Abstract
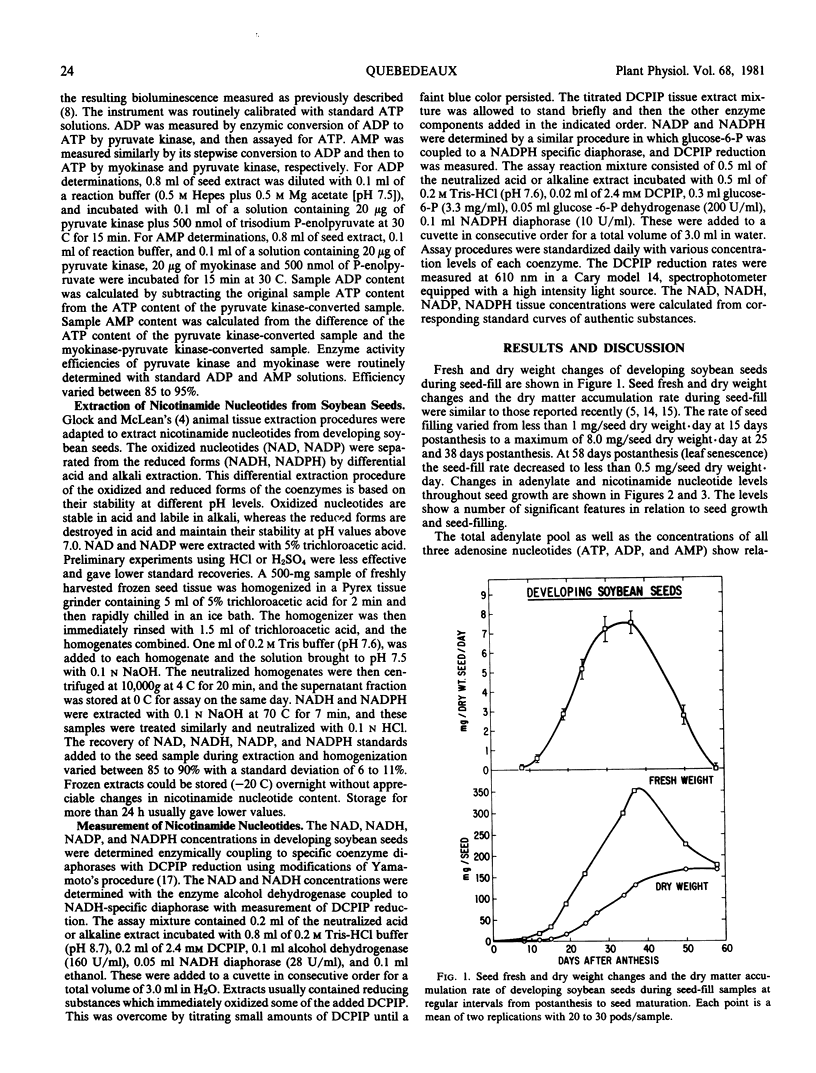
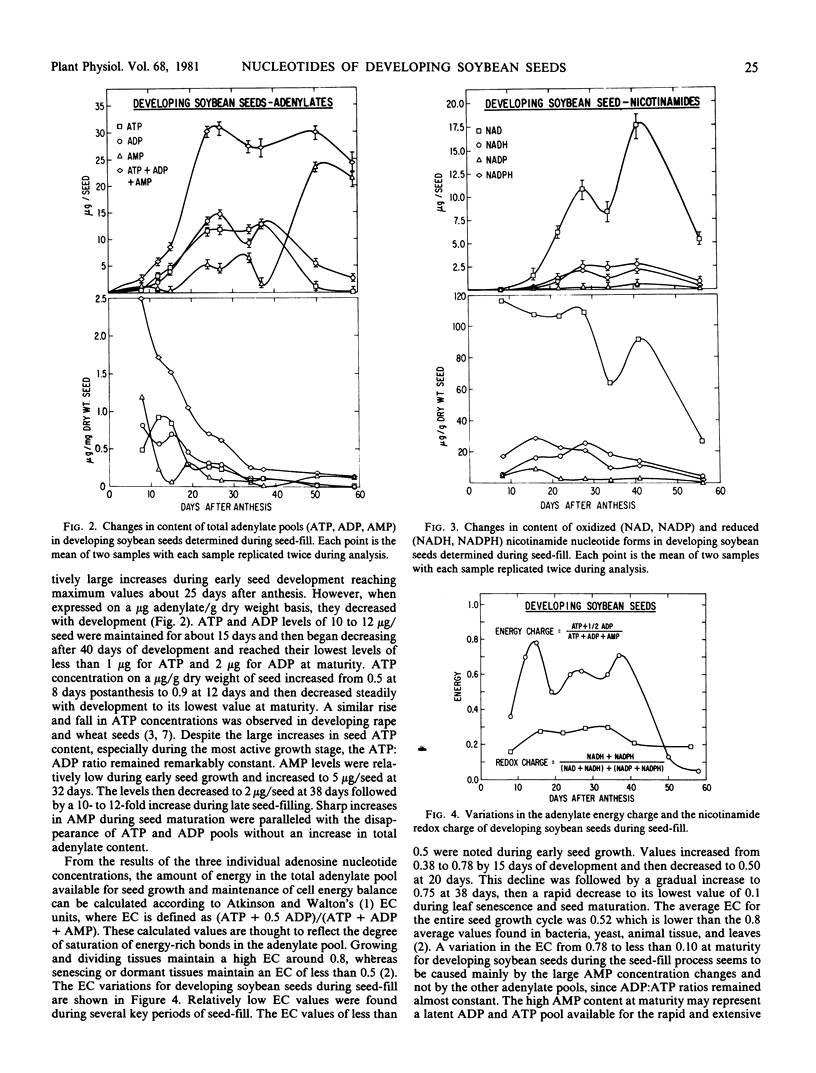
Profiles of adenylate and nicotinamide nucleotides in soybean seeds were determined during seed-fill. The ATP content per seed increased during the early seed-filling stages to a level of 10 to 12 micrograms per seed. Seed ATP decreased after 40 days of development and reached its lowest level of less than 1 microgram at maturity. The ATP:ADP ratios were relatively constant at all seed development stages. Sharp increases in AMP levels during the late seed-fill stages were paralleled with a disappearance of ATP and ADP pools resulting in a reduced seed energy charge. Energy charge varied from the highest value of 0.78 at mid-seed-fill to less than 0.10 at maturity.
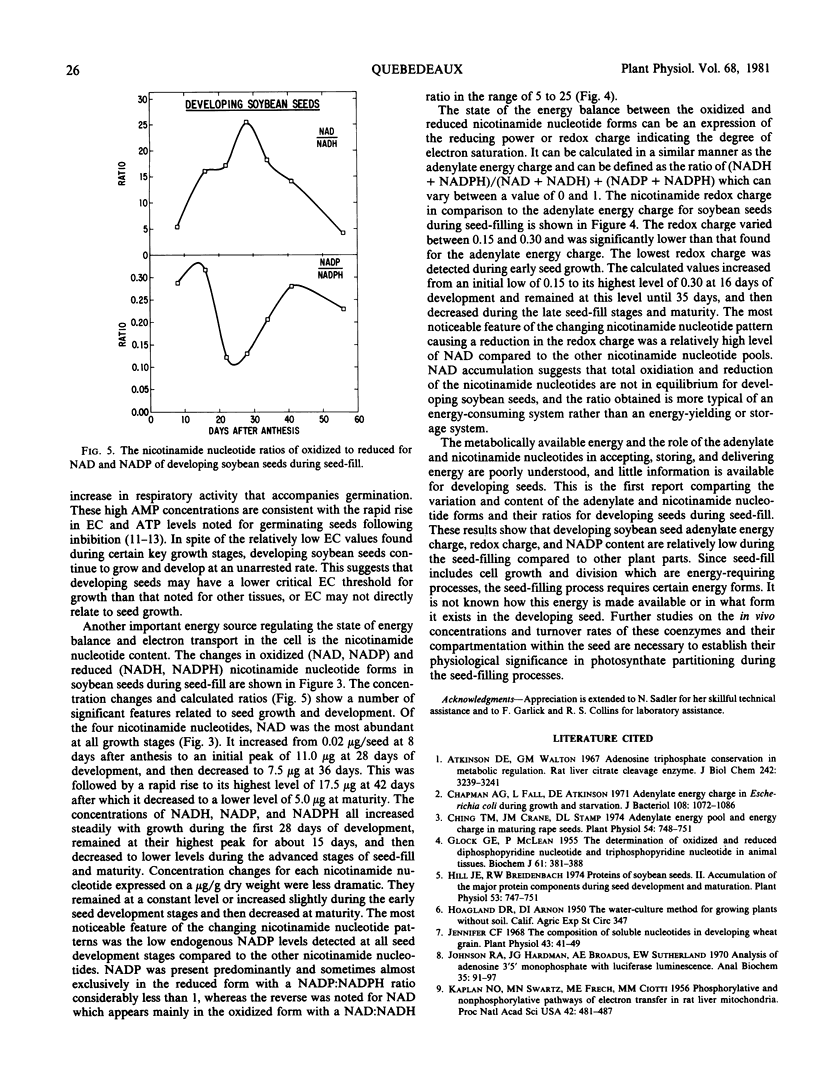
Of the oxidized (NAD, NADP) and reduced (NADH, NADPH) nicotinamide nucleotide forms, NAD was the most abundant. Levels as high as 17.5 micrograms per seed were observed during the mid-seed-filling stages. NADP was found almost exclusively in the reduced form with a NADP: NADPH ratio of less than 0.35, whereas the reverse was noted for NAD which was found mainly in the oxidized form with a NAD:NADH ratio in the range of 5 to 25. NADP was detected in low concentrations compared to the other adenylate and nicotinamide nucleotides. The nicotinamide redox charge defined as (NADH + NADPH)/(NAD + NADH) + (NADP + NADPH) was calculated to express the state of the energy balance between the oxidized and reduced nicotinamide nucleotide forms. The nicotinamide redox charge varied between 0.15 and 0.30 during seed development and was significantly lower than that found for the adenylate energy charge.