In the acute myeloid leukemia (AML)-associated chromosomal translocation t(8;21), the hematopoietic master regulator RUNX1 (also known as AML1, CBFα2 or PEBP2αB) located on chromosome 21 is fused to almost the entire ETO gene (also known as MTG8 or RUNX1T1) on chromosome 8, thereby generating the fusion protein RUNX1/ETO. RUNX1/ETO is a strong repressor of myeloid differentiation functioning via the dysregulation of genes connected to hematopoietic stem cell (HSC) maturation.1 In this study, we analyzed whether RUNX1/ETO also regulates genes of the cell adhesion molecule family that play an important role in HSC homeostasis.
To assess adhesion molecules regulated by RUNX1/ETO, we lentivirally expressed a truncated leukemogenic form,2 RUNX1/ETOtr, in lineage negative hematopoietic murine bone marrow progenitor cells (lin-mBM) and analyzed for changes in adhesion molecule expression using flow cytometry. While CXCR family members remained unaffected, RUNX1/ETOtr specifically increased integrin α4, α5 and β1 subunit expression levels, which form the integrins VLA-4 and VLA-5 (Figure 1A and data not shown). Since the ex vivo culture of primary lin- mBM cells was associated with cellular differentiation potentially impelling integrin expression levels, we analyzed RUNX1/ETOtr-mediated regulation of VLA-4 and VLA-5 in the multipotent hematopoietic progenitor cell line FDCP-mix. As expected, RUNX1/ETOtr was able to up-regulate the cell surface expression levels of the integrin α4, α5 and β1 subunits (Figure 1B and C). Moreover, RUNX1/ETOtr up-regulated α4, α5 and β1 at the mRNA level in FDCP-mix cells while the DNA binding-defective mutant RUNX1/ETOtr(L148D) failed, thereby suggesting that the DNA binding capability of RUNX1/ETOtr is required for the observed integrin upregulation (Figure 1D). Of note, integrin expression levels directly correlated to RUNX1/ETOtr expression levels as estimated using eGFP (Figure 1E), indicating that the observed integrin upregulation is RUNX1/ETOtr dose-dependent. Similar results were obtained with transduced murine lin- BM cells (Figures 1F). Expression of the full length RUNX1/ETO also up-regulated the integrin subunits in FDCP-mix and lin- mBM cells, albeit to a lesser extent (Figure 1G and data not shown). To analyze VLA-4 adhesion molecule expression in an in vivo RUNX1/ETO model, we utilized a recently described leukemia model generated via transplantation of RUNX1/ETOtr-expressing lin- mBM cells into lethally irradiated syngenic mice. As shown by others,3,4 RUNX1/ETOtr enhanced the clonogenic potential and inhibited the differentiation of lin- mBM cells ex vivo, enhanced colony formation in the spleens of lethally irradiated and transplanted syngeneic mice, and induced a leukemia phenotype characterized by an enlarged spleen and the appearance of myeloblasts in the spleen (Online Supplementary Figure S1A-J). Bone marrow and spleen cells isolated from diseased and control mice were further analyzed for cell surface adhesion molecule expression using flow cytometry. Indeed, we found increased expression levels of α4, α5 and β1 subunits on bone marrow cells of diseased mice (Figure 1H). Of note, expression of the integrin αL subunit was down-regulated as shown previously in RUNX1/ETO positive cells,5 whereas the expression of integrin β2 and the chemokine receptor CXC family members CXCR3, CXCR4 and CXCR5 remained unaltered. A similar regulation pattern was observed in spleen cells (data not shown). In silico analyses with published chip-sequencing and gene expression array sets also identified VLA-4 integrin subunits as potential RUNX1/ETO targets and upregulation of α4 and β1 in human primary t(8;21)-positive (t(8;21)+) AML (Online Supplementary Figure S2).
Figure 1.
