Abstract
The prognosis of patients with primary mediastinal large B-cell lymphoma has improved over recent years. However, the optimal treatment strategy including the role of radiotherapy remains unknown. We retrospectively analyzed the clinical outcomes of 345 patients with newly diagnosed primary mediastinal large B-cell lymphoma in Japan. With a median follow up of 48 months, the overall survival at four years for patients treated with R-CHOP (n=187), CHOP (n=44), DA-EPOCH-R (n=9), 2nd- or 3rd-generation regimens, and chemotherapy followed by autologous stem cell transplantation were 90%, 67%, 100%, 91% and 92%, respectively. Focusing on patients treated with R-CHOP, a higher International Prognostic Index score and the presence of pleural or pericardial effusion were identified as adverse prognostic factors for overall survival in patients treated with R-CHOP without consolidative radiotherapy (IPI: hazard ratio 4.23, 95% confidence interval 1.48–12.13, P=0.007; effusion: hazard ratio 4.93, 95% confidence interval 1.37–17.69, P=0.015). Combined with the International Prognostic Index score and the presence of pleural or pericardial effusion for the stratification of patients treated with R-CHOP without radiotherapy, patients with lower International Prognostic Index score and the absence of effusion comprised approximately one-half of these patients and could be identified as curable patients (95% overall survival at 4 years). The DA-EPOCH-R regimen might overcome the effect of these adverse prognostic factors. Our simple indicators of International Prognostic Index score and the presence of pleural or pericardial effusion could stratify patients with primary mediastinal large B-cell lymphoma and help guide selection of treatment.
Introduction
Primary mediastinal large B-cell lymphoma (PMBL) is characterized by distinct clinical, pathological and genetic features and comprises a subtype of diffuse large B-cell lymphoma (DLBCL) according to the current World Health Organization (WHO) classification.1 The disease is more common in younger females and often presents with bulky mediastinal mass without extrathoracic spread and pleural or pericardial effusion.2–5
Prior to the introduction of rituximab, the outcomes of patients treated with anthracycline-containing chemotherapies, including cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP), had a suboptimal progression-free survival (PFS) of 38%-52%.5,6 Several retrospective analyses revealed that the outcomes of the 2nd- and 3rd-generation chemotherapeutic regimens, such as methotrexate, leucovorin, doxorubicin, cyclophosphamide, vincristine, bleomycin and prednisolone (MACOP-B), might be superior to those of CHOP regimens.5,7–10 High-dose chemotherapy followed by autologous stem cell transplantation (HDT/ASCT) was also associated with encouraging results (PFS >75% for newly diagnosed PMBL patients).3,11,12 These reports indicate that intensive regimens might be beneficial in a certain proportion of PMBL patients.
In the rituximab era, the combination of rituximab and chemotherapy has improved outcomes in various subtypes of B-cell lymphoma.13–22 In the literature, more than 80% of patients with PMBL receiving immunochemotherapy with or without radiotherapy (RT) also achieved long-term overall survival (OS).17–22 Despite the outstanding advances with R-CHOP, 20%-30% of patients still experience progression or relapse and have poor outcomes. Moreover, approximately 80% of long-term survivors treated with R-CHOP required consolidative RT for residual mediastinal disease.20–23 Considering late adverse events induced by the mediastinal RT, namely the increased risk of secondary breast cancer and cardiac toxicity, the risk of RT should be minimized, especially for younger patients.24–26
Recently, Dunleavy et al. reported excellent outcomes for dose-adjusted etoposide, cyclophosphamide, doxorubicin, vincristine, prednisolone and rituximab (DA-EPOCH-R) when restricting candidates for RT according to the results of positron-emission tomography/computed tomography (PET/CT).27 Although outcomes were reported from a phase II trial, the regimen might be a promising treatment strategy to reduce the risk of RT. Meanwhile, the DA-EPOCH-R regimen is somewhat complicated and expensive, requiring continuous infusion for 96 h in each cycle and frequent evaluation of complete blood counts. Considering R-CHOP-based regimens without RT could provide curative potential for a significant proportion of PMBL patients without hospitalization,19,21 it would, therefore, be beneficial to identify the subset of patients that could be cured with this treatment strategy.
The goal of the present multicenter co-operative retrospective study in Japan was to investigate the optimal treatment strategy for PMBL patients by evaluating the clinical outcomes in response to various treatments and to assess a risk-stratified treatment strategy to minimize the risk of late adverse events in PMBL patients.
Methods
Patients
A total of 363 patients with PMBL newly diagnosed between May 1986 and September 2012 at one of any of the 65 participating hospitals in Japan were retrospectively analyzed. We registered consecutive patients who were diagnosed with PMBL at each institution in accordance with the WHO classification.1 The time period during which we could collect the clinical data from each institution varied due to the differences in the length of time medical records are kept there. Medical record data since the 1980s were collected from three institutions, while data since the 1990s and 2000s were available from 10 and 65 institutions, respectively. In this study, PMBL was defined as patients with a dominant mass within the anterior mediastinum, irrespective of the tumor size. In addition, a central pathological review was performed by a hematopathologist (SN) for 196 patients for whom histological paraffin-embedded tissue materials could be provided. Eighteen of the 363 patients were excluded from analysis due to disease other than PMBL (n=10) by central pathological review or due to the absence of important clinical information (n=8). For the remaining patients who were not available for the central review, the histological diagnosis of PMBL was re-confirmed by a pathologist at each institution, according to the current WHO classification. Therefore, 345 patients were finally analyzed for the present study. Patients were treated according to each institution’s treatment standards. The study protocol was approved by the institutional review boards of Nagoya Daini Red Cross Hospital where this study was organized and of each participating hospital. The study complied with all the provisions of the Declaration of Helsinki.
