Abstract
The pathogenesis of bone resorption in β-thalassemia major is multifactorial and our understanding of the underlying molecular and cellular mechanisms remains incomplete. Considering the emerging importance of the endocannabinoid/endovanilloid system in bone metabolism, it may be instructive to examine a potential role for this system in the development of osteoporosis in patients with β-thalassemia major and its relationship with iron overload and iron chelation therapy. This study demonstrates that, in thalassemic-derived osteoclasts, tartrate-resistant acid phosphatase expression inversely correlates with femoral and lumbar bone mineral density, and directly correlates with ferritin levels and liver iron concentration. The vanilloid agonist resiniferatoxin dramatically reduces cathepsin K levels and osteoclast numbers in vitro, without affecting tartrate-resistant acid phosphatase expression. The iron chelators deferoxamine, deferiprone and deferasirox decrease both tartrate-resistant acid phosphatase and cathepsin K expression, as well as osteoclast activity. Taken together, these data show that transient receptor potential vanilloid type 1 activation/desensitization influences tartrate-resistant acid phosphatase expression and activity, and this effect is dependent on iron, suggesting a pivotal role for iron overload in the dysregulation of bone metabolism in patients with thalassemia major. Our applied pharmacology provides evidence for the potential of iron chelators to abrogate these effects by reducing osteoclast activity. Whether iron chelation therapy is capable of restoring bone health in humans requires further study, but the potential to provide dual benefits for patients with β-thalassemia major –preventing iron-overload and alleviating associated osteoporotic changes – is exciting.
Introduction
Osteopenia and osteoporosis (OP) are responsible for substantial morbidity in adult patients with β-thalassemia major (TM), affecting 40–50% of this population and conferring a significantly increased risk of fractures.1–4 The pathogenesis of OP is multifactorial and includes environmental (diet and lifestyle), iatrogenic (drugs), acquired (bone marrow expansion, hemochromatosis, hepatitis, deficiency of growth hormone or insulin growth factor I, and hypogonadism) and genetic factors.1–7 The relative contribution of these factors to TM-OP is uncertain, and the role of iron overload and iron chelation therapy is the subject of increasing interest. Interestingly, reports have shown different responses to bisphosphonate treatment between patients with TM and those with thalassemia intermedia: in a prospective study, individuals with TM were shown to have high turnover bone disease and responded more favorably to treatment with pamidronate and hormone replacement therapy, as compared with patients with thalassemia intermedia.8 The pathological mechanisms of disturbed bone integrity may, therefore, be different between TM and thalassemia intermedia.
Iron deposition in bone impairs osteoid maturation, inhibits mineralization, and reduces the bone metabolism unit tensile strength, resulting in focal osteomalacia.9–11 Three iron chelation therapies have been approved for clinical use: deferoxamine (DFO), deferiprone (DFP) and deferasirox (DFX).12 DFO inhibits DNA synthesis, osteoblast and fibroblast proliferation, and collagen formation, and enhances osteoblast apoptosis.13 The effects of DFP and DFX on bone metabolism and health have not been studied.
Even after restoring hemoglobin levels, adequate hormone replacement, and effective iron chelation with normalization of iron status, TM patients continue to show imbalanced bone turnover, with an increased resorptive phase resulting in seriously diminished bone mineral density.14–16 The increased osteoclast activity seems to be at least partially a consequence of an imbalance in the receptor–activator of the nuclear factor-kappa B ligand (RANKL)/osteoprotegerin system, and the overproduction of cytokines involved in osteoclast differentiation and function.17–21 However, no correlation has been found between RANKL or osteoprotegerin levels and bone mineral density of the lumbar spine or the femoral neck.18 Conversely, we have shown a direct correlation between bone mineral density and the levels of expression of tartrate-resistant acid phosphatase (TRAP).22 Interestingly, TRAP is an iron-phosphoesterase and its activation is associated with the redox state of the di-iron metal center or proteolytic cleavage in an exposed loop domain, due to the cysteine proteinase cathepsin K.23 Both TRAP and cathepsin K are considered markers of osteoclast activity. The endocannabinoid/endovanilloid system has recently been considered a potential therapeutic target for bone disease.22,24–31 We have previously reported that human osteoclasts express the functional transient receptor potential vanilloid type 1 (TRPV1) channel together with the cannabinoid receptors type 1 and 2 (CB1/CB2), and metabolic enzymes for the two most studied endocannabinoids; anandamide (AEA) and 2-arachydonoyl-glycerol (2-AG). Cannabinoid/vanilloid agonists alone, or in combination with selective antagonists, are able to modulate osteoclast formation and activity.22,30 Consistent with data from rodents, which suggest that this system may represent a novel therapeutic target for treating diseases characterized by abnormal bone resorption,31 we have also previously shown that expression of TRPV1 and of CB1 versus CB2 is dramatically modified in osteoclasts from osteoporotic post-menopausal women.22 Therefore, considering the emerging role of the endocannabinoid/endovanilloid system in bone metabolism, and specifically in the pathophysiology of OP, we evaluated the possible influence of this system in the development of TM-induced OP and specifically its relationship with iron overload and chelation therapy.
Methods
Patients
Twenty-nine patients with β-TM (32% male; mean age 32.1±3.9 years) and 40 controls (40% male; mean age 33±4.3 years) attending the Department of Woman, Child and of General and Specialist Surgery at the Second University of Naples (SUN) were recruited. All patients gave informed written consent to participation in the study, which was approved by the SUN Ethics Committee, in accordance with the Declaration of Helsinki. None of the patients had thyroid disease, diabetes mellitus, malabsorption or hypoparathyroidism (Online Supplementary Table S1). All patients received regular transfusions, maintaining pretransfusion hemoglobin levels at approximately 9.5 g/dL. Twenty-four patients had been splenectomized. None of the participants had received bisphosphonates or steroid/estrogenic treatments for at least 3 years prior to recruitment.
Iron overload was assessed by measurement of mean serum ferritin levels over the last 10 years, and estimation of liver iron concentration (LIC) by T2* magnetic resonance imaging. The LIC was calculated from liver T2* images (TE 0.99 – 16.50 ms) using the previously described formula [1/(T2*/1000)] × 0.0254 + 0.202.8,32 LIC results were expressed as milligrams of iron per gram of liver (dry weight) and 1.8 mg/g was considered the upper normal limit. Magnetic resonance imaging T2* values were also expressed in milliseconds for additional analysis. The bone mineral density of the lumbar spine (L1–L4) and the femoral neck was determined by dual energy X-ray absorptiometry using a bone densitometer (Hologic, QDR 4500, Hologic Inc., Bedford, MA, USA). Normal (Z-score between 0 and 1), osteopenic (Z-score between −1 and −2.5) and osteoporotic (Z-score below −2.5) groups were defined using the standard World Health Organization criteria.33 According to these criteria, 14 patients had OP, nine had osteopenia and six had normal bone mineral density. Laboratory methods are summarized below and detailed in Online Supplement 1.