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E., Walton G. M. Adenosine triphosphate conservation in metabolic regulation. Rat liver citrate cleavage enzyme. J Biol Chem. 1967 Jul 10;242(13):3239–3241. [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M., Crane J. M. Adenylate energy pool and energy charge in maturing rape seeds. Plant Physiol. 1974 Nov;54(5):748–751. doi: 10.1104/pp.54.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., MCLEAN P. The determination of oxidized and reduced diphosphopyridine nucleotide and triphosphopyridine nucleotide in animal tissues. Biochem J. 1955 Nov;61(3):381–388. doi: 10.1042/bj0610381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. E., Breidenbach R. W. Proteins of Soybean Seeds: II. Accumulation of the Major Protein Components during Seed Development and Maturation. Plant Physiol. 1974 May;53(5):747–751. doi: 10.1104/pp.53.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner C. F. The composition of soluble nucleotides in the developing wheat grain. Plant Physiol. 1968 Jan;43(1):41–49. doi: 10.1104/pp.43.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Hardman J. G., Broadus A. E., Sutherland E. W. Analysis of adenosine 3',5'-monophosphate with luciferase luminescence. Anal Biochem. 1970 May;35(1):91–97. doi: 10.1016/0003-2697(70)90014-x. [DOI] [PubMed] [Google Scholar]
