Abstract
Background: We conducted a meta-analysis in order to investigate whether circulating adiponectin, an insulin-sensitizing hormone produced by adipocytes, is associated with breast cancer risk.
Methods: A systematic literature search was performed in PubMed, Medline, EMBASE, ISI Web of Knowledge and the Cochrane Library. The summary relative risk (SRR) was calculated by pooling the different study-specific estimates using the random effect models. Meta-regression, subgroup and sensitivity analyses were carried out to investigate between-study heterogeneity and to test publication bias.
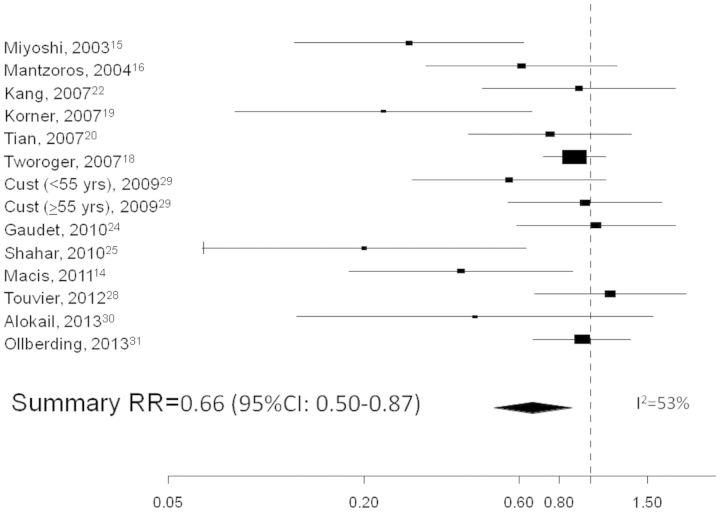
Results: Data from 15 observational studies, published between 2003 and April 2013 for a total of 4249 breast cancer cases, were analysed. The SRR for the ‘highest’ vs ‘lowest’ adiponectin levels indicated a 34% reduction in breast cancer risk [95% confidence interval (CI): 13%–50%]. Between-study heterogeneity was not substantial (I2 = 53%). Ten studies were included in the dose-response analysis: the SRR for an increase of 3 µg/ml of adiponectin corresponded to a 5% risk reduction (95% CI: 1%–9%). The comparison between ‘highest’ and ‘lowest’ levels of adiponectin showed an inverse association in postmenopausal women (SRR = 0.80; 95% CI: 0.63–1.01) and an indication of an inverse relationship in premenopausal women (SRR = 0.72, 95% CI: 0.30–1.72). No evidence of publication bias was found.
Conclusions: Low circulating adiponectin levels are associated with an increased breast cancer risk. However, properly designed studies are needed to confirm the role of adiponectin as breast cancer biomarker, and clinical trials should be performed to identify those interventions that may be effective in modulating adiponectin levels and reducing breast cancer risk.
Keywords: Circulating adiponectin, breast cancer risk, biomarker
Key Messages.
The results of our meta-analysis of the epidemiology literature indicate an association between low circulating adiponectin levels and increased breast cancer risk.
No indication of publication bias or substantial between-study heterogeneity was found.
Properly designed studies are needed to confirm the role of adiponectin as a biomarker of breast cancer risk.
Introduction
Obesity is a well-recognized risk factor for postmenopausal breast cancer,1–3 whereas controversial findings have been reported in premenopausal women. A large meta-analysis showed an inverse association between body mass index (BMI) and the incidence of premenopausal breast cancer.4 On the other hand, recent data from two large prevention studies have demonstrated that premenopausal women with a high BMI are at increased risk for developing breast cancer.5
The biological mechanisms linking obesity and cancer risk have not been fully elucidated, but it is now clear that adipose tissue is not only an energy storage site, since it also plays an active role in systemic energy balance and in other physiological pathways through the production and secretion of adipokines.6
Adiponectin is an insulin-sensitizing hormone produced by adipocytes, together with other adipokines, for the maintenance of metabolic homeostasis. Interestingly, this hormone appears to have anti-inflammatory, anti-atherogenic, anti-angiogenic and anti-diabetic properties.7 Serum adiponectin levels are reduced in obesity and increased after severe weight loss.8,9 Low levels of adiponectin are also closely linked to insulin resistance and hyperinsulinaemia,10 which were demonstrated to be positively associated with breast cancer risk11,12 and poor outcomes in women with early breast cancer.13
Collectively, this evidence leads to the hypothesis that adiponectin may act as a molecular mediator linking excess adiposity with carcinogenesis.7
We recently found that low levels of adiponectin in premenopausal women at high risk for breast cancer increased the risk of breast neoplastic events by 12% (P = 0.03).14 Notably, we also confirmed the association between adiponectin, obesity and insulin resistance, since we observed an inverse correlation of adiponectin with BMI and the Homeostasis Model Assessment (HOMA) index, a measure of insulin resistance.