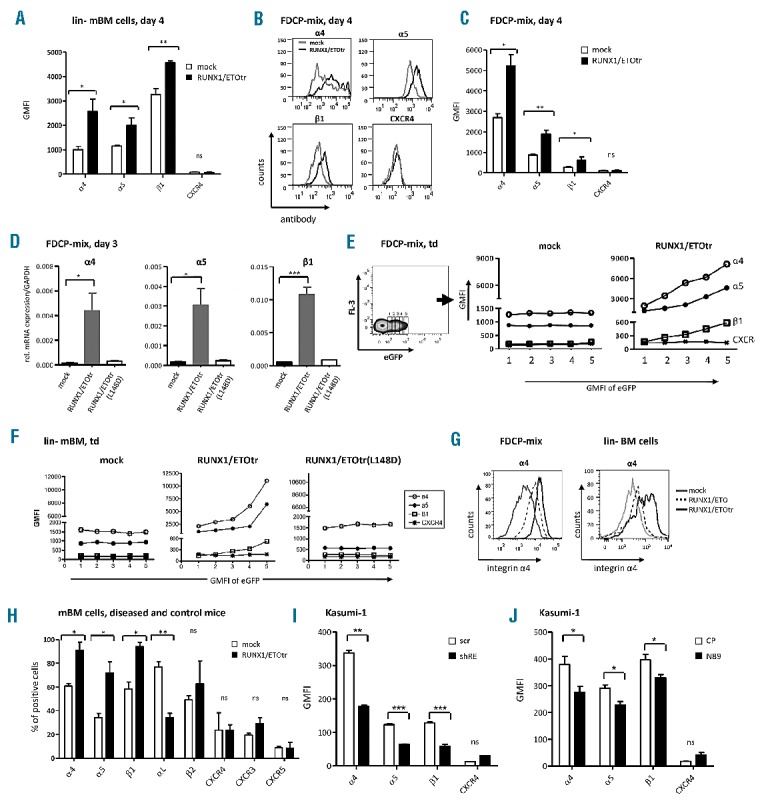
RUNX1/ETO up-regulates integrin α4, α5 and β1 subunit expression in hematopoietic progenitor cells. (A) lin- mBM cells were transduced with RUNX1/ETOtr and mock control lentiviral vectors and measured for integrin α4, α5 and β1 subunit and CXCR4 expression using flow cytometry on Day 4 after transduction. (B) Integrin subunit expression in transduced FDCP-mix cells at Day 4 after transduction. (C) Quantitative analysis of (B). (D) mRNA expression of the integrin α4, α5 and β1 subunits amplified using qPCR by the corresponding integrin primers (Applied Biosystems, Darmstadt, Germany) and normalized to GAPDH. Total RNA from FDCP-mix cells was isolated using an RNeasy micro kit (Qiagen, Hilden, Germany) on Day 3 after transduction. Next, 500 ng of RNA was reverse transcribed into cDNA using superscript III reverse transcriptase (Invitrogen, Darmstadt, Germany). (E and F) Representative examples of integrin α4, α5 and β1 subunit expression levels in transduced FDCP-mix and lin- mBM cells shown for different eGFP expression levels. (G) Integrin α4 subunit cell surface expression of FDCP-mix and lin- BM cells four days after transduction. (H) Analysis of adhesion molecule expression in bone marrow of mock- and RUNX1/ETOtr-lin- mBM cells transplanted mice. (I) Expression levels of α4, α5 and β1 integrin subunits and CXCR4 in t(8;21) positive Kasumi-1 cells transduced with lentiviral vectors expressing scramble control (scr) or shRNA targeting the breakpoint region of RUNX1/ETO (shRE) as measured by FACS. (J) Expression of α4, α5 and β1 integrin subunits and CXCR4 in Kasumi-1 cells transduced with lentiviral vectors expressing control peptides (CP) or RUNX1/ETO inhibitory peptides (N89) as measured by FACS. n=3; mean±SD. *P<0.05; **P<0.01; ***P<0.001; ns: not significant. GMFI: geometrical mean fluorescence intensity.
