Abstract
Natural killer cells are the first lymphocyte subset to reconstitute, and play a major role in early immunity after allogeneic hematopoietic stem cell transplantation. Cells expressing the activating receptor NKG2C seem crucial in the resolution of cytomegalovirus episodes, even in the absence of T cells. We prospectively investigated natural killer-cell reconstitution in a cohort of 439 adult recipients who underwent non-T-cell-depleted allogeneic hematopoietic stem cell transplantation between 2005 and 2012. Freshly collected blood samples were analyzed 3, 6, 12 and 24 months after transplantation. Data were studied with respect to conditioning regimen, source of stem cells, underlying disease, occurrence of graft-versus-host disease, and profiles of cytomegalovirus reactivation. In multivariate analysis we found that the absolute numbers of CD56bright natural killer cells at month 3 were significantly higher after myeloablative conditioning than after reduced intensity conditioning. Acute graft-versus-host disease impaired reconstitution of total and CD56dim natural killer cells at month 3. In contrast, high natural killer cell count at month 3 was associated with a lower incidence of chronic graft-versus-host disease, independently of a previous episode of acute graft-versus-host disease and stem cell source. NKG2C+CD56dim and total natural killer cell counts at month 3 were lower in patients with reactivation of cytomegalovirus between month 0 and month 3, but expanded greatly afterwards. These cells were also less numerous in patients who experienced later cytomegalovirus reactivation between month 3 and month 6. Our results advocate a direct role of NKG2C-expressing natural killer cells in the early control of cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation.
Introduction
Natural killer (NK) cells were first described as cytotoxic lymphocytes that do not require prior sensitization to kill tumor cells. Their dual role, both as a main component of the innate immunity and as a modulator of the adaptive immunity, is now well documented.1 NK cells are of major importance in allogeneic hematopoietic stem cell transplantation (HSCT) because of their ability to recognize and destroy abnormal leukocytes, such as leukemic cells.2 They are the first subset of lymphocytes that reconstitute after allogeneic HSCT, with an early expansion of the cytokine-producing CD56bright NK cell subset. We found that this is followed by the sequential expansion of an intermediate CD56brightCD16low subset, before the development of the dominant CD56dim subset, characterized by its higher cytotoxic activity.3
Transplant and disease-related factors can directly influence the upcoming immune reconstitution. Pre-transplant factors such as the source of stem cells, the conditioning regimen (myeloablative conditioning versus reduced intensity conditioning), and the underlying disease (malignant versus non-malignant) greatly influence the speed and the strength of immune reconstitution after allogeneic HSCT. This is of utmost importance, as fast and robust immune reconstitution may affect the clinical outcome in terms of graft-versus-host disease (GvHD), graft-versus-leukemia effect and the occurrence of opportunistic infections or viral reactivation. In addition, through their alloreactivity against recipient dendritic cells, donor-derived NK cells may well forestall GvHD.4
Despite great progresses made in the diagnosis and management of GvHD, the outcome of allogeneic HSCT remains compromised by a high morbidity rate with dysfunction of various organs and poor quality of life. Kim and colleagues associated the onset of acute GvHD with impaired immune reconstitution and delayed recovery of total NK cells at 1 month post-transplantation in a cohort of 59 children.5 However, to the best of our knowledge, no study has addressed the potential impact of acute GvHD on the recovery of NK cell subsets or the potential consequences of NK cell reconstitution on chronic GvHD and clinical outcome in a large cohort of adult patients.
Cytomegalovirus (CMV) is another major cause of morbidity in CMV-seropositive allogeneic HSCT recipients. We and others have shown the key role of both CD8+ and CD4+ CMV-specific T cells in the resolution of CMV reactivation after allogeneic HSCT.6–9 The marked susceptibility to herpesvirus in cases of NK cell deficiency is supporting evidence of the critical role of NK cells in host responses against viral infections.10,11 NK cell function is regulated by a diverse array of inhibitory and activating receptors, including killer cell immunoglobulin-like receptors, NKG2A, NKG2C, NKG2D, and natural cytotoxic receptors, defining functionally distinct subsets of NK cells within the total population.12 Guma and colleagues reported increased proportions of CD94/NKG2C+ NK cells from healthy CMV-seropositive donors, suggesting that viral infection may shape the NK receptor repertoire.13 They also showed that direct in vitro stimulation using virus-infected fibroblasts elicits a preferential expansion of CD94/NKG2C+ NK cells from CMV-seropositive healthy donors.14 In a case report of a child with severe combined immunodeficiency infected with CMV, it was noted that the infection was controlled after the expansion of potent NKG2C+ NK cells, indicating a direct role of this subset of cells in anti-CMV immunity.15
In this study, we prospectively evaluated the reconstitution of NK cell subsets in a cohort of 439 consecutive recipients of non-T-cell-depleted allogeneic HSCT, excluding those receiving cord blood transplants and children under the age of 15. Our first aim was to evaluate the influences of stem cell source, conditioning regimen and primary disease on NK subset reconstitution. We then assessed the impact of NK cell subset reconstitution on clinical outcomes, especially the occurrence of chronic GvHD and transplant-related mortality. Finally, we evaluated the interplay between the kinetics of the reconstitution of NK cell subsets and reactivation of CMV.