The first observation linking adiponectin to breast cancer was reported by Miyoshi et al.15 who suggested that low serum adiponectin levels were associated with an increased breast cancer risk and possibly with a more aggressive breast cancer phenotype. Over the past decade, many authors have investigated the association between adiponectin and breast cancer in case-control15–31 and cohort studies.32,33 We have conducted a meta-analysis in order to verify whether a systematic revision of the literature and a summary relative risk (SRR) estimate of published data, with quantitative assessment of between-study heterogeneity, supports the role of circulating adiponectin as a biomarker of breast cancer risk.
Methods
Pre-defined protocol
A systematic literature search and a quantitative analysis were planned, conducted and reported according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines.34
Literature search
Published reports were identified from the following databases, using validated search strategies: PubMed (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi); Medline (Ovid Technologies, Inc., New York, 1950-29 April 2011); EMBASE (Elsevier, Amsterdam, The Netherlands, 1980-29 April 2011); ISI Web of Knowledge (Thomson Scientific Technical Support, New York, 1945-4 May 2011), and the Cochrane collaboration databases (http://www.cochrane.org). All searches were carried out using the MeSH terms ‘breast neoplasms’ or ‘breast cancer’, each combined with one of the following: ‘adiponectin’, ‘ACDC’, ‘ADPN’, ‘APM1’, ‘APM-1’, ‘GBP28’, ‘ACRP30’ or ‘ADIPOQ’. We also performed manual searches of the references cited in our retrieved articles and in earlier reviews on the topic.
Study selection
Primary inclusion criteria were case-control and cohort studies published as an original article, and the report of relative risk estimates or crude data for adiponectin serum levels. Ecological studies, case reports, reviews not including original data, and editorials were not considered eligible. No language or geographical restriction was applied.
After assessing whether the titles of the identified articles could meet our primary inclusion criteria, we checked the relevance of the corresponding abstracts and retrieved full copies of the manuscripts; these were then independently read by at least two investigators.
We then selected only those studies that: (i) reported sufficient information to estimate the relative risk and the 95% confidence intervals (CI) for the different quantiles (or at least for the upper quantile), used to categorize adiponectin serum levels (odds ratios, relative risks or crude data, and corresponding standard errors, variance, confidence intervals or P-values of the significance of the estimates); and (ii) were independent and did not duplicate previously published results. In the case of multiple articles on the same population, results from the study including the largest number of subjects were used.
Finally, studies with the following features were excluded: (i) studies reporting breast cancer recurrence risk estimates among cancer patients; and (ii) studies reporting breast cancer mortality risk estimates. Review articles not reporting original data were also excluded, although they were checked for useful references.
A standardized data-collection protocol was used for gathering the relevant information from each selected article.
Data extraction
Data were extracted by one investigator using a pre-defined database. The resulting table was then verified by a second investigator and by the statistician who performed the meta-analysis. Laboratory assays used to measure adiponectin levels were recorded. The most adjusted relative risk estimate was always used. Data extraction also included key study characteristics such as the type of stratified analysis performed (e.g. by menopausal status), and the confounding factors that were adjusted for in the analysis.
Statistical analysis
Since cancer is a relatively rare disease, we ignored the distinction between the various estimates of relative risk (i.e. odds ratio (OR), rate ratio, risk ratio), and all measures were interpreted as relative risk.35 Every measure of association, adjusted for the maximum number of confounding variables, and the corresponding confidence intervals, were transformed into log relative risks. The corresponding variance was calculated using the formula proposed by Greenland.35 When no estimates were given, crude estimates were calculated from tabular data. Woolf’s formula was used to evaluate the standard error of the log relative risk. The measure of heterogeneity I2 was considered in order to compare between-study heterogeneity. It can be interpreted as the percentage of total variation across studies that is attributable to heterogeneity: larger values of I2 indicate greater heterogeneity.36
First of all, we computed the SRR estimates for the ‘highest’ vs the ‘lowest’ category of baseline adiponectin concentration. When the information was available, we also calculated the summary estimates of the dose-response effect of adiponectin levels on breast cancer risk. The procedure is based on two steps: first, a linear model was fitted within each study to estimate the relative risk per unit of adiponectin increase. When sufficient information was published (i.e. the number of subjects at each serum level category), the model was fitted according to the method proposed by Greenland and Longnecker.37 This method provides the natural logarithm of the relative risk, and an estimate of its standard error, taking into account that the estimates for separate categories are referred to the same reference category. When the number of subjects in each serum level category was not available from the publications, coefficients were calculated discounting the correlation between the estimates of risk at the separate exposure levels.
Second, the SRR was estimated by pooling the different study-specific estimates using the random effect models as described by van Houwelingen et al.,38 with summary effect size obtained from the estimation of maximum likelihood. Confidence intervals were computed assuming an underlying t-distribution and using PROC MIXED in SAS [SAS Institute Inc. SAS Windows version (8.02), 1999, Cary, NC].
Publication bias was graphically assessed using a funnel plot and the Macaskill test,39 which is more powerful when less than 20 estimates are included in the analysis.
The final summary relative risks represent the breast cancer risk associated with 1 or 3 µg/ml increment of adiponectin.
Heterogeneity and sensitivity analyses
To assess the influence of possible sources of bias, we followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist for observational epidemiological studies.40 Accordingly, using meta-regression, we evaluated between-study heterogeneity by assessing the influence of different study features, such as study population and study design, assessment of adiponectin level, suitability of statistical methods and appropriate reporting of results.