Human cell cultures
Osteoclasts were differentiated from peripheral blood mononuclear cells as previously described.22,30 Control osteoclast cultures were harvested in the presence of 10% serum from thalassemic patients (TS), instead of 10% fetal bovine serum.
Reverse transcriptase polymerase chain reaction and real-time quantitative polymerase chain reaction
Levels of expression of TRAP, cathepsin K, TRPV1 (transcript variants 1 and 3), CB1 (isoforms a and b), CB2, fatty acid amide hydrolase (FAAH), N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD), diacylglycerol lipase alpha (DAGL-α), monoacylglycerol lipase (MAGL), cannabinoid receptor interacting protein 1a and the housekeeping β-actin gene were analyzed using realtime polymerase chain reaction (PCR) and quantitative PCR.
Tartrate-resistant alkaline phosphatase assay
The ACP method was used as previously described.22,30 TRAP-positive multinucleated osteoclasts were counted, using an optical microscope, in at least three different wells for each treatment group.
Western blot and immunofluorescence
TRPV1 channels in total lysates from osteoclast cultures were analyzed by western blot experiments. Immunofluorescence was used to identify TRPV1, TRAP, and vimentin.
Drugs and treatments
Osteoclasts were treated with resiniferatoxin (5 μM) for 48 h after day 21 (full differentiation) or differentiated in the presence of DFO (10 μM), DFP (20 μM) or DFX (5 μM) from the first day of culture until they were fully differentiated into osteoclasts. Osteoclasts derived from healthy subjects were treated with ammonium iron (III) citrate, FAC (50 μM), from day 7 (second change of medium) until they were fully differentiated into osteoclasts (day 21). They were then treated with DFO (10 μM), DFP (20 μm), DFX (5 μm) or vehicle (water) from day 15. Control osteoclasts were harvested in 10% TS medium from day 1, and either treated or not treated with DFO (10 μM), DFX (5 μM) or vehicle (water) from day 15. RNA was extracted or a TRAP assay was performed 48 h after resiniferatoxin treatment or on day 22.
Endocannabinoid measurements
Osteoclasts were homogenized in chloroform/methanol/TRIS-HCl 50 mM pH 7.4 (2:1:1, v/v) containing 10 pmol of [2H]8-AEA, [2H]4- palmitoylethanolamide (PEA) and [2H]4-oleoylethanolamide (OEA), and [2H]5-2-AG as internal deuterated standards. The extract was purified and the eluted fraction containing AEA and 2-AG was analyzed as previously described.34,35
Results
TRAP is up-regulated in thalassemic osteoclasts and correlates directly with bone loss
Reverse transcriptase PCR analysis for the osteoclast markers TRAP and cathepsin K showed that cells in culture were osteoclasts. As expected, both the enzymes TRAP and cathepsin K were over-expressed in osteoclasts derived from TM patients with respect to levels in osteoclasts from healthy controls (Figure 1A).
Figure 1.
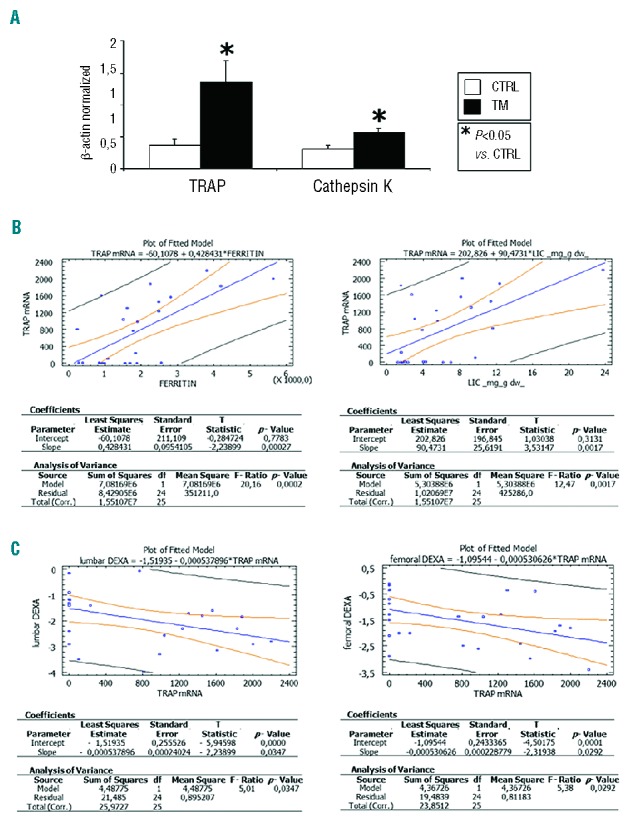
(A) Osteoclastic markers TRAP and cathepsin K are up-regulated in thalassemic osteoclasts. Levels of the markers of bone resorption, TRAP and cathepsin K, are significantly higher in osteoclasts derived from subjects with TM, as compared with controls. Data were acquired from human in vitro osteoclasts by real time PCR normalized for the housekeeping gene β-actin. Data are presented as mean ± SD. One-way ANOVA followed by the Student Neuman-Keuls post hoc test were used for the statistical analysis. P<0.05 was considered statistically significant. (B) TRAP levels directly correlate with ferritin and liver iron concentration. The figures show the regression analysis performed using StatGraph software between ferritin and LIC values with TRAP mRNA levels in 25 out of 29 patients. (C) Lumbar and femoral bone mass loss are directly associated with TRAP mRNA levels. The figures show the regression analysis performed using StatGraph software between lumbar and femoral Z-score associated dual energy X-ray absorptiometry values with TRAP mRNA levels in 25 out of 29 patients.
Interestingly, ferritin and LIC levels in TM subjects were directly associated with TRAP expression. Simple regression analyses between ferritin or LIC and TRAP mRNA levels were statistically significant (P=0.0002 and P=0.0017, respectively) (Figure 1B). Regression analysis was also performed between magnetic resonance imaging T2* values expressed as milliseconds with TRAP mRNA levels in 25 out of 29 patients (Online Supplementary Figure S1) and confirmed that correlations remained statistically significant (P=0.0184). Moreover, TRAP levels inversely correlated with lumbar and femoral bone mineral density (P=0.0347 and P=0.0292, respectively) (Figure 1C).