- Kaplan N. O., Swartz M. N., Frech M. E., Ciotti M. M. PHOSPHORYLATIVE AND NONPHOSPHORYLATIVE PATHWAYS OF ELECTRON TRANSFER IN RAT LIVER MITOCHONDRIA. Proc Natl Acad Sci U S A. 1956 Aug;42(8):481–487. doi: 10.1073/pnas.42.8.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Cohen H. P. Measurement of adenosine triphosphate content of crayfish stretch receptor cell preparations. Anal Biochem. 1968 Sep;24(3):531–540. doi: 10.1016/0003-2697(68)90161-9. [DOI] [PubMed] [Google Scholar]
- Moreland D. E., Hussey G. G., Shriner C. R., Farmer F. S. Adenosine Phosphates in Germinating Radish (Raphanus sativus L.) Seeds. Plant Physiol. 1974 Oct;54(4):560–563. doi: 10.1104/pp.54.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obendorf R. L., Marcus A. Rapid Increase in Adenosine 5'-Triphosphate during Early Wheat Embryo Germination. Plant Physiol. 1974 May;53(5):779–781. doi: 10.1104/pp.53.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quebedeaux B., Chollet R. Growth and Development of Soybean (Glycine max [L.] Merr.) Pods: CO(2) Exchange and Enzyme Studies. Plant Physiol. 1975 Apr;55(4):745–748. doi: 10.1104/pp.55.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quebedeaux B., Sweetser P. B., Rowell J. C. Abscisic Acid Levels in Soybean Reproductive Structures during Development. Plant Physiol. 1976 Sep;58(3):363–366. doi: 10.1104/pp.58.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. NAD Kinase in Higher Plants. Plant Physiol. 1966 Mar;41(3):523–528. doi: 10.1104/pp.41.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. Pyridine Nucleotide Content in the Higher Plant. Effect of Age of Tissue. Plant Physiol. 1963 Jan;38(1):45–54. doi: 10.1104/pp.38.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