Methods
Patients’ characteristics and blood samples
We prospectively studied 439 adult patients over the age of 15, who underwent allogeneic HSCT from an HLA-identical sibling (n=237), an HLA-matched, unrelated donor (n=151) or an HLA-mismatched, unrelated donor (n=51). All recipients received a non-T-cell-depleted allogeneic transplant at the Hematology and Bone Marrow Transplantation Unit, Saint-Louis Hospital (Paris, France) between 2005 and 2012. The source of stem cells was unmanipulated bone marrow or peripheral blood stem cells. The characteristics of the patients and their transplants are presented in Online Supplementary Table S1.
All patients gave their informed consent to participation in this study, which was approved by the Institutional Review Board of Saint-Louis Hospital and performed in accordance with the Declaration of Helsinki.
Flow cytometry and monoclonal antibodies
Lymphocyte eight-color immunophenotyping was performed on freshly collected whole blood samples, using a FACS Canto II flow cytometer and FACS DIVA software (BD Biosciences, Le Pont de Claix, France). The absolute lymphocyte count was determined using the TruCount system (BD Biosciences) with anti-CD3-FITC, -CD8-PE, -CD45-PerCP, -CD4-APC, -CCR7-PECy7 and -C45RA-APC monoclonal antibodies (BD Multitest, BD Biosciences).
Total NK cells, CD56bright and CD56dim NK cell populations were defined using the gating strategy shown in Online Supplementary Figure S1.
Virological monitoring
Patients were monitored for CMV infection once weekly using quantitative real-time polymerase chain reaction analysis of whole blood collected in EDTA, as previously described.16,17
CMV reactivation was defined as a CMV viral load of over 1000 copies/mL. Pre-emptive antiviral therapy was given when the viral load reached this threshold on two successive determinations.
Definition of the main clinical outcomes
Acute GvHD and chronic GvHD were diagnosed and graded according to published criteria.18 The incidence of acute GVHD grades 0–1 and grades 2–4 was 51.8% and 48.2%, respectively.
Transplant-related mortality was defined as death after complete remission and its cumulative incidence was determined. Relapses and deaths resulting from progressive disease were competing events. Patients lost to follow-up were censored at the last known point of study.
Statistical analyses
Recipient and transplant-related characteristics such as source of stem cells, conditioning regimen, underlying disease, recipient age, recipient/donor CMV serological status, and acute GvHD were defined as risk factors that could potentially influence the post-HSCT outcomes of interest in this study, i.e. NK reconstitution, transplant-related mortality, chronic GvHD, and CMV reactivation.
Univariate and multivariate methods developed by Fine and Gray for competing risk studies19 were used to estimate the impact of NK cell counts at month 3 and other risk factors on these outcomes.
All tests were two-sided, with a type I error rate fixed at 0.05. All analyses were performed using SPSS 20 and R 2.6.2 statistical software (R packages “cmprsk” for competing risks, The R Foundation for Statistical Computing, Vienna, Austria).