Immunohistochemistry
Immunohistochemistry was performed using formalin-fixed, paraffin-embedded tissue sections using the avidin-biotin peroxidase complex method. Monoclonal antibodies targeting the following proteins were used: CD20, CD30, CD3, CD10, BCL6, MUM1 and CD15 (Dako). In addition, programmed cell death ligand-1 (PDL1) was evaluated, as previously described.28 To evaluate PDL1, we used a polyclonal rabbit antibody for CD274 (ab82059; Abcam) according to the manufacturer’s instructions. The cut-off values for these markers were 20% for CD30, and 30% for Bcl-6, MUM1 and PDL1.29–31
Treatment
Initial treatments were performed based on the physicians’ decisions at each institution, as there had been no uniform treatment guidelines for PMBL in Japan. Patients who received CHOP or a CHOP-like regimen, with or without rituximab, were categorized and analyzed as the R-CHOP or CHOP group, respectively. Patients who received 2nd-/3rd-generation treatments were categorized and analyzed as the 2nd-/3rd-generation regimen group, irrespective of the use of rituximab. Patients who received the DA-EPOCH-R regimen27 were analyzed as the DA-EPOCH-R group. Patients who underwent consolidative HDT/ASCT after initial therapy were analyzed as the HDT/ASCT group, irrespective of the use of rituximab. CHOP- or R-CHOP-based regimens were mainly selected in 46 institutions. Physicians at six institutions selected 2nd-/3rd-generation chemotherapeutic regimens other than CHOP- or R-CHOP-based regimens as the first-line treatment. HDT/ASCT as the first-line treatment was performed at 13 institutions. Consolidative RT was performed according to the treatment strategy used at each institution.
Response assessment
Clinical data were collected from case report forms. In principle, an effusion was evaluated by CT and/or echocardiography, as per the usual pre-treatment evaluation. Responses were evaluated by each investigator in accordance with the 1999 International Workshop Criteria.32
Statistical analysis
Overall survival was defined as the period from diagnosis to death or last follow up. Progression-free survival (PFS) was defined as the period from diagnosis to disease progression, relapse, death from any cause, or last date of follow up. Patients who did not achieve a complete remission (CR) or partial response (PR) were considered to have primary refractory disease. Early relapse was defined as relapse occurring less than 12 months after diagnosis. PFS and OS were analyzed using Kaplan-Meier methods and results were compared using the log rank test. Univariate and multivariate Cox regression analyses were performed to assess the effects of prognostic factors. Multivariate analysis was built with a forward/backward, step-wise method using threshold values for removal from and addition to the model of P=0.20 and P=0.05, respectively. The individual factors of IPI were entered into the model in multivariate analysis. All probability values were two-sided and had an overall significance level of 0.05. Statistical analyses were performed with Stata SE 12 software (StataCorp LP, College Station, TX, USA).
Results
Patients’ characteristics
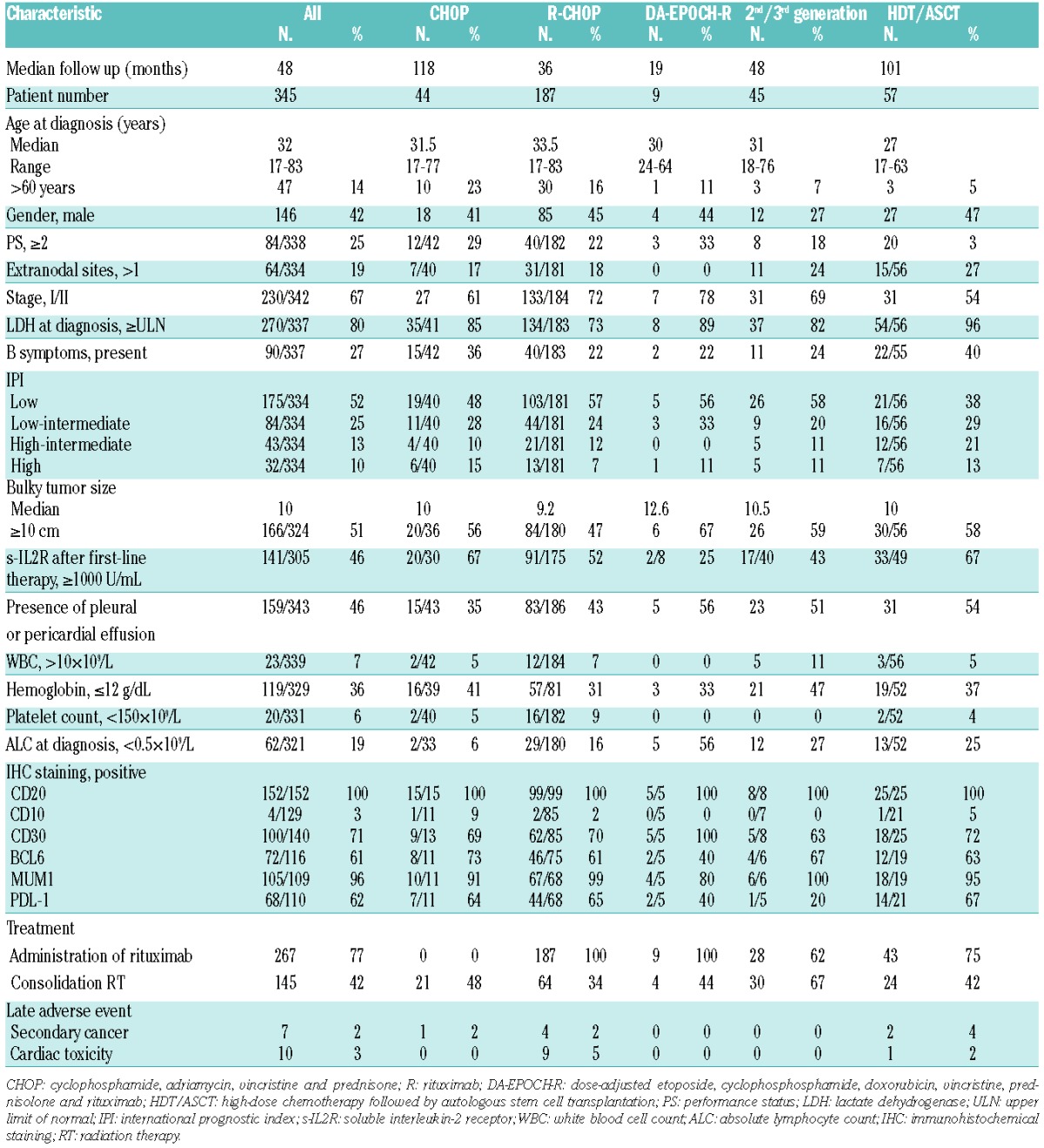
Patients’ characteristics are summarized in Table 1. Median age was 32 years (range 17–83 years) and females were predominant (58%). The median diameter of mediastinal mass was 10 cm (range 3–32 cm). Stage I/II disease, low-risk disease according to the International Prognostic Index (IPI), and performance status (PS) 0/1 were also predominant (67%, 52% and 75%, respectively). The presence of pleural or pericardial effusion, elevated lactate dehydrogenase (LDH) level and more than one extranodal lesion were observed in 46%, 80% and 9% of patients, respectively. For the patients who had extranodal involvement, major extranodal sites were lung (n=44), effusion (n=49) and cardiac (n=28). Pathological features are listed in Table 1. Lymphoma cells in all patients expressed CD20. Further, CD30, BCL6, and MUM1 expression was detected in 71%, 61%, and 96%, respectively. PDL1 was expressed in 62% of 110 evaluable patients.