TRPV1 channels, CB1/CB2 receptors, FAAH, NAPE-PLD, DAGL-α and MAGL mRNA are expressed in osteoclasts from patients with thalassemia major
We confirmed the presence of mature mRNA for TRPV1 and CB1/CB2 receptors, as well as for the endocannabinoid/endovanilloid metabolic enzymes: NAPE-PLD, FAAH, DAGL-α and MAGL (Figure 2A). Although both the long and short isoforms of CB1, namely CB1a and CB1b, were expressed, CB1b levels were higher than those observed in osteoclasts derived from control subjects. The expression of CB2 was lower in osteoclasts from TM patients than in osteoclasts from healthy subjects. TRPV1 expression was significantly up-regulated in TM osteoclasts, with a higher expression of the variant 1 (VR1-1) relative to variant 3 (VR1-3) (Figure 2A). Both the enzymes for the synthesis and the catabolism of anandamide (NAPE-PLD and FAAH, respectively) and for the synthesis of 2-AG, DAGLα, were significantly decreased in TM patients, whereas the enzyme for the degradation of 2-AG, MAGL, was significantly increased (Figure 2A). Accordingly, we found significantly lower levels of both AEA and 2-AG in osteoclasts from TM patients (Figure 2B). We also measured the levels of the two AEA homologs, OEA and PEA, which with AEA share the ability to activate TRPV1. Significantly, the levels of OEA were decreased, while those of PEA were increased in osteoclasts from TM patients (Figure 2B).
Figure 2.
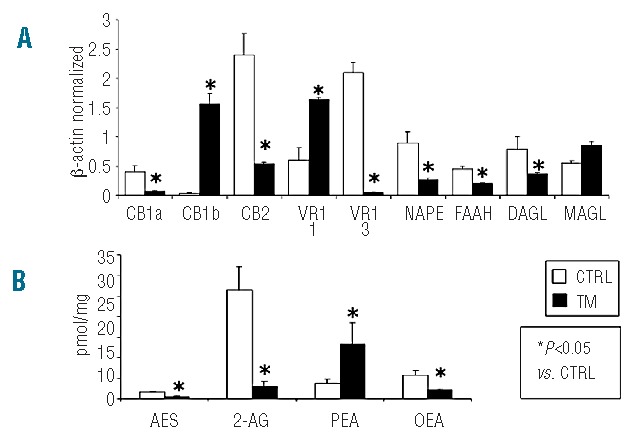
(A) TRPV1 channels, CB1/CB2 receptors, FAAH, NAPE-PLD, DAGLα and MAGL mRNA are expressed in thalassemic human osteoclasts in culture. Data were collected from human in vitro osteoclasts by PCR starting from 250 ng of total mRNA for the RT reaction and were normalized for the housekeeping gene β-actin. Data are presented as mean ± SD. One-way ANOVA followed by the Student Neuman-Keuls post hoc test was used for the statistical analysis. P<0.05 was considered statistically significant. VR1 = TRPV1. 1, 3 = isoform 1, 3. (B) Measurement of the levels of endocannabinoids (AEA and 2-AG), oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) in osteoclasts. Osteoclasts from thalassemic patients contained measurable amounts of AEA (0.54±0.17 pmol/mg extract), 2-AG (2.95±1.26 pmol/mg), and OEA (2.09±0.44 pmol/mg), which were significantly lower in comparison to those found in control-derived osteoclasts [AEA (1.6±0.23 pmol/mg extract), 2-AG (26.4±5.82 pmol/mg), and OEA (5.7±1.14 pmol/mg)]. Conversely, thalassemic-derived osteoclasts contained higher amounts of PEA (13.38±5.11 pmol/mg), with respect to control-derived osteoclasts [PEA (3.8±0.91 pmol/mg)]. Data are presented as mean±SEM from four to six experiments.
TRPV1 channel stimulation decreases cathepsin K without modifying TRAP and increases CB1/CB2 receptors and TRPV1 channel expression
Treatment with the selective TRPV1 channel agonist resiniferatoxin (5 μM) did not significantly affect TRAP mRNA levels in TM osteoclasts. Nevertheless, resiniferatoxin significantly reduced cathepsin K mRNA levels in the cells (Figure 3A).
Figure 3.

TRPV1 channel stimulation decreases cathepsin K without modifying TRAP, increases CB1/CB2 receptors and TRPV1 channel expression, and decreases the number of TRAP-positive osteoclasts. (A) Resiniferatoxin (RTX) (5 μM) significantly reduces the levels of cathepsin K, with no change in TRAP expression levels. (B) RTX (5 μM) induces a significant increase in CB1 and CB2 expression and, accordingly, an increase of the cannabinoid receptor interacting protein 1A, as well as a significant increase in the vanilloid receptor TRPV1, in particular isoform 3. Pharmacological treatment with RTX (5 μM) also significantly modulates expression levels of the AEA metabolic enzymes, increasing NAPE-PLD and reducing FAAH, but with no change in the levels of expression of the 2-AG metabolic enzymes, DAGL-α and MAGL. Data were collected from human in vitro osteoclasts by PCR starting from 250 ng of total mRNA for the RT reaction and have been normalized for the gene β-actin. Data are presented as mean ± SD. One-way ANOVA followed by the Student Neuman-Keuls post hoc test was used for the statistical analysis. P<0.05 was considered statistically significant. (C) Treatment with the vanilloid agonist RTX (5 μM) significantly reduces the number of osteoclasts derived from osteoporotic TM patients but not those derived from non-osteoporotic TM subjects. Treatment with the vanilloid antagonist I-RTX (2 5 μM) reduces osteoclast numbers in both osteoporotic and non-osteoporotic TM subjects. Data are presented as mean ± SEM. One-way ANOVA followed by the Student Neuman-Keuls post hoc test was used for the statistical analysis. P<0.05 was considered statistically significant.
Treatment of TM-derived osteoclasts with resiniferatoxin significantly increased the expression of CB1, CB2 and the levels of cannabinoid receptor interacting protein 1a mRNA. In addition, resiniferatoxin also enhanced the expression of TRPV1 variant 3 (mainly expressed in healthy osteoclasts), but did not significantly alter the expression of variant 1. Resiniferatoxin also changed AEA metabolic enzymes, significantly reducing FAAH and increasing NAPE-PLD, but did not have significant effects on 2-AG enzymes, DAGL or MAGL (Figure 3B).
TRPV1 stimulation decreases the number of TRAP-positive osteoclasts
We counted TRAP-positive cells in osteoclast cultures from TM patients with OP and in those without OP/osteopenia. Treatment with resiniferatoxin (5 μM) decreased the number of TRAP-positive multinucleated (n≥3) osteoclasts in TM OP patients, whereas no effect was detected in the other TM subjects. We did, however, observe reductions in the number and activity of osteoclasts in both groups when the cells were treated with the TRPV1 antagonist iodio-resiniferatoxin (2.5 μM), suggesting that the effect of the agonist described above was due to activation and subsequent desensitization of the channel (Figure 3C).