Results
Early reconstitution of natural killer cell subsets is dependent on the conditioning regimen and the occurrence of acute graft-versus-host disease
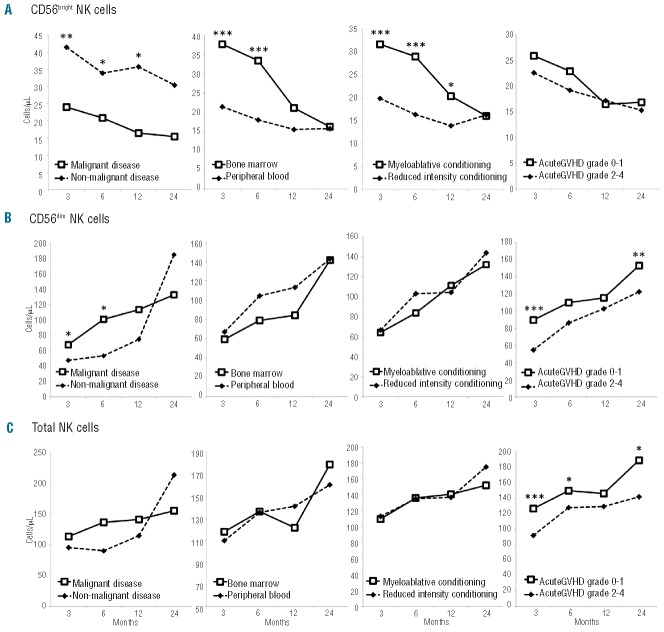
NK cell counts and subsets were determined at months 3, 6, 12 and 24 after allogeneic HSCT. Our results confirmed previous data showing that early NK cell reconstitution is due to a substantial expansion of the CD56bright population at month 3 and also showed that this population remains increased over a year following transplantation. HLA compatibility was not associated with any difference in NK cell subset count at any time point (data not shown). We analyzed the reconstitution of CD56bright, CD56dim and total NK cells in different transplantation settings, with regard to the underlying primary disease, the conditioning regimen, the source of stem cells and the occurrence of acute GvHD. Absolute CD56bright NK cell counts were significantly increased at months 3 and 6 in patients treated for non-malignant diseases. Similar findings were observed in those transplanted with bone marrow and in patients who received a myeloablative conditioning regimen (Figure 1A). A multivariate analysis by ANOVA showed that the conditioning regimen was the decisive factor influencing CD56bright subset counts at month 3 (P=0.008, Table 1).
Figure 1.
Kinetics of NK cell subset reconstitution after allogeneic HSCT. The median absolute counts of NK cell subsets (cells/μL) are shown at months 3, 6, 12 and 24. (A) CD56bright NK cells. (B) CD56dim NK cells. (C) Total NK cells. *P<0.05; **P≤0.01; ***P≤0.001.
Table 1.
Multivariate analysis of variance (ANOVA test) of parameters influencing NK cell subset counts at month 3.

CD56dim and total NK cell counts at months 3 and 6 were not statistically different between patients with either underlying malignant or non-malignant disease, transplanted with either bone marrow or peripheral blood stem cells, and conditioned with either a myeloablative or reduced intensity regimen (Figure 1B, C, three left panels). However, they were significantly lower in patients who had had acute GvHD grades 2–4 (P=0.0001 at month 3 and P=0.043 at month 6, Figure 1B,C, right panels). Although myeloablative conditioning was a factor associated with a higher risk of acute GvHD than was reduced intensity conditioning (myeloablative conditioning = 58% versus reduced intensity conditioning = 41%, P=0.00005), acute GvHD was shown to be the decisive factor influencing total NK and CD56dim subset counts at month 3 in a multivariate analysis by ANOVA (P=0.0001 and P=0.001, respectively, Table 1).
Finally, absolute counts of NK subsets reached similar values at month 24, regardless of the pre-transplant settings.
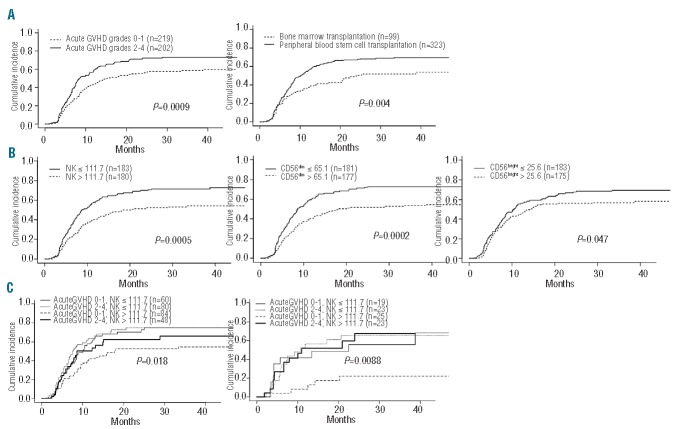
Absolute natural killer cell count is an independent risk factor for chronic graft-versus-host disease
As expected,20–23 the cumulative incidence of chronic GvHD was significantly higher in the group of patients who had had acute GvHD (grades 2–4 versus grades 0–1, P=0.0009, Figure 2A). The risk of chronic GvHD was also higher in patients who received peripheral blood stem cell grafts than in those who received bone marrow grafts (P=0.004, Figure 2A). We did not find any association between the CD34+ cell dose or the conditioning regimen and the incidence of chronic GvHD (data not shown).
Figure 2.
Risk factors for chronic GvHD. Cumulative incidence of chronic GvHD is displayed according to (A) the severity of acute GvHD and the source of stem cells, and (B) the absolute counts of NK cell subsets at month 3. (C) Combinatorial analysis of the risk factors for chronic GvHD in patients transplanted with peripheral blood stem cells (left panel) and with bone marrow (right panel).