Table 1.
Patients’ characteristics.
Treatment regimen
In all, 267 patients received rituximab-containing chemotherapy. CHOP and R-CHOP chemotherapy groups consisted of 44 and 187 patients, respectively. DA-EPOCH-R chemotherapy was administered to 9 patients. In the 2nd-/3rd-generation regimen group (n=45), 28 patients received MACOP-B with (n=18) or without (n=10) rituximab, 15 patients received cyclophosphamide, vincristine, bleomycin, etoposide, doxorubicin and prednisolone (CyclOBEAP)33 with (n=12) or without (n=3) rituximab, and 2 patients received vincristine, cyclophosphamide, doxorubicin, ranimustine, vindesine, etoposide carboplatin and prednisone (JCOG-LSG15 study regimen).34 In the HDT/ASCT group (n=57), 43 patients received rituximab-containing chemotherapy as the initial chemotherapy. Consolidative RT was given to 42% of all patients. After approval of the use of rituximab for DLBCL in Japan in 2003, the use of rituximab-containing regimens rapidly increased, as shown in Online Supplementary Table S1. There was a moderate decrease in the use of HDT/ASCT and radiation therapy after initial treatment. The DA-EPOCH-R regimen was selected in the latest period.
Clinical outcomes
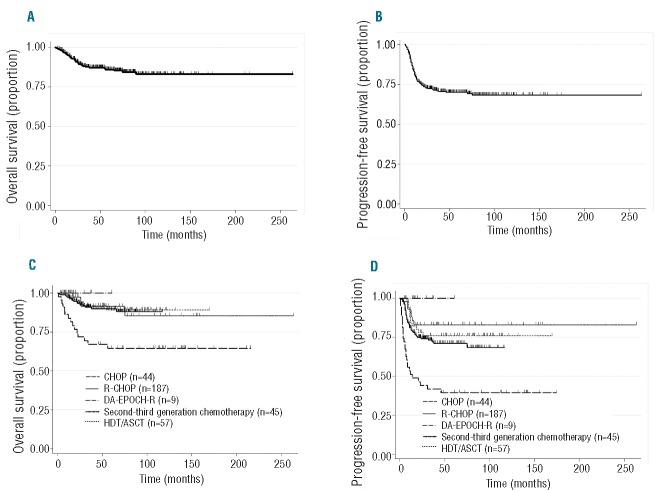
With a median follow up of 48 months in surviving patients, the OS and PFS at four years were 87% and 70%, respectively (Figure 1A and B). The OS and PFS of patients treated with rituximab-containing chemotherapy were superior to those of patients receiving chemotherapy without rituximab (4-year OS: 91% vs. 77%, P<0.001; 4-year PFS: 75% vs. 54%, respectively, P<0.001). There was no difference in the risk of central nervous system (CNS) relapse between patients treated with and patients treated without rituximab as first-line treatment (3.8% vs. 1.3%; P=0.251). The OS at four years for patients treated with CHOP, R-CHOP, DA-EPOCH-R, the 2nd-/3rd-generation regimens, and HDT/ASCT was 67%, 90%, 100%, 91% and 92%, respectively, with median follow-up durations of 118 months, 36 months, 19 months, 48 months and 101 months, respectively (P<0.001) (Figure 1C); PFS at four years was 40%, 71%, 100%, 83% and 76%, respectively (P<0.001) (Figure 1D).
Figure 1.
Survival of patients with primary mediastinal large B-cell lymphoma. (A) Overall survival (OS) of all patients with primary mediastinal large B-cell lymphoma (PMBL). (B) Progression-free survival (PFS) of all patients with PMBL. (C) OS of patients with PMBL treated with CHOP (n=44), R-CHOP (n=188), DA-EPOCH-R (n=9), 2nd- or 3rd-generation regimens (n=45), and HDT/ASCT (n=57). (D) PFS of patients with PMBL treated with CHOP (n=44), R-CHOP (n=188), DA-EPOCH-R (n=9), 2nd- or 3rd-generation regimens (n=45), and HDT/ASCT (n=57). CHOP: cyclophosphamide, adriamycin, vincristine and prednisone; R: rituximab; DA-EPOCH-R: dose-adjusted etoposide, cyclophosphosphamide, doxorubicin, vincristine, prednisolone and rituximab; HDT/ASCT: high-dose chemotherapy followed by autologous stem cell transplantation.
Secondary malignancies and cardiac toxicity developed after treatment in 7 and 10 patients, respectively. The median age of these 17 patients was 62 years. Seven of 17 patients received RT or ASCT as first-line treatment. In addition, 3 of 7 patients who developed secondary malignancies received RT during the initial series of treatment. Among the secondary malignancies, myelodysplastic syndrome (MDS) or acute myeloblastic leukemia (AML) was reported in 2 patients. The patient who developed MDS received HDT/ASCT as a first-line treatment. The patient who developed AML received CHOP as a first-line treatment and ICE as a salvage treatment. Among the 187 patients treated with R-CHOP, 9 experienced cardiac toxicity, and 4 developed a secondary cancer. The median time to development of a secondary malignancy was 40.5 months (range 9–200 months).
