Abstract
Background: Some studies have suggested that overweight is associated with lower mortality, but these results may be affected by reverse causality. We analysed how body mass index (BMI) in young adulthood is associated with mortality in the general population and after the diagnoses of coronary heart disease (CHD), stroke and cancer.
Methods: BMI was measured at an average age of 18 years in 734 438 Swedish men born in 1950–65. Diagnoses of CHD, stroke and cancer as well as all-cause mortality were derived from registers covering the whole population, up to 31 December 2010. The follow-up of 24.56 million person-years included 33 067 cases of mortality and 19 843 CHD, 13 578 stroke and 27 365 cancer diagnoses. Hazard ratios (HR) [with 95% confidence intervals (CI)] were estimated by the Cox proportional hazards model.
Results: Higher mortality in the whole cohort (HR = 1.26, 1.21–1.32) as well as after the diagnosis of CHD (HR = 1.33, 1.09–1.63) or cancer (HR = 1.13, 1.01–1.25) was found in moderately overweight men (BMI 25.0–27.4 kg/m2) as compared with normal weight men (BMI 20.1–22.4 kg/m2); for stroke patients the result for the same BMI categories was not statistically significant (HR = 1.17, 0.94–1.45). Mortality increased with increasing weight status and was highest in obese men (BMI >30 kg/m2): HR = 2.17 (2.02–2.34) for the whole cohort, 2.35 (1.81–3.05) after the diagnosis of CHD, 2.08 (1.56–2.77) after stroke and 1.68 (1.40–2.01) after cancer.
Conclusions: Even moderate overweight in young adulthood increases all-cause mortality and mortality after the diagnosis of CHD, stroke and cancer in men. Preventing overweight in young adulthood remains as an important public health issue.
Keywords: Overweight, mortality, fatality, cardiovascular diseases, cancer
Key Messages.
Not only obesity but also overweight among men in young adulthood was associated with higher all-cause mortality in the total cohort as well as higher mortality risk after hospitalization for coronary heart disease, stroke and cancer as compared with normal-weight men.
Previous studies reporting better survival probabilities related to overweight as compared with normal weight are likely to be caused by underlying diseases affecting weight and mortality.
Even slight overweight in young adulthood increases mortality risk in middle age and is thus an important public health problem.
Introduction
The prevalence of obesity has strongly increased worldwide over recent decades. In 2008, 10% of the world population was estimated to be obese, meaning that this prevalence has increased 2-fold since 1980.1 Obesity is an important risk factor of atherosclerotic coronary artery disease2 and also increases the risk of stroke3 and several cancers.4 Even though these disease groups represent the most important causes of death in industrialized countries,5 the association between body mass index (BMI) and all-cause mortality is still under debate. A recent meta-analyses based on 141 studies found that even though obesity (BMI ≥30 kg/m2) was associated with increased mortality, overweight (BMI 25.0–29.9 kg/m2) was associated with lower mortality, as compared with the normal weight category (BMI 18.5–24.9 kg/m2).6 A previous study also found that intentional weight loss was associated with higher mortality, further questioning whether high BMI is always a risk factor for mortality.7 However, other large studies have reported higher mortality related to overweight.8,9 Further, studies based on BMI in childhood or young adulthood have reported largely linear associations between BMI and later mortality with only weak evidence of higher all-cause mortality as well as CVD and cancer mortality in the lowest BMI categories.10–13 Thus, this question is still open.
The possible beneficial effects of overweight on total mortality, at least in the presence of certain clinical conditions, have attracted substantial attention in previous scientific literature. Lower mortality in overweight patients as compared with normal weight patients has been found in diabetes,14 coronary artery disease15 and chronic heart failure.16,17 These results seem to provide some evidence that the beneficial effects of overweight on all-cause mortality may be due to better opportunities to survive severe medical conditions. Studies from the USA and The Netherlands also found that the medical treatment of patients with CVD was higher standard in the overweight than in the normal weight group, which may contribute to the lower mortality of the overweight patients.18,19 These results have given rise to the so-called ‘obesity paradox’, suggesting that moderate overweight may increase chances of survival even when the well-known health risks related to obesity are taken into account.20 Since high BMI is a risk factor for cardiovascular diseases, the nature or severity of this category of diseases may also be different in lean compared with obese patients.
