gy+other group, and 22 days (range 15–42 days) in the TBI<10Gy+other or non-TBI group. (B) Cumulative incidence of platelet recovery 100 days after CBT was 82% (95%CI: 74%–87%) in the TBI≥10Gy+Ara-C+CY group, 85% (95%CI: 74%–92%) in the TBI≥10Gy+Ara-C/G-CSF+CY group, 58% (95%CI: 48%–66%) in the TBI≥10Gy+other group, and 47% (95%CI: 25%–62%) in the TBI<10Gy+other or non-TBI group. Median times to platelet recovery were 46 days (range 28–168 days) in the TBI≥10Gy+Ara-C+CY group, 45.5 days (range 27–263 days) in the TBI≥10Gy+Ara-C/G-CSF+CY group, 48 days (range 20–249 days) in the TBI≥10Gy+other group, and 51 days (range 22–109 days) in the TBI<10Gy+other or non-TBI group. (C) Cumulative incidence of TRM at one year was 21% (95%CI: 15%–27%) in the TBI≥10Gy+Ara-C+CY group, 15% (95%CI: 8%–23%) in the TBI≥10Gy+Ara-C/G-CSF+CY group, 27% (95%CI: 20%–35%) in the TBI≥10Gy+other group, and 27% (95%CI: 14%–42%) in the TBI<10Gy+other or non-TBI group. (D) Cumulative incidence of relapse at three years was 37% (95%CI: 28%–46%) in the TBI≥10Gy+Ara-C+CY group, 18% (95%CI: 10%–27%) in the TBI≥10Gy+Ara-C/G-CSF+CY group, 30% (95%CI: 22%–37%) in the TBI≥10Gy+other group, and 36% (95%CI: 20%–52%) in the TBI<10Gy+other or non-TBI group. (E) Probability of disease-free survival at three years was 40% (95%CI: 31%–49%) for the TBI≥10Gy+Ara-C+CY group, 64% (95%CI: 52–74%) for the TBI≥10Gy+Ara-C/G-CSF+CY group, 38% (95%CI: 30%–47%) for the TBI≥10Gy+other group, and 36% (95%CI: 20%–51%) for the TBI<10Gy+other or non-TBI group. (F) Probability of overall survival was 52% (95%CI: 42%–60%) for the TBI≥10Gy+Ara-C+CY group, 70% (95%CI: 57%–79%) for the TBI≥10Gy+Ara-C/G-CSF+CY group, 45% (95%CI: 36%–54%) for the TBI≥10Gy+other group, and 39% (95%CI: 22%–55%) for the TBI<10Gy+other or non-TBI group. Median period of follow up for survivors (n=261) in the entire cohort was 24 months (range 1–122 months) after CBT.
Table 2.
Multivariate analysis of transplant outcomes.
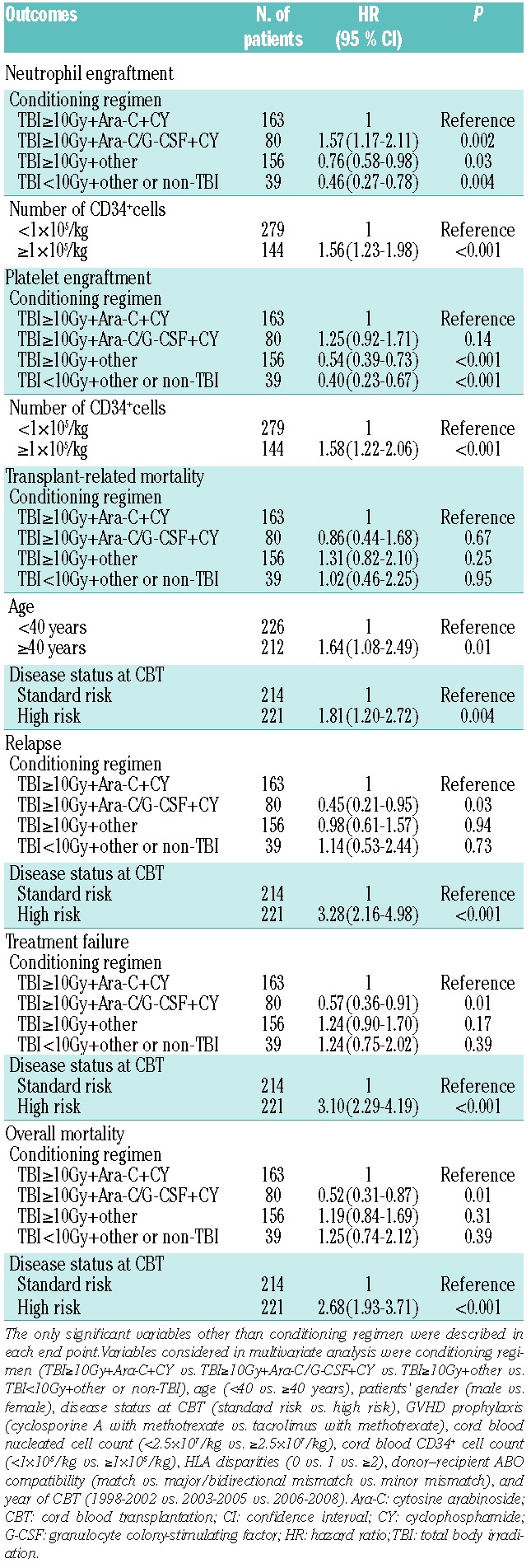