To evaluate the stability of the pooled estimates, we also examined changes in results after the exclusion of specific studies.
Meta-regressions and subgroup analyses were carried out to quantify the between-study heterogeneity. Heterogeneity was investigated by considering all the possible factors that could influence the estimates (publication year, study design, geographical location, assays used for adiponectin determination, serum adiponectin levels before breast cancer diagnosis, adjustment for confounding factors and population characteristics). Finally, analyses by menopausal status were also conducted.
Results
The flow diagram for study inclusion in the meta-analysis is shown in Figure 1. A total of 22 articles were retrieved and checked for relevance in terms of intervention, population studied, and reporting of breast cancer incidence/mortality data. All articles were in English. Seven articles were not included for the following reasons: Chen et al.,17 Gulcelik et al.41 and Dalamaga et al.42 because no information was published to calculate the risk estimate; Duggan et al.32 because their endpoint was breast cancer mortality; Al-Delaimy et al.26 and Oh et al.33 because their endpoint was breast cancer recurrence among breast cancer patients; and Al Awadhi et al.27 because contradictory results were reported in the tables (reference 27: Table III and Table IV).
Figure 1.
Flow diagram of study selection.
Furthermore, since Mantzoros and colleagues16 presented the OR related to a change in serum adiponectin levels by one marginal quintile of the storage duration-adjusted measurements, we re-calculated it from the original crude data in order to be consistent with the risk estimates derived from the other studies.
Overall, we included estimates from 15 studies that presented data on the association between adiponectin and breast cancer risk.14–16,18–25,28–31 The characteristics of these studies are summarized in Table 1. All articles were published between 2003 and April 2013. We included one cohort study (57 cases and a total of 235 subjects),14 eight hospital-based case-control studies (944 cases and 859 controls),15,16,19–23,25 one population-based case-control study (56 cases and 53 controls)30 and five nested case-control studies (3192 cases and 4130 controls)18,24,28,29,31 for a total of 4249 breast cancer events. Six studies were conducted in Europe,14,16,19,23,28,29 three in the USA,18,24 one in Japan,15 one in China,21 one in Taiwan,20 one in Malaysia,25 one in Korea22 and one in Saudi Arabia.30 In six studies,14,18,24,28,29,31 blood samples were taken before breast cancer diagnosis. Circulating adiponectin levels were measured by enzyme-linked immunosorbent assay in eight studies,14,15,21–23,25,28,31 by a radioimmunoassay in five studies,16,18–20,29 and by means of a multiplex assay in two studies.24,30 In only one article was the published OR not adjusted for the BMI,25 but the authors stated that there were no differences in BMI between cases and controls. Furthermore, the OR from Mantzoros et al.16 was not adjusted for any confounder, and we had to re-calculate it. Seven studies presented the estimates by menopausal status.14,15,18,20,24,29,31 Ten studies were included in the dose-response analysis.14,16,19,21,23–25,28,29,31
Table 1.
Characteristics of studies included in the meta-analysis
| Study (year) | Country | Study design | No cases/controls | Mean age (years) cases/controls | Menopausal status (N) | Assay method | Adiponectin levels (µg/ml) (cases/controls) | RR (95% CI) for ‘highest’ vs ‘lowest’ category of adiponectin or dose-response (DR) | Adjustments and matching |
|---|---|---|---|---|---|---|---|---|---|
| Miyoshi et al.15 (2003) | Japan | Case-control (HB) | 102/100 | 54.0/52.8 | 97 pre 105 post | ELISA | 7.57/8.83 | 0.27 (0.12–0.62) | Age, family history, age at menarche, parity and BMI |
| Mantzoros et al.16 (2004) | Greece | Case-control (HB) | 174/167 | NR | 93 pre 248 post | RIA | 16.7/17.4 | 0.61 (0.31–1.20) | None (OR re-calculated from crude data) |
| Hou et al.21 (2007)a | China | Case-control (HB) | 80/50 | 48/49 | 69 pre 61 post | ELISA | 8.60/10.37 | 0.805 (0.704–0.921) (DR) | Matched for age. resistin, leptin, BMI, waist circumference, triglycerides, HDL-c, LDL-c and fasting blood glucose |
| Kang et al.22 (2007) | Korea | Case-control (HB) | 41/43 | 47.4/47.8 | 35 pre 49 post | ELISA | 6.93/7.60 | 0.92 (0.46–1.81) | Age, BMI, menopausal status, fasting blood glucose and resistin |
