Abstract
A proportion of patients with aplastic anemia who are treated with immunosuppressive therapy develop clonal hematologic disorders, including post-aplastic anemia myelodysplastic syndrome. Many will proceed to allogeneic hematopoietic stem cell transplantation. We identified 123 patients with post-aplastic anemia myelodysplastic syndrome who from 1991 through 2011 underwent allogeneic hematopoietic stem cell transplantation, and in a matched-pair analysis compared outcome to that in 393 patients with de novo myelodysplastic syndrome. There was no difference in overall survival. There were no significant differences with regard to 5-year probabilities of relapse, non-relapse mortality, relapse-free survival and overall survival; these were 14%, 40%, 46% and 49% for post-aplastic anemia myelodysplastic syndrome, and 20%, 33%, 47% and 49% for de novo myelodysplastic syndrome, respectively. In multivariate analysis, relapse (hazard ratio 0.71; P=0.18), non-relapse mortality (hazard ratio 1.28; P=0.18), relapse-free survival (hazard ratio 0.97; P=0.80) and overall survival (hazard ratio 1.02; P=0.88) of post-aplastic anemia myelodysplastic syndrome were similar to those of patients with de novo myelodysplastic syndrome. Cytogenetic risk was independently associated with overall survival in both groups. Thus, transplant success in patients with post-aplastic anemia myelodysplastic syndrome was similar to that in patients with de novo myelodysplastic syndrome, and cytogenetics was the only significant prognostic factor for post-aplastic anemia myelodysplastic syndrome patients.
Introduction
Immunosuppressive therapy (IST) and hematopoietic stem cell transplantation (HSCT) are the two main treatment strategies for patients with aplastic anemia (AA). While in most patients AA is immune-mediated and amenable to IST, younger patients with HLA-identical siblings often undergo HSCT as first-line therapy. Older individuals, on the other hand, and those without HLA-matched related donors are generally given IST, typically with antithymocyte globulin (ATG) with or without the addition of cyclosporine.1,2 Response rates with IST have ranged from 40% to 70%, and many responding patients are surviving long-term with normal or close to normal blood cell counts, not requiring further therapy.3–5 However, some patients, particularly among those who do not achieve sustained responses to IST, will show clonal evolution and present clinically with manifestations of paroxysmal nocturnal hemoglobinuria (PNH) or myelodysplastic syndrome (post-AA MDS) that may evolve to acute myeloid leukemia.6 Transformation to MDS has been reported in 1.7–15% of patients over observation periods of 5–11 years.7 Improvements in supportive care allow for prolonged survival of non-responding patients, even without HSCT, and the evolution of clonal hematopoietic disorders.
The pathogenesis of MDS (or acute myeloid leukemia) in AA patients may be related to immune dysregulation, possibly enhanced by IST, and increased growth factor drive in response to the proliferative demands during the disease course.7 Excessive telomere shortening leading to acquisition of chromosomal instability may contribute to clonal evolution.8 It is not clear whether MDS developing on that background differs from de novo MDS. However, immune-dysregulation and pro-inflammatory signals associated with the immune mechanism of AA may lead to a marrow environment in post-AA MDS patients, which differs from that in patients with de novo MDS. These differences may affect the prognosis and may impact the outcome of HSCT.
Allogeneic HSCT is a powerful therapeutic option for patients with MDS, including those with “secondary” MDS, be it due to prior cytotoxic therapy or related to an antecedent hematologic disorder such as AA.9–12 A previous analysis of transplant outcome in patients with therapy-related MDS showed survival rates of 19–21% at five years, depending on disease status at transplantation,10 while another study reported survival rates of 20–48% at three years.12 These studies focused on MDS patients who had received prior cytotoxic chemotherapy or radiation for various malignant diseases. A recent study of 17 children with AA who developed MDS or AML following IST reported a 5-year event-free survival of 41%.13 However, there no large-scale study of transplantation for post-AA MDS has been made, and there are no data comparing results to patients with de novo MDS. Thus, in the present study, we analyzed transplant outcomes of post-AA MDS patients, carried out a matched-pair analysis including patients with de novo MDS, and determined prognostic factors for transplant outcome.
Methods
Study population
In the databases of the Center for International Bone Marrow Transplantation Research (CIBMTR) and the Fred Hutchinson Cancer Research Center, we identified 123 patients transplanted from 1991 through 2011 for MDS developing after treatment of aplastic anemia with IST (post-AA MDS). Cases of marrow failure/AA possibly associated with constitutional/inherited disorders were excluded. The time interval from the diagnosis of AA to the establishment of the diagnosis of MDS was 4–491 months (median 34 months). The MDS categories comprised refractory anemia (RA), refractory anemia with ring sideroblasts (RARS), refractory anemia with excess blasts (RAEB), and refractory anemia with excess blasts in transformation (RAEB-t). The data bases did not allow for categorization according to the WHO classification, nor was it possible in this retrospective analysis to categorize patients according to the recently proposed five cytogenetic risk groups as used in the revised International Prognostic Scoring System (IPSS-R).14 Patients with AML (>30% marrow myeloblasts) and CMML were excluded. We conducted a matched pair analysis, matching cases to control on factors shown to influence transplantation outcomes. Overall, 1473 patients with de novo MDS were reported to the CIBMTR during the study period. Patients with MDS related to cytotoxic therapy, Fanconi anemia and other inherited marrow failure syndromes were excluded from the study. Cases were matched to controls from this cohort for age (< 10 years vs. ≥ 10 years), disease status at transplantation (RA/RARS vs. RAEB/RAEB-t), donor type (HLA-identical sibling vs. other relative vs. matched unrelated donor vs. mismatched unrelated donor), year of transplant (1991–1999 vs. 2000–2011), and conditioning regimen intensity (myeloablative vs. reduced intensity). Cases and controls were matched to their respective age as close as possible: the difference in age of cases and controls was 0–1 year for 56%, 2–5 years for 30%, and 6–10 years for the remaining 14%. For this latter sub-group, the median age for cases was 23 years, compared to 29 years for controls.