Among patients in the entire cohort, the cumulative incidence of TRM at 100 days and at one year was 17% (95%CI: 13%–20%) and 22% (95%CI: 18%–26%), respectively. There was no significant difference in cumulative incidence of TRM at one year among the four groups in univariate analysis (P=0.19) (Figure 1C). Multivariate analysis of TRM, adjusting for other variables, showed no significant difference between the TBI≥10Gy+Ara-C+CY group and the TBI≥10Gy+Ara-C/G-CSF+CY group (P=0.67), TBI≥10Gy+other group (P=0.25), or TBI<10Gy+other or non-TBI group (P=0.95) (Table 2). The cumulative incidence of relapse at three years was 30% (95%CI: 25%–35%) in the entire cohort. There was no significant difference in cumulative incidence of relapse at three years among the four groups (P=0.05) (Figure 1D). In multivariate analysis, the hazard risk of relapse was lower in the TBI≥10Gy+Ara-C/G-CSF+CY group (P=0.03), but not in the TBI≥10Gy+other group (P=0.94) and TBI<10Gy+other or non-TBI group (P=0.73) compared with the TBI≥10Gy+Ara-C+CY group (Table 2).
Among the entire cohort, the probability of disease-free survival (DFS) and overall survival (OS) at three years was 44% (95%CI: 39%–49%) and 52% (95%CI: 46%–57%), respectively. There was a significant difference in the probability of DFS at three years among the four groups in univariate analysis (P=0.001) (Figure 1E). The probability of DFS at three years was significantly better in the TBI≥10Gy+Ara-C/G-CSF+CY group compared with the TBI≥10Gy+Ara-C+CY group (P=0.02), the TBI≥10Gy+other group (P=0.002) and TBI<10Gy+other or non-TBI group (P=0.006). Multivariate analysis showed significantly decreased rates of treatment failure in the TBI≥10Gy+Ara-C/G-CSF+CY group compared with the TBI≥10Gy+Ara-C+CY group (P=0.01) (Table 2). In univariate analysis, there was a significant difference in the probability of OS at three years among the four groups (P=0.001) (Figure 1F). Multivariate analysis showed significantly decreased overall mortality in the TBI≥10Gy+Ara-C/G-CSF+CY group compared with the TBI≥10Gy+Ara-C+CY group (P=0.01) (Table 2). We also analyzed a subgroup of patients with standard risk (n=214) or high risk (n=221) at CBT. In standard-risk patients, the hazard risk of overall mortality (P=0.04), treatment failure (P=0.01) and relapse (P=0.002) was significantly lower in the TBI≥10Gy+Ara-C/G-CSF+CY group compared with the TBI≥10Gy+Ara-C+CY group, while that of high-risk patients was not (Online Supplementary Table S1 and Figures S1 and S2).