| Korner et al.19 (2007) | Greece | Case-control (HB) | 74/76 | 62.5/55.6 | 33 pre 117 post | RIA | 9.1/11.3 | 0.23 (0.08–0.66) | Age, BMI, age at menarche, parity, menopausal status, family history, insulin and leptin |
| Tian et al.20 (2007) | Taiwan | Case-control (HB) | 244/244 | 51.5/48.4 | 282 pre 206 post | RIA | NR | 0.75 (0.42–1.34) | Age at enrolment, date at enrolment, fasting status, menopausal status, BMI and WHR |
| Tworoger et al.18 (2007) | USA | Nested case-control (PB) | 1477/2196 | NHS: 57.1/58.1 NHSII: 45.4/45.1 | 822 pre 2167 postb | RIA | NHS: 14.4/14.8 NHSII: 16.7/15.6 | 0.89 (0.71–1.11) | Matched for age, menopausal status,, month/year of blood collection. Adjusted for BMI at age 18, weight change from age 18 to blood draw, family history, history of benign breast disease, duration of HRT use, age at first birth/parity and age at menarche |
| Cust et al.29 (2009) | Sweden | Nested case-control (PB) | 561/561 | 52.5/NR | 334 post | RIA | 6.9/6.6 | <55 yrs: 0.56 (0.28–1.11); ≥55 yrs: 0.96 (0.55–1.65) | Matched for age and date at blood sampling. Adjusted for BMI and HRT use |
| Hancke et al.23 (2010)a | Germany | Case-control (HB) | 159/41 | 59.51/49 | 65 pre 135 post | ELISA | 18.53/17.77 | 1.005 (0.945–1.0699) (DR) | Age, BMI, menopausal status, HRT, family history |
| Gaudet et al.24 (2010) | USA | Nested case-control (PB) | 230/231 | NR | All post | Linco 3-plex Human Adipokine Panel | NR/23.388 | 1.04 (0.59–1.83) | Age at reference, BMI, parity, age at first full-term birth, age at menopause, and current HRT use |
| Shahar et al.25 (2010) | Malaysia | Case-control (HB) | 70/138 | 47.3/46.2 | 142 pre 66 post | ELISA | 11.9/15.2 | 0.2 (0.000–0.6) | Matched for age and menopausal status. Adjusted for age at first pregnancy, family history, smoking status, alcohol intake, OC, HRT use. BMI was not different among cases and controls |
| Macis et al.14 (2012) | Italy | Cohort | 57/235 | 45/46 | All pre | ELISA | At baseline: 9.8/12 New breast events: 8.1/10.9 | 0.39 (0.18–0.88)c 0.918 (0.864–0.976) (DR) | Age, disease status at baseline (Gail vs DIN), BMI |
| Touvier et al.28 (2013) | France | Nested case-control (PB) | 218/436 | 49.2/51.5 | NR | ELISA | 13.8/11.0 | 1.15 (0.67–1.97) | Age, BMI, height, SU.VI.MAX intervention group, alcohol intake, physical activity, smoking status, educational level, hs-CRP, sICAM-1, sVCAM-1 soluble E-selectin, MCP-1, leptin |
| Alokail et al.30 (2013) | Saudi Arabia | Case-control (PB) | 56/53 | 43.1/46.4 | 78 pre 31 post | Multiplex assay | 14.8/ 19.1 | 0.44 (0.12–1.5) | Matched for age and BMI. Adjusted for age at menarche and menopausal status |
| Ollberding et al.31 (2013) | USA | Nested case-control (PB) | 706/706 | 67.8/67.8 | 706 post | ELISA | 8.9/ 10 | 0.94 (0.66–1.32) | Matched for age, ethnicity, date of blood draw, and HRT use; adjusted for BMI, CRP, history of diabetes, and history of hypertension |
BMI, body mass index; CI, confidence interval; DIN, ductal intraepithelial neoplasia; DR, dose response; ER, estrogen receptor; HB, hospital based; HDL-c, high density lipoprotein cholesterol; HRT, hormone replacement therapy; hs-CRP, high-sensitivity C-reactive protein; LDL-c, low density lipoprotein cholesterol; NHS, Nurses’ Health Study; NR, not reported; MCP-1, monocyte chemoattractant protein 1; OC, oral contraceptive; PB, population based; PR, progesterone receptor; pre, premenopause; post, postmenopause; RR, relative risk or odds ratio; sICAM-1, soluble intercellular adhesion molecule 1; SU.VI.MAX, Supplémentation en Vitamines et Minéraux Antioxydants; sVCAM-1, soluble vascular adhesion molecule 1; WHR, waist to hip ratio.
aThese papers only reported dose-response relationship between adiponectin and breast cancer risk.
bThe following subjects were excluded from the analysis: 171 cases and 214 controls with unknown menopausal status; 132 cases and 167 controls who were premenopausal at blood collection time point and postmenopausal at cancer diagnosis time point.
c We re-calculated the OR adjusting for BMI in order to be consistent with adjustments of other studies and to reduce between-study heterogeneity.
Summary estimates
The SRR for the ‘highest’ vs the ‘lowest’ circulating adiponectin levels indicate a 34% reduction in breast cancer risk (95% CI: 13%–50%), as shown in the forest plot (Figure 2). An inverse relationship was also found when considering the dose-response relationship: the SRR was 0.982 (95% CI: 0.967–0.998) for an increase of 1 unit of adiponectin, which corresponds to a 5% reduction in risk (95% CI: 1%–9%) for an increase of 3 µg/ml.
Figure 2.
Forest plot of the ‘highest’ vs the ‘lowest’ category of circulating adiponectin and breast cancer risk.