Further we asked if VLA-4 and VLA-5 integrin upregulation is maintained by RUNX1/ETO in the transformed human leukemia cell line Kasumi-1, derived from a t(8;21)+ AML patient. Kasumi-1 cells, which express RUNX1/ETO and to a lesser extent RUNX1/ETOtr,4 bear high levels of VLA-4 whereas the integrin αL subunit is absent in these cells. We specifically down-regulated RUNX1/ETO via lentivirally delivered shRNA targeting the RUNX1/ETO breakpoint sequences (shRE), which are present in both full length and truncated forms (Online Supplementary Figure S3). At Day 4 after transduction with vectors co-expressing shRE and eGFP, α4, α5 and β1 expression levels were significantly reduced as assessed using flow cytometry, while CXCR4 levels remained unaltered (Figure 1I). Similar results were obtained with NHR2 competitive peptides (N89) (Figure 1J), which also interfere with both RUNX1/ETO forms by disrupting RUNX1/ETO tetramer formation.6 These results suggest that integrin subunit expression remains dependent on continuous RUNX1/ETO expression and function in transformed human t(8;21) positive leukemic cells. Similarly, VLA-4 expression levels in Kasumi-1 cells decreased after enforced expression of the nuclear repressor protein promyelocytic leukemia zinc finger (PLZF) which has been recently shown to repress the transcription of VLA-4 in hematopoietic progenitors.7 Interestingly, RUNX1/ETO has been described as a dominant negative inhibitor of PLZF,8 thereby suggesting that RUNX1/ETO could further activate VLA-4 expression by inhibiting the repressive function of PLZF on VLA-4 transcription (Online Supplementary Figure S1K and L).
We finally examined whether RUNX1/ETO-triggered α4, α5 and β1 upregulation induces changes in cellular adhesion and migration behavior. In fact, RUNX1/ETOtr activated the adhesion of lin- mBM cells to the natural VLA-4 ligand, VCAM-1, under increasing rates of shear stress. Similar results were obtained for human leukemic t(8;21) positive Kasumi-1 cells compared to mobilized human hematopoietic CD34+ progenitor cells (Figure 2A), showing that RUNX1/ETO activates VLA-4-dependent cellular adhesion. However, adhesion to plasma fibronectin, harboring binding sites exclusively for VLA-5, was reduced (data not shown). Furthermore, adhesion of Kasumi-1 cells to VCAM-1-coated surfaces was reduced in the presence of N89 compared to control peptide (CP) expressing cells (Figure 2B). Interaction of Kasumi-1 cells to VCAM-1 additionally induced cell spreading as confirmed by cytoplasmic actin staining with phalloidin and cell nucleus staining with DAPI (data not shown), thereby indicating that VLA-4/VCAM-1 triggers changes in cytoskeletal dynamics. Therefore, we analyzed whether RUNX1/ETOtr also activates cellular migration. CP and N89 transduced Kasumi-1 cells were analyzed using human umbilical vein endothelial cell (HUVEC) transendothelial migration assay towards SDF-1α. Compared to control peptide-expressing Kasumi-1 cells N89 expressing counterparts show reduced migration ability towards SDF-1α, the natural ligand of the chemokine receptor CXCR4, which is critical for appropriate VLA-4 activation (Figure 2C). Expression of RUNX1/ETOtr also increased the directional migratory capacity of lin- mBM cells towards SDF-1α (Figure 2D). Moreover, the migratory capacity of t(8;21) positive Kasumi-1 cells towards SDF-1α across a HUVEC monolayer was almost completely inhibited by VLA-4 blocking monoclonal antibodies (Figure 2E). These results show that RUNX1/ETO induces adhesion and migration of leukemic cells via the VLA-4 integrin. As SDF-1α is produced at high levels in the bone marrow niche, RUNX1/ETO-induced VLA-4 upregulation and activation might allow strong cell recruitment and attachment of leukemic cells within the bone marrow niche. Accordingly, as reported recently, VLA-4 and CD44, which is also up-regulated by RUNX1/ETO,9 contribute to side population functionality of hematopoietic progenitor cells co-cultured with mesenchymal stromal cells.10
Figure 2.