To assess the influence of NK reconstitution on subsequent occurrence of chronic GvHD, we separated the patients into two groups according to whether their NK cell subset counts at month 3 were below or above the median of the entire cohort. Figure 2B shows a side-by-side comparison of these groups in terms of cumulative incidence of chronic GvHD. The occurrence of chronic GvHD was less frequent among patients with total NK, CD56dim and, to a lesser extent, CD56bright NK cell counts above the median at month 3 (P=0.0005, P=0.0002 and P=0.047, respectively). A multivariate analysis using a Fine-Gray regression model confirmed that the occurrence of acute GvHD grades 2–4, the source of stem cells and the total NK cell count at month 3 were all independent risk factors for the development of chronic GvHD (P=0.018, P=0.013 and P=0.012, respectively). The relative risk of chronic GvHD associated with a NK cell count below the median (<111.7/μL) at month 3 was 1.8, while the relative risk of chronic GvHD associated with previous occurrence of acute GvHD was 1.7.
In a combinatorial analysis presented in Figure 2C, we showed that these three factors had a cumulative impact on the incidence of chronic GvHD. The group of patients with the lowest risk of chronic GvHD, far below that of any other group of patients, was that with the triple combination of a NK cell count at month 3 above the median, a bone marrow transplantation and absence or low grade acute GvHD.
Natural killer cell count reliably stratifies the risk of treatment-related mortality
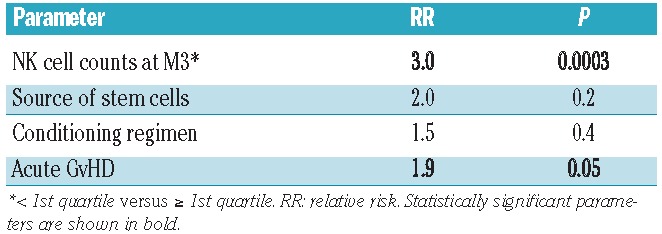
In our cohort of 439 patients, the cumulative incidence of treatment-related mortality was significantly higher in patients with grades 2–4 acute GvHD than in those with grade 0 or 1 (P=0.0088). To assess the influence of NK cell counts at month 3 on treatment-related mortality, we distinguished patients with total NK cell counts below or above the median. We observed no significant difference between the two groups (data not shown). Nonetheless, as illustrated in Figure 3A, the cumulative incidence of treatment-related mortality was much higher in the group of patients with total NK cell counts in the lowest quartile (P=0.0005), and this was observed regardless of the acute GvHD grade (Figure 3B,C). No significant difference was observed between all three other quartiles. This result was supported by a multivariate analysis taking into account total NK cell count at month 3, the source of stem cells, the type of conditioning regimen and the occurrence of acute GvHD. Patients with NK cell counts at month 3 in the lowest quartile had a three times greater risk of transplant-related mortality than that of patients in all the other quartiles (P=0.0003, Table 2). The same result was observed concerning CD56dim (P=0.00053) but not CD56bright NK cells (P=NS).
Figure 3.

NK cell counts at month 3 and treatment-related mortality (TRM). Cumulative incidence of TRM is displayed according to absolute counts of total NK cells at month 3 (lower quartile < 62.8/μL) in the entire cohort (A), in patients who developed acute GvHD grades 0–1 (B), and in patients who developed acute GvHD grades 2–4 (C).
Table 2.
Multivariate analysis (Fine and Gray competing risk model) of risk factors for treatment-related mortality.
Cytomegalovirus reactivation and natural killer cell reconstitution
Two-hundred and forty-eight patients were CMV-seropositive before transplantation and 61.5% of them had a CMV reactivation before month 3. We assessed whether NK cell count at month 3 was associated with previous CMV reactivation. Our data showed that total NK cell and CD56dim NK cell counts were decreased in patients with previous CMV reactivation (P=0.01 and P=0.036, respectively), whereas CD56bright NK cell count was not significantly differ ent. The difference was even more significant when considering NKG2C+ subsets whereas the counts of NK cells expressing NKG2A were similar regardless of CMV reactivation status (Figure 4A). CMV-seropositive recipients (R+) who did not develop CMV reactivation before month 3 had higher counts of NKG2C+ NK cells at month 3 (P=0.004), whereas patients with CMV reactivation had low levels, similar to those in seronegative recipients (R-) transplanted from seronegative donors (D-) (Figure 4B). In the case of CMV reactivation, NKG2C+ NK cell count increased rapidly and reached higher values at month 12 than that in patients without CMV reactivation. The kinetics of NKG2C+ CD56dim NK cells was then evaluated according to CMV reactivation and to the serological status of donor/recipient pairs. In the absence of CMV reactivation (i.e. viral load below 1000 copies/mL), CMV-seropositive patients who received an allogeneic HSCT from seropositive donors (D+/R+) had higher counts of NKG2C+ CD56dim NK cells at month 3 than had patients with CMV reactivation (P=0.003). Strikingly, NKG2C+ CD56dim NK cell count was significantly higher at month 3 in D+/R+ patients than in D−/R+ patients in whom CMV was not reactivated (P=0.03) (Figure 4C). NKG2C+ NK cell count remained low and stable in the absence of CMV reactivation before month 3 in D−/R+ patients. Similarly to total NKG2C+ NK cells, the NKG2C+ CD56dim NK subset markedly increased after CMV reactivation, and during the 12 months of follow up.