Patients’ characteristics and clinical outcomes in the R-CHOP group
Detailed characteristics of patients in the R-CHOP group are shown in Online Supplementary Table S2. We divided this group into four subgroups according to the disease status after R-CHOP or R-CHOP-like regimen and the presence or absence of consolidative RT: namely, R-CHOP+RT with residual mass, R-CHOP+RT in CR, R-CHOP with residual mass and R-CHOP in CR. Among the 187 patients in the R-CHOP group, 64 patients received consolidative RT after R-CHOP (Online Supplementary Table S3). Elderly age and higher IPI score were less common in those who received consolidative RT. Thirty-three of 64 patients received consolidative RT with residual mass after R-CHOP, while 31 of 64 patients received RT in CR after R-CHOP. Among the remaining 123 patients without consolidative RT, 34 patients did not achieve CR after R-CHOP, and 89 patients were in CR after R-CHOP, respectively. Among 34 patients with residual mass who were treated with R-CHOP, 16 patients developed progressive disease (PD), and 4 patients received follow up without RT based on the negative findings on PET/CT after the initial series of treatment. Of 89 patients who achieved a CR after R-CHOP but did not receive RT, 14 patients experienced relapse. Among these 14 patients, 9 developed the relapsed disease in their mediastinum, while the remaining 5 relapsed in other sites. The OS and PFS at four years of patients receiving consolidative RT were 100% and 85%, respectively, in the group with residual mass, and 96% and 90%, respectively, in the group in CR (OS: P=0.15; PFS: P=0.80) (Online Supplementary Figures S1 and S2). Meanwhile, the OS and PFS at four years of patients who did not receive consolidative RT were 64% and 35%, respectively, in the group with residual mass without disease progression, and 95% and 77%, respectively, in the group in CR (OS: P<0.001; PFS: P<0.001). Taken together, these data indicate that a significant proportion of patients achieving CR after R-CHOP can be cured without consolidative RT.
Prognostic factors and survival for patients treated with R-CHOP and without consolidative radiotherapy
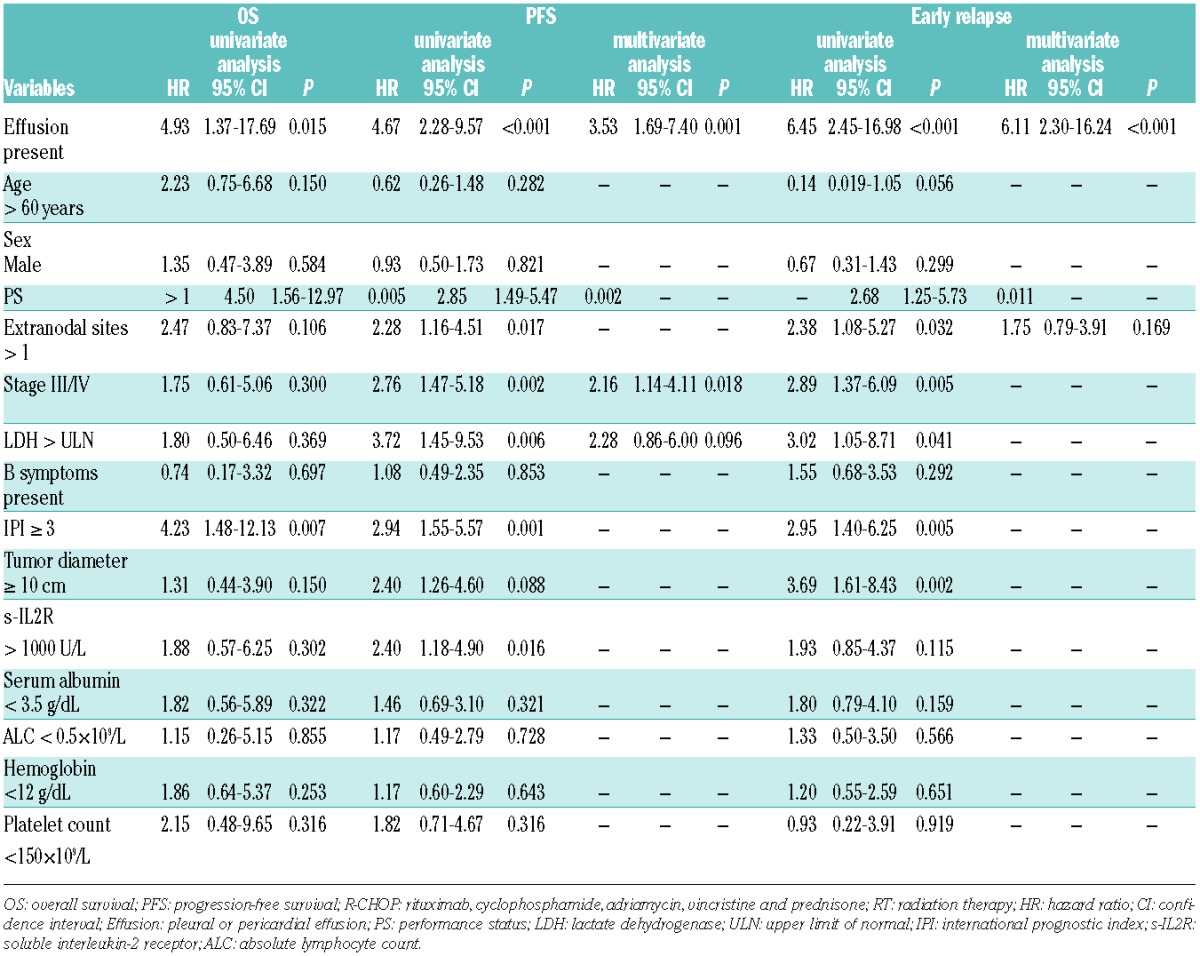
One hundred and twenty-three patients receiving R-CHOP without consolidative RT were analyzed. The analysis of potential prognostic factors is shown in Table 2. On univariate analysis, the presence of pleural or pericardial effusion, performance status (PS) over 1 and higher IPI were adverse prognostic factors for OS, and the presence of pleural or pericardial effusion, advanced stage, extranodal involvement, PS, LDH, soluble interleukin-2 receptor (sIL-2R), and higher IPI were adverse prognostic factors for PFS. On multivariate analysis, we could not identify significant prognostic factors for OS. The presence of pleural or pericardial effusion [hazard ratio (HR), 3.53; 95% confidence interval (CI), 1.69–7.40; P=0.001] and advanced stage (stage III/IV; HR, 2.16; 95%CI: 1.14–4.11; P=0.018) were identified as adverse prognostic factors for PFS. As almost all the patients with progression after R-CHOP developed disease within 12 months after diagnosis, we performed Cox regression analyses to determine the predictive factors for primary refractory or early relapse within 12 months after diagnosis. On multivariate analysis, only the presence of pleural or pericardial effusion was predictive of primary refractory or early relapse within 12 months (HR, 6.11; 95%CI: 2.30–16.24; P<0.001). In this cohort, only 5 (8%) of 65 patients without pleural or pericardial effusion experienced primary refractory or early relapse within 12 months; meanwhile, 25 (43%) of 58 patients with pleural or pericardial effusion (P<0.001) had refractory or early relapsed disease.