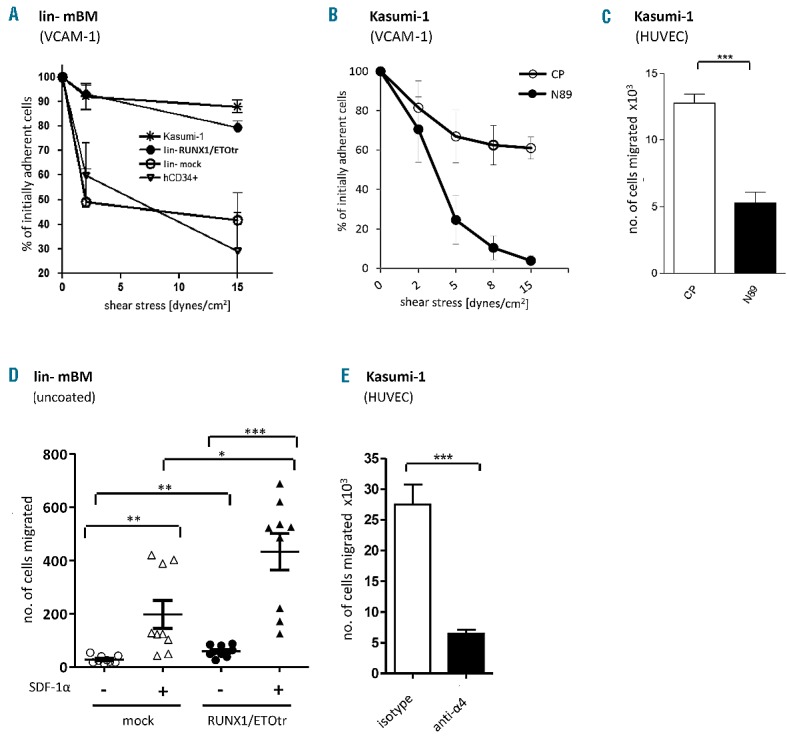
RUNX1/ETO activates VLA-4-dependent adhesion and migration of hematopoietic progenitor cells. (A) The adhesion efficiency of transduced lin- mBM cells, wild-type Kasumi-1 and mobilized human hematopoietic CD34+ cells on recombinant VCAM-1 (2 mg/μL; R&D Systems)-coated surfaces under shear stress was assessed using a parallel plate flow chamber. Before applying shear stress, the cells were incubated on recombinant VCAM-1-coated surfaces for 3 min without any additional stimulation. (B) Adhesion efficiency of transduced Kasumi-1 cells expressing control peptides (CP) and RUNX1/ETO inhibiting peptides (N89) on recombinant VCAM-1 (2 mg/μL; R&D Systems)-coated surfaces under shear stress was assessed using a parallel plate flow chamber. (C) Migration capacities of transduced Kasumi-1 cells were studied using transendothelial migration assay on Day 4 after transduction across a HUVEC monolayer towards SDF-1α. N89 expressing Kasumi-1 cells (black bar) show reduced migration ability towards SDF-1α as compared to control peptide (CP) expressing Kasumi-1 cells (white bar). (D) The migratory capacity of transduced lin- mBM cells towards SDF-1α was examined using transwell migration assays. SDF-1α (100 ng/μL) was aliquoted into the lower transwell chamber. Next, 1×105 cells were seeded on the upper chamber separated by a polycarbonate membrane with a pore size of 5 μm (Corning, Steinheim, Germany). The migrated cells in the lower chamber were quantified after 4 h of incubation at 37°C. (E) Migratory capacity of Kasumi-1 cells treated with isotype or anti-α4 blocking antibodies after overnight culture across a HUVEC monolayer towards SDF-1α. Data shown as mean±SD. *P<0.05; ** P<0.01; ***P<0.001; ns: not significant. VCAM-1: vascular cell adhesion molecule 1; SDF-1α: stromal derived factor 1α.