We performed a subgroup analysis by menopausal status. The published ORs by adiponectin levels are reported in Table 2. Four studies presented the estimates in premenopausal women for a total of 566 cases, and six studies reported the estimates in postmenopausal women for a total of 2281 cases. We found a trend for an inverse association comparing the ‘highest’ vs the ‘lowest’ levels of adiponectin in postmenopausal women (SRR = 0.80; 95% CI: 0.63-1.01) and an indication of an inverse relationship in premenopausal women (SRR = 0.72; 95% CI: 0.30-1.72).
Table 2.
Relationship between adiponectin levels and breast cancer risk by menopausal status
| Study | Menopausal status | Adiponectin levels (µg/ml) | Cases/controls (No.) | OR |
|---|---|---|---|---|
| Miyoshi et al.15 | Premenopausal women | >10.6 | 6/12 | 1.00 (reference) |
| 6.9–10.6 | 25/16 | 3.41 (0.99–11.66) | ||
| ≤6.9 | 21/17 | 3.46 (0.89–13.50) | ||
| Postmenopausal women | >10.6 | 11/21 | 1.00 (reference) | |
| 6.9–10.6 | 11/17 | 1.94 (0.51–7.44) | ||
| ≤6.9 | 28/17 | 3.90 (1.23–12.44) | ||
| Tian et al.20 | Premenopausal women | ≤13.37 | 111/106 | 1.00 (reference) |
| >13.37 | 30/35 | 0.84 (0.46–1.52) | ||
| Postmenopausal women | ≤15.69 | 90/77 | 1.00 (reference) | |
| >15.69 | 13/25 | 0.55 (0.23–0.97) | ||
| Tworoger et al.18, a | Premenopausal women | ≤9.3; ≤17.4; ≤12.0 | 316/506 | 1.00 (reference) |
| 9.3–11.7; 17.4–20.6; 12.0–15.6 | 1.05 (0.69–1.59) | |||
| 11.7–13.8; 20.6–23.2; 15.6–19.3 | 1.12 (0.70–1.80) | |||
| >13.8; >23.2; >19.3 | 1.30 (0.80–2.10) | |||
| Postmenopausal women | ≤9.3; ≤17.4; ≤12.0 | 858/1309 | 1.00 (reference) | |
| 9.3–11.7; 17.4–20.6; 12.0–15.6 | 0.91 (0.69–1.21) | |||
| 11.7–13.8; 20.6–23.2; 15.6–19.3 | 0.97 (0.73–1.27) | |||
| >13.8; >23.2; >19.3 | 0.73 (0.55–0.98) | |||
| Cust et al.29 | Postmenopausal women | <4.9 | 105/116 | 1.00 (reference) |
| 4.9–9.1 | 123/110 | 1.21 (0.80–1.83) | ||
| >9.1 | 106/108 | 0.96 (0.55–1.65) | ||
| Gaudet et al.24 | Postmenopausal women | 0.626–19.535 | 54/62 | 1.00 (reference) |
| 19.536–25.758 | 57/57 | 1.02 (0.59–1.72) | ||
| 25.759–32.921 | 59/53 | 1.18 (0.68–2.08) | ||
| 32.922–73.588 | 60/59 | 1.04 (0.59–1.83) | ||
| Macis et al.14 | Premenopausal women | <7.2 | 21/59 | 1.00 (reference) |
| 7.2–10.00 | 16/58 | 0.73 (0.38–1.40) | ||
| 10.0–14.00 | 11/59 | 0.54 (0.26–1.12) | ||
| >14.00 | 9/59 | 0.39 (0.18–0.88) | ||
| Ollberding et al.31 | Postmenopausal women | ≤5.7 | 182/177 | 1.00 (reference) |
| 5.8–10.0 | 210/176 | 1.18 (0.87–1.58) | ||
| 10.1–16.1 | 158/176 | 0.92 (0.67–1.27) | ||
| >16.1 | 156/177 | 0.94 (0.66–1.32) |
aAdiponectin levels were determined in different batches.
We used subgroup, meta-regression and sensitivity analyses to evaluate the influence of single studies or fa ctors that might modify this association, such as age, menopausal status, adjustment for BMI, study design, publication year, country, assay method, and assessment of adiponectin serum levels before diagnosis. None of the factors or single studies significantly affected the SRRs. In Table 3 we report the SRR from subgroup analyses performed by study design, assay methods and adjustment for confounders that may have introduced considerable heterogeneity into the study results. The SRR did not substantially change between case-control and nested case-control and/or cohort studies (P = 0.20), between ELISA and other assay methods (P = 0.76), or whether an adjustment for menopausal status was carried out for risk estimates (P = 0.72). However, interestingly, the reduction in risk was greater among case-control studies than among cohorts or nested case-control studies. Furthermore, sensitivity analyses excluding studies with particular features, e. g. those presenting risk estimates unadjusted for any confounder, did not change our results.
Table 3.
Summary risk estimates from subgroup analyses
| N. estimates | SRR | Low | Up | P | ||
|---|---|---|---|---|---|---|
| Study design | CC | 7 | 0.48 | 0.29 | 0.81 | 0.20 |
| NCC and CO | 7 | 0.88 | 0.73 | 1.07 | ||
| Assay method | ELISA | 7 | 0.58 | 0.32 | 1.03 | 0.76 |
| No | 6 | 0.82 | 0.65 | 1.03 | ||
| Risk estimate adjustmentsa | For menopausal status | 6 | 0.61 | 0.35 | 1.06 | 0.72 |
| No | 3 | 0.62 | 0.15 | 2.57 |
SRR, summary relative risk; CC, case-control; NCC, nested case-control; CO, cohort; ELISA, enzyme-linked immunosorbent assay.
aAdjustments or matching; risk adjustment for menopausal status was evaluated for studies including pre- and post-menopausal women.