However, from the public health point of view, the key issue is whether overweight really offers health benefits or whether it is rather a proxy indicator of a lack of underlying diseases with a negative effect on survival per se. The severity of the main diagnosed disease and that of other underlying disease also affecting survival can lead to weight loss and thus a spurious association between overweight and lower mortality. Thus, taking this reverse causality into account is difficult. In this study we aim to avoid the possibility of reverse causality by using a very large population-based cohort of Swedish men whose BMI was measured in early adulthood, minimizing the potential effects of underlying diseases on BMI. As compared with previous studies using BMI in young adulthood, the strength of our data is not only the large size but also the possibility to identify major disease diagnoses. Thus, we can analyse not only total mortality but also survival after major disease diagnoses.
Methods
The baseline measures were taken during the conscription examination in Sweden. In our study cohort born in 1950–65, the conscription examination predated active military service and was mandatory by law for all male Swedish citizens. However, those with a severe handicap or a chronic disease were exempted from the examination, based on a certificate issued by a physician. Age at the time of the conscription examination varied from 16 to 25 years, but only 1.4% of the conscripts were younger than 17 or older than 20 years. Thus our study cohort is likely to be mainly free from diseases affecting BMI.
BMI was computed from height and weight measured in underwear, dividing weight (kg) by squared height (m2). The World Health Organization (WHO) classification was used as the basis of our BMI classification because it has been used in most previous studies.21 However, since our dataset is large and permits a more detailed BMI classification, we split both normal and overweight categories. Thus, we used seven BMI categories: less than 18.5 kg/m2 (underweight), 18.5–20.0 kg/m2 (lower normal weight), 20.1–22.4 kg/m2 (normal weight), 22.5–24.9 kg/m2 (upper normal weight), 25.0–27.4 kg/m2 (lower overweight), 27.5–29.9 kg/m2 (upper overweight), and 30 kg/m2 or more (obese). In total we had 746 752 participants at baseline, and for 734 438 participants we also had measures of systolic blood pressure (SBP), diastolic blood pressure (DBP) and muscle strength. Occupational socioeconomic position was derived from censuses in 1980, 1985 and 1990 and classified into seven categories (higher-level non-manuals, middle-level non-manuals, lower-level non-manuals, farmers, skilled workers, unskilled workers, no previous occupation). Education was based on entries in the Swedish Register of Education for the period 1990–2010 and classified into five categories (higher education, secondary education, basic education, less than basic education and missing). More detailed information on the measurements has been reported previously in this journal.22
We then identified diagnoses of coronary heart disease (CHD) and stroke from the Swedish Hospital Discharge Register, using the first hospitalization for each, and the first registered cancer diagnosis from the Cancer Register, and linked them to the baseline data available from the Swedish Military Service Conscription Register. During the follow-up to 31 December 2010, the Eighth, Ninth, and Tenth Revisions of the International Classification of Diseases (ICD) were used for CHD (410–414 for ICD-8 and ICD-9 and I20–I25 for ICD-10) and stroke diagnoses (430–438, 344 for ICD-8, 430–438, 342, 344 for ICD-9 and I60–I66, G45 for ICD-10). For cancer diagnoses, ICD-9 was used during the whole follow-up period (140–209). We then followed up the mortality of all participants, based on linkage with the Swedish Cause of Death Register. Hospital discharge, cancer and cause-of-death registers cover the entire Swedish population. The register linkages were done using the unique personal identity numbers assigned to all Swedish citizens a few days after birth. Thus, we repeated all mortality analyses in four cohorts: one including all participants with follow-up starting from the conscription examination; and three sub-cohorts for those having diagnoses of CHD, stroke and cancer with follow-up starting from the date of diagnosis. In total we had 33 067 cases of mortality and 19 843 diagnoses of CHD, 13 578 diagnoses of stroke and 27 365 diagnoses of cancer during the 24.56 million person-years of follow-up. The participants were 45 to 60 years of age at the end of follow-up on 31 December 2010. The median age was 43 years at mortality, 49 years at CHD diagnosis, 48 years at stroke diagnosis and 47 years at cancer diagnosis.