Table 2.
Risk factors for overall survival, progression-free survival and early relapse for patients treated with R-CHOP without consolidative radium therapy.
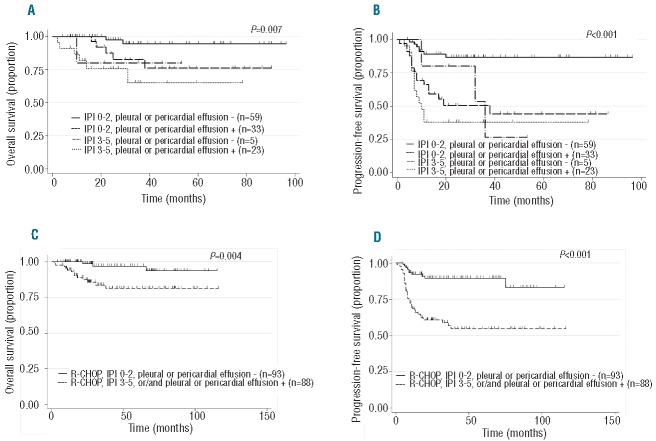
As IPI and the presence of pleural or pericardial effusion were prognostic factors for OS on univariate analysis, and these were not related (correlation coefficient = 0.39), the OS and PFS in patients receiving R-CHOP without RT were analyzed according to these prognostic factors. The OS and PFS in patients receiving R-CHOP without RT were analyzed according to the presence of pleural or pericardial effusion and IPI. As expected (Figure 2A and B), the best OS and PFS were observed in patients with low IPI and without pleural or pericardial effusion. The OS and PFS at four years of these 58 patients were 95% and 87%, respectively. Meanwhile, based on individual factors of LDH, B symptom, and pleural or pericardial effusion identified on multivariate analysis for PFS, the OS and PFS were also analyzed (Online Supplementary Figures S3 and S4). Although the OS and PFS could be well stratified, the number of patients categorized into the well stratified low-risk category was lower than that of patients under the stratification using IPI and effusion. Taken together, these data indicate that a significant proportion of patients with low IPI and without pleural or pericardial effusion at the time of diagnosis can be potentially cured by the R-CHOP regimen without consolidative RT.
Figure 2.
Survival of patients with primary mediastinal large B-cell lymphoma according to the International Prognostic Index and the presence pleural or pericardial effusion. (A) Overall survival (OS) of patients with primary mediastinal large B-cell lymphoma (PMBL) treated with R-CHOP without radiation therapy (RT) according to the international prognostic index (IPI) and the presence pleural or pericardial effusion. (B) Progression-free survival (PFS) of patients with PMBL treated with R-CHOP without RT according to the IPI and the presence pleural or pericardial effusion. (C) OS of patients with PMBL treated with R-CHOP according to the IPI and the presence pleural or pericardial effusion. (D) PFS of patients with PMBL treated with R-CHOP according to the IPI and the presence pleural or pericardial effusion. R-CHOP: rituximab, cyclophosphamide, adriamycin, vincristine and prednisone; RT: radiation therapy.
Meanwhile, the treatment should be considered for patients with higher IPI and the presence of pleural or pericardial effusion. As shown in Figure 2C and D, the outcomes of R-CHOP were not satisfactory in patients with higher IPI and/or the presence of pleural or pericardial effusion (4-year OS: 97% vs. 81%, P=0.004; 4-year PFS: 89% vs. 54%, P<0.001, respectively).
Discussion
It is important to establish a more effective and less toxic standard treatment for PMBL, as affected patients tended to be young and can be cured when properly treated. The present study investigated a larger cohort than other studies and indicated that almost all PMBL patients with lower IPI and the absence of the pleural or pericardial effusion could be cured by the R-CHOP regimen without consolidative RT. Considering the excellent outcomes of the recent promising regimen DA-EPOCH-R, reported by Dunleavy et al.,27 the initial treatment regimen for PMBL could be stratified according to our simple indicators of IPI score and the presence of pleural or pericardial effusion; DA-EPOCH-R or R-CHOP could be selected for high- or low-risk PMBL patients, respectively.
Consistent with other studies, patients who received rituximab-containing chemotherapies showed better outcomes.17–22,27,35 HDT/ASCT and 2nd-/3rd-generation regimens that were more intensive and that have been historically used as first-line treatment for PMBL resulted in better outcomes than those seen in response to CHOP chemotherapy.11,17,18,36 In the present study, similar OS and PFS was observed among patients treated with a 2nd-/3rd-generation regimen, HDT/ASCT, and R-CHOP. This suggests that R-CHOP regimen might have curative potential in a significant proportion of PMBL patients without utilizing 2nd-/3rd-generation regimen or HDT/ASCT and thereby avoiding their associated toxicities.