Immunocytochemistry shows increased expression of TRPV1 channels in activated osteoclasts from patients with thalassemia major
Immunolabeling of the TRPV1 channel revealed increased expression in osteoclasts from TM OP patients as compared with the expression in osteoclasts derived from non-OP TM subjects. Vimentin staining also revealed an increased number of giant and multinucleated osteoclasts in TM-derived plates (Figure 4A). The mean number of nuclei in osteoclasts was higher in TM OP patients than in TM non-OP patients (16±6 versus 5±2). Western blot analysis of total osteoclast lysate demonstrated that osteoclasts from TM patients with OP had a significant increase in TRPV1 protein expression (Figure 4B and C).
Figure 4.
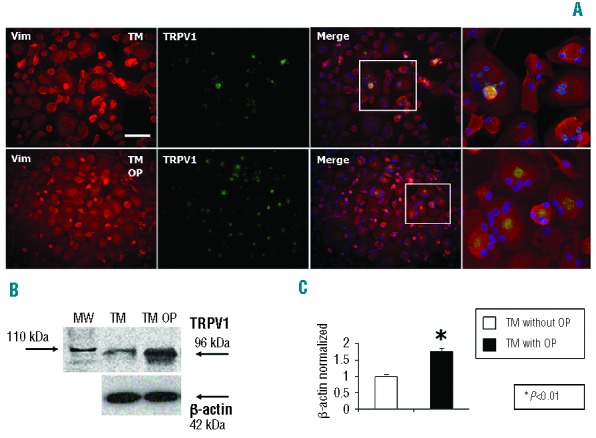
Immunohistochemistry shows increased expression of TRPV1 channels in TM activated osteoclasts. (A) Immunolabeling of the TRPV1 channel shows increased expression in TM subjects, in comparison with controls. Vimentin (Vim) staining also revealed an increased number of giant osteoclasts in TM-derived plates. Scale = 100 μm. (B–C) Western blot analysis for the TRPV1 channel in total osteoclast lysate, normalized with respect to β-actin, showed a significant increase in TRPV1 protein expression in OP-derived osteoclasts, which was attenuated by treatment with the vanilloid agonist resiniferatoxin (RTX) (5 μM). Data are presented as mean ± SD. One-way ANOVA followed by the Student Neuman-Keuls post hoc test was used for the statistical analysis. P<0.05 was considered statistically significant. MW=molecular weight.
Deferoxamine affects the expression of TRAP and cathepsin K and number of TRAP-positive osteoclasts in osteoporotic patients with thalassemia major
Osteoporotic TM-derived cell cultures were treated with DFO (10 μM). Both the biomarkers TRAP and cathepsin K were reduced in osteoclasts exposed to DFO, compared with vehicle-treated osteoclasts (Figure 5A). Moreover, DFO significantly reduced the number of TRAP-positive multinucleated (n≥3) cells, as revealed by the TRAP assay (t=4.280 P=0.002), and abolished the ability of resiniferatoxin (5 μM) to significantly reduce the number of osteoclasts (Figure 5B). Of note, DFO had a cytotoxic effect and no more than 30% of osteoclasts (all TRAP-positive) survived when exposed to the dose of DFO chosen in this study.
Figure 5.
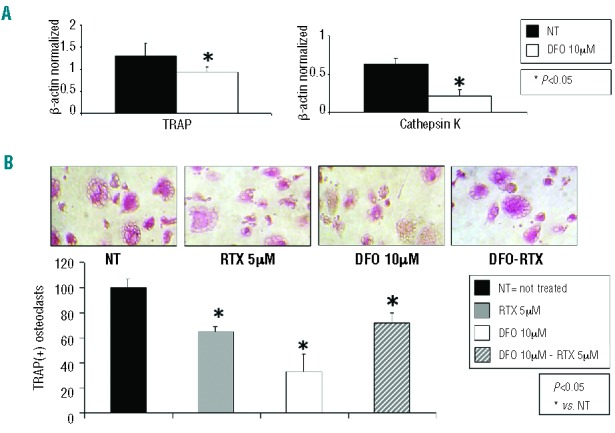
(A) Deferoxamine (DFO) affects the expression of TRAP and cathepsin K in TM osteoclasts. Treatment with DFO (10 μM) drastically reduces expression of both TRAP and cathepsin K biomarkers. Data were acquired from human in vitro osteoclasts by real time PCR starting from 100 ng of total mRNA for the RT reaction, and have been normalized for the housekeeping gene β-actin. Data are presented as a mean ± SD. One-way ANOVA followed by the Student Neuman-Keuls post hoc test was used for the statistical analysis. P<0.05 was considered statistically significant. NT = water or DMSO 0.01%. (B) DFO affects number of TRAP-positive osteoclasts. DFO (10 μM) is able to reduce osteoclast numbers, while treatment of osteoclasts differentiated in the presence of DFO with resiniferation (RTX) was not able to reduce the number of TRAP(+) multinucleated (n≥3) cells. Data are presented as a mean ± SD from n=6 for each group. One-way ANOVA followed by the Student Neuman-Keuls post hoc test was used for the statistical analysis. P<0.05 was considered statistically significant. NT = water or DMSO 0.01%.
Osteoclast activity and number are affected by different iron chelators
TRAP assays showed that DFO (10 μM) induced an approximately 70–80% reduction in osteoclast activity (Figure 6A), although this effect occurred in parallel with induction of cell death (Figure 6B). Treatment with comparable doses of two orally active chelators, DFP (20 μM) and DFX (5 μM) had significantly less cytotoxic effect (approximately 55% and 45% of osteoclasts surviving, respectively) than had DFO (t=-2.824 P=0.011 and t=-2.300 P=0.034, respectively) (Figure 6B). Interestingly, while DFP and DFO reduced TRAP-positive osteoclast numbers in a comparable manner (60% versus 70%; P>0.05), DFX reduced osteoclast activity significantly more than either DFO (90% versus 70%, t=−2.655 P=0.016) or DFP (90% versus 60%, t=3.473 P=0.003) (Figure 6A).
Figure 6.
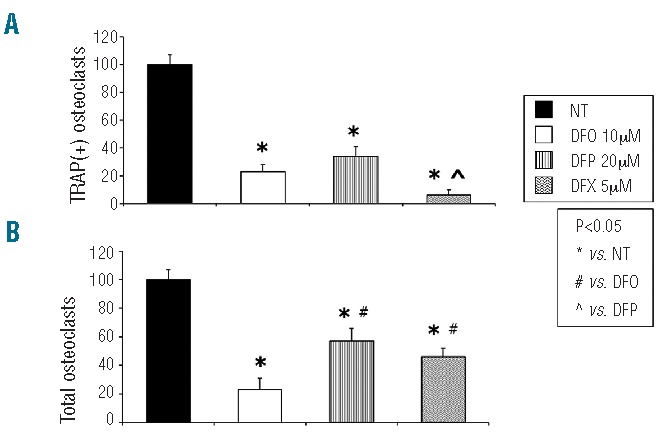
Cytotoxicity and osteoclast activation effects of different iron chelators. (A) Treatment with DFO (10 μM), DFP (20 μM) and DFX (5 μM) reduced numbers of TRAP-positive osteoclasts. The effect exerted by DFX was comparable to that of DFO and significantly greater than that of DFP. (B) DFO-induced reductions in osteoclast activity ocurred in parallel with cellular death. Conversely, treatment with DFP (20 μM) and DFX (5 μM) showed less cytotoxic effects. The effects on cell viability of these two iron chelating agents did not differ significantly. Data are presented as a mean ± SD from n=10 for each group. One-way ANOVA followed by the Student Neuman-Keuls post hoc test was used for the statistical analysis. P<0.05 was considered statistically significant.