The data were analysed using the Cox proportional hazards model. We estimated hazard ratios (HR) with 95% confidence intervals (CI) for total mortality first after the conscription examination and then after the diagnoses of CHD, stroke and cancer. Emigrants were identified from the Swedish population register and censored on the date of emigration. All-cause mortality was used instead of cause-specific mortality, which is also available in the Swedish Cause of Death Register, because we wanted to analyse the association between BMI and total survival after diagnosis. Age at the conscription examination was adjusted for when analysing mortality in the whole cohort and age at the time of diagnosis was adjusted for when analysing survival after diagnosis. Further, all models were adjusted for the conscription office. Swedish conscripts are required to enlist at the conscription office closest to their residence, and thus adjusting the results for the conscription office takes possible geographical variation in Sweden into account. We further stratified birth year into 5-year categories, thus allowing different baseline hazards rates in each 5-year category. The basic model (Model 1) was thus adjusted for age at baseline (age at conscription for total mortality and age at diagnosis for mortality after CHD, stroke and cancer diagnoses), conscription office and birth cohort. To test the shape of the association, we also calculated the basic model by using restricted cubic splines with four knots placed at the 5th, 35th, 65th and 95th percentiles and present these results as a graph. We then adjusted the results for the three muscle strength measures used as proxy indicators of body composition in early adulthood (Model 2), then additionally for DBP and SBP (Model 3) and finally also for education and socio-economic position (Model 4). Proportional hazards assumptions were tested for BMI both by testing the correlation of Schoenfeld residuals with follow-up time and comparing Kaplan–Meier curves. We did not find any evidence of violations for mortality after the diagnosis of CHD (P = 0.44), stroke (P = 0.66) or cancer (P = 0.39), and the Kaplan–Meier curves were parallel. For total mortality (regardless of a prior diagnosis of CHD, stroke or cancer) the testing of Schoenfeld residuals indicated a violation of the proportional hazards assumption (P < 0.0001). However, the Kaplan–Meier curves appeared parallel, and this statistically significant result is likely to be due to the high statistical power in these analyses (the Kaplan–Meier curves are not shown but are available from the corresponding author). All analyses were done with Stata statistical software, version 11.0 for Windows.
Results
Table 1 presents the baseline characteristics of the participants and mortality as well as the incidence rates during the follow-up by BMI categories. Mean BMI was low in this young cohort [21.4 kg/m2, standard deviation (SD) = 2.72], and 42% of the participants were in the normal weight category. SBP and DBP increased monotonically from the underweight to the obese category. As expected, a monotonic increase with BMI was also found in all strength measures. The proportion of overweight and obesity was lowest in men with higher education (6%) and higher-level non-manuals (5.1%) and highest in men with less than basic education (11.5%) and unskilled workers (11.6%). Mortality was lowest in the normal weight category and then increased up to the obese category. There was also a monotonic increase in mortality with decreasing BMI between the normal and underweight categories, with mortality in the underweight category being intermediate between that in the upper and the lower overweight categories. The same pattern was also found for stroke hospitalization incidence. However for CHD hospitalization, the incidence increased from the lower normal weight category to the obese category. For cancer, a somewhat higher incidence was found in the overweight and obese categories as compared with the normal and underweight categories, but the differences were smaller than for CHD and stroke.
Table 1.