Late toxicities are another important issue to consider when weighing the benefits of different curative regimens. In the current study, 17 patients had late adverse events (secondary cancer, n=7; cardiac toxicity, n=10). Previous reports indicated that RT to the mediastinum significantly increased the risk of breast cancer and cardiac toxicity.24–26,37 Although longer follow up is required to evaluate for late toxicities, we investigated whether we could omit the consolidative RT from the current treatment strategies. We analyzed the outcomes of patients treated with R-CHOP without consolidative RT, and identified higher IPI and the presence of pleural or pericardial effusion as adverse risk factors for OS. Moreover, the presence of the effusion was identified as an adverse risk factor for early relapse. Considering that previous studies had reported that the presence of pleural effusion was associated with poor outcomes in patients with PMBL and Hodgkin lymphoma,21,38 our results might be universal. Our simple indicators could identify patients who could be cured in response to R-CHOP without consolidative RT; however, patients with these factors comprised only approximately one-half of patients receiving R-CHOP. This means the remaining patients should be treated with an alternative regimen. The fact that excellent outcomes were seen in patients with higher IPI and the presence of the effusion receiving DA-EPOCH-R regimen in this study, as well as in another recent report,27 suggests that it may be reasonable to use this approach in high-risk PMBL patients. A prospective trial of this strategy is warranted.
Another approach to stratify PMBL patients is currently being investigated in Europe. The prospective IELSG-37 trial is investigating whether consolidative RT could be omitted according to the presence or absence of FDG-PET or PET/CT findings after the initial series of treatments. In clinical practice, we frequently encounter patients in whom it is difficult to judge FDG-PET positivity.39,40 Unfortunately, we could not evaluate the role of PET/CT in this study because of retrospective settings. Meanwhile, the very recent report from the IELSG-26 study clarified the role of PET/CT after treatment in PMBL patients.41 Considering the difficulty of re-biopsy of the suspected mediastinal mass after treatment, using the optimal cut-off value on PET/CT after treatment reported by IELSG could be an important tool to assess the risk of treatment failure.
This study has several limitations. First, its retrospective nature might have unrecognized biases and the results should be interpreted with care. Regarding evaluation of response, evaluation of the residual mass might have been heterogeneous at each institution because of the retrospective setting. Therefore, the CR rate in our study could be over-estimated. Second, patients received various treatment regimens and consolidative RT according to each institution’s preferred strategy; thus, treatment outcomes might have been over-estimated or under-estimated. In particular, patients who did not receive consolidative RT might have had clinical indicators that physicians considered favorable, resulting in an overestimation of the clinical outcomes in response to R-CHOP without consolidative RT. However, in the present analysis, the proportion of patients with higher IPI and with the presence of effusion was not low in patients who did not receive consolidative RT compared with that in patients who did receive RT. This suggests that the base-line characteristics and outcomes of patients without consolidative RT were not necessarily favorable and that they might not have been over-estimated. Finally, we carried out a central pathological review for only 196 patients. We tried to collect as much pathological histological paraffin-embedded tissue materials as possible. However, in some cases, sufficient materials were not available because they were too old. In addition, the period during which data could be submitted differed because clinical data were kept for different lengths of time at the different institutions. Therefore, the number of institutions who could submit clinical data in the 1980s and 1990s was smaller than in the 2000s: 10 and 65 institutions before and after the year 2000, respectively. Furthermore, although gene expression or methylation profiling can help to diagnose PMBL correctly, for the moment we cannot use these tools in routine clinical practice. Further study of the utility of these biological tools is necessary to improve diagnosis and management of this disease.
In conclusion, the present study demonstrated that IPI and the presence of pleural or pericardial effusion were adverse prognostic factors for risk stratification of PMBL patients treated with R-CHOP. R-CHOP without consolidative RT can achieve a high rate of cure for approximately one-half of PMBL patients, while alternative regimens, including DA-EPOCH-R, should be offered to the remaining patients. Prospective studies to validate these prognostic factors and a risk-adopted treatment strategy are warranted.
Acknowledgments