Figure 4.
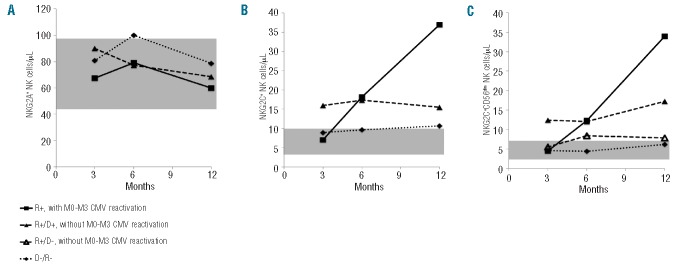
Reconstitution kinetics of NKG2C+ NK cells. (A) and (B) Counts of total NKG2A+ and NKG2C+ NK cells (cells/μL) (respectively) are shown at months 3, 6 and 12, according to the occurrence of CMV reactivation between month 0 (M0) and month 3 (M3). (C) Counts of NKG2C+ CD56dim NK cells (cells/μL) are shown according to the serological status of donor/recipient pairs and CMV reactivation. “D−/R−” stands for CMV-seronegative recipients, transplanted from CMV-seronegative donors with no evidence of CMV infection. The gray area indicates the interquartile range in a blood donor population (n = 45).
Finally, being aware of the known increased risk of CMV reactivation after a previous one, and to get insight into whether NK cell count at month 3 might be predictive of subsequent CMV reactivation, we separated patients into two other groups depending on the occurrence of CMV reactivation between month 3 and month 6. Our results showed that NKG2C+ total and CD56dim NK cell counts were significantly increased at month 3 in patients who did not experience any subsequent reactivation (P=0.011 and P=0.007, respectively, Online Supplementary Figure S2A–C).
T-cell-mediated immunity is an essential host process in the control of CMV reactivation. We found a strong correlation between CD56dim NK cell and total CD8+ T-cell counts, at month 3 and afterwards, only in the case of CMV reactivation. This correlation reached a higher significance with activated CD25+CD8+ T-cells (P<0.0001) (Online Supplementary Figure S3) and, to a lesser extent, with terminally differentiated memory CD8+ T-cells (P=0.01, data not shown). These data suggest that CMV may condition the magnitude and the shape of both innate and adaptive immunity.
Discussion
Outcomes in the first few months following allogeneic HSCT remain compromised by the risk of acute GvHD and infections. In particular, the possibility of reactivation of latent viruses such as Epstein-Barr virus24 and CMV25 has been associated with the long-lasting immunodeficiency that follows transplantation. In this context, NK cells are the first lymphoid subset to be replenished and their numbers become normal within 1–2 months after HSCT,26 so their impact on outcome is likely to be predominant early after HSCT. We have previously reported that NK cell frequency in lymphocytes – but not their absolute number – is higher in HSCT recipients during the first 3 months after transplant than it is in HSCT donors.3 In this single-center, longitudinal study, we analyzed the association between the reconstitution of NK subsets and outcome of allogeneic HSCT in the largest cohort of non-T-cell-depleted hematopoietic transplant recipients studied so far, to the best of our knowledge.