Taken together our data reveal a direct link between RUNX1/ETO and integrin VLA-4 expression and VLA-4-mediated adhesion and migration capabilities, which might play an important role in RUNX1/ETO-induced AML development. The inducible knockdown of VLA-4 subunits in de novo developing RUNX1/ETOtr triggered AML in mice might uncover a role of VLA-4 for the function of RUNX1/ETOtr during leukemia development. However, our results demonstrate for the first time that an AML-associated gene product directly controls integrin VLA-4 expression and function.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
Funding: the authors were supported by research grants from the German Cancer Aid (108695 to RH), the Jose’ Carreras Leukemia Foundation (DJCLS R 12/28, to CW and RH) and the LOEWE Center for Cell and Gene Therapy Frankfurt funded by the “Hessian Ministry of Higher Education, Research and the Arts” (CGT III L 4-518/17.004 to JL).
References
- 1.Peterson LF, Zhang DE. The 8;21 translocation in leukemogenesis. Oncogene. 2004;23(24):4255–62. [DOI] [PubMed] [Google Scholar]
- 2.Yan M, Burel SA, Peterson LF, Kanbe E, Iwasaki H, Boyapati A, et al. Deletion of an AML1-ETO C-terminal NcoR/SMRT-interacting region strongly induces leukemia development. Proc Natl Acad Sci USA. 2004;101(49):17186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hug BA, Lee SY, Kinsler EL, Zhang J, Lazar MA. Cooperative function of Aml1-ETO corepressor recruitment domains in the expansion of primary bone marrow cells. Cancer Res. 2002;62(10):2906–12. [PubMed] [Google Scholar]
- 4.Yan M, Kanbe E, Peterson LF, Boyapati A, Miao Y, Wang Y, et al. A previously unidentified alternatively spliced isoform of t(8;21) transcript promotes leukemogenesis. Nat Med. 2006;12(8):945–9. [DOI] [PubMed] [Google Scholar]
- 5.Puig-Kroger A, Sanchez-Elsner T, Ruiz N, Andreu EJ, Prosper F, Jensen UB, et al. RUNX/AML and C/EBP factors regulate CD11a integrin expression in myeloid cells through overlapping regulatory elements. Blood. 2003;102(9):3252–61. [DOI] [PubMed] [Google Scholar]
- 6.Wichmann C, Chen L, Heinrich M, Baus D, Pfitzner E, Zörnig M, et al. Targeting the oligomerization domain of ETO interferes with RUNX1/ETO oncogenic activity in t(8;21)-positive leukemic cells. Cancer Res. 2007;67(5):2280–9. [DOI] [PubMed] [Google Scholar]
- 7.Quaranta MT, Spinello I, Testa U, Mariani G, Diverio D, Foa R, et al. PLZF-mediated control on VLA-4 expression in normal and leukemic myeloid cells. Oncogene. 2006;25(3):399–408. [DOI] [PubMed] [Google Scholar]
- 8.Melnick A, Carlile GW, McConnell MJ, Polinger A, Hiebert SW, Licht JD. AML-1/ETO fusion protein is a dominant negative inhibitor of transcriptional repression by the promyelocytic leukemia zinc finger protein. Blood. 2000;96(12):3939–47. [PubMed] [Google Scholar]
- 9.Peterson LF, Wang Y, Lo MC, Yan M, Kanbe E, Zhang DE. The multi-functional cellular adhesion molecule CD44 is regulated by the 8;21 chromosomal translocation. Leukemia. 2007;21(9):2010–9. [DOI] [PubMed] [Google Scholar]
- 10.Malfuson JV, Boutin L, Clay D, Thepenier C, Desterke C, Torossian F, et al. SP/drug efflux functionality of hematopoietic progenitors is controlled by mesenchymal niche through VLA-4/CD44 axis. Leukemia. 2014;28(4):853–64. [DOI] [PubMed] [Google Scholar]