Between-study heterogeneity was not substantial (I2 = 53%) and we did not find any evidence of publication bias (data not shown).
Discussion
The results of this meta-analysis indicate an inverse association between circulating adiponectin levels and breast cancer risk, showing a 34% reduction in risk for the ‘highest’ vs the ‘lowest’ circulating adiponectin levels and a 5% reduction in risk for an increase of 3 µg/ml of adiponectin in the dose-response analysis. We observed a moderate level of heterogeneity across the studies included in the meta-analysis (I2 = 53%) and no evidence of publication bias, suggesting a good reliability of our results. The three studies that increased the between-study heterogeneity are those that showed strongly inverse associations.15,19,25
In order to investigate the influence of study features, we identified several characteristics such as study design, assay methods for the analysis of adiponectin, and population characteristics, as factors that may add variability to the results. We evaluated their influence on between-study heterogeneity through meta-regression and subgroup analyses. We did not observe any difference among all the analysed factors. However, the inverse association between adiponectin and breast cancer was attenuated in the nested case-control and cohort studies compared with case-control studies. Even though we found no substantial heterogeneity and none of the analysed factors introduced important variability, in order to be conservative we calculated our summary estimates using the random effect models that take into account between-study variations.
Increasing evidence in the literature supports the association between adiponectin and breast cancer risk.
Two previous meta-analyses on this topic43,44 were recently published. Ye et al.43 presented summary standard mean difference (SMD) values of circulating adiponectin levels between breast cancer cases and controls. They found that serum total adiponectin concentrations were significantly lower in patients with breast cancer, with a pooled SMD of –0.39 µg/ml (95% CI: −0.618– −0.161, P = 0.001). They did not find a significant difference among premenopausal women; however, the reported estimates were not adjusted for confounders and SMD cannot be interpreted as a risk measure. Overall, Liu et al.44 did not find a significant breast cancer increased risk comparing the ‘highest’ vs the ‘lowest’ adiponectin levels. However, in our meta-analysis we summarized a higher number of studies (15 vs 13), events (4249 vs 3578) and comparable estimates. Indeed, we excluded a study that did not compare ‘highest’ vs ‘lowest’ adiponectin levels21 and a study with inconsistent data.27 Thus, we were able to increase the statistical power of the analysis with lower heterogeneity compared with Liu et al.44
Interestingly, adiponectin levels have been reported to be correlated with factors such as obesity and insulin resistance which have been associated with breast cancer risk and prognosis.5,45–47 Indeed, adiponectin levels have been shown to be inversely related to both BMI14,15,17,18,20,21,23,25,27,29,31,32,48 and HOMA index.14,27,32 Adiponectin levels have also been inversely associated with C-reactive protein, estradiol, dehydroepiandrosterone, estrone and testosterone,18,24,30–32 and positively linked to follicle-stimulating hormone, sex hormone-binding globulin and high-density lipoprotein cholesterol,14,24,27,30 which have been associated with breast cancer risk and progression.
In our analysis by menopausal status, we only found a weak inverse association between adiponectin levels and postmenopausal breast cancer risk. The association between adiponectin and premenopausal breast cancer risk was in the same direction, but it was not significant probably due to the limited number of subjects analysed.
Many authors have investigated whether the relationship between adiponectin and breast cancer varied by tumour stage or grade, size, molecular subtypes or lymph node metastasis status.15,18–23,29,31,33,41,49 It was not possible to perform subgroup meta-analyses because most of the authors did not publish sufficient information to calculate the ORs. However, some authors reported a consistent inverse association between adiponectin levels and lymph node metastasis,21,22 tumour grade, or stage.15,21,41 Interestingly, an increased risk of ER-negative breast cancer in patients with lower adiponectin levels has been reported.15,22 In a study on breast cancer patients, a 2.82-fold higher risk of breast cancer recurrence was found in ER/PR-negative but not in ER/PR-positive breast cancer patients with the lowest adiponectin levels.33
Recently, Duggan et al.50 reported that adiponectin levels above the median were associated with a 61% decrease in risk of breast cancer mortality, although no significant effect on all-cause mortality was observed.
Different mechanisms of action by which adiponectin may reduce the risk of breast cancer development and progression have been proposed and reviewed.51,52 These include the reduction of aromatase activity and local estrogen production resulting in cell proliferation decrease, the reduction of cell motility and angiogenesis, the enhancement of cell differentiation, and the induction of apoptosis with consequent inhibition of tumour growth.51,52 Many pharmacological and non-pharmacological interventions have been shown to influence adiponectin levels.