The authors would like to thank following physicians for providing patient cases; Yoshinobu Kanda, MD of Saitama Medical Center, Jichi Medical University, Yoshihiro Yakushizin, MD of Ehime University Hospital, Yasunori Nakagawa, MD of the Japanese Red Cross Medical Center, Taro Masunari, MD of Chugoku Central Hospital, Yoshitoyo Kagami, MD of Toyota Kosei Hospital, Kazunori Ohnishi, MD of Hamamatsu University Hospital, Naoe Goto, MD of Gifu University Hospital, Norifumi Tsukamoto, MD of Gunma University Hospital, Noriyasu Fukushima, MD of Saga University Hospital, Shigeru Kusumoto, MD of Nagoya City University Hospital, Yoshitaka Imaizumi, MD of Nagasaki University Hospital, Koji Kato, MD of Kyushu University Hospital, Takenori Takahata, MD of Hirosaki University Hospital, Yasufumi Masaki, MD of Kanazawa Medical University Hospital, Akiyo Yoshida, MD of Kanazawa University Hospital, Masanobu Nakata, MD of Sapporo Hokuyu Hospital, Akinao Okamoto, MD of Fujita Health University Hospital, Ryosuke Shirasaki, MD of Teikyo University Hospital, Ichiro Hanamura, MD of Aichi Medical University Hospital, Kensuke Naito, MD of Hamamatsu Medical Center, Shingo Kurahashi, MD of Japanese Red Cross Nagoya First Hospital, Masahide Yamamoto, MD of Medical Hospital of Tokyo Medical and Dental University, Junji Suzumiya, MD of Shimane University Hospital, Hirokazu Nagai, MD of Nagoya Medical Center, Masahiro Yokoyama, MD of Cancer Institute Hospital, Yuichi Hasegawa, MD of University of Tsukuba Hospital, Katsuhiro Miura, MD of Nihon University Itabashi Hospital, Kensuke Usuki, MD of NTT Medical Center Tokyo, Naokuni Uike, MD of Kyushu Cancer Center, Shin Fujisawa, MD of Yokohama City University Medical Center, Yasushi Takamatsu, MD of Fukuoka University Hospital, Akinori Nishikawa, MD of Wakayama Medical University Hospital, Naoto Tomita, MD of Yokohama City University Hospital, Hideki Tsujimura, MD of Chiba Cancer Center, Takashi Miyagi, MD of Heart Life Hospital, Katsuya Fujimoto, MD of Hokkaido University Hospital, Senji Kasahara, MD of Gifu Municipal Hospital, Atsushi Wakita, MD of Nagoya City West Medical Center, Michihide Tokuhira, MD of Saitama Medical Center, Takahiko Utsumi, MD of Shiga Medical Center for Adults, Kazuhito Yamamoto, MD of Aichi Cancer Center, Kunio Kitamura, MD of Ichinomiya Municipal Hospital, Toshimasa Kamoda, MD of Tenri Hospital, Kana Miyazaki, MD of Mie University Hospital, Keiichiro Mihara, MD of Hiroshima University Hospital, Yoshiko Inoue, MD of Kumamoto Medical Center, Masatoshi Kanno, MD of Nara Medical University Hospital, Kazutaka Sunami, MD of Okayama Medical Center, Noriko Usui, MD of Third Hospital, Jikei University School of Medicine, and Yoshiharu Maeda, MD of Komagome Hospital.; and We thank Tomomitsu Hotta, MD, of the National Cancer Center for critical reading of the manuscript. We also thank the patients, physicians, nurses, and staff members who participated in this multicenter trial for their excellent cooperation.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work was supported by the Hematological Malignancy Clinical Study Group and the National Cancer Center Research and Development Fund (23-A-17).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press;. 2008. [Google Scholar]
- 2.Cazals-Hatem D, Lepage E, Brice P, Ferrant A, d’Agay MF, Baumelou E, et al. Primary mediastinal large B-cell lymphoma. A clinicopathologic study of 141 cases compared with 916 nonmediastinal large B-cell lymphomas, a GELA (“Groupe d’Etude des Lymphomes de l’Adulte”) study. Am J Surg Pathol. 1996;20(7):877–88. [DOI] [PubMed] [Google Scholar]
- 3.Lazzarino M, Orlandi E, Paulli M, Strater J, Klersy C, Gianelli U, et al. Treatment outcome and prognostic factors for primary mediastinal (thymic) B-cell lymphoma: a multicenter study of 106 patients. J Clin Oncol. 1997;15(4):1646–53. [DOI] [PubMed] [Google Scholar]
- 4.van Besien K, Kelta M, Bahaguna P. Primary mediastinal B-cell lymphoma: a review of pathology and management. J Clin Oncol. 2001;19(6):1855–64. [DOI] [PubMed] [Google Scholar]
- 5.Zinzani PL, Martelli M, Bertini M, Gianni AM, Devizzi L, Federico M, et al. Induction chemotherapy strategies for primary mediastinal large B-cell lymphoma with sclerosis: a retrospective multinational study on 426 previously untreated patients. Haematologica. 2002;87(12):1258–64. [PubMed] [Google Scholar]
- 6.Hamlin PA, Portlock CS, Straus DJ, Noy A, Singer A, Horwitz SM, et al. Primary mediastinal large B-cell lymphoma: optimal therapy and prognostic factor analysis in 141 consecutive patients treated at Memorial Sloan Kettering from 1980 to 1999. Br J Haematol. 2005;130(5):691–9. [DOI] [PubMed] [Google Scholar]
- 7.Martelli MP, Martelli M, Pescarmona E, De Sanctis V, Donato V, Palombi F, et al. MACOP-B and involved field radiation therapy is an effective therapy for primary mediastinal large B-cell lymphoma with sclerosis. Ann Oncol. 1998;9(9):1027–9. [DOI] [PubMed] [Google Scholar]
- 8.Todeschini G, Ambrosetti A, Meneghini V, Pizzolo G, Menestrina F, Chilosi M, et al. Mediastinal large-B-cell lymphoma with sclerosis: a clinical study of 21 patients. J Clin Oncol. 1990;8(5):804–8. [DOI] [PubMed] [Google Scholar]
- 9.Zinzani PL, Martelli M, Bendandi M, De Renzo A, Zaccaria A, Pavone E, et al. Primary mediastinal large B-cell lymphoma with sclerosis: a clinical study of 89 patients treated with MACOP-B chemotherapy and radiation therapy. Haematologica. 2001; 86(2):187–91. [PubMed] [Google Scholar]
- 10.Zinzani PL, Martelli M, Magagnoli M, Pescarmona E, Scaramucci L, Palombi F, et al. Treatment and clinical management of primary mediastinal large B-cell lymphoma with sclerosis: MACOP-B regimen and mediastinal radiotherapy monitored by (67)Gallium scan in 50 patients. Blood. 1999;94(10):3289–93. [PubMed] [Google Scholar]
- 11.Sehn LH, Antin JH, Shulman LN, Mauch P, Elias A, Kadin ME, et al. Primary diffuse large B-cell lymphoma of the mediastinum: outcome following high-dose chemotherapy and autologous hematopoietic cell transplantation. Blood. 1998;91(2):717–23. [PubMed] [Google Scholar]
- 12.Cairoli R, Grillo G, Tedeschi A, Gargantini L, Marenco P, Tresoldi E, et al. Efficacy of an early intensification treatment integrating chemotherapy, autologous stem cell transplantation and radiotherapy for poor risk primary mediastinal large B cell lymphoma with sclerosis. Bone Marrow Transplant. 2002;29(6):473–7. [DOI] [PubMed] [Google Scholar]
- 13.Witzens-Harig M, Ho AD, Kuhnt E, Trneny M, Rieger M, Osterborg A, et al. Primary Mediastinal B Cell Lymphoma Treated with CHOP-Like Chemotherapy with or without Rituximab: 5-Year Results of the Mabthera International Trial Group (MInT) Study. ASH Annual Meeting Abstracts. 2012;120(21):1612. [Google Scholar]