Anti-leukemia effects of allo-HSCT consist of leukemia eradication by both a conditioning regimen of chemotherapy with or without radiation and the graft-versus-leukemia (GvL) effect. Since relapse is the most common cause of death after allo-HSCT, an intensified conditioning regimen or enhancement of GvL effects is needed to reduce the incidence of relapse. Because of the difficulty in controlling the degree of GvL effects, an intensified conditioning regimen has been extensively analyzed. The several improvements to a typical conditioning regimen have included the addition of other agents to a standard myeloablative regimen, a dose escalation of drugs or TBI, or administration of drugs other than CY. Among these, the addition of other agents to a standard myeloablative regimen has been the most commonly used.5,6 In fact, several studies have reported a decrease in the incidence of relapse following intensified conditioning, but with a higher TRM, and no improvement in survival was achieved.5,7 Furthermore, the effect of adding high-dose Ara-C to a TBI/CY myeloablative conditioning regimen is controversial.7 However, all of these studies analyzed patients receiving BM or mobilized PB stem cell transplantation from related or unrelated donors. This finding was not confirmed in CBT. In our study, neutrophil and platelet engraftment was significantly higher in the TBI≥10Gy+Ara-C+CY group compared with the TBI≥10Gy+other group, suggesting that the addition of Ara-C to TBI/CY was beneficial in terms of stable engraftment, but not for survival in CBT for AML.
Granulocyte colony-stimulating factor was originally identified as an agent for stimulation of neutrophil production. Although G-CSF is most commonly used to reduce the duration of neutropenia after chemotherapy, it is also commonly used for hematopoietic stem cell (HSC) mobilization for HSCT. Although the mechanism of HSC mobilization is not clearly understood, G-CSF could disrupt the contact between HSC in a BM niche, leading to HSC migration. In a mouse bone marrow transplantation (BMT) model, G-CSF prior to low-dose irradiation enhanced donor HSC engraftment.14 This effect might be mainly due to the migration of recipient HSC from a BM niche by G-CSF treatment before transplantation. In fact, our data showed that neutrophil engraftment was significantly higher in the TBI≥10Gy+Ara-C/G-CSF+CY group compared with the TBI≥10Gy+Ara-C+CY group. These data suggest that the effect of the addition of G-CSF to a conditioning regimen could enhance neutrophil engraftment after CBT.
It has been reported that the administration of G-CSF increased the susceptibility of the cell-cycle-specific agent Ara-C in leukemia cells in vitro and in a xenograft model.8,9,15 In clinical studies, several regimens have attempted to demonstrate the efficacy of concomitant use of G-CSF with chemotherapy for newly diagnosed AML.10,11 We hypothesized that the addition of G-CSF to a conditioning regimen might improve outcome in an allo-HSCT setting. In our study, relapse was significantly lower in the TBI≥10Gy+Ara-C/G-CSF+CY group compared with the TBI≥10Gy+Ara-C+CY group. In a subgroup analysis, the effect of a G-CSF combination regimen for reduced relapse was significant in standard-risk but not high-risk patients. This is similar to a previous prospective randomized study of concomitant use of G-CSF with chemotherapy by Löwenberg et al.11 Further studies are required to confirm which subgroup of patients with AML could benefit from a G-CSF combination regimen in CBT to reduce the incidence of relapse.