Baseline characteristics (mean and SD or proportion), mortality and coronary heart disease, stroke and cancer diagnoses (incidence rates and number of cases) according to baseline BMI
| Characteristics |
Baseline BMI (kg/m2) |
||||||
|---|---|---|---|---|---|---|---|
| Underweight | Lower normal weight | Normal weight | Upper normal weight | Lower overweight | Upper overweight | Obese | |
| (<18.5) | (18.5–20.0) | (20.1–22.4) | (22.5–24.9) | (25.0–27.4) | (27.5–29.9) | (≥30) | |
| Baseline anthropometric characteristics: means (SD) | |||||||
| BMI (kg/m2) | 17.7 (0.69) | 19.3 (0.42) | 21.2 (0.71) | 23.5 (0.69) | 26.0 (0.73) | 28.6 (0.67) | 32.6 (2.66) |
| DBP (mmHg) | 68 (9.3) | 68.4 (9.4) | 69 (9.6) | 69 (9.8) | 71 (10.1) | 72 (10.4) | 74 (10.8) |
| SBP (mmHg) | 125 (10.8) | 127 (10.8) | 128 (10.9) | 130 (10.9) | 132 (10.9) | 134 (11.4) | 137 (11.3) |
| Elbow flexion strength (N) | 308 (59) | 346 (65) | 383 (74) | 418 (83) | 436 (90) | 447 (94) | 457 (99) |
| Hand grip strength (N) | 551 (86) | 590 (88) | 623 (93) | 650 (100) | 656 (105) | 657 (110) | 660 (112) |
| Knee extension strength (N) | 455 (87) | 505 (92) | 552 (102) | 595 (113) | 613 (120) | 627 (127) | 641 (134) |
| Socioeconomic variables, % | |||||||
| Education | |||||||
| Higher education | 9.4 | 21.3 | 44.7 | 18.6 | 4.3 | 1.1 | 0.6 |
| Secondary education | 10.1 | 20.6 | 40.6 | 19.1 | 6.2 | 2.0 | 1.4 |
| Basic education | 10.8 | 20.8 | 38.5 | 18.7 | 7.0 | 2.4 | 1.8 |
| Less than basic education | 11.1 | 20.3 | 38.4 | 18.7 | 7.2 | 2.5 | 1.8 |
| Missing | 9.2 | 20.7 | 41.9 | 18.7 | 6.0 | 2.0 | 1.5 |
| Social position, % | |||||||
| Higher-level non-manuals | 10.4 | 22.5 | 44.8 | 17.2 | 3.7 | 0.9 | 0.5 |
| Middle-level non-manuals | 9.8 | 21.2 | 43.3 | 18.9 | 4.8 | 1.3 | 0.7 |
| Lower-level non-manuals | 10.5 | 21.1 | 41.7 | 18.6 | 5.4 | 1.6 | 1.1 |
| Farmers | 8.8 | 19.9 | 41.5 | 19.9 | 6.5 | 2.1 | 1.3 |
| Skilled workers | 9.5 | 20.0 | 40.5 | 19.7 | 6.6 | 2.2 | 1.5 |
| Unskilled workers | 10.3 | 20.4 | 38.7 | 18.8 | 7.1 | 2.5 | 2.0 |
| No previous occupation | 10.6 | 21.2 | 41.8 | 18.2 | 5.0 | 1.8 | 1.4 |
| Incidence rates per 10 000 person-years (number of cases) | |||||||
| Mortality | 14.71 (3691) | 13.16 (6810) | 12.6 (12,789) | 13.2 (6031) | 15.3 (2120) | 20.0 (873) | 25.5 (753) |
| CHD | 7.05 (1760) | 7.01 (3613) | 7.30 (7402) | 9.08 (4123) | 12.23 (1683) | 16.38 (708) | 18.99 (554) |
| Stroke | 5.72 (1430) | 5.34 (2753) | 5.18 (5255) | 5.48 (2494) | 7.03 (970) | 8.08 (351) | 11.08 (325) |
| Cancer | 11.19 (2784) | 11.12 (5707) | 11.12 (11,236) | 11.22 (5078) | 11.98 (1647) | 12.87 (557) | 12.16 (356) |
| Mean age at baseline (years) | 18.4 | 18.4 | 18.4 | 18.5 | 18.5 | 18.5 | 18.5 |
| Median follow-up time (years) | 35.2 | 34.8 | 34.1 | 33.6 | 33.2 | 33.0 | 31.9 |
| Million person-years | 2.51 | 5.17 | 10.18 | 4.57 | 1.39 | 0.44 | 0.30 |
| Number of participants | 73 325 | 15 2954 | 30 4873 | 138 382 | 42313 | 13 404 | 9187 |
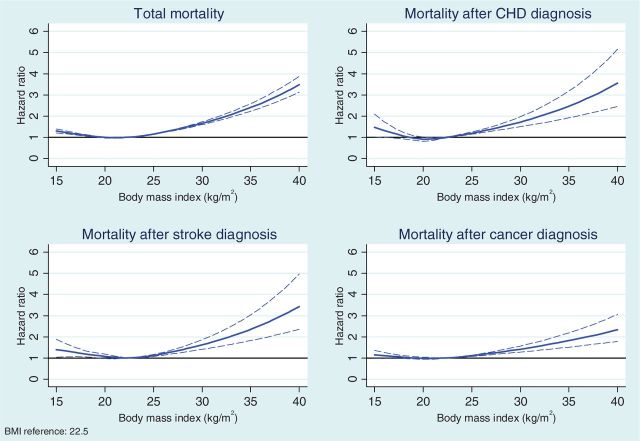
Table 2 presents HRs by BMI categories for mortality after conscription and after diagnoses of CHD, stroke and cancer. In Model 1 adjusted only for age at the time of conscription or diagnosis and for conscription office, total mortality and mortality after diagnoses of CHD and cancer increased monotonically up to the obese category when compared with the normal weight category. For mortality after stroke, the increase occurred after the upper normal weight category. HRs in the overweight categories were substantially higher as compared with the normal weight categories and they were highest in the obese category. In the underweight category, the mortality was higher than in the normal weight category for all mortality outcomes, but the HR was statistically significant only for total mortality, and the HR for mortality was at approximately at the same level as seen in the upper normal weight category. The same J-shaped association was confirmed when we estimated the shape of these association using splines (Figure 1).