Iron overload of osteoclasts derived from healthy people increases TRAP expression and activity
Molecular levels of TRAP, revealed by real-time PCR in osteoclast cultures derived from healthy subjects, were significantly increased (approximately 25 fold) after FAC-induced iron overload. The co-application from day 15 of DFO (10 μM), DFP (20 μM) or DFX (5 μM), fully reversed this effect. Whereas DFO-treated iron-overloaded osteoclasts expressed higher TRAP levels than vehicle-treated cells, DFX and DFP completely abolished FAC-induced TRAP over-expression, and decreased TRAP levels with respect to vehicle. However, only treatment with DFX had a significant effect (90% reduction versus vehicle) (Figure 7A).
Figure 7.

Iron overload induced in-vitro affects TRAP expression levels and osteoclast number. (A) FAC-induced iron overload significantly increased the expression levels of TRAP. The co-application of DFX (5 μM), DFP (20 μM) or DFO (10 μM) reversed this effect. Data were acquired from human in vitro osteoclasts by real time PCR starting from 100 ng of total mRNA for the reverse transcriptase reaction and have been normalized for the housekeeping gene β-actin. (B) FAC-induced iron overload increased the number and size of TRAP(+) osteoclasts. Co-treatment with DFX (5 μM), DFP (20 μM) or DFO (10 μM) reversed this effect. Vehicle = water. Data are presented as a mean±SD. A t- test was used for the statistical analysis. P<0.05 was considered statistically significant.
TRAP assays revealed that in vitro iron-overload, using FAC (50 μM), induced an increase in both osteoclast number (more than 40%) and size. Co-treatment from day 15 with DFO (10 μM), DFP (20 μM) or DFX (5 μM) fully reversed this effect, with DFX demonstrating the largest effect (10%, 60% and 70%, respectively) (Figure 7B).
Serum from patients with thalassemia major increases the number of TRAP-positive osteoclasts from healthy controls
In-vitro iron overload of osteoclasts from healthy subjects using serum from TM patients induced an increase in the number of TRAP-positive cells (Figure 8). This increase in percentage of active osteoclasts was significant (TS versus control, t=3.453, n=3, P=0.03) and was significantly reversed following treatment with DFX, but not with DFO (TS-DFX versus TS t=−4.079, n=3, P=0.015; TS-DFO versus TS t=−1.88, n=3, P=0.132). Moreover, control-derived osteoclasts treated with chelators showed increased activity (DFO versus control t=1.394, n=3, P=0.05; DFX versus control t=2.465, n=3, P=0.17).
Figure 8.
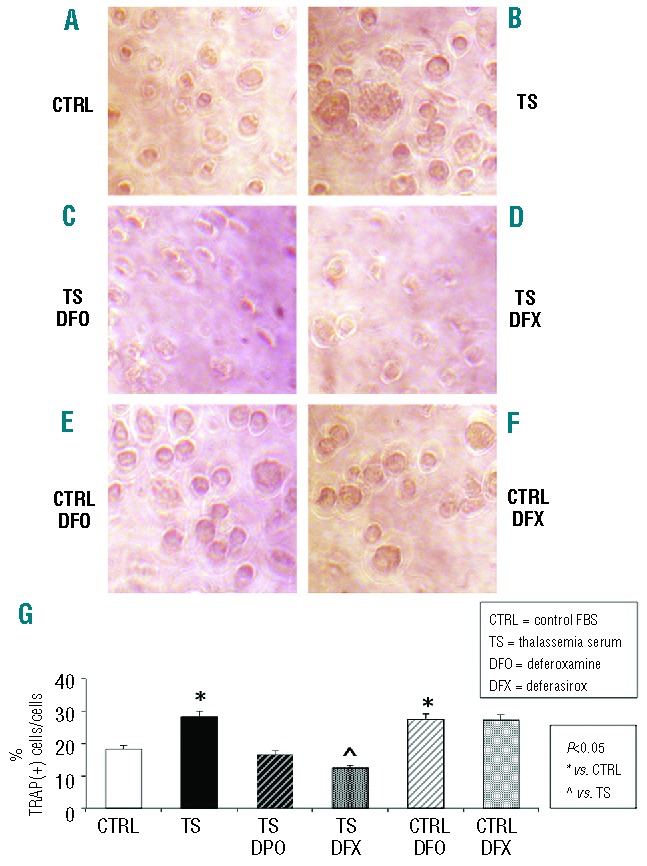
Thalassemia serum increases the number of TRAP(+) osteoclasts from healthy controls. (A) TRAP assay performed on untreated osteoclast culture from healthy subjects. (B) Serum from thalassemic patients (TS) significantly increases the percentage of TRAP(+) cells (TS vs. CTRL n=3, t=3.453, P=0.03). (C) DFO (10μM) reverses the effect induced by TS (TS-DFO vs. TS n=3, t=−1.88, P= 0.132). (D) DFX (5 μM) significantly reverses the effect induced by TS (TS-DFX vs. TS n=3, t= −4.079, P=0.015). (E) DFO (10 μM) induces an increase in TRAP(+) cells (DFO vs. CTRL n=3, t=1.394, P=0.048). (F) DFX (5 μM) induces an increase in TRAP(+) cells with respect to CTRL (DFX vs. CTRL n=3, t=2.465, P=0.17. (G) Graph showing the percentage of TRAP(+) cells with respect to the total cell number for each treatment (CTRL=10% fetal bovine serum-containing medium; TS=10% thalassemia sera-containing medium; Data are presented as mean±SD. A t-test was used for the statistical analysis. P<0.05 was considered statistically significant.