It was reported that weight loss by a low calorie diet combined with physical exercise induced an increase in adiponectin levels ranging from 18% to 48%.53 However, a weight loss greater than 7% is needed to increase adiponectin levels with calorie intake restriction.53,54 Similarly, medical interventions for weight loss, such as orlistat or bariatric surgery, have been shown to increase adiponectin levels.54 Daily intake of fish or omega-3 supplements and dietary fibre supplementation have been reported to increase adiponectin levels by about 14-60% and 60-115%, respectively.53 Among pharmacological interventions that influence adipose tissue functions, some anti-diabetic, anti-hypertensive, anti-inflammatory and lipid-lowering drugs have been shown to increase adiponectin levels.54 A review of molecular pathways related to adiponectin signalling in cancer, illustrating potential key components for therapeutic intervention, was recently published.55
Interestingly, most of the interventions that increase adiponectin levels have been also associated with reduced breast cancer risk.
Our results suggest an intriguing association between low levels of circulating adiponectin and increased breast cancer risk. However, this is a meta-analysis of observational studies, which may have not been completely controlled for confounders. Further ad hoc studies should be designed to investigate the association between adiponectin and breast cancer risk. We need to establish the optimal level of adiponectin or the increase in adiponectin concentration that can have a protective effect against breast cancer. In addition, clinical trials should be carried out to identify those interventions that may increase circulating adiponectin levels and reduce breast cancer risk.
Overall, these data suggest a possible role of adiponectin as a biomarker of breast cancer risk, which could be helpful in identifying subjects at high risk for breast cancer occurrence who may benefit from preventive treatments.
Conflict of interest: None declared.
References
- 1.Van Den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 2000;152:514–27. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: The Women's Health Initiative (United States). Cancer Causes Control 2002;13:741–51. [DOI] [PubMed] [Google Scholar]
- 3.Lahmann PH, Hoffmann K, Allen N, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer and nutrition (EPIC). Int J Cancer 2004;111:762–71. [DOI] [PubMed] [Google Scholar]
- 4.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78. [DOI] [PubMed] [Google Scholar]
- 5.Cecchini RS, Costantino JP, Cauley JA, et al. Body mass index and the risk for developing invasive breast cancer among high-risk women in Nsabp P-1 and Star Breast Cancer Prevention Trials. Cancer Prev Res (Phila) 2012;5:583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell 2004;116:337–50. [DOI] [PubMed] [Google Scholar]
- 7.Barb D, Pazaitou-Panayiotou K, Mantzoros CS. Adiponectin: A link between obesity and cancer. Expert Opin Investig Drugs 2006;15:917–31. [DOI] [PubMed] [Google Scholar]
- 8.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999;257:79–83. [DOI] [PubMed] [Google Scholar]
- 9.Christiansen T, Paulsen SK, Bruun JM, Ploug T, Pedersen SB, Richelsen B. Diet-induced weight loss and exercise alone and in combination enhance the expression of adiponectin receptors in adipose tissue and skeletal muscle, but only diet-induced weight loss enhanced circulating adiponectin. J Clin Endocrinol Metab 2010;95:911–19. [DOI] [PubMed] [Google Scholar]
- 10.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001;86:1930–35. [DOI] [PubMed] [Google Scholar]
- 11.Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 2009;101:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabat GC, Kim M, Caan BJ, et al. Repeated measures of serum glucose and insulin in relation to postmenopausal breast cancer. Int J Cancer 2009;125:2704–10. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol 2002;20:42–51. [DOI] [PubMed] [Google Scholar]
- 14.Macis D, Gandini S, Guerrieri-Gonzaga A, et al. Prognostic effect of circulating adiponectin in a randomized 2 x 2 trial of low-dose tamoxifen and fenretinide in premenopausal women at risk for breast cancer. J Clin Oncol 2012;30:151–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyoshi Y, Funahashi T, Kihara S, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res 2003;9:5699–704. [PubMed] [Google Scholar]
- 16.Mantzoros C, Petridou E, Dessypris N, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab 2004;89:1102–07. [DOI] [PubMed] [Google Scholar]
- 17.Chen DC, Chung YF, Yeh YT, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett 2006;237:109–14. [DOI] [PubMed] [Google Scholar]
- 18.Tworoger SS, Eliassen AH, Kelesidis T, et al. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab 2007;92:1510–16. [DOI] [PubMed] [Google Scholar]
- 19.Korner A, Pazaitou-Panayiotou K, Kelesidis T, et al. : Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J Clin Endocrinol Metab 2007;92:1041–48. [DOI] [PubMed] [Google Scholar]
- 20.Tian YF, Chu CH, Wu MH, et al. Anthropometric measures, plasma adiponectin, and breast cancer risk. Endocr Relat Cancer 2007;14:669–77. [DOI] [PubMed] [Google Scholar]
- 21.Hou WK, Xu YX, Yu T, et al. Adipocytokines and breast cancer risk. Chin Med J (Engl) 2007;120:1592–96. [PubMed] [Google Scholar]