Table 2.
Hazard ratios (HR) with 95% confidence intervals (CI) of total mortality and mortality after diagnosis of coronary heart disease, stroke and cancer
| Weight (BMI) |
Model 1 |
Model 2 |
Model 3 |
Model 4 |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Total mortality | ||||||||
| Underweight (<18.5) | 1.11 | 1.07–1.15 | 1.05 | 1.01–1.09 | 1.05 | 1.01–1.09 | 1.03 | 0.99–1.08 |
| Lower normal weight (18.5–20.0) | 1.02 | 0.99–1.05 | 1.00 | 0.97–1.03 | 1.00 | 0.97–1.03 | 0.97 | 0.94–1.00 |
| Normal weight (20.1–22.4) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Upper normal weight (22.5–24.9) | 1.08 | 1.05–1.12 | 1.10 | 1.07–1.14 | 1.10 | 1.06–1.13 | 1.09 | 1.05–1.12 |
| Lower overweight (25.0–27.4) | 1.26 | 1.21–1.32 | 1.29 | 1.23–1.35 | 1.27 | 1.21–1.33 | 1.19 | 1.13–1.25 |
| Upper overweight (27.5–29.9) | 1.66 | 1.55–1.78 | 1.70 | 1.58–1.82 | 1.66 | 1.54–1.78 | 1.51 | 1.40–1.62 |
| Obese (≥30) | 2.17 | 2.02–2.34 | 2.22 | 2.06–2.40 | 2.14 | 1.98–2.30 | 1.91 | 1.77–2.07 |
| Mortality after coronary heart disease | ||||||||
| Underweight (<18.5) | 1.19 | 0.97–1.47 | 1.13 | 0.91–1.41 | 1.14 | 0.92–1.42 | 1.12 | 0.90–1.39 |
| Lower normal weight (18.5–20.0) | 0.99 | 0.83–1.18 | 0.96 | 0.81–1.15 | 0.97 | 0.82–1.16 | 0.98 | 0.82–1.17 |
| Normal weight (20.1–22.4) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Upper normal weight (22.5–24.9) | 1.12 | 0.95–1.31 | 1.14 | 0.97–1.34 | 1.14 | 0.97–1.34 | 1.12 | 0.95–1.31 |
| Lower overweight (25.0–27.4) | 1.33 | 1.09–1.63 | 1.37 | 1.11–1.68 | 1.34 | 1.09–1.65 | 1.27 | 1.04–1.57 |
| Upper overweight (27.5–29.9) | 1.96 | 1.53–2.51 | 2.02 | 1.57–2.60 | 1.97 | 1.52–2.54 | 1.85 | 1.43–2.38 |
| Obese (≥30) | 2.35 | 1.81–3.05 | 2.43 | 1.86–3.18 | 2.29 | 1.74–3.01 | 2.08 | 1.58–2.74 |
| Mortality after stroke | ||||||||
| Underweight (<18.5) | 1.12 | 0.93–1.35 | 1.08 | 0.89–1.31 | 1.08 | 0.89–1.31 | 1.01 | 0.83–1.23 |
| Lower normal weight (18.5–20.0) | 1.15 | 1.00–1.34 | 1.13 | 0.98–1.32 | 1.14 | 0.98–1.32 | 1.12 | 0.96–1.31 |
| Normal weight (20.1–22.4) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Upper normal weight (22.5–24.9) | 0.98 | 0.83–1.15 | 0.99 | 0.84–1.17 | 0.99 | 0.84–1.16 | 0.97 | 0.82–1.14 |
| Lower overweight (25.0–27.4) | 1.17 | 0.94–1.45 | 1.18 | 0.95–1.47 | 1.17 | 0.94–1.46 | 1.10 | 0.88–1.37 |
| Upper overweight (27.5–29.9) | 1.67 | 1.23–2.25 | 1.71 | 1.27–2.32 | 1.68 | 1.24–2.29 | 1.61 | 1.18–2.19 |
| Obese (≥30) | 2.08 | 1.56–2.77 | 2.14 | 1.59–2.87 | 2.07 | 1.53–2.79 | 1.83 | 1.35–2.47 |
| Mortality after cancer | ||||||||
| Underweight (<18.5) | 1.05 | 0.96–1.14 | 1.01 | 0.92–1.10 | 1.01 | 0.92–1.11 | 0.98 | 0.90–1.08 |
| Lower normal weight (18.5–20.0) | 1.02 | 0.95–1.09 | 1.00 | 0.94–1.07 | 1.00 | 0.94–1.07 | 0.98 | 0.92–1.06 |
| Normal weight (20.1–22.4) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Upper normal weight (22.5–24.9) | 1.07 | 0.99–1.14 | 1.08 | 1.00–1.16 | 1.08 | 1.00–1.16 | 1.05 | 0.98–1.13 |