Discussion
The molecular and cellular mechanisms responsible for the pathogenesis of bone resorption in TM remain poorly understood. Osteoblast dysfunction is recognized as a critical pathogenic mechanism, and serum levels of osteocalcin are decreased in patients with TM. There is also evidence of increased osteoclast activation in TM patients.6,18 Accordingly, elevated markers of bone resorption have been found in TM subjects.14,36 Endocrinopathies are common in TM patients, and these may also contribute to osteopenia-OP syndrome, particularly hypogonadotrophic hypogonadism.37 Anti-resorption therapies, such as bisphosphonates in combination with hormone replacement regimens, lead to improvements in bone integrity in thalassemic patients.16 With the aim of examining a homogenous group of patients and limiting explanatory variables, we recruited patients without thyroid disease, diabetes mellitus, malabsorption, or hypoparathyroidism or on steroid/estrogenic treatment (Online Supplementary Table S1). Previous studies have, however, shown that patients with TM show relatively less impressive improvements in spinal bone mineral density following hormone replacement therapy, as compared to individuals with premature ovarian failure, suggesting mechanisms of osteoporosis independent of hypogonadism.16
In this study, we found that the expression of TRAP and cathepsin K mRNA in TM-derived osteoclasts was increased and correlated significantly with a reduction in femoral and lumbar bone mineral density (Figure 1). This suggests a role for activated osteoclasts in the pathogenesis of TM-related bone resorption. Interestingly, enhancement of TRAP directly and significantly correlated with ferritin levels and LIC, confirming a key role for iron overload in the pathogenesis of TM-associated bone disease. LIC was calculated from liver T2* images (TE 0.99 – 16.50 ms) using a previously described formula [1/(T2*/1000)] × 0.0254 + 0.202.32 Different magnetic resonance imaging parameters have been used to indicate liver iron levels,32,38,39 specifically including R2 measurements, which have been validated for LIC. Nonetheless, TRAP mRNA correlated with T2* units independently of the calibration equation in this study (see Online Supplementary Figure S2 for the regression analysis).
Our data also implicate the endocannabinoid/endovanilloid system in activation of osteoclasts. Osteoclasts from patients with TM express higher levels of CB1 and TRPV1 receptors, as compared with osteoclasts from healthy controls (Figure 2). This is consistent with previous findings in osteoclasts derived from women with post-menopausal OP.22 Considering the profound influence of hormones on growth and remodeling, it would have been interesting to examine gender differences in our study, but the relatively small sample size precluded this.
Previous reports have shown that CB1 and TRPV1 play a cooperative excitatory role in stimulating osteoclast activity,24–27 whereas CB2 activation is inhibitory.28,29, 40–42 This is accompanied by a pattern of alterations in endocannabinoid levels – with a reduction in those with highest efficacy at CB2 receptors (2-AG), and an increase in those which activate TRPV1 (PEA).43 Thus, increased TRPV1/CB1 receptors with decreased CB2 receptor activity may represent one of the molecular events underpinning the development of OP. Consistent with this, we found that CB1b and TRPV1 isoform 1 were strongly over-expressed in osteoclasts from thalassemic patients, whereas the CB1a and TRPV1-3 isoforms were present at lower levels (Figure 2). Although these receptor isoforms seem to exhibit similar pharmacology, they may have different functions in cell physiology.44 Indeed, the majority of our knowledge of the cannabinoid receptors is in relation to their ligands and receptor binding sites, second messengers or signal transduction mechanisms, and postreceptor intracellular protein-protein interactions. Little, however, is known about regulation of CB1 or CB2 gene expression. The levels and anatomical distribution of CB1 mRNA and protein are developmental stage-specific and can be dysregulated in several pathological conditions.45 Moreover, exposure to a variety of challenges may profoundly alter CB1 and CB2 gene expression and mRNA levels.22,30,40,46,47
Given the potential anti-osteoporotic effects of TRPV1 pharmacological stimulation/desensitization,22 we treated osteoclasts from TM patients with the TRPV1 agonist resiniferatoxin, which led to an increase in CB2 receptor expression and a dramatic reduction of cathepsin K (Figure 3). This occurred in the absence of any significant change in TRAP mRNA levels (Figure 3A). Treatment with resiniferatoxin did, however, lead to a reduction in the number of TRAP-positive osteoclasts (Figure 3C). This could potentially be explained if iron overload in TM prevents TRPV1 activation from further modifying TRAP levels - which are directly regulated by iron at the gene transcription level.48 Indeed, TRAP is an iron phosphatase, thus the presence of iron overload, which is characteristic of patients with TM, may activate TRAP transcription per se. On the other hand, the activation of TRAP protein is associated with proteolytic cleavage in an exposed loop domain due to cathepsin K,23 which was found to be significantly decreased in resiniferatoxin-treated TM-derived osteoclasts (Figure 3A). Accordingly, we found that iron overload of healthy-derived osteoclasts, obtained by FAC or using thalassemia serum, induced an increase in osteoclast number, size and activity, and increased levels of TRAP. These effects were completely abolished by the chelating agents DFX, DFP and DFO (Figures 7 and 8). It should be noted that the concentration of FAC used in these experiments was supraphysiological, and likely exceeded the binding capacity of the chelators tested. The abrogation of TRAP levels seen in response to the chelating agents (Figure 7B) may, therefore, occur through alternative mechanisms.
To investigate the involvement of iron overload further, we differentiated osteoclasts from osteoporotic thalassemic subjects in the presence of the iron chelating agent DFO. In support of the above hypothesis, DFO-treated osteoclasts showed decreased levels of the osteoclast biomarkers, TRAP and cathepsin K (Figure 5). Resiniferatoxin treatment of DFO-exposed osteoclasts from TM subjects resulted in slight increases in both osteoclast number and activity, similar to those of osteoclasts derived from healthy subjects.30 These data indicate that the ability of TRPV1 activation/desensitization to influence TRAP expression and activity is dependent on iron, suggesting a central role for iron overload in the dysregulation of bone metabolism in TM patients.
In agreement with previous studies on the importance of iron in the function of the hematopoietic stem cell lineage,49,50 we observed a pronounced cytotoxicity in DFO-treated osteoclast cultures. Consequently, we decided to test two other iron chelators, DFP and DFX. Both of these oral chelators had less effect on cell viability than had DFO (P<0.05) (Figure 6B), and DFX additionally showed stronger effects in term of reduction in osteoclast activity (P<0.05) (Figure 6A). Although it is not possible to draw conclusions on the potential efficacy of different chelating agents on preventing TM-associated bone resorption in this experimental, in-vitro setting, given the large differences in pharmacokinetics between DFX and DFO (plasma-protein binding of 99% and 10%, respectively), it is quite possible that differences may exist between these agents in this respect. In our cultures DFX binds to fetal bovine serum proteins and the bonds may attenuate the cytotoxicity of DFX.