- 22.Kang JH, Yu BY, Youn DS. Relationship of serum adiponectin and resistin levels with breast cancer risk. J Korean Med Sci 2007;22:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancke K, Grubeck D, Hauser N, Kreienberg R, Weiss JM. Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Res Treat 2010;119:367. [DOI] [PubMed] [Google Scholar]
- 24.Gaudet MM, Falk RT, Gierach GL, et al. Do adipokines underlie the association between known risk factors and breast cancer among a cohort of United States women? Cancer Epidemiol 2010;34:580–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahar S, Salleh RM, Ghazali AR, Koon PB, Mohamud WN. Roles of adiposity, lifetime physical activity and serum adiponectin in occurrence of breast cancer among Malaysian women in Klang Valley. Asian Pac J Cancer Prev 2010;11:61–66. [PubMed] [Google Scholar]
- 26.Al-Delaimy WK, Flatt SW, Natarajan L, et al. Igf1 and risk of additional breast cancer in the WHEL Study. Endocr Relat Cancer 2011;18:235–44. [DOI] [PubMed] [Google Scholar]
- 27.Al Awadhi SA, Al Khaldi RM, Al RT, Kapila K, Mojiminiyi OA. Associations of adipokines & insulin resistance with sex steroids in patients with breast cancer. Indian J Med Res 2012;135:500–05. [PMC free article] [PubMed] [Google Scholar]
- 28.Touvier M, Fezeu L, Ahluwalia N, et al. Association between prediagnostic biomarkers of inflammation and endothelial function and cancer risk: a nested case-control study. Am J Epidemiol 2013;177:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cust AE, Stocks T, Lukanova A, et al. The influence of overweight and insulin resistance on breast cancer risk and tumour stage at diagnosis: a prospective study. Breast Cancer Res Treat 2009;113:567–76. [DOI] [PubMed] [Google Scholar]
- 30.Alokail MS, Al-Daghri N, Abdulkareem A, et al. Metabolic syndrome biomarkers and early breast cancer in Saudi women: evidence for the presence of a systemic stress response and/or a pre-existing metabolic syndrome-related neoplasia risk? BMC Cancer 2013;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ollberding NJ, Kim Y, Shvetsov YB, et al. Prediagnostic leptin, adiponectin, C-reactive protein, and the risk of postmenopausal breast cancer. Cancer Prev Res (Phila) 2013;6:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duggan C, Irwin Ml, Xiao L, et al. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol 2011;29:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh SW, Park CY, Lee ES, et al. Adipokines, insulin resistance, metabolic syndrome, and breast cancer recurrence: a cohort study. Breast Cancer Res 2011;13:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) Group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 35.Greenland S: Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 37.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–09. [DOI] [PubMed] [Google Scholar]
- 38.Van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;21:589–624. [DOI] [PubMed] [Google Scholar]
- 39.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med 2001;20:641–54. [DOI] [PubMed] [Google Scholar]
- 40.Von EE, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–49. [DOI] [PubMed] [Google Scholar]
- 41.Gulcelik MA, Colakoglu K, Dincer H, Dogan L, Yenidogan E, Gulcelik NE. Associations between adiponectin and two different cancers: breast and colon. Asian Pac J Cancer Prev 2012;13:395–98. [DOI] [PubMed] [Google Scholar]
- 42.Dalamaga M, Karmaniolas K, Papadavid E, Pelekanos N, Sotiropoulos G, Lekka A. Hyperresistinemia is associated with postmenopausal breast cancer. Menopause 2013;20:845–51. [DOI] [PubMed] [Google Scholar]
- 43.Ye J, Jia J, Dong S, et al. : Circulating adiponectin levels and the risk of breast cancer: a meta-analysis. Eur J Cancer Prev 2014;23:158–65. [DOI] [PubMed] [Google Scholar]
- 44.Liu Ly, Wang M, Ma ZB, et al. The role of adiponectin in breast cancer: a meta-analysis. PLoS One 2013;8:E73183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol 2012;30:164–71. [DOI] [PubMed] [Google Scholar]
- 46.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 2003;95:1218–26. [DOI] [PubMed] [Google Scholar]
- 47.Gunter MJ, Hoover DR, Yu H, et al. : Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 2009;101:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maskarinec G, Woolcott C, Steude JS, Franke AA, Cooney RV. The relation of leptin and adiponectin with breast density among premenopausal women. Eur J Cancer Prev 2010;19:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Wang Y. Adiponectin and breast cancer. Med Oncol 2010;28:1288–95. [DOI] [PubMed] [Google Scholar]
- 50.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr 2007;86:S858–66. [DOI] [PubMed] [Google Scholar]
- 51.Fabian CJ. Adiponectin: a risk biomarker and attractive target for chemoprevention. J Clin Oncol 2012;30:124–26. [DOI] [PubMed] [Google Scholar]
- 52.Jarde T, Perrier S, Vasson MP, Caldefie-Chezet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer 2011;47:33–43. [DOI] [PubMed] [Google Scholar]
- 53.Silva FM, De Almeida JC, Feoli AM: Effect of diet on adiponectin levels in blood. Nutr Rev 2011;69:599–612. [DOI] [PubMed] [Google Scholar]
- 54.Westerink J, Visseren FL. Pharmacological and non-pharmacological interventions to influence adipose tissue function. Cardiovasc Diabetol 2011;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vansaun MN. Molecular pathways: adiponectin and leptin signaling in cancer. Clin Cancer Res 2013;19:1926–32. [DOI] [PMC free article] [PubMed] [Google Scholar]