| Lower overweight (25.0–27.4) | 1.13 | 1.01–1.25 | 1.14 | 1.03–1.27 | 1.15 | 1.03–1.28 | 1.09 | 0.98–1.22 |
| Upper overweight (27.5–29.9) | 1.39 | 1.19–1.63 | 1.42 | 1.21–1.66 | 1.42 | 1.21–1.66 | 1.26 | 1.07–1.49 |
| Obese (≥30) | 1.68 | 1.40–2.01 | 1.71 | 1.42–2.06 | 1.71 | 1.42–2.06 | 1.54 | 1.27–1.86 |
Model 1, age at baseline, conscription office, and birth cohort; Model 2, Model 1 + elbow flexion, hand grip and knee extension strength; Model 3, Model 2 + systolic blood pressure and diastolic blood pressure; Model 4, Model 3 + own education and occupation-based socio-economic position.
Figure 1.
Association between BMI and mortality in the total cohort and after the diagnosis of coronary heart disease, stroke and cancer; 95% confidence intervals are shown with dotted lines. The results are adjusted for age at baseline, conscription office and birth cohort.
We then adjusted the model for possible confounding factors (Table 2). Adjusting the results for muscle strength (Model 2), with further adjustment for DBP and SBP (Model 3) had only little effect on the associations between the BMI categories and mortality. Further adjustment of education and social position somewhat decreased the differences in mortality between BMI categories. However, the monotonically increasing pattern of mortality was still found after the normal weight (total mortality and mortality after the diagnosis of CHD or cancer) or upper normal weight category (mortality after the diagnosis of stroke), and most of the HR differences remained statistically significant.
Discussion
Based on our results, not only obesity but also overweight in early adulthood increases mortality after the diagnoses of CHD, stroke and cancer. Muscle strength used as a proxy indicator of body composition, SBP and DBP had only little effect on these associations in our cohort. Socioeconomic factors somewhat explained the differences in mortality between the BMI categories, but overweight and obesity were still found to be associated with higher mortality even after an adjustment for education and social position. These results are different from, but not necessarily in contrast to, the previous findings showing lower survival among overweight patients with cardiovascular diseases as compared with normal weight patients.14–17 In these previous studies, BMI was measured at the time of the cardiovascular diagnoses, whereas in our data it was measured decades before the diagnoses in a population mainly free from all diseases potentially affecting BMI. Thus, a likely reason for these inconsistent results is that high BMI is a risk factor for survival by itself, but reverse causality due to disease-related weight loss may reverse this association when BMI is measured close to the time of the cardiovascular event.