In summary, this study provides evidence that osteoclast activity is increased by elevated levels of ferritin and LIC, as demonstrated by the biomarker TRAP and cathepsin levels, which together are correlated with reduced bone mineral density. The expression of TRPV1 channels, and the positive balance of CB1 versus CB2 cannabinoid receptors in TM osteoclasts appear to play a cooperative role in stimulating osteoclasts. Indeed, exposing healthy osteoclasts to serum from TM patients led to an increase in TRAP levels. Pharmacological stimulation of TRPV1 increased activation of osteoclasts in healthy subjects,30 while TRPV1 antagonism and desensitization can induce over-expression of the protective CB2 cannabinoid receptor - which acts as an “anti-inflammatory” counterpart receptor system in osteoporotic conditions.22 Activation in TM subjects did not alter TRAP levels, but there was a signal to reduced levels following treatment with iron-chelating agents, suggesting that TRPV1 activation and desensitization are dependent on iron. Treatment with iron chelators was associated with reduced levels of the osteoclast markers TRAP and cathepsin K.
For the first time, we demonstrate that iron overload, through up-regulation of TRAP expression, causes overactivity of osteoclasts in TM. Normal levels of activity can be restored with chelation therapy, opening up the possibility that oral chelation therapy could have a role in alleviating TM-associated OP.
Acknowledgments
The authors would like to thank all patients and their families for their participation in this study. This work was partially supported by the Italian Medicines Agency (AIFA) Project 2005, the national authority responsible for drug regulation in Italy (FARM5JJASJ) and by the Department of Women, Children and of General and Specialist Surgery at the Second University of Naples (Grant on Normal and Pathological Hematopoiesis). We thank Novartis for providing the DFX. The authors thank Kaivan Khavandi MD for medical editorial assistance with this manuscript. Financial support for editorial assistance was provided by Novartis Pharmaceuticals.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Skordis N, Toumba M. Bone disease in thalassaemia major: recent advances in pathogenesis and clinical aspects. Pediatr Endocrinol Rev. 2011;8(Suppl 2):300–6. [PubMed] [Google Scholar]
- 2.Haidar R, Musallam KM, Taher AT. Bone disease and skeletal complications in patients with beta thalassemia major. Bone. 2011;48(3):425–32. [DOI] [PubMed] [Google Scholar]
- 3.Toumba M, Skordis N. Osteoporosis syndrome in thalassaemia major: an overview. J Osteoporos. 2010;2010:537673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terpos E, Voskaridou E. Treatment options for thalassemia patients with osteoporosis. Ann NY Acad Sci. 2010;1202:237–43. [DOI] [PubMed] [Google Scholar]
- 5.Vogiatzi MG, Macklin EA, Fung EB, Cheung AM, Vichinsky E, Olivieri N, et al. Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res. 2009;24 (3):543–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voskaridou E, Terpos E. New insights into the pathophysiology and management of osteoporosis in patients with beta thalassaemia. Br J Haematol. 2004;127(2):127–39. [DOI] [PubMed] [Google Scholar]
- 7.Perrotta S, Cappellini MD, Bertoldo F, Servedio V, Iolascon G, D’Agruma L, et al. Osteoporosis in beta-thalassaemia major patients: analysis of the genetic background. Br J Haematol. 2000;111(2):461–6. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee R, Shah FT, Davis BA, Byers M, Sooranna D, Bajoria R, et al. Prospective study of histomorphometry, biochemical bone markers and bone densitometric response to 18 pamidronate in beta-thalassaemia presenting with osteopenia-osteoporosis syndrome. Br J Haematol. 2012;159 (4):462–71. [DOI] [PubMed] [Google Scholar]
- 9.Mahachoklertwattana P, Sirikulchayanonta V, Chuansumrit A, Karnsombat P, Choubtum L, Sriphrapradang A, et al. Bone histomorphometry in children and adolescents with beta-thalassemia disease: ironassociated focal osteomalacia. J Clin Endocrinol Metab. 2003;88(8):3966–72. [DOI] [PubMed] [Google Scholar]
- 10.Valenti L, Varenna M, Fracanzani AL, Rossi V, Fargion S, Sinigaglia L. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos Int. 2009;20(4):549–55. [DOI] [PubMed] [Google Scholar]
- 11.Isomura H, Fujie K, Shibata K, Inoue N, Iizuka T, Takebe G, et al. Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology. 2004;197 (2):93–100. [DOI] [PubMed] [Google Scholar]
- 12.Maggio A, Filosa A, Vitrano A, Aloj G, Kattamis A, Ceci A, et al. Iron chelation therapy in thalassemia major: a systematic review with meta-analyses of 1520 patients included on randomized clinical trials. Blood Cells Mol Dis. 2011;47(3):166–75. [DOI] [PubMed] [Google Scholar]
- 13.De Sanctis V, Pinamonti A, Di Palma A, Sprocati M, Atti G, Gamberini MR, et al. Growth and development in thalassaemia major patients with severe bone lesions due to desferrioxamine. Eur J Pediatr. 1996;155 (5):368–72. [DOI] [PubMed] [Google Scholar]
- 14.Voskaridou E, Kyrtsonis MC, Terpos E, Skordili M, Theodoropoulos I, Bergele A, et al. Bone resorption is increased in young adults with thalassaemia major. Br J Haematol. 2001;112(1):36–41. [DOI] [PubMed] [Google Scholar]
- 15.Voskaridou E, Terpos E, Spina G, Palermos J, Rahemtulla A, Loutradi A, et al. Pamidronate is an effective treatment for osteoporosis in patients with beta-thalassaemia. Br J Haematol. 2003;123(4):730–7. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee R, Katz M, Bajoria R. Use of hormone replacement therapy for correction of high turnover bone disease in hypogonadal beta-thalassemia major patients presenting with osteoporosis: comparison with idiopathic premature ovarian failure. Hemoglobin. 2011;35(56):6538. [DOI] [PubMed] [Google Scholar]
- 17.Dresner Pollack R, Rachmilewitz E, Blumenfeld A, Idelson M, Goldfarb AW. Bone mineral metabolism in adults with beta-thalassaemia major and intermedia. Br J Haematol. 2000;111(3):902–7. [PubMed] [Google Scholar]
- 18.Morabito N, Gaudio A, Lasco A, Atteritano M, Pizzoleo MA, Cincotta M, et al. Osteoprotegerin and RANKL in the pathogenesis of thalassemia-induced osteoporosis: new pieces of the puzzle. J Bone Miner Res. 2004;19(5):722–7. [DOI] [PubMed] [Google Scholar]
- 19.Voskaridou E, Terpos E. Osteoprotegerin to soluble receptor activator of nuclear factor kappa-B ligand ratio is reduced in patients with thalassaemia-related osteoporosis who receive vitamin D3. Eur J Haematol. 2005; 74(4):359–61. [DOI] [PubMed] [Google Scholar]
- 20.Voskaridou E, Anagnostopoulos A, Konstantopoulos K, Stoupa E, Spyropoulou E, Kiamouris C, et al. Zoledronic acid for the treatment of osteoporosis in patients with beta-thalassemia: results from a single-center, randomized, placebo-controlled trial. Haematologica. 2006;91(9):1193–202. [PubMed] [Google Scholar]
- 21.Morabito N, Russo GT, Gaudio A, Lasco A, Catalano A, Morini E, et al. The “lively” cytokines network in beta-thalassemia major-related osteoporosis. Bone. 2007;40 (6):1588–94. [DOI] [PubMed] [Google Scholar]
- 22.Rossi F, Bellini G, Luongo L, Torella M, Mancusi S, De Petrocellis L, et al. The endovanilloid/endocannabinoid system: a new potential target for osteoporosis therapy. Bone. 2011;48(5):997–1007. [DOI] [PubMed] [Google Scholar]
- 23.Ljusberg J, Wang Y, Lang P, Norgard M, Dodds R, Hultenby K, et al. Proteolytic excision of a repressive loop domain in tartrate-resistant acid phosphatase by cathepsin K in osteoclasts. J Biol Chem. 2005;280(31): 28370–81. [DOI] [PubMed] [Google Scholar]
- 24.Bab I, Zimmer A, Melamed E. Cannabinoids and the skeleton: from marijuana to reversal of bone loss. Ann Med. 2009;41(8):560–7. [DOI] [PubMed] [Google Scholar]
- 25.Bab I, Ofek O, Tam J, Rehnelt J, Zimmer A. Endocannabinoids and the regulation of bone metabolism. J Neuroendocrinol. 2008;20 (Suppl 1):69–74. [DOI] [PubMed] [Google Scholar]
- 26.Idris AI, van ’t Hof RJ, Greig IR, Ridge SA, Baker D, Ross RA, et al. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med. 2005;11(7): 774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Idris AI, Sophocleous A, Landao-Bassonga E, Canals M, Milligan G, Baker D, et al. Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metab. 2009;10(2):139–47. [DOI] [PubMed] [Google Scholar]
- 28.Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA. 2006;103(3):696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Idris AI, Sophocleous A, Landao-Bassonga E, van’t Hof RJ, Ralston SH. Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology. 2008;149(11):5619–26. [DOI] [PubMed] [Google Scholar]
- 30.Rossi F, Siniscalco D, Luongo L, De Petrocellis L, Bellini G, Petrosino S, et al. The endovanilloid/endocannabinoid system in human osteoclasts: possible involvement in bone formation and resorption. Bone. 2009; 44(3):476–84. [DOI] [PubMed] [Google Scholar]
- 31.Idris AI, Landao-Bassonga E, Ralston SH. The TRPV1 ion channel antagonist capsazepine inhibits osteoclast and osteoblast differentiation in vitro and ovariectomy induced bone loss in vivo. Bone. 2010;46 (4):1089–99. [DOI] [PubMed] [Google Scholar]
- 32.Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106(4):1460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organization technical report series. 1994;843:1–129. [PubMed] [Google Scholar]
- 34.Bisogno T, Sepe N, Melck D, Maurelli S, De Petrocellis L, Di Marzo V. Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochemical J. 1997;322(Pt 2):671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–4. [DOI] [PubMed] [Google Scholar]
- 36.Angelopoulos NG, Goula A, Katounda E, Rombopoulos G, Kaltzidou V, Kaltsas D, et al. Markers of bone metabolism in eugonadal female patients with beta-thalassemia major. Pediatr Hematol Oncol. 2007;24(7): 481–91. [DOI] [PubMed] [Google Scholar]
- 37.Chatterjee R, Bajoria R. Osteopenia-osteoporosis syndrome in patients with thalassemia: understanding of type of bone disease and response to treatment. Hemoglobin. 2009;33 (Suppl 1):S136–8. [DOI] [PubMed] [Google Scholar]
- 38.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22 (23):2171–9. [DOI] [PubMed] [Google Scholar]
- 39.Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA, et al. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood. 2009; 113(20):4853–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossi F, Bellini G, Luongo L, Mancusi S, Torella M, Tortora C, et al. The 17-betaoestradiol inhibits osteoclast activity by increasing the cannabinoid CB2 receptor expression. Pharmacol Res. 2013;68(1):7–15. [DOI] [PubMed] [Google Scholar]
- 41.Rossi F, Bellini G, Alisi A, Alterio A, Maione S, Perrone L, et al. Cannabinoid receptor type 2 functional variant influences liver damage in children with non-alcoholic fatty liver disease. PloS One. 2012;7(8):e42259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi F, Bellini G, Tolone C, Luongo L, Mancusi S, Papparella A, et al. The cannabinoid receptor type 2 Q63R variant increases the risk of celiac disease: implication for a novel molecular biomarker and future therapeutic intervention. Pharmacol Res. 2012;66 (1):88–94. [DOI] [PubMed] [Google Scholar]
- 43.Izzo AA, Piscitelli F, Capasso R, Marini P, Cristino L, Petrosino S, et al. Basal and fasting/refeeding-regulated tissue levels of endogenous PPAR-alpha ligands in Zucker rats. Obesity. 2010;18(1):55–62. [DOI] [PubMed] [Google Scholar]
- 44.Straiker A, Wager-Miller J, Hutchens J, Mackie K. Differential signalling in human cannabinoid CB1 receptors and their splice variants in autaptic hippocampal neurones. Br J Pharmacol. 2012;165(8):2660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laprairie RB, Kelly ME, Denovan-Wright EM. The dynamic nature of type 1 cannabinoid receptor (CB(1)) gene transcription. Br J Pharmacol. 2012;167(8):1583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El Rawas R, Thiriet N, Nader J, Lardeux V, Jaber M, Solinas M. Early exposure to environmental enrichment alters the expression of genes of the endocannabinoid system. Brain Res. 2011;1390:80–9. [DOI] [PubMed] [Google Scholar]
- 47.Hong S, Zheng G, Wu X, Snider NT, Owyang C, Wiley JW. Corticosterone mediates reciprocal changes in CB 1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat. Gastroenterology. 2011;140(2):627–37e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sooampon S, Manokawinchoke J, Pavasant P. Transient receptor potential vanilloid-1 regulates osteoprotegerin/RANKL homeostasis in human periodontal ligament cells. J Periodontal Res. 2013;48(1):22–9. [DOI] [PubMed] [Google Scholar]
- 49.Kramer JL, Baltathakis I, Alcantara OS, Boldt DH. Differentiation of functional dendritic cells and macrophages from human peripheral blood monocyte precursors is dependent on expression of p21 (WAF1/CIP1) and requires iron. Br J Haematol. 2002;117 (3):727–34. [DOI] [PubMed] [Google Scholar]
- 50.Furukawa Y. Cell cycle control during hematopoietic cell differentiation. Hum Cell. 1997;10(3):159–64. [PubMed] [Google Scholar]