Definitions
Cytogenetic risk was graded into good, intermediate, and poor, based on the IPSS.14 Time of engraftment was defined as the first of three consecutive days with neutrophil counts that exceeded 0.5 ×109/L. Acute and chronic graft-versus-host disease (GvHD) were defined and graded according to standard criteria.15,16 Overall survival (OS) was defined as survival irrespective of disease status, and relapse-free survival (RFS) as survival without evidence of relapse at any point in time. Relapse incidence (RI) was defined as cumulative incidence of disease recurrence and non-relapse mortality (NRM) was defined as the occurrence of death without prior evidence of relapse.
Statistical analysis
The probabilities of hematopoietic recovery, acute and chronic GvHD, NRM and relapse were calculated using the cumulative incidence estimator.17 The probabilities of OS and RFS were calculated with the Kaplan-Meier estimator.18 Cox multivariate models19 were constructed to test whether there were any differences in outcome between the groups, adjusting for other factors that might influence outcome after HSCT. The main effect (post-AA MDS vs. de novo MDS) was held in all steps of model building regardless of the level of significance. Variables tested are shown in Tables 1 and 2. All variables that attained P-values of 0.05 or under were retained in the final model. All P-values are two-sided. Analyses were performed with SAS v.9.3 software (SAS, Cary, NC, USA).