In addition to CHD and stroke diagnoses, we found an association between increased mortality and overweight or obesity after a cancer diagnosis. The results on cancer are consistent with a previous study which showed that obese patients had an increased risk of mortality after a diagnosis of colorectal cancer as compared with normal weight patients when using BMI measured 7 years before the diagnosis.23 In our cohort, overweight and obesity were associated with an increased risk of cancer, but these associations were weaker than the risk of CHD, and the overweight participants had only a slightly higher incidence of cancer than the normal weight participants.
We also found that not only mortality after diagnoses but also mortality in the whole cohort was lowest in the normal weight category. Our results on total mortality are broadly consistent with the results of two large meta-analyses based on pooled individual-based data which showed that the nadir of mortality was within the BMI category of 20.0–25.0 kg/m2 and increased linearly at higher BMI levels.8,9 These results, on the other hand, contrast with the meta-analysis based on estimates extracted from published papers which showed that the lowest mortality was seen in the overweight category (BMI 25.0–29.9 kg/m2).6 In addition to underlying diseases resulting in weight loss, another possible explanation is the effect of smoking. Even though the increased risk of mortality in the overweight category as compared with the normal weight category was found in all participants in the two individual-based meta-analyses, the difference was stronger in non-smokers.8,9 We had access to information on smoking for a large sub-cohort of men. In our previous study using this sub-cohort, we did not find any interaction effect between smoking and BMI when predicting all-cause mortality.24 This result was expected because the cohort was young at baseline, and thus the participants who were smokers had not been smoking for very long before the measurement of BMI. Therefore the role of smoking in this young cohort is very different in comparison with that in middle-age cohorts where long-time smoking may have decreased BMI or BMI may have increased after the quitting of smoking, thus affecting the association between BMI and mortality.
The mean BMI in our cohort was low, reflecting the young age of the participants at baseline. BMI has been found to increase in a monotonic fashion from young adulthood to middle age.25 A large part of this weight gain is due to the accumulation of fat mass, which is stored in men particularly in the abdominal cavity.26 Thus, our study participants are likely to have been heavier at the time of disease onset but may have subsequently lost weight because of the effect of illness. These results indicate that even relatively minor overweight in young adulthood is a risk factor for further mortality, probably because many of these men are likely to become obese in middle age.
In addition to the early age at baseline, our study differed from previous research because the participants were relatively young at the end of follow-up in spite of the long duration of follow-up. Therefore, we cannot rule out the possibility that many of the men who were in the normal BMI range in young adulthood might have become moderately obese in mid -life and experienced increased mortality in that time of life. By contrast, previous research suggests that high BMI in old age is associated with better health outcomes, due to higher muscle mass rather than fat mass.27 Thus more detailed anthropometrical measures enabling fat mass to be differentiated from muscle mass, in addition to a longer follow-up time, would be needed to analyse this issue correctly.
Our data have strengths, but also limitations. Our main strength is that we have BMI measured in early adulthood and thus minimally affected by underlying diseases which may cause reverse causality. Since the conscription examination was mandatory under Swedish law in our study cohorts, our baseline data are not prone to self-selection, and because of the universal healthcare system in Sweden, our follow-up data on hospitalization, cancer diagnoses and mortality also cover the whole Swedish population. Further, we have measures of muscle strength and blood pressure at baseline and also register-based information on education and occupational socioeconomic position used as covariates in this study. A limitation of our data is that the study participants were relatively young at the end of the follow-up; therefore, our results cannot be generalized to old age. Moreover, we do not have repeated measures of BMI after young adulthood and thus cannot study how different trajectories of weight change are associated with survival. Most men increase in weight during ageing, but those with diseases may lose weight. Further, our data include only men, and thus further studies are needed to analyse whether similar results can be obtained for women.
In conclusion, our results show that overweight and obesity in early adulthood are risk factors for all-cause mortality and decrease the chances of survival after the diagnosis of CHD, stroke and cancer in later life. Overweight in young adulthood thus remains an important public health problem in terms of all-cause mortality in the general population as well as survival after CHD, stroke and cancer.