These data are consistent with our previous findings in 43 patients who underwent HLA-matched non-T-cell-depleted HSCT, showing that the early post-transplant period is dominated by CD56bright NK cells.3 Here, we clearly demonstrate that CD56bright NK cell count depended on the conditioning regimen and was higher among patients who received myeloablative conditioning. This result may be explained by a greater availability of the bone marrow, allowing primary expansion of the cells within a poor cellular environment. We also show that persistently low CD56dim NK cell counts were associated with the occurrence of grade 2–4 acute GvHD. As the CD56bright subset was not affected, we therefore observed a profound defect in CD56dim NK cells during acute GvHD, the mechanism of which remains to be elucidated. In addition, low total NK cell count at month 3 was associated with a higher risk of chronic GvHD and constituted another independent risk factor, together with the source of stem cells and occurrence of acute GvHD.27–29 The favorable impact of NK cells on GvHD has been extensively reported both in mice and humans receiving mismatched hematopoietic transplants or alloreactive infused NK cells.2,30 NK cells are assumed to protect against GvHD by inhibiting alloreactive donor T cells and to support graft-versus-leukemia effects in mouse models.31,32 Based on a study of 43 patients, Chang and colleagues suggested that NK cell count could be used to predict clinical outcomes after unmanipulated haploidentical transplantation.33 Only a few studies have investigated the role of NK cells in HLA-matched allogeneic HSCT.34 A higher dose of infused donor-derived NK cells was found to be associated with a lower incidence of chronic GvHD.35 In another study, low total NK cell counts were detected at months 3, 6 and 12 after transplantation in patients with extensive chronic GvHD, but due to the limited cohort of patients, multivariate analysis especially taking into account previous acute GvHD could not be performed.36
We show here that low NK cell count at month 3 was strongly associated with an increased risk of treatment-related mortality, which is mainly due to chronic GvHD and infections. This relationship was not linear as only patients with NK cell counts in the lowest quartile (< 62.8/mL) had a three-fold increased risk of treatment-related mortality.
NK cells provide a first line of defense against several types of viruses including CMV. Although these cells have traditionally been classified as cells of the innate immune system, compelling evidence indicates that they share many similarities with cytotoxic T lymphocytes. Consistent studies have shown that CMV stimulates the expansion of a specific CD56dim NKG2C+ NK cell subset in different settings. Levels of NK cells expressing NKG2C were increased in patients experiencing acute CMV infection after solid organ transplantation37,38 and expansion of potent NKG2C+ NK cells was evidenced in CMV-reactivating recipients of allogeneic HSCT or unrelated CMV-naïve cord blood grafts.39–41 In addition, recovery of NK cell function has been correlated with protection against CMV.42 Although the studies providing this information were conducted on relatively small cohorts, the results indicate that the reconstitution of NK cells after allogeneic HSCT may be decisive in the early control of CMV reactivation. NK cells from mice challenged with murine CMV undergo all four phases of an immune response against a pathogen: clonal-like expansion, involving the Ly49H+ NK cell subset, contraction and then a memory-like phase, with heightened responses to re-challenge by the same antigen.43 Similarly, human CMV has been reported to induce a persistent reconfiguration of the NK cell compartment, with the expansion of NK cells bearing the NKG2C receptor, in healthy individuals as well as in patients with different pathologies.13,44,45
This expansion of activated and differentiated NKG2C+ NK cells was recently described upon additional encounters with viruses, such as Hantavirus, human immunodeficiency virus, hepatitis B virus and hepatitis C virus.46–48 However, the mechanism by which CMV drives NKG2C+ NK cells is unknown, and the ligand for NKG2C remains elusive. In our study, patients with CMV reactivation before month 3 had significantly fewer CD56dim NKG2C+ NK cells at month 3 but their counts increased significantly afterwards. Strikingly, in the absence of CMV reactivation (or in case of viral loads below 1000 copies/mL), the counts of this NK subset were higher in seropositive patients who received HSCT from seropositive donors (R+/D+) than in D−/R+ and D−/R− patients. This observation may be related to the fact that CMV viral loads below the therapeutic threshold are sufficient to provide low levels of chronic stimulation in D+/R+ recipients, allowing the expansion of transplantable NKG2C+ NK cells with memory-like properties, as previously suggested.40 We, therefore, hypothesize that a threshold level of NKG2C+ NK cells above normal values in the first 3 months after allogeneic HSCT may protect against CMV reactivation. In turn, in the case of CMV reactivation, the proliferation of NKG2C+ NK cells is boosted.
To our knowledge, this is the first study analyzing the reconstitution of CD56bright, CD56dim, and total NKG2C+ NK cells with regard to acute and chronic GvHD, CMV reactivation and treatment-related mortality in allogeneic HSCT settings. Our data highlight the favorable role of early, efficient NK cell reconstitution after allogeneic HSCT on clinical outcome. Determination of NK cell subset counts is a simple flow cytometry analysis feasible on a routine basis to monitor post-transplant immune reconstitution. We suggest its use to help in stratifying the risk of chronic GvHD, treatment-related mortality and CMV reactivation.