Table 1.
Patients’ and disease characteristics in myelodysplastic syndrome patients evolving from aplastic anemia (post-AA MDS) and de novo MDS patients for matched pair analysis.
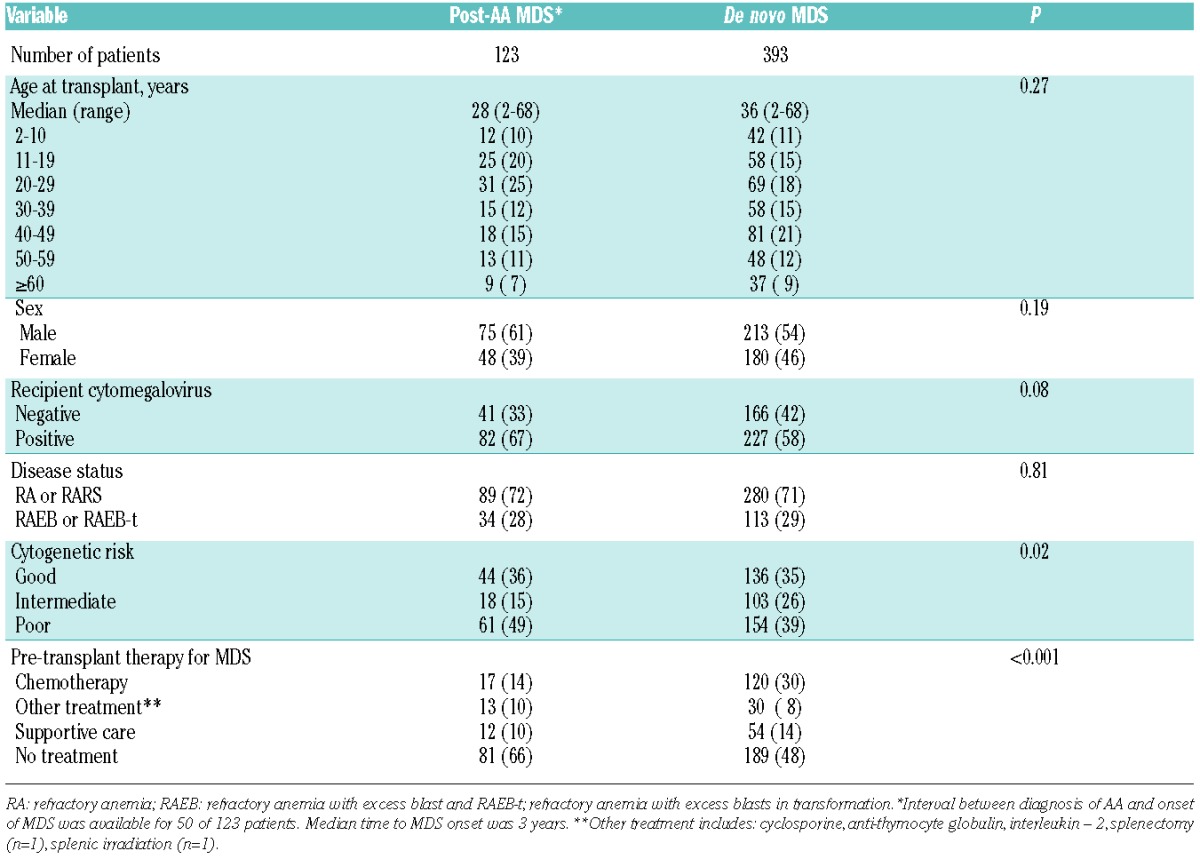
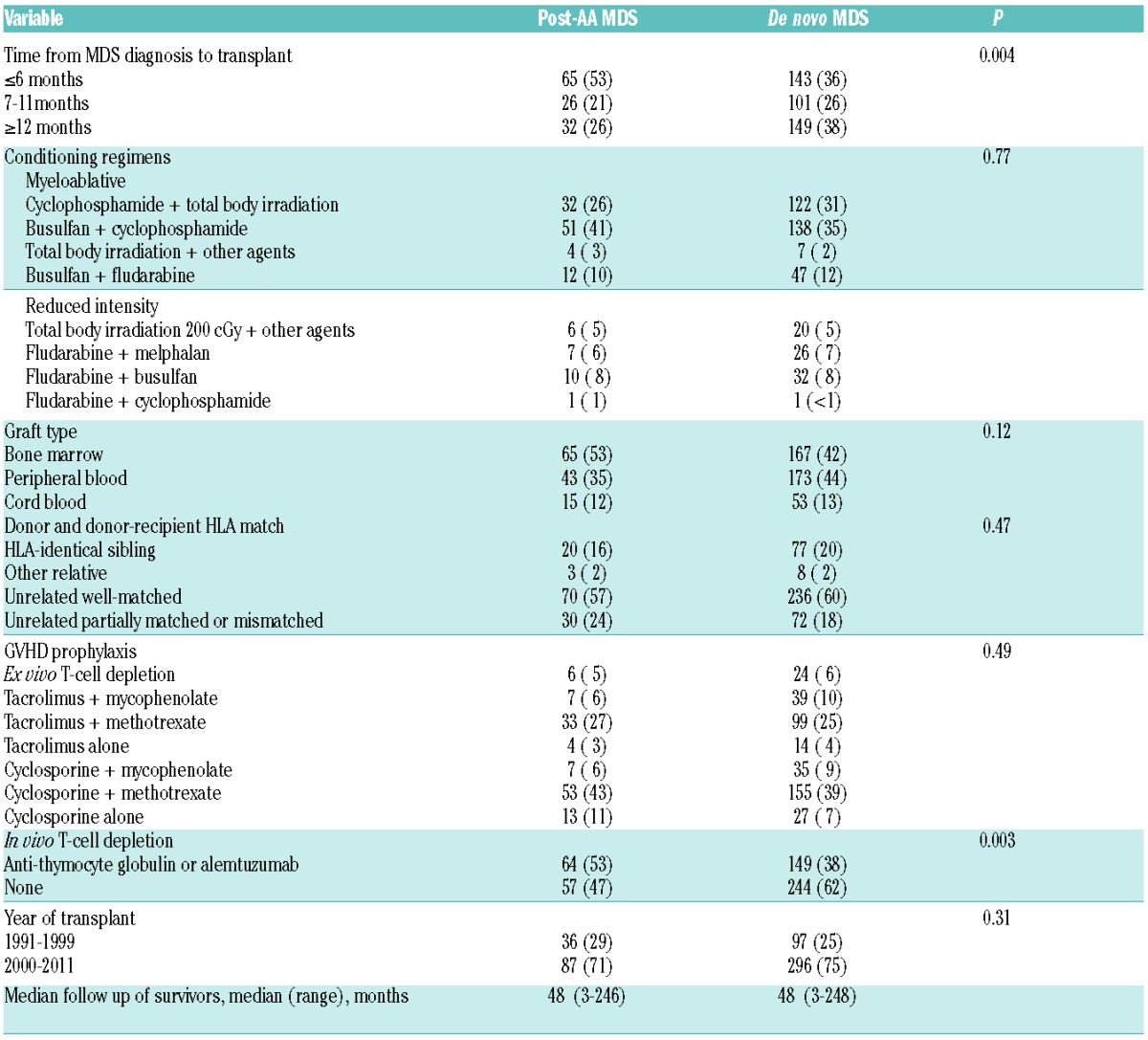
Table 2.
Transplant characteristics in patients with post-AA MDS and de novo MDS.
Results
Patients’, disease, and transplant characteristics
Patients’ and disease characteristics of post-AA MDS and de novo MDS patients are summarized in Table 1. Median age was 28 years (range 2–68 years) in post-AA MDS patients, and 36 years (range 2–68 years) in de novo MDS patients. The majority of patients (72% with post-AA MDS, and 71% with de novo MDS) had less than 5% marrow myeloblasts at transplantation. The cytogenetic risk was good in 44 patients (36%), intermediate in 18 (15%), and poor in 61 patients (49%) in the post-AA MDS cohort; 46 of those 61 patients with poor risk cytogenetics had chromosome 7 abnormalities. There were more patients with poor risk cytogenetics and fewer patients with intermediate risk cytogenetics in the post-AA cohort than in the de novo cohort (P=0.02). Seventy-two percent of children with poor-risk cytogenetics had RA at transplantation compared to 65% of older patients with poor-risk cytogenetics. Patients with post-AA MDS were less likely to have received any treatment for MDS prior to transplantation (42 of 123; 34%) compared to patients with de novo MDS (204 of 393; 52%).