Funding
This work was supported by the Academy of Finland (grant number 266592) and the Swedish Council for Health, Working Life and Welfare Social Research (grant number 2010-1828).
Conflict of interest: None declared.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marinou K, Tousoulis D, Antonopoulos AS, Stefanadi E, Stefanadis C. Obesity and cardiovascular disease: from pathophysiology to risk stratification. Int J Cardiol 2010;138:3–8. [DOI] [PubMed] [Google Scholar]
- 3.Strazzullo P, D'Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke 2010;41:e418–26. [DOI] [PubMed] [Google Scholar]
- 4.Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci 2012;1271:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorensen TI, Rissanen A, Korkeila M, Kaprio J. Intention to lose weight, weight changes, and 18-y mortality in overweight individuals without co-morbidities. PLoS Med 2005;2:e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitlock G, Lewington S, Sherliker P, et al. Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010;363:2211–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker JL, Olsen LW, Sørensen TIA. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med 2007;357:2329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SY, Wilkens LR, Murphy SP, Monroe KR, Henderson BE, Kolonel LN. Body mass index and mortality in an ethnically diverse population: the Multiethnic Cohort Study. Eur J Epidemiol 2012;27:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okasha M, McCarron P, McEwen J, Davey Smith G. Body mass index in young adulthood and cancer mortality: a retrospective cohort study. J Epidemiol Community Health 2002;56:780–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjørge T, Engeland A, Tverdal A, Davey Smith G. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol 2008;168:30–37. [DOI] [PubMed] [Google Scholar]
- 14.Carnethon MR, De Chavez PJ, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA 2012;308:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet 2006;368:666–78. [DOI] [PubMed] [Google Scholar]
- 16.Kenchaiah S, Pocock SJ, Wang D, et al. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007;116:627–36. [DOI] [PubMed] [Google Scholar]
- 17.Oreopoulos A, McAlister FA, Kalantar-Zadeh K, et al. The relationship between body mass index, treatment, and mortality in patients with established coronary artery disease: a report from APPROACH. Eur Heart J 2009;30:2584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinberg BA, Cannon CP, Hernandez AF, Pan W, Peterson ED, Fonarow GC. Medical therapies and invasive treatments for coronary artery disease by body mass: the “obesity paradox” in Get With The Guidelines database. Am J Cardiol 2007;100:1331–35. [DOI] [PubMed] [Google Scholar]
- 19.Schenkeveld L, Magro M, Oemrawsingh RM, et al. The influence of optimal medical treatment on the ‘obesity paradox', body mass index and long-term mortality in patients treated with percutaneous coronary intervention: a prospective cohort study. BMJ Open 2012;2:e000535.-2011-000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doehner W, Clark A, Anker SD. The obesity paradox: weighing the benefit. Eur Heart J 2010;31:146–48. [DOI] [PubMed] [Google Scholar]
- 21.WHO Expert Committee. Physical Status: The Use and Interpretation of Anthropometry. Geneva: WHO, 1995. [PubMed] [Google Scholar]
- 22.Silventoinen K, Magnusson PK, Tynelius P, Batty GD, Rasmussen F. Association of body size and muscle strength with incidence of coronary heart disease and cerebrovascular diseases: a population-based cohort study of one million Swedish men. Int J Epidemiol 2009;38:110–18. [DOI] [PubMed] [Google Scholar]
- 23.Campbell PT, Newton CC, Dehal AN, Jacobs EJ, Patel AV, Gapstur SM. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol 2012;30:42–52. [DOI] [PubMed] [Google Scholar]
- 24.Neovius M, Sundstrom J, Rasmussen F. Combined effects of overweight and smoking in late adolescence on subsequent mortality: nationwide cohort study. BMJ 2009;338:b496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hjelmborg JB, Fagnani C, Silventoinen K, et al. Genetic influences on growth traits of BMI: a longitudinal study of adult twins. Obesity (Silver Spring) 2008;16:847–52. [DOI] [PubMed] [Google Scholar]
- 26.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013;93:359–404. [DOI] [PubMed] [Google Scholar]
- 27.Landi F, Zuccala G, Gambassi G, et al. Body mass index and mortality among older people living in the community J Am Geriatr Soc 1999;47:1072–76. [DOI] [PubMed] [Google Scholar]