Acknowledgments
This work was supported by INSERM and AP-HP (Translational Research Grant in Biology 2010, #RTB10002). The authors would like to thank the technicians of the Laboratory of Immunology of Saint-Louis Hospital for their invaluable contribution to this work.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Campbell KS, Hasegawa J. Natural killer cell biology: an update and future directions. J Allergy Clin Immunol. 2013;132(3):536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–100. [DOI] [PubMed] [Google Scholar]
- 3.Dulphy N, Haas P, Busson M, Belhadj S, Peffault de Latour R, Robin M, et al. An unusual CD56(bright) CD16(low) NK cell subset dominates the early posttransplant period following HLA-matched hematopoietic stem cell transplantation. J Immunol. 2008;181(3):2227–37. [DOI] [PubMed] [Google Scholar]
- 4.Della Chiesa M, Falco M, Muccio L, Bertaina A, Locatelli F, Moretta A. Impact of HCMV infection on NK cell development and function after HSCT. Front Immunol. 2013;4:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HO, Oh HJ, Lee JW, Jang PS, Chung NG, Cho B, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation in children: a single institution study of 59 patients. Korean J Pediatr. 2013;56(1):26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gratama JW, van Esser JW, Lamers CH, Tournay C, Lowenberg B, Bolhuis RL, et al. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98(5):1358–64. [DOI] [PubMed] [Google Scholar]
- 7.Moins-Teisserenc H, Busson M, Scieux C, Bajzik V, Cayuela JM, Clave E, et al. Patterns of cytomegalovirus reactivation are associated with distinct evolutive profiles of immune reconstitution after allogeneic hematopoietic stem cell transplantation. J Infect Dis. 2008;198(6):818–26. [DOI] [PubMed] [Google Scholar]
- 8.Pourgheysari B, Piper KP, McLarnon A, Arrazi J, Bruton R, Clark F, et al. Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT. Bone Marrow Transplant. 2009;43(11):853–61. [DOI] [PubMed] [Google Scholar]
- 9.Tormo N, Solano C, Benet I, Clari MA, Nieto J, de la Camara R, et al. Lack of prompt expansion of cytomegalovirus pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells is associated with rising levels of pp65 antigenemia and DNAemia during pre-emptive therapy in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2010;45(3):543–9. [DOI] [PubMed] [Google Scholar]
- 10.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320(26):1731–5. [DOI] [PubMed] [Google Scholar]
- 11.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4(15):1545–58. [DOI] [PubMed] [Google Scholar]
- 12.Cheent K, Khakoo SI. Natural killer cells: integrating diversity with function. Immunology. 2009;126(4):449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104(12):3664–71. [DOI] [PubMed] [Google Scholar]
- 14.Guma M, Budt M, Saez A, Brckalo T, Hengel H, Angulo A, et al. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107(9):3624–31. [DOI] [PubMed] [Google Scholar]
- 15.Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, Roosnek E. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112(3):914–5. [DOI] [PubMed] [Google Scholar]
- 16.Gouarin S, Vabret A, Scieux C, Agbalika F, Cherot J, Mengelle C, et al. Multicentric evaluation of a new commercial cytomegalovirus real-time PCR quantitation assay. J Virol Methods. 2007;146(1–2):147–54. [DOI] [PubMed] [Google Scholar]
- 17.Schnepf N, Scieux C, Resche-Riggon M, Feghoul L, Xhaard A, Gallien S, et al. Fully automated quantification of cytomegalovirus (CMV) in whole blood with the new sensitive Abbott RealTime CMV assay in the era of the CMV international standard. J Clin Microbiol. 2013;51(7):2096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigorito AC, Campregher PV, Storer BE, Carpenter PA, Moravec CK, Kiem HP, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114(3):702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446): 496–509. [Google Scholar]
- 20.Blaise D, Kuentz M, Fortanier C, Bourhis JH, Milpied N, Sutton L, et al. Randomized trial of bone marrow versus lenograstim-primed blood cell allogeneic transplantation in patients with early-stage leukemia: a report from the Societe Francaise de Greffe de Moelle. J Clin Oncol. 2000;18(3):537–46. [DOI] [PubMed] [Google Scholar]
- 21.Morton J, Hutchins C, Durrant S. Granulocyte-colony-stimulating factor (G-CSF)-primed allogeneic bone marrow: significantly less graft-versus-host disease and comparable engraftment to G-CSF-mobilized peripheral blood stem cells. Blood. 2001;98(12):3186–91. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002;100(3): 761–7. [DOI] [PubMed] [Google Scholar]