Transplant characteristics are summarized in Table 2. Post-AA MDS patients had a shorter interval from MDS diagnosis to transplantation (median 6 months vs. 9 months for de novo MDS; P=0.004). Post-AA MDS patients were less likely to have received chemotherapy for MDS prior to transplantation (14% vs. 31%; P<0.0001). Most transplants were from unrelated donors (84% in post-AA MDS; 80% with de novo MDS). The source of stem cells was similar for post-AA MDS and de novo MDS patients: bone marrow (53% vs. 42%), peripheral blood (35% vs. 44%), and cord blood (12% vs. 13%). More patients with post-AA MDS received in vivo T-cell depletion with ATG or alemtuzumab (53% vs. 38% with de novo MDS; P=0.003).
Engraftment and graft-versus-host disease
There was no difference in hematologic recovery or graft failure rates between the groups. The median time to neutrophil engraftment was 18 days for post-AA MDS, and 17 days for de novo MDS. The Day-28 cumulative incidence of neutrophil recovery was 87% (95%CI: 79–92) for post-AA MDS and 89% (95%CI: 85–92) for de novo MDS (P=0.51).
There was a trend towards higher cumulative incidence of GvHD among post-AA MDS patients but this did not reach statistical significance. The Day-100 cumulative incidence of grade II–IV acute GvHD was 52% (95%CI: 43–61) and 47% (95%CI: 42–52), respectively (P=0.29) (Figure 1A), and the 5-year cumulative incidence of chronic GvHD was 55% (95%CI: 45–64) and 46% (95%CI: 41–51), respectively (P=0.11) (Figure 1B).
Figure 1.
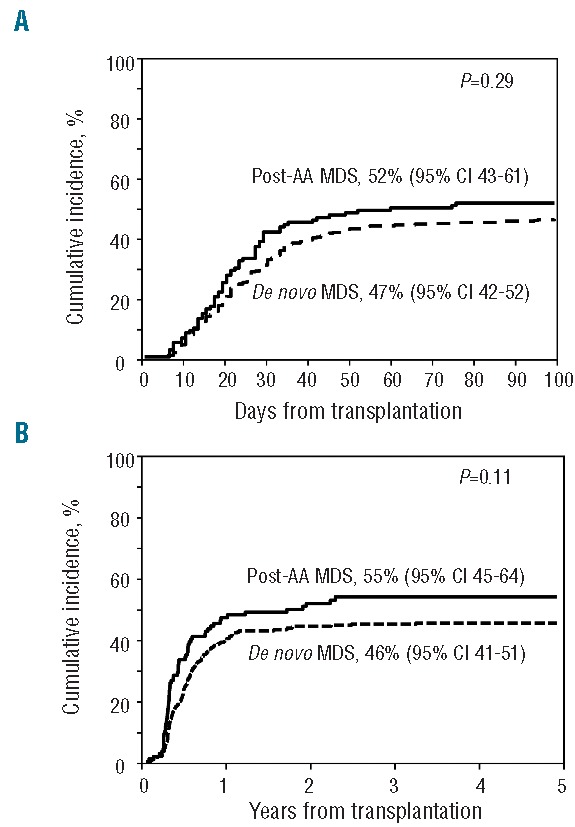
Cumulative incidence of acute (A) and chronic (B) graft-versus-host disease in post aplastic anemia myelodysplastic syndrome and de novo myelodysplastic syndrome patients after allogeneic hematopoietic stem cell transplantation.
Relapse and non-relapse mortality
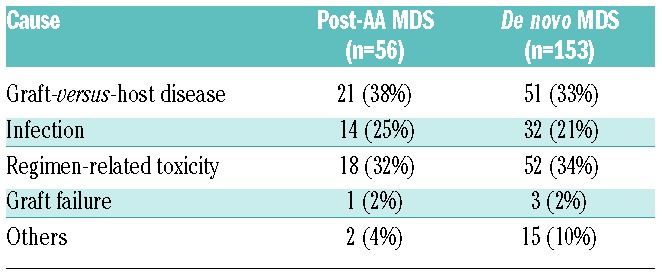
With a median follow up of four years, the 5-year cumulative incidence of relapse was 14% for post-AA MDS patients and 20% for de novo MDS (Figure 2A). The difference was of borderline significance in univariate analysis (P=0.07), but multivariate analysis indicated that post-AA MDS was not independently associated with lower risk of relapse once pre-transplant therapy had been accounted for (HR 0.71; 95%CI: 0.44–1.17; P=0.18) (Table 3). Relapse risk was higher in patients who received chemotherapy prior to transplantation compared to those who received none (HR 1.94; 95%CI: 1.23–3.07; P=0.005). Compared to patients who did not receive any treatment, there was no significant difference in relapse risk between patients who received treatment that did not include chemotherapy (HR 1.26; 95%CI: 0.60–2.64; P=0.54) and those who received supportive care (HR 1.43; 95%CI: 0.74–2.77; P=0.28). The 5-year cumulative incidence of NRM was 40% for post-AA MDS and 33% for de novo MDS (P=0.16) (Figure 2B). By multivariate analysis, NRM was non-significantly higher in post-AA MDS patients (HR 1.28; 95%CI: 0.89–1.83; P=0.18) (Table 3). There was no significant difference in the causes of NRM between groups, which were mainly GvHD, infection, and regimen-related toxicity (Table 4). There was a suggestion that post-AA MDS patients died more frequently from GvHD and infection than did de novo MDS patients. Graft failure did not significantly contribute to NRM in either group. Cytogenetic risk was a significant predictor for NRM (P=0.01).
Figure 2.
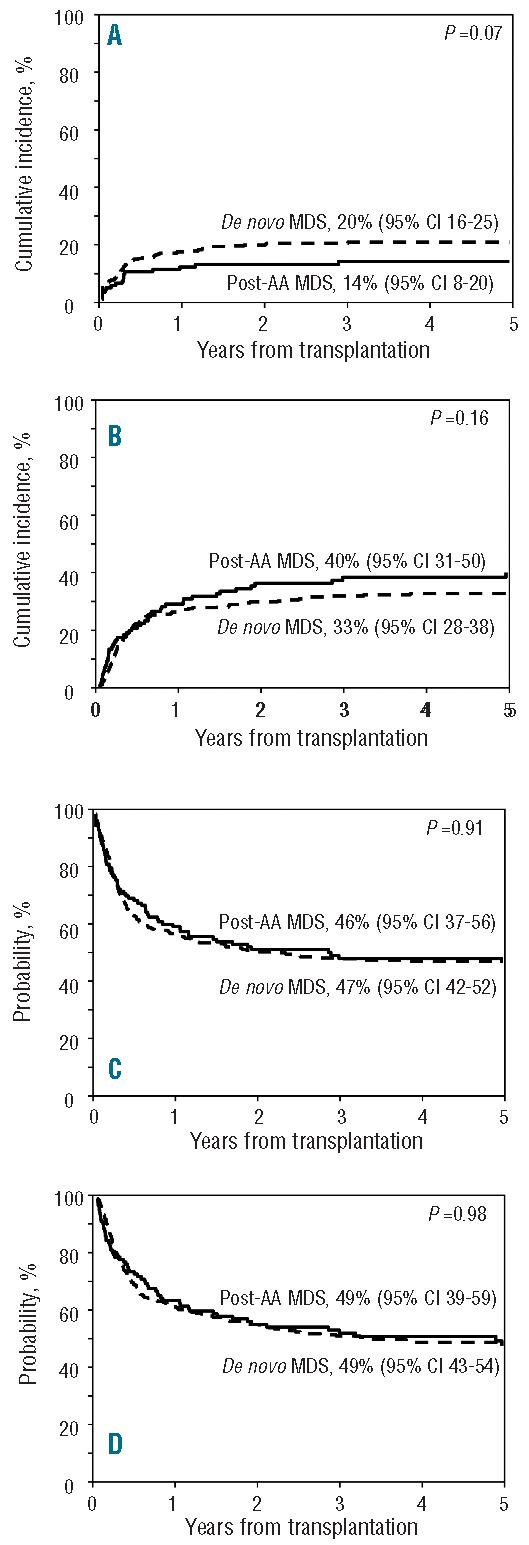
Probability of relapse (A), non-relapse mortality (B), relapse-free survival (C) and overall survival (D) of post aplastic anemia myelodysplastic syndrome and de novo myelodysplastic syndrome patients after allogeneic hematopoietic stem cell transplantation.
Table 3.
Multivariate analysis of prognostic factors for transplant outcomes.
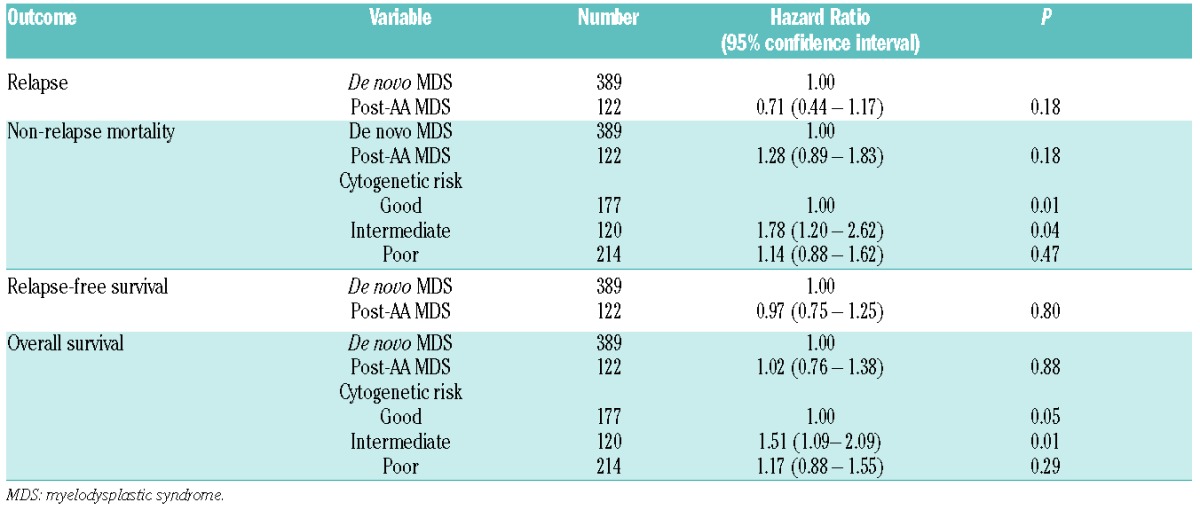
Table 4.
Causes of non-relapse mortality among post-AA MDS and de novo MDS patients.
Relapse-free and overall survival
Relapse-free survival (46% vs. 47%; P=0.91) (Figure 2C) and OS (49% for both groups; P=0.98) (Figure 2D) were similar for both groups; the lower relapse risk among post-AA MDS patients was counterbalanced by a slightly higher NRM, although this difference did not attain the level of significance set for this study. Multivariate analysis confirmed that post-AA MDS by itself was not an independent predictor of RFS (HR 0.97; 95%CI: 0.75–1.25; P=0.80), and no other independent factor was identified. Similarly, post-AA MDS was not associated with OS (HR 1.02; 95%CI: 0.76–1.38; P=0.88), while cytogenetic risk was a predictor for OS (P=0.05).
Prognostic factors for transplant outcome in patients with post-AA MDS
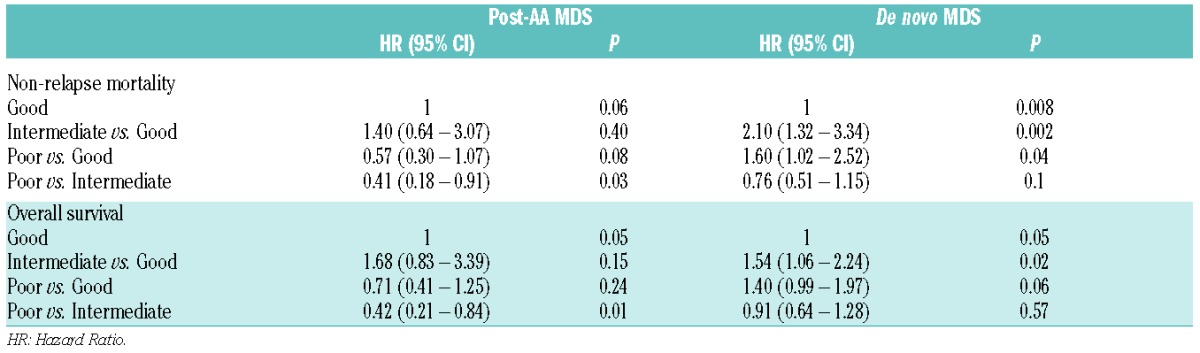
Among patients with post-AA MDS, multivariate subset analysis identified cytogenetic risk category as the only independent prognostic factor for OS (P=0.05) and NRM (P=0.06), although the level of significance for NRM was marginal (Table 5). It was of note, however, and unexpected that NRM and OS tended to be more favorable among patients with poor risk than among those with intermediate-risk cytogenetics. Patients with intermediate-risk cytogenetics had higher mortality risks compared to those with good-risk cytogenetics, although this difference did not reach statistical significance. Subset analyses of patients with de novo MDS also identified cytogenetic risk category as the only independent prognostic factor for OS (P=0.05) and NRM (P=0.008) (Table 5). In these patients, intermediate-risk and poor-risk cytogenetics were associated with higher NRM and lower OS compared to good-risk cytogenetics. No specific predictors for relapse and RFS were identified.
Table 5.
Cytogenetics as prognostic factor for overall survival (subgroup analysis).
Subset analyses
In addition, we performed two subset analyses, one addressing the potential impact of the transplant period, and one aimed at delineating possible differences between pediatric and adult patients. In the first subset analysis, the study population was restricted to patients transplanted between 2000 and 2011 to exclude the possibility that the effects of recent changes in transplantation strategies on outcome may have been obscured by the inclusion of patients transplanted over the course of two decades. Consistent with the main analysis, there were no differences in overall survival (HR 0.87; 95%CI: 0.59–1.29; P=0.49), non-relapse mortality (HR 1.09; 95%CI: 0.68–1.75; P=0.72), relapse (HR 0.67; 95%CI: 0.36–1.25; P=0.21) and relapse-free survival (HR 0.88; 95%CI: 0.62–1.25; P=0.49) between patients with post-AA MDS and de novo MDS. The second subset analysis included patients 20 years of age or older. Consistent with the main analysis, there were no differences in overall survival (HR 0.98; 95%CI: 0.68–1.41; P=0.92), non-relapse mortality (HR 1.27; 95%CI: 0.82–1.98; P=0.29), relapse (HR 0.61; 95%CI: 0.34–1.08; P=0.09) and relapse-free survival (HR 0.94; 95%CI: 0.68–1.28; P=0.69) between patients with post-AA MDS and de novo MDS. For older patients, intermediate risk (HR 1.84; 95%CI: 1.31–2.57; P=0.0004) and poor-risk cytogenetics (HR 1.66; 95%CI: 1.22–2.26; P=0.001) were associated with lower overall survival compared to those with good-risk cytogenetics.
Discussion
A large proportion of patients with post-AA MDS were transplanted successfully from related or unrelated donors, with a 5-year RFS of 46%, and 5-year OS of 49%, success rates similar to those in ‘matched’ patients with de novo MDS, even after adjusting for other potential prognostic factors. While there was a clinical impression that patients with post-AA MDS relapsed less frequently (P=0.05 in univariate analysis), once pre-transplant therapy (administered after the diagnosis of MDS was established) was included in the analysis, there was no significant difference between the two cohorts. As there was a trend in the reverse direction for NRM, survival rates for the two cohorts were basically identical. Higher NRM among patients with post-AA MDS could have been due to a longer overall disease course (AA followed by MDS), leading to the development of additional comorbidities. Also, the different pathogenesis and an altered immune-environment in post-AA MDS compared to patients with de novo MDS might contribute to differences in the various end points. One might further speculate that there could have been an enrichment for patients with shortened telomeres in the post-AA MDS cohort, related to the prolonged course after IST failure, a consideration of potential interest as recent studies suggest that shorter telomeres were independently associated with higher NRM in patients with hematologic malignancies.20
We did not include GvHD, a post-transplant risk factor, in multivariate analysis since the study was focused on pre-transplant variables. There was a trend towards higher cumulative incidence rates of both acute and chronic GvHD in post-AA MDS, which may have been related to a higher proportion of HLA-mismatched unrelated donor transplants, a pattern that would be consistent with the observed higher rate of NRM and lower incidence of relapse among post-AA MDS patients due to a stronger graft-versus-MDS effect.
The prognosis of therapy-related MDS evolving after chemotherapy or radiotherapy is worse than that of de novo MDS, presumably because of the higher frequency of poor-risk cytogenetics and molecular mutations than are observed in de novo MDS patients.11,21 In the present cohort of post-AA MDS patients, the proportion with poor-risk cytogenetics was also higher than in patients with de novo MDS, even though prior treatment consisted of IST rather than cytotoxic modalities. This observation suggests the possibility that IST and, possibly, factors predisposing to AA, contributed to the evolution of the karyotype. However, the overall outcome of post-AA MDS was comparable to that of de novo MDS and likely better than the outcome in patients with MDS evolving after chemo- or radiotherapy as observed in prior studies.10,12 Results in patients with post-AA MDS in the present analysis were similar to results in a smaller cohort of comparable patients reported previously from the FHCRC with a 5-year RFS of 47%.11 It was unexpected that the karyotype should have a different impact in patients with post-AA MDS than in patients with de novo MDS, and that the outcome should be comparable, despite a greater proportion of patients with poor-risk cytogenetics in the post-AA MDS group. A subset analysis indicated, however, that in adults, there was no difference in the impact of cytogenetic risk between post-AA MDS and de novo MDS, suggesting that the unexpected pattern (of intermediate- vs. poor-risk cytogenetics) was related to outcome in pediatric and adolescent patients. A greater proportion of patients aged 20 years and younger with poor-risk cytogenetics was transplanted in RA compared to those older than 20 years, in support of bone marrow blast counts, in addition to cytogenetic risk, being a predictor of transplant outcome. Furthermore, there was a larger proportion of patients in the de novo MDS cohort who had received chemotherapy before HSCT (in efforts to induce remission), and it is likely that patients considered to be at the highest risk of relapse were the most likely to receive pre-HSCT chemotherapy. In addition, a recent analysis in a large cohort of unselected patients with MDS showed that pre-transplant chemotherapy was an adverse risk factor for post-HSCT outcome.22.23 These data, therefore, suggest some differences compared to results in patients with MDS after cytotoxic chemotherapy or radiotherapy, where outcome was strictly related to the karyotype.10,11
The present results do raise questions with regard to the timing of HSCT in patients with AA. Long-term outcome of patients with severe AA undergoing transplantation from HLA-matched siblings has been shown to be superior to IST, at least in patients up to approximately 40 years of age.23,24 Even transplants from unrelated donors in patients with AA who have failed to respond to IST, but have not developed MDS, have been carried out with increasing success, with survival probabilities in the range of 50–80%,25–28 clearly superior to the results in post-AA MDS patients presented here. Those data are of particular relevance since many patients with AA are receiving IST because HLA-matched related donors are not available, and 82% of post-AA MDS patients in the present study eventually received HSCT from unrelated donors. Quite likely, therefore, it would be advantageous to transplant AA patients who fail to achieve sustained responses to IST before MDS develops. Patients who do respond to IST should be monitored closely for any changes in hematopoietic parameters and be transplanted as early as possible if there is evidence of clonal disease evolution. However, the retrospective nature of the present analysis precludes a more definitive statement with regard to the timing of transplantation. A longitudinal registration study to evaluate the fate of patients who receive IST is warranted.
In summary, patients who developed MDS following IST for AA had a probability of survival similar to patients with de novo MDS. Long-term survival may be slightly better than that of patients with treatment-related MDS following cytotoxic therapy.