- 14.Shimada K, Matsue K, Yamamoto K, Murase T, Ichikawa N, Okamoto M, et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26(19):3189–95. [DOI] [PubMed] [Google Scholar]
- 15.Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008; 9(2):105–16. [DOI] [PubMed] [Google Scholar]
- 16.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–42. [DOI] [PubMed] [Google Scholar]
- 17.Savage KJ, Al-Rajhi N, Voss N, Paltiel C, Klasa R, Gascoyne RD, et al. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol. 2006;17(1):123–30. [DOI] [PubMed] [Google Scholar]
- 18.Zinzani PL, Stefoni V, Finolezzi E, Brusamolino E, Cabras MG, Chiappella A, et al. Rituximab combined with MACOP-B or VACOP-B and radiation therapy in primary mediastinal large B-cell lymphoma: a retrospective study. Clin Lymphoma Myeloma. 2009;9(5):381–5. [DOI] [PubMed] [Google Scholar]
- 19.Rieger M, Osterborg A, Pettengell R, White D, Gill D, Walewski J, et al. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol. 2011;22(3):664–70. [DOI] [PubMed] [Google Scholar]
- 20.Tai WM, Quah D, Yap SP, Tan SH, Tang T, Tay KW, et al. Primary mediastinal large B-cell lymphoma: optimal therapy and prognostic factors in 41 consecutive Asian patients. Leuk Lymphoma. 2011;52(4):604–12. [DOI] [PubMed] [Google Scholar]
- 21.Savage KJ, Yenson PR, Shenkier T, Klasa R, Villa D, Goktepe O, et al. The Outcome of Primary Mediastinal Large B-Cell Lymphoma (PMBCL) in the R-CHOP Treatment Era. ASH Annual Meeting Abstracts. 2012;120(21):303. [Google Scholar]
- 22.Vassilakopoulos TP, Pangalis GA, Katsigiannis A, Papageorgiou SG, Constantinou N, Terpos E, et al. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone with or without radiotherapy in primary mediastinal large B-cell lymphoma: the emerging standard of care. Oncologist. 2012;17(2):239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu LM, Fang H, Wang WH, Jin J, Wang SL, Liu YP, et al. Prognostic significance of rituximab and radiotherapy for patients with primary mediastinal large B-cell lymphoma receiving doxorubicin-containing chemotherapy. Leuk Lymphoma. 2013; 54(8):1684–90. [DOI] [PubMed] [Google Scholar]
- 24.Galper SL, Yu JB, Mauch PM, Strasser JF, Silver B, Lacasce A, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117(2):412–8. [DOI] [PubMed] [Google Scholar]
- 25.Henderson TO, Amsterdam A, Bhatia S, Hudson MM, Meadows AT, Neglia JP, et al. Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med. 2010; 152(7):444–55; W144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieder C, Schill S, Kneschaurek P, Molls M. Comparison of three different mediastinal radiotherapy techniques in female patients: Impact on heart sparing and dose to the breasts. Radiother Oncol. 2007;82(3):301–7. [DOI] [PubMed] [Google Scholar]
- 27.Dunleavy K, Pittaluga S, Maeda LS, Advani R, Chen CC, Hessler J, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368(15):1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto W, Nakamura N, Tomita N, Ishii Y, Takasaki H, Hashimoto C, et al. Clinicopathological analysis of mediastinal large B-cell lymphoma and classical Hodgkin lymphoma of the mediastinum. Leuk Lymphoma. 2013;54(5):967–72. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto W, Nakamura N, Tomita N, Ishii Y, Takasaki H, Hashimoto C, et al. Clinicopathological analysis of mediastinal large B-cell lymphoma and classical Hodgkin lymphoma of the mediastinum. Leuk Lymphoma. 2013;54(5):967–72. [DOI] [PubMed] [Google Scholar]
- 30.Hu S, Xu-Monette ZY, Balasubramanyam A, Manyam GC, Visco C, Tzankov A, et al. CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2013; 121(14):2715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–82. [DOI] [PubMed] [Google Scholar]
- 32.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999; 17(4):1244. [DOI] [PubMed] [Google Scholar]
- 33.Niitsu N, Okamoto M, Aoki S, Okumura H, Yoshino T, Miura I, et al. Multicenter phase II study of the CyclOBEAP (CHOP-like + etoposide and bleomycin) regimen for patients with poor-prognosis aggressive lymphoma. Ann Hematol. 2006;85(6):374–80. [DOI] [PubMed] [Google Scholar]
- 34.Yamada Y, Tomonaga M, Fukuda H, Hanada S, Utsunomiya A, Tara M, et al. A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br J Haematol. 2001;113(2):375–82. [DOI] [PubMed] [Google Scholar]
- 35.Barth TF, Leithauser F, Joos S, Bentz M, Moller P. Mediastinal (thymic) large B-cell lymphoma: where do we stand? Lancet Oncol. 2002;3(4):229–34. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez J, Conde E, Gutierrez A, Garcia-Ruiz JC, Lahuerta JJ, Varela MR, et al. Front-Line Autologous Stem Cell Transplantation (ASCT) in Primary Mediastinal Large B-Cell Lymphoma: The GEL-TAMO Experience. ASH Annual Meeting Abstracts. 2006;108(11):3056–. [Google Scholar]
- 37.Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Wells WA, Winter JN, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;366(5):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter BD, Dhakal S, Voci S, Goldstein NP, Constine LS. Pleural effusions in patients with Hodgkin lymphoma: clinical predictors and associations with outcome. Leuk Lymphoma. 2014;55(8):1822–6. [DOI] [PubMed] [Google Scholar]
- 39.Filippi AR, Piva C, Giunta F, Bello M, Chiappella A, Caracciolo D, et al. Radiation therapy in primary mediastinal B-cell lymphoma with positron emission tomography positivity after rituximab chemotherapy. Int J Radiat Oncol Biol Phys. 2013; 87(2):311–6. [DOI] [PubMed] [Google Scholar]
- 40.Woessmann W, Lisfeld J, Burkhardt B. Therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;369(3):282. [DOI] [PubMed] [Google Scholar]
- 41.Martelli M, Ceriani L, Zucca E, Zinzani PL, Ferreri AJ, Vitolo U, et al. [18F]fluorodeoxyglucose positron emission tomography predicts survival after chemoimmunotherapy for primary mediastinal large B-cell lymphoma: results of the International Extranodal Lymphoma Study Group IELSG-26 Study. J Clin Oncol. 2014; 32(17):1769–75. [DOI] [PubMed] [Google Scholar]