- 23.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clave E, Agbalika F, Bajzik V, Peffault de Latour R, Trillard M, Rabian C, et al. Epstein-Barr virus (EBV) reactivation in allogeneic stem-cell transplantation: relationship between viral load, EBV-specific T-cell reconstitution and rituximab therapy. Transplantation. 2004;77(1):76–84. [DOI] [PubMed] [Google Scholar]
- 25.Mori T, Kato J. Cytomegalovirus infection/disease after hematopoietic stem cell transplantation. Int J Hematol. 2010; 91(4):588–95. [DOI] [PubMed] [Google Scholar]
- 26.Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol. 2012; 19(4):324–35. [DOI] [PubMed] [Google Scholar]
- 27.Atkinson K, Horowitz MM, Gale RP, van Bekkum DW, Gluckman E, Good RA, et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood. 1990;75(12): 2459–64. [PubMed] [Google Scholar]
- 28.Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28(3):250–9. [PubMed] [Google Scholar]
- 29.Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone Marrow Transplant. 2001;28(2):121–9. [DOI] [PubMed] [Google Scholar]
- 30.Velardi A, Ruggeri L, Mancusi A, Burchielli E, Perruccio K, Aversa F, et al. Clinical impact of natural killer cell reconstitution after allogeneic hematopoietic transplantation. Semin Immunopathol. 2008;30(4):489–503. [DOI] [PubMed] [Google Scholar]
- 31.Asai O, Longo DL, Tian ZG, Hornung RL, Taub DD, Ruscetti FW, et al. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest. 1998; 101(9):1835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115(21):4293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang YJ, Zhao XY, Huang XJ. Effects of the NK cell recovery on outcomes of unmanipulated haploidentical blood and marrow transplantation for patients with hematologic malignancies. Biol Blood Marrow Transplant. 2008;14(3):323–34. [DOI] [PubMed] [Google Scholar]
- 34.Pidala J, Sarwal M, Roedder S, Lee SJ. Biologic markers of chronic GVHD. Bone Marrow Transplant. 2014;49(3):324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larghero J, Rocha V, Porcher R, Filion A, Ternaux B, Lacassagne MN, et al. Association of bone marrow natural killer cell dose with neutrophil recovery and chronic graft-versus-host disease after HLA identical sibling bone marrow transplants. Br J Haematol. 2007;138(1):101–9. [DOI] [PubMed] [Google Scholar]
- 36.Abrahamsen IW, Somme S, Heldal D, Egeland T, Kvale D, Tjonnfjord GE. Immune reconstitution after allogeneic stem cell transplantation: the impact of stem cell source and graft-versus-host disease. Haematologica. 2005;90(1):86–93. [PubMed] [Google Scholar]
- 37.Hadaya K, de Rham C, Bandelier C, Bandelier C, Ferrari-Lacraz S, Jendly S, et al. Natural killer cell receptor repertoire and their ligands, and the risk of CMV infection after kidney transplantation. Am J Transplant. 2008;8(12):2674–83. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci USA. 2011;108(36):14725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Della Chiesa M, Falco M, Podesta M, Locatelli F, Moretta L, Frassoni F, et al. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus? Blood. 2012;119(2):399–410. [DOI] [PubMed] [Google Scholar]
- 40.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012;189(10): 5082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119(11):2665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barron MA, Gao D, Springer KL, Patterson JA, Brunvand MW, McSweeney PA, et al. Relationship of reconstituted adaptive and innate cytomegalovirus (CMV)-specific immune responses with CMV viremia in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49(12):1777–83. [DOI] [PubMed] [Google Scholar]
- 43.Sun JC, Lanier LL. The natural selection of herpesviruses and virus-Specific NK cell receptors. Viruses. 2009;1(3):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malmberg KJ, Beziat V, Ljunggren HG. Spotlight on NKG2C and the human NK-cell response to CMV infection. Eur J Immunol. 2012;42(12):3141–5. [DOI] [PubMed] [Google Scholar]
- 45.Muntasell A, Vilches C, Angulo A, Lopez-Botet M. Adaptive reconfiguration of the human NK-cell compartment in response to cytomegalovirus: a different perspective of the host-pathogen interaction. Eur J Immunol. 2013;43(5):1133–41. [DOI] [PubMed] [Google Scholar]
- 46.Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beziat V, Dalgard O, Asselah T, Halfon P, Bedossa P, Boudifa A, et al. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol. 2012;42(2): 447–57. [DOI] [PubMed] [Google Scholar]
- 48.Thomas R, Low HZ, Kniesch K, Jacobs R, Schmidt RE, Witte T. NKG2C deletion is a risk factor of HIV infection. AIDS Res Hum Retroviruses. 2012;28(8):844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]