Footnotes
Funding
The CIBMTR which is supported by Public Health Service Grant/Cooperative Agreement U24–CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government. *Corporate Members. S-Y K and HJD were supported by grants HL036444, HL095999 and CA015704.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Scheinberg P. Aplastic anemia: therapeutic updates in immunosuppression and transplantation. Hematology Am Soc Hematol Educ Program. 2012;292–300. [DOI] [PubMed] [Google Scholar]
- 2.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012; 120(6):1185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H. Antithymocyte globulin with or without cyclosporine A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood. 2003; 101(4):1236–42. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld S, Follman D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcomes. JAMA. 2003; 289(9):1130–5. [DOI] [PubMed] [Google Scholar]
- 5.Osugi Y, Yagasaki H, Sako M, Kosaka Y, Taga T, Ito T, et al. Antithymocyte globulin and cyclosporine for treatment of 44 children with hepatitis associated aplastic anemia. Haematologica. 2007;92(12): 1687–90. [DOI] [PubMed] [Google Scholar]
- 6.Kojima S, Ohara A, Tsuchida M, Kudoh T, Hanada R, Okimoto Y, et al. Risk factors for evolution of acquired aplastic anemia into myelodysplastic syndrome and acute myeloid leukemia after immunosuppressive therapy in children. Blood. 2002; 100(3):786–90. [DOI] [PubMed] [Google Scholar]
- 7.Afable MG, Tiu RV, Maciejewski JP. Clonal evolution in aplastic anemia. Hematology Am Soc Hematol Educ Program. 2011; 2011:90–5. [DOI] [PubMed] [Google Scholar]
- 8.Scheinberg P, Cooper JN, Sloand EM, Wu CO, Calado RT, Young NS. Association of telomere length of peripheral blood leukocytes with hematopoietic relapse, malignant transformation, and survival in severe aplastic anemia. JAMA. 2010;304(12): 1358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oosterveld M, Muus P, Suciu S, Koller C, Verhoef G, Labar B, et al. Chemotherapy only compared to chemotherapy followed by transplantation in high risk myelodysplastic syndrome and secondary acute myeloid leukemia; two parallel studies adjusted for various prognostic factors. Leukemia. 2002;16(9):1615–21. [DOI] [PubMed] [Google Scholar]
- 10.Litzow MR, Tarima S, Perez WS, Bolwell BJ, Cairo MS, Camitta BM, et al. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010; 115(9):1850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang C, Storer BE, Scott BL, Bryant EM, Shulman HM, Flowers ME, et al. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. Blood. 2007;110(4):1379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroger N, Brand R, van Biezen A, Zander A, Dierlamm J, Niederwiesser D, et al. Risk factors for therapy-related myelodysplastic syndrome and acute myeloid leukemia treated with allogeneic stem cell transplantation. Haematologica. 2009;94(4):542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimi A, Strahm B, Baumann I, Strahm B, Baumann I, Furlan I, et al. Hematopoietic stem cell transplantation in children and young adults with secondary myelodysplastic syndrome and acute myeloid leukemia following aplastic anemia. Biol Blood Marrow Transplant. 2014;20(3):425–9. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Marnero G, Sole F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980; 69(2):204–17. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 17.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–06. [DOI] [PubMed] [Google Scholar]
- 18.Klein JP, Moeschberger ML. Survival Analysis: Statistical Methods for Censored and Truncated Data. 2nd ed New York, NY: Springer-Verlag; 2003. [Google Scholar]
- 19.Cox DR. Regression model and life tables. J R Stat Soc B. 1972;34(2):187–200. [Google Scholar]
- 20.Peffault de Latour R, Calado RT, Busson M, Abrams J, Adoui N, Robin M, et al. Age-adjusted recipient pretransplantation telomere length and treatment-related mortality after hematopoietic stem cell transplantation. Blood. 2012;120(16):3353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christiansen DH, Andersen MK, Pedersen-Bjergaard J. Mutations with loss of heterozygosity of p53 are common in therapy related myelodysplasia and acute myeloid leukemia after exposure to alkylating agents and significantly associated with deletion or loss of 5q, a complex karyotype, and a poor prognosis. J Clin Oncol. 2001; 19(5):1405–13. [DOI] [PubMed] [Google Scholar]
- 22.Deeg HJ, Scott BL, Fang M, Shulman HM, Gyurkocza B, Myerson D, et al. Five-group cytogenetic risk classification, monosomal karyotype and outcomes after hematopoietic cell transplantation for MDS or acute leukemia evolving from MDS. Blood 2012; 120(7):1398–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peinemann F, Grouven U, Kroger N, Bartel C, Pittler MH, Lange S. First-line matched related donor hematopoietic stem cell transplantation compared to immunosuppressive therapy in acquired severe aplastic anemia. PLoS One. 2011;6(4):e18572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viollier R, Socie G, Tichelli A, Bacigalupo A, Korthof ET, Marsh J, et al. Recent improvement in outcome of unrelated donor transplantation for aplastic anemia. Bone Marrow Transplant. 2008;41(1):45–50. [DOI] [PubMed] [Google Scholar]
- 26.Eapen M, Le Rademacher J, Antin JH, Champlin RE, Carreras J, Fay J, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118(9):2618–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maury S, Balere-Appert ML, Chir Z, Boiron JM, Galambrun C, Yakouben K, et al. Unrelated stem cell transplantation for severe acquired aplastic anemia: improved outcome in the era of high-resolution HLA matching between donor and recipient. Haematologica. 2007;92(5):589–96. [DOI] [PubMed] [Google Scholar]
- 28.Lee JW, Cho BS, Lee SE, Eom KS, Kim YJ, Kim HJ, et al. The outcome of unrelated hematopoietic stem cell transplants with total body irradiation (800 cGy) and cyclophosphamide (120 mg/kg) in adult patients with acquired severe aplastic anemia. Biol Blood Marrow Transplant. 2011; 17(1):101–8. [DOI] [PubMed] [Google Scholar]