In conclusion, our data show that the addition of G-CSF-combined Ara-C to a TBI+CY conditioning regimen resulted in a significantly higher incidence of neutrophil engraftment and significantly better DFS and OS, and a reduced relapse rate in CBT for AML. Although these findings should be confirmed in prospective studies, a G-CSF-combined myeloablative conditioning regimen promotes better engraftment and survival results in CBT for AML.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Takahashi S, Iseki T, Ooi J, Tomonari A, Takasugi K, Shimohakamada Y, et al. Single-institute comparative analysis of unrelated bone marrow transplantation and cord blood transplantation for adult patients with hematologic malignancies. Blood. 2004; 104(12):3813–20. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi S, Ooi J, Tomonari A, Konuma T, Tsukada N, Oiwa-Monna M, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109(3):1322–30. [DOI] [PubMed] [Google Scholar]
- 3.Atsuta Y, Suzuki R, Nagamura-Inoue T, Taniguchi S, Takahashi S, Kai S, et al. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood. 2009;113(8):1631–8. [DOI] [PubMed] [Google Scholar]
- 4.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11(7):653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanda Y, Sakamaki H, Sao H, Okamoto S, Kodera Y, Tanosaki R, et al. Effect of conditioning regimen on the outcome of bone marrow transplantation from an unrelated donor. Biol Blood Marrow Transplant. 2005;11(11):881–9. [DOI] [PubMed] [Google Scholar]
- 6.Mengarelli A, Iori A, Guglielmi C, Romano A, Cerretti R, Torromeo C, et al. Standard versus alternative myeloablative conditioning regimens in allogeneic hematopoietic stem cell transplantation for high-risk acute leukemia. Haematologica. 2002;87(1):52–8. [PubMed] [Google Scholar]
- 7.Riddell S, Appelbaum FR, Buckner CD, Stewart P, Clift R, Sanders J, et al. High-dose cytarabine and total body irradiation with or without cyclophosphamide as a preparative regimen for marrow transplantation for acute leukemia. J Clin Oncol. 1988;6(4):576–82. [DOI] [PubMed] [Google Scholar]
- 8.Miyauchi J, Kelleher CA, Wang C, Minkin S, McCulloch EA. Growth factors influence the sensitivity of leukemic stem cells to cytosine arabinoside in culture. Blood. 1989;73(5):1272–8. [PubMed] [Google Scholar]
- 9.Takahashi S, Okamoto SI, Shirafuji N, Ikebuchi K, Tani K, Shimane M, et al. Recombinant human glycosylated granulocyte colony-stimulating factor (rhG-CSF)-combined regimen for allogeneic bone marrow transplantation in refractory acute myeloid leukemia. Bone Marrow Transplant. 1994;13(3):239–45. [PubMed] [Google Scholar]
- 10.Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, Vekemans MC, et al. Favorable effect of priming with granulocyte colony-stimulating factor in remission induction of acute myeloid leukemia restricted to dose escalation of cytarabine. Blood. 2012; 119(23):5367–73. [DOI] [PubMed] [Google Scholar]
- 11.Löwenberg B, van Putten W, Theobald M, Gmür J, Verdonck L, Sonneveld P, et al. Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med. 2003;349(8):743–52. [DOI] [PubMed] [Google Scholar]
- 12.Mori T, Tanaka M, Kobayashi T, Ohashi K, Fujisawa S, Yokota A, et al. Prospective multicenter study of single-unit cord blood transplantation with myeloablative conditioning for adult patients with high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2013;19(3):486–91. [DOI] [PubMed] [Google Scholar]
- 13.Konuma T, Kato S, Ooi J, Oiwa-Monna M, Ebihara Y, Mochizuki S, et al. Single-unit cord blood transplantation after granulocyte colony-stimulating factor-combined myeloablative conditioning for myeloid malignancies not in remission. Biol Blood Marrow Transplant. 2014; 20(3):396–401. [DOI] [PubMed] [Google Scholar]
- 14.Mardiney M, 3rd, Malech HL. Enhanced engraftment of hematopoietic progenitor cells in mice treated with granulocyte colony-stimulating factor before low-dose irradiation: implications for gene therapy. Blood. 1996;87(10):4049–56. [PubMed] [Google Scholar]
- 15.Saito Y, Uchida N, Tanaka S, Suzuki N, Tomizawa-Murasawa M, Sone A, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010; 28(3